Thermal Resilience of Feeding Kinematics May Contribute to the Spread of Invasive Fishes in Light of Climate Change
Abstract
:1. Introduction
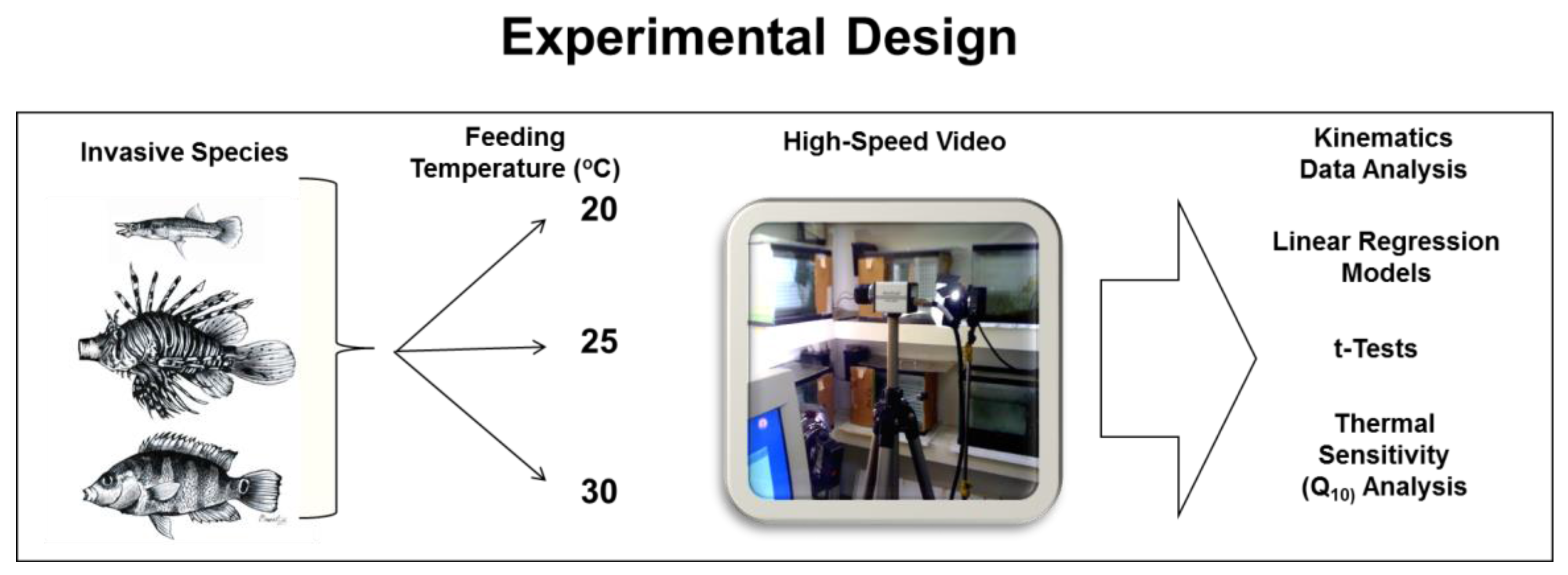
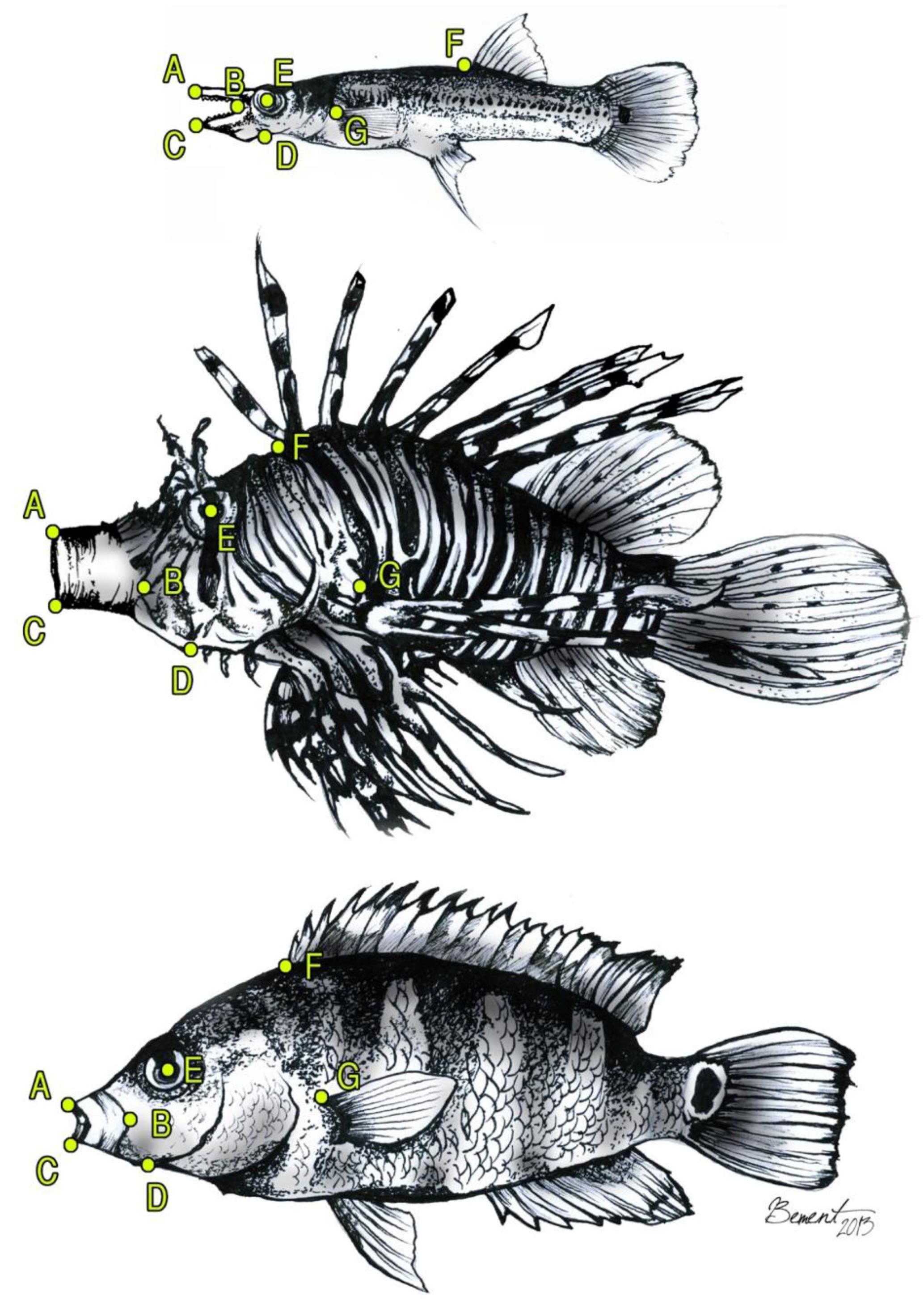
2. Materials and Methods
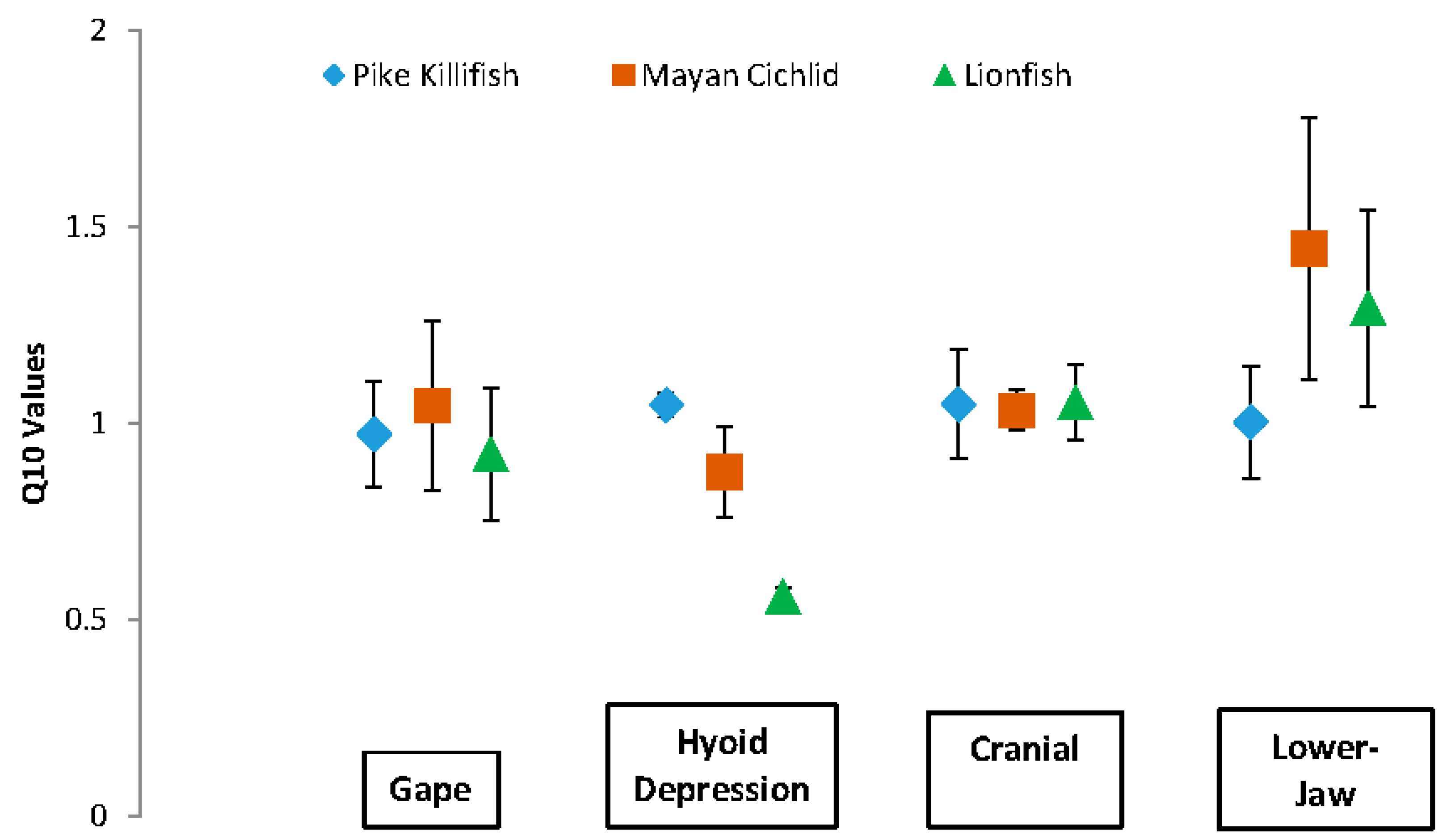
3. Results
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Davis, M.B.; Shaw, R.G. Range shifts and adaptive responses to Quaternary climate change. Science 2001, 292, 673–679. [Google Scholar] [CrossRef] [PubMed]
- Baker, C.; Starger, C.J.; McClanahan, T.R.; Glynn, P.W. Coral reefs: Corals’ adaptive response to climate change. Nature 2004. [Google Scholar] [CrossRef] [PubMed]
- Portner, H.O.; Farrell, A.P. Physiology and climate change. Nature 2008, 322, 690–692. [Google Scholar]
- Portner, H.O. Integrating climate-related stressor effects on marine organisms: Unifying principles linking molecule to ecosystem-level changes. Mar. Ecol. Prog. Ser. 2012, 470, 273–290. [Google Scholar] [CrossRef]
- Huey, R.B.; Kingsolver, J.G. Evolution of resistance to high temperature in ectotherms. Am. Nat. 1993, 142, S21–S46. [Google Scholar] [CrossRef]
- Chown, S.L.; Gaston, K.J.; Robinson, D. Macrophysiology: Large-scale patterns in physiological traits and their ecological implications. Func. Ecol. 2004, 18, 159–167. [Google Scholar] [CrossRef]
- Helmouth, B.; Kingsolver, J.G.; Carrington, E. Biophysics, physiological ecology, and climate change: Does mechanism matter? Ann. Rev. Physiol. 2005, 67, 177–201. [Google Scholar] [CrossRef] [PubMed]
- Deutsch, C.A.; Tewksbury, J.J.; Huey, R.B.; Sheldon, K.S.; Ghalambor, C.K.; Haak, D.C.; Martin, P.R. Impacts of climate warming on terrestrial ectotherms across latitude. Proc. Natl. Acad. Sci. USA 2008, 105, 6668–6672. [Google Scholar] [CrossRef] [PubMed]
- Huey, R.B.; Deutsch, C.A.; Tewksbury, J.J.; Vitt, L.J.; Hertz, P.E.; Perez, J.A.; Garland, T. Why tropical forest lizards are vulnerable to climate warming. Proc. R. Soc. B 2009, 276, 1939–1948. [Google Scholar] [CrossRef] [PubMed]
- Kearney, M.R.; Porter, W.P. Mechanistic niche modeling: Combining physiological and spatial data to predict species ranges. Ecol. Lett. 2009, 12, 334–350. [Google Scholar] [CrossRef] [PubMed]
- Dillion, M.E.; Wang, G.; Huey, R.B. Global metabolic impacts of recent climate warming. Nature 2010, 467, 704–706. [Google Scholar] [CrossRef] [PubMed]
- Doak, D.F.; Morris, W.F. Demographic compensation and tipping points in climate-induced range shifts. Nature 2010, 467, 959–962. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.L.; Hewitt, N.; Klenk, N.; Bazely, D.R.; Yan, N.; Wood, S.; Henriques, I.; MacLellan, J.I.; Lipsig-Mumme, C. Effects of climate change on the distribution of invasive alien species in Canada: A knowledge synthesis of range change projections in a warming world. Environ. Rev. 2012, 20, 1–16. [Google Scholar] [CrossRef]
- Perrings, C.; Dehnen-Shmutz, K.; Touza, J.; Williamson, M. How to manage biological invasions under globalization. Trends Ecol. Evol. 2005, 20, 212–215. [Google Scholar] [CrossRef] [PubMed]
- Hellmann, J.J.; Byers, J.E.; Bierwagen, B.G.; Dukes, J.S. Five potential consequences of climate change for invasive species. Conserv. Biol. 2008, 22, 534–543. [Google Scholar] [CrossRef] [PubMed]
- Shafland, P.L.; Pestrak, J.M. Lower lethal temperatures for fourteen nonnative fishes in Florida. Environ. Biol. Fishes 1982, 7, 149–156. [Google Scholar] [CrossRef]
- Rome, L.C.; Sosnicki, A.A. The influence of temperature on mechanics of red muscle in carp. J. Physiol. 1990, 427, 151–169. [Google Scholar] [CrossRef] [PubMed]
- Wintzer, A.P.; Motta, P.J. The effects of temperature on prey-capture kinematics of the bluegill (Lepomis macrochirus): Implications for feeding studies. Can. J. Zool. 2004, 82, 794–799. [Google Scholar] [CrossRef]
- DeVries, M.S.; Wainwright, P.C. The effects of acute temperature change on prey capture kinematics in largemouth bass, Micropterus salmoides. Copeia 2006, 3, 437–444. [Google Scholar] [CrossRef]
- Cossins, A.R.; Bowler, K. Temperature Biology of Animals; Chapman and Hall (Methuen): New York, NY, USA, 1987. [Google Scholar]
- Clarke, A.; Johnston, N.M. Scaling of metabolic rate with body mass and temperature in teleost fish. J. Anim. Ecol. 1999, 68, 893–905. [Google Scholar] [CrossRef]
- Gillooly, J.F.; Brown, J.H.; West, G.B.; Savage, V.M. Effects of size and temperature on metabolic rate. Science 2001, 293, 2248–2251. [Google Scholar] [CrossRef] [PubMed]
- Josephson, R.K. Contraction dynamics and power output of skeletal muscle. Annu. Rev. Physiol. 1993, 55, 527–546. [Google Scholar] [CrossRef] [PubMed]
- Watabe, S. Temperature plasticity of contractile proteins in fish muscle. J. Exp. Biol. 2002, 205, 2231–2236. [Google Scholar] [PubMed]
- Malek, R.L.; Sajadi, H.; Abraham, J.; Grundy, M.A.; Gerhard, G.S. The effects of temperature reduction on gene expression and oxidative stress in skeletal muscle from adult zebrafish. Comp. Biochem. Physiol. Part C: Toxicol. Pharmacol. 2004, 138, 363–373. [Google Scholar] [CrossRef] [PubMed]
- Johnston, I.A.; Temple, G.K. Thermal plasticity of skeletal muscle phenotype in ectothermic vertebrates and its significance for locomotory behavior. J. Exp. Biol. 2002, 205, 2305–2322. [Google Scholar] [PubMed]
- Crawshaw, L.I.; Ackerman, R.A.; White, F.N.; Heath, M.E. Metabolic and acid-base changes during selection of warmer water by cold-acclimated fish. Am. J. Physiol. 1982, 242, R157–R161. [Google Scholar] [PubMed]
- Lemons, D.E.; Crawshaw, L.I. Behavioral and metabolic adjustments to low temperatures in largemouth bass (Micropterus salmoides). Physiol. Zool. 1985, 58, 175–180. [Google Scholar] [CrossRef]
- Clark, D.S.; Green, J.M. Seasonal variation in temperature preference of juvenile Atlantic cod (Gadus morhua), with evidence supporting an energetic basis for their diel vertical migration. Can. J. Zool. 1991, 69, 1302–1307. [Google Scholar] [CrossRef]
- Rome, L.C.; Swank, D.M.; Coughlin, D.J. The influence of temperature on power production during swimming. II. Mechanics of red muscle fibers in vivo. J. Exp. Biol. 2000, 203, 333–345. [Google Scholar] [PubMed]
- Herbing, I. Effects of temperature on larval fish swimming performance: The importance of physics to physiology. J. Fish Biol. 2002, 61, 865–876. [Google Scholar] [CrossRef]
- Lee, C.G.; Farrell, A.P.; Lotto, A.; MacNutt, M.J.; Hinch, S.G.; Healey, M.C. The effect of temperature on swimming performance and oxygen consumption in adult sockeye (Oncorhynchus nerka) and coho (O. kisutch) salmon stocks. J. Exp. Biol. 2003, 206, 3239–3251. [Google Scholar] [CrossRef] [PubMed]
- Green, B.S.; Fisher, R. Temperature influences swimming speed, growth and larval duration in coral reef fish larvae. J. Exp. Mar. Biol. Ecol. 2004, 299, 115–132. [Google Scholar] [CrossRef]
- Sloan, T.J.; Turingan, R.G. Invariant feeding kinematics of two trophically distinct invasive Florida fishes, Belonesox belizanus and Cichlasoma urophthalmus across environmental temperature regimes. Int. J. Biol. 2012, 4, 117–126. [Google Scholar] [CrossRef]
- Turingan, R.G.; Sloan, T.J. Modeling the relationship between environmental temperature and feeding performance in Florida (USA) nonnative fishes, with implications for invasive-species response to climate change. Annu. Rev. Res. Biol. 2014, 4, 121–132. [Google Scholar] [CrossRef]
- Belshe, J.F. Observations of an Introduced Tropical Fish (Belonesox belizanus) in Southern Florida; University of Miami: Coral Gables, FL, USA, 1961. [Google Scholar]
- Miley, W.W. Ecological Impact of the Pike Killifish, Belonesox belizanus, Kner, (Poeciliidae) in Southern Florida. Master Thesis, Florida Atlantic University, Boca Raton, FL, USA, 1978. [Google Scholar]
- Anderson, R. Geographic variation and aspects of the life history of Belonesox belizanus Kner (Pisces: Poeciliidae) from Central America. Master Thesis, University of Central Florida, Orlando, FL, USA, 1980. [Google Scholar]
- Kerfoot, J.R.; Lorenz, J.J.; Turingan, R.G. Environmental correlates of the abundance and distribution of Belonesox belizanus in a novel environment. Environ. Biol. Fish 2011, 92, 125–139. [Google Scholar] [CrossRef]
- Florida Wildlife Commission. Available online: http://myfwc.com/ (accessed on 15 December 2012).
- National Oceanic and Atmospheric Administration. National Oceanographic Data Center. Available online: http://www.nodc.noaa.gov/dsdt/cwtg/all.html (accessed on 15 December 2012).
- Hubbs, C. Fishes of the Yucatan Peninsula. Carnegie Inst. Wash. Publ. 1936, 457, 157–287. [Google Scholar]
- Rosen, D.E.; Bailey, R.M. The poeciliid fishes (Cyprinodontiformes): Their structure, zoogeography, and systematics. Bull. Am. Mus. Nat. Hist. 1963, 126, 1–146. [Google Scholar]
- Greven, H.; Brenner, M. Further notes on dentition and prey capture of the Pike killifish Belonesox belizanus (Poeciliidae). Bull. Fish Biol. 2008, 10, 97–103. [Google Scholar]
- Ferry-Graham, L.A.; Hernandez, L.P.; Gibb, A.; Pace, C. Unusual kinematics and jaw morphology associated with piscivory in the poeciliid, Belonesox belizanus. Zoology 2010, 113, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Harms, C.A.; Turingan, R.G. Dietary flexibility despite behavioral stereotypy contributes to successful invasion of the pike killifish, Belonesox belizanus, in Florida, USA. Aquat. Invasions 2012, 7, 547–553. [Google Scholar] [CrossRef]
- FishBase Species Database. Available online: www.FISHBASE.org (accessed on 15 December 2012).
- Whitfield, P.E.; Hare, J.A.; David, A.W.; Harter, S.L.; Munoz, R.C.; Addison, C.M. Abundance estimates of the Indo-Pacific lionfish Pterois volitans/miles complex in the western North Atlantic. Biol. Invasions 2007, 9, 53–64. [Google Scholar] [CrossRef]
- Hamner, R.M.; Freshwater, D.W.; Whitfield, P.E. Mitochondrial cytochrome b analysis reveals two invasive lionfish species with strong founder effects in the western Atlantic. J. Fish Biol. 2007, 71, 214–222. [Google Scholar] [CrossRef]
- Morris, J.A.; Akins, J.L. Feeding ecology of invasive lionfish (Pterois volitans) in the Bahamian archipelago. Environ. Biol. Fishes 2009, 86, 389–398. [Google Scholar] [CrossRef]
- Morris, J.A. The biology and ecology of Indo-Pacific lionfish. Dissertation, North Carolina State University, Raleigh, NC, USA, 2009. [Google Scholar]
- Albins, M.A.; Hixon, M.A. Invasive Indo-Pacific lionfish Pterois volitans reduce recruitment of Atlantic coral-reef fishes. Mar. Ecol. Prog. Ser. 2008, 367, 233–238. [Google Scholar] [CrossRef]
- Pfeiffenberger, J.A. Modulation and Scaling of Prey Capture Kinematics Through Ontogeny in Invasive Indo-Pacific Lionfish, Pterois volitans/miles complex. Ph.D. Thesis, Florida Institute of Technology, Melbourne, FL, USA, 2012. [Google Scholar]
- Miller, R.R. Geographical distribution of Central American freshwater fishes. Copeia 1966, 4, 773–802. [Google Scholar] [CrossRef]
- Martinez-Palacios, C.A.; Ross, L.G.; Rosado-Vallado, M. The effects of salinity on the survival and growth of juvenile Cichlasoma urophthalmus. Aquaculture 1990, 91, 65–75. [Google Scholar] [CrossRef]
- Stauffer, J.R.; Boltz, S.E. Effect of salinity on the temperature preference and tolerance of age-0 Mayan cichlids. Trans. Am. Fish. Soc. 1994, 123, 101–107. [Google Scholar] [CrossRef]
- Schofield, P.J.; Loftus, W.F.; Fontaine, J.A. Salinity effects on behavioural response to hypoxia in the non-native Mayan cichlid Cichlasoma urophthalmus from Florida Everglades wetlands. J. Fish Biol. 2009, 7, 149–156. [Google Scholar]
- Martinez-Palacios, C.A.; Ross, L.G. The feeding ecology of Central American cichlid Cichlasoma urophthalmus (Gunther). J. Fish Biol. 1988, 33, 665–670. [Google Scholar] [CrossRef]
- Bergmann, G.T.; Motta, P.J. Diet and morphology through ontogeny of the nonindigenous Mayan cichlid “Cichlasoma (Nandopsis)” urophthalmus (Gunther 1862) in southern Florida. Environ. Biol. Fishes 2005, 72, 205–211. [Google Scholar] [CrossRef]
- Chavez-Lopez, R.; Peterson, M.S.; Brown-Peterson, N.; Morales-Gomez, A.A.; Franco-Lopez, J. Ecology of the Mayan cichlid, Cichlasoma urophthalmus, in the Alvarado Lagoonal system, Veracruz, Mexico. Gulf Caribb. Res. 2005, 17, 123–131. [Google Scholar] [CrossRef]
- Hellig, C.J.; Kerschbaumer, M.; Sefc, K.M.; Koblmuller, S. Allometric shape change of the lower pharyngeal jaw correlates with a dietary shift to piscivory in a cichlid fish. Naturwissenschaften 2010, 97, 663–672. [Google Scholar] [CrossRef] [PubMed]
- Hulsey, C.D. Function of a key morphological innovation: Fusion of the cichlid pharyngeal jaw. Proc. R. Soc. B 2006, 273, 669–675. [Google Scholar] [CrossRef] [PubMed]
- Schmidt-Nielsen, K. Animal Physiology: Adaptation and Environment; Cambridge University Press: Cambridge, UK, 1997. [Google Scholar]
- Invasive Species and Climate Change. Available online: www.invasivespecies.gov (accessed on 15 December 2012).
- Rahel, F.J.; Olden, J.D. Assessing the effects of climate change on aquatic invasive species. Conserv. Biol. 2008, 22, 521–533. [Google Scholar] [CrossRef] [PubMed]
- Crowder, L.B. Character displacement and habitat shift in a native cisco in Southeastern Lake Michigan: Evidence for competition? Copeia 1984, 4, 878–883. [Google Scholar] [CrossRef]
- Douglas, M.E.; Marsh, P.C.; Minckley, W.L. Indigenous fishes of western North America and the hypothesis of competitive displacement: Medafulgida (Cyprinidae) as a case study. Copeia 1994, 1, 9–19. [Google Scholar] [CrossRef]
- Gozlan, R.E.; St-Hilaire, S.; Feist, S.W.; Martin, P.; Kent, M.L. An emergent infectious disease threatens European fish biodiversity. Nature 2005. [Google Scholar] [CrossRef] [PubMed]
- Ogutu-Ohwayo, R. The decline of the native fishes of lakes Victoria and Kyoga (East Africa) and the impact of introduced species, especially the Nile perch, Lates niloticus, and the Nile tilapia, Oreochromis niloticus. Environ. Biol. Fish 1990, 27, 81–96. [Google Scholar] [CrossRef]
- Mooney, H.A.; Cleland, E.E. The evolutionary impact of invasive species. Proc. Natl. Acad. Sci. USA 2001, 98, 5446–5451. [Google Scholar] [CrossRef] [PubMed]
- Perry, W.L.; Lodge, D.M.; Feder, J.L. Importance of hybridization between indigenous and nonindigenous freshwater species: An overlooked threat to North American biodiversity. Syst. Biol. 2002, 51, 255–275. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Pathak, A.K.; Lakra, W.S. Invasion of an exotic fish—Common carp, Cyprinuscarpio L. in the Ganga River, India and its impacts. Acta Inchthyol. Piscat. 2010, 40, 11–19. [Google Scholar] [CrossRef]
- Sato, M.; Kawaguchi, Y.; Nakajima, J.; Mukai, T.; Shimatani, Y.; Onikura, N. A review of the research on introduced freshwater fishes: New perspectives, the need for research, and management implications. Landsc. Ecol. Eng. 2010, 6, 99–108. [Google Scholar] [CrossRef]
- McKinney, M.L.; Lockwood, J.L. Biotic homogenization: A few winners replacing many losers in the next mass extinction. Trends Ecol. Evol. 1999, 14, 450–453. [Google Scholar] [CrossRef]
- Bacheler, N.M.; Neal, J.W.; Noble, R.L. Diet overlap between native bigmouth sleepers (Gobiomorus dormitory) and introduced predatory fishes in a Puerto Rico reservoir. Ecol. Freshwater Fish 2004, 13, 111–118. [Google Scholar] [CrossRef]
- Rahel, F.J. Biogeographic barriers, connectivity and homogenization of freshwater faunas: It’s a small world after all. Freshwater Biol. 2007, 52, 696–710. [Google Scholar] [CrossRef]
- Weinstein, M.R.; Litt, M.; Kertesz, D.A.; Wyper, P.; Rose, D.; Coulter, M.; McGreer, A.; Facklam, R.; Ostach, C.; Willey, B.M.; et al. Invasive infections due to a fish pathogen, Streptococus iniae. S. iniae study group. N. Engl. J. Med. 1997, 337, 589–594. [Google Scholar] [CrossRef] [PubMed]
- Britton, J.R.; Davies, G.D.; Brazier, M.; Pinder, A.C. A case study on the population ecology of a topmouth gudgeon (Pseudorasbora parva) population in the UK and the implications for native fish communities. Aquat. Conserv. 2006, 17, 749–759. [Google Scholar] [CrossRef]
- Hochachka, P.W.; Somero, G.N. Biochemical Adaptation: Mechanisms and Processes in Physiological Evolution; Oxford University Press: Oxford, UK, 2002. [Google Scholar]
- Adams, S.M.; McLean, R.B.; Parrotta, J.A. Energy partitioning in largemouth bass under conditions of seasonality of seasonally fluctuating prey availability. Trans. Am. Fish. Soc. 1982, 111, 549–558. [Google Scholar] [CrossRef]
- Cochran, P.A.; Adelman, I.R. Seasonal aspects of daily ration and diet of largemouth bass, Micropterus salmoides, with an evaluation of gastric evacuation rates. Environ. Biol. Fishes 1982, 7, 265–275. [Google Scholar] [CrossRef]
- Garcia-Berthou, E.; Moreno-Amich, R. Food of introduced pumpkinseed sunfish: Ontogenetic diet shift and seasonal variation. J. Fish Biol. 2000, 57, 29–40. [Google Scholar] [CrossRef]
- Liem, K.F. Modulatory multiplicity in the functional repertoire of the feeding mechanism in cichlids. I. Piscivores. J. Morphol. 1978, 158, 323–360. [Google Scholar] [CrossRef]
- Liem, K.F. Adaptive significance of intra- and interspecific differences in the feeding repertoires of cichlid fishes. Am. Zool. 1980, 20, 295–314. [Google Scholar] [CrossRef]
- Lauder, G.V. Patterns of evolution in the feeding mechanism of actinopterygian fishes. Am. Zool. 1982, 22, 275–285. [Google Scholar] [CrossRef]
- Wainwright, P.C.; Lauder, G.V. Feeding biology of sunfishes: Patterns of variation in the feeding mechanism. Zool. J. Linn. Soc. 1986, 88, 217–228. [Google Scholar] [CrossRef]
- Turingan, R.G.; Wainwright, P.C. Morphological and functional bases of durophagy in the queen triggerfish, Balistes vetula (Pisces, tetraodontiformes). J. Morphol. 1993, 215, 101–118. [Google Scholar] [CrossRef]
- Ferry-Graham, L.A.; Lauder, G.V. Aquatic prey capture in ray-finned fishes: A century of progress and new directions. J. Morphol. 2001, 248, 99–119. [Google Scholar] [CrossRef] [PubMed]
- Westneat, M.W. Evolution of levers and the linkages in the feeding mechanism of fishes. Integr. Comp. Biol. 2004, 44, 378–389. [Google Scholar] [CrossRef] [PubMed]
- Deban, S.M.; Lappin, A.K. Thermal effects on the dynamics and motor control of ballistic prey capture in toads: Maintaining high performance at low temperature. J. Exp. Biol. 2011, 214, 1333–1346. [Google Scholar] [CrossRef] [PubMed]
- Deban, S.M.; Richardson, J.C. Cold-blooded snipers: Thermal independence of ballistic tongue projection in the salamander Hydromantes platycephalus. J. Exp. Zool. 2011, 315, 618–630. [Google Scholar] [CrossRef] [PubMed]
- Navas, C.A.; James, R.S.; Wakeling, J.M.; Kemp, K.M.; Johnston, I.A. An integrative study of the temperature dependence of whole animal and muscle performance during jumping and swimming in the frog Rana temporaria. J. Comp. Physiol. B 1999, 169, 588–596. [Google Scholar] [CrossRef] [PubMed]
- Christian, K.A.; Tracy, C.R. The effect of thermal environment on the ability of hatchling Galapagos land iguanas to avoid predation during dispersal. Oecologia 1981, 49, 218–223. [Google Scholar] [CrossRef]
- Bennett, A.F. Thermal dependence of muscle function. Am. J. Physiol. 1984, 247, R217–R229. [Google Scholar] [PubMed]
- Marsh, R.L.; Bennet, A.F. Thermal dependence of contractile properties of skeletal muscle from the lizard, Sceleporus occidentalis with comments on the methods for fitting and comparing force-velocity curves. J. Exp. Biol. 1986, 126, 63–77. [Google Scholar] [PubMed]
- Sandusky, E.P.; Deban, S.M. Temperature effects on the biomechanics of prey capture in the frog Rana pipiens. J. Exp. Zool. 2012, 317A, 595–607. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, M.H.; Farley, C.T.; Full, R.J.; Koehl, M.A.R.; Kram, R.; Lehman, S. How animals move: An integrative view. Science 2000, 288, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Grimby, L.; Hannerz, J.; Hedman, B. The fatigue and voluntary discharge properties of single motor units in man. J. Physiol. 1981, 316, 545–554. [Google Scholar] [CrossRef] [PubMed]
- Aagaard, P.; Simonson, E.B.; Andersen, J.L.; Magnusson, P.; Dyhre-Poulsen, P. Increased rate of force development and neural drive of human skeletal muscle following resistance training. J. Appl. Physiol. 2002, 93, 1318–1326. [Google Scholar] [CrossRef] [PubMed]
- Johnston, I.A.; Davison, W.; Goldspink, G. Energy metabolism of carp swimming muscles. J. Comp. Physiol. 1997, 114, 203–216. [Google Scholar] [CrossRef]
- Rome, L.C.; Loughna, P.T.; Golspink, G. Muscle fibre recruitment as a function of swim speed and muscle temperature in carp. Am. J. Physiol. 1984, 247, R272–R279. [Google Scholar] [PubMed]
- Rome, L.C. Influence of temperature on muscle recruitment and muscle function in vivo. Am. J. Physiol. 1990, 259, R210–R222. [Google Scholar] [PubMed]
- Peplowski, M.M.; Marsh, R.L. Work and power output in the hindlimb muscles of Cuban tree frogs Oesteopilu septentrionalis during jumping. J. Exp. Biol. 1997, 200, 2861–2870. [Google Scholar] [PubMed]
- Bojsen-Moller, J.; Magnusson, S.P.; Rasmussen, L.R.; Kjaer, M.; Aagaard, P. Muscle performance during maximal isometric and dynamic contractions is influenced by the stiffness of the tendinous structures. J. Appl. Physiol. 2005, 99, 986–994. [Google Scholar] [CrossRef] [PubMed]
- Konow, N.; Azizi, E.; Roberts, T.J. Muscle power attenuation by tendon during energy dissipation. Proc. R. Soc. B 2012, 279, 1108–1113. [Google Scholar] [CrossRef] [PubMed]
- Boutilier, R.G.; Glass, M.L.; Heisler, N. Blood gases, and extracellular/intracellular acid-base status as a function of temperature in the anuran amphibian Xenopus laevis and Bufo marinus. J. Exp. Biol. 1987, 130, 13–25. [Google Scholar]
- Renaud, J.M.; Stevens, E.D. The extent of short-term and long-term compensation to temperature shown by frog and toad Sartorius muscle. J. Exp. Biol. 1984, 108, 57–75. [Google Scholar]
- Vogel, S. Life in Moving Fluids, the Physical Biology of Flow, 2nd ed.; Princeton University Press: Princeton, NJ, USA, 1994; p. 467. [Google Scholar]


| Kinematics | Species | a | b | r2 | p |
|---|---|---|---|---|---|
| Gape | P | 0.245 | 0.0343 | 0.0734 | 0.163 |
| L | 1.797 | −0.0357 | 0.0859 | 0.130 | |
| M | 1.821 | −0.0376 | 0.0893 | 0.122 | |
| Hyoid | P | 0.393 | −1.14 × 10−3 | 4.53 × 10−4 | 0.914 |
| Depression | L | 0.738 | −0.0162 | 0.0853 | 0.131 |
| M | 0.980 | −0.0267 | 0.227 | 0.010 | |
| Cranial | P | 11.385 | −0.243 | 0.0419 | 0.296 |
| Rotation | L | 8.808 | −0.107 | 9.38 × 10−3 | 0.624 |
| M | −1.817 | 0.368 | 0.101 | 0.099 | |
| Lower-Jaw | P | 13.365 | −0.179 | 0.0286 | 0.390 |
| Rotation | L | −3.568 | 0.610 | 0.233 | 0.009 |
| M | 8.089 | 0.0664 | 3.61 × 10−3 | 0.761 |
| Kinematics | Species | Mean Q10 | t-Statistic | Df | p |
|---|---|---|---|---|---|
| Gape | P | 0.973 | −7.6174 | 3 | 0.005 |
| L | 0.922 | −6.4448 | 3 | 0.008 | |
| M | 1.046 | −4.3859 | 3 | 0.022 | |
| Hyoid | P | 1.047 | −30.0273 | 3 | 8.113 × 10−5 |
| L | 0.559 | −67.1036 | 3 | 7.239 × 10−6 | |
| M | 0.876 | −9.8617 | 3 | 0.002 | |
| Cranial | P | 1.049 | −6.8275 | 3 | 0.006 |
| Rotation | L | 1.054 | −9.7247 | 3 | 0.002 |
| M | 1.033 | −19.0769 | 3 | 3.145 × 10−4 | |
| Lower-Jaw | P | 1.004 | −6.9719 | 3 | 0.006 |
| Rotation | L | 1.294 | −2.8106 | 3 | 0.063 |
| M | 1.444 | −1.6617 | 3 | 0.195 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Turingan, R.; Sloan, T. Thermal Resilience of Feeding Kinematics May Contribute to the Spread of Invasive Fishes in Light of Climate Change. Biology 2016, 5, 46. https://doi.org/10.3390/biology5040046
Turingan R, Sloan T. Thermal Resilience of Feeding Kinematics May Contribute to the Spread of Invasive Fishes in Light of Climate Change. Biology. 2016; 5(4):46. https://doi.org/10.3390/biology5040046
Chicago/Turabian StyleTuringan, Ralph, and Tyler Sloan. 2016. "Thermal Resilience of Feeding Kinematics May Contribute to the Spread of Invasive Fishes in Light of Climate Change" Biology 5, no. 4: 46. https://doi.org/10.3390/biology5040046
APA StyleTuringan, R., & Sloan, T. (2016). Thermal Resilience of Feeding Kinematics May Contribute to the Spread of Invasive Fishes in Light of Climate Change. Biology, 5(4), 46. https://doi.org/10.3390/biology5040046





