The Potential of Non-Provitamin A Carotenoids for the Prevention and Treatment of Non-Alcoholic Fatty Liver Disease
Abstract
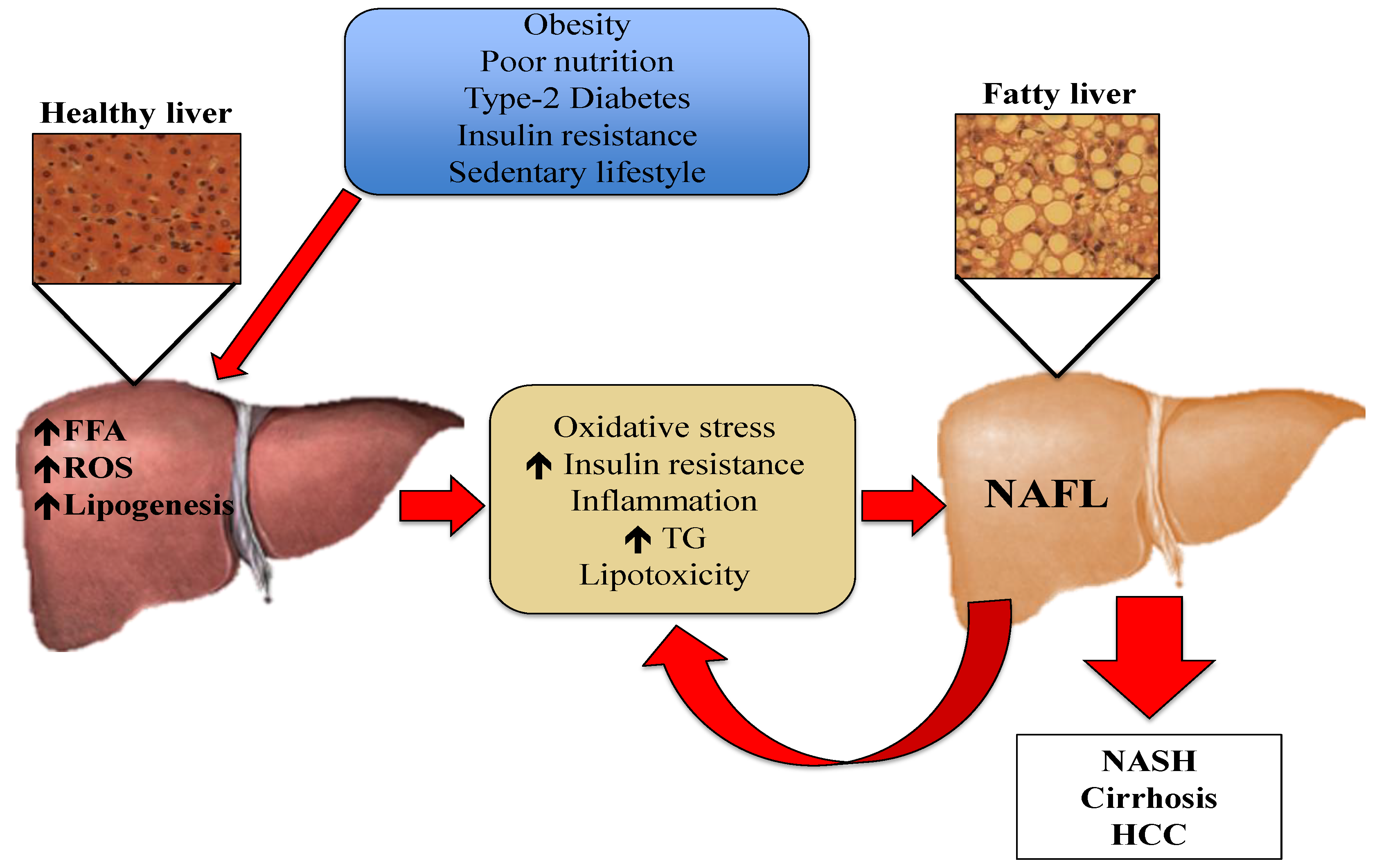
:1. Introduction
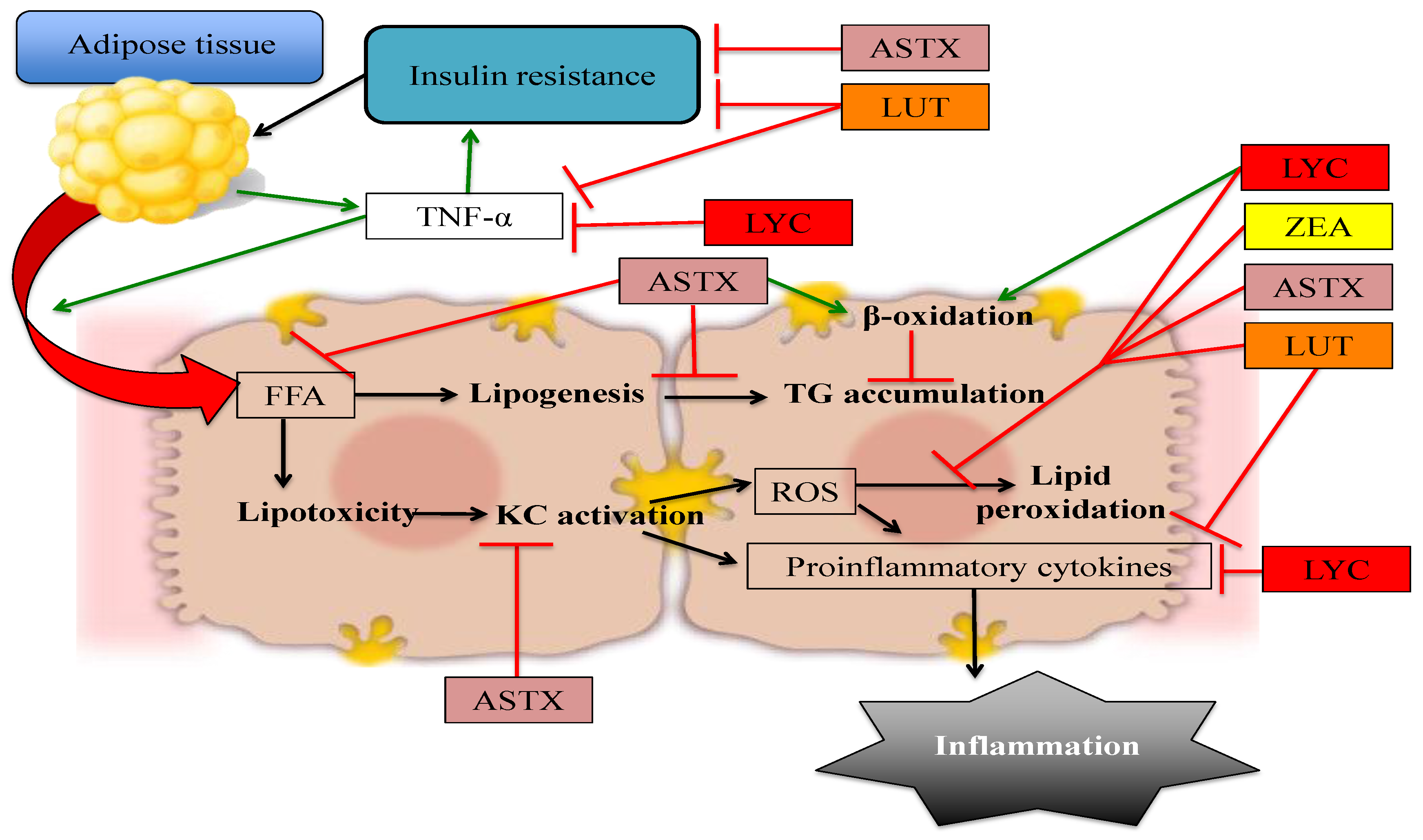
2. Astaxanthin
3. Lutein and Zeaxanthin
4. Lycopene
5. Conclusions
Author Contributions
Conflicts of Interest
References
- Yilmaz, B.; Sahin, K.; Bilen, H.; Bahcecioglu, I.H.; Bilir, B.; Ashraf, S.; Halazun, K.J.; Kucuk, O. Carotenoids and non-alcoholic fatty liver disease. Hepatobiliary Surg. Nutr. 2015, 4, 161–171. [Google Scholar] [PubMed]
- Puri, P.; Baillie, R.A.; Wiest, M.M.; Mirshahi, F.; Choudhury, J.; Cheung, O.; Sargeant, C.; Contos, M.J.; Sanyal, A.J. A lipidomic analysis of nonalcoholic fatty liver disease. Hepatology 2007, 46, 1081–1090. [Google Scholar] [CrossRef] [PubMed]
- Tolman, K.G.; Dalpiaz, A.S. Treatment of non-alcoholic fatty liver disease. Ther. Clin. Risk Manag. 2007, 3, 1153–1163. [Google Scholar] [PubMed]
- Krawczyk, M.; Bonfrate, L.; Portincasa, P. Nonalcoholic fatty liver disease. Best Pract. Res. Clin. Gastroenterol. 2010, 24, 695–708. [Google Scholar] [CrossRef] [PubMed]
- Browning, J.D.; Szczepaniak, L.S.; Dobbins, R.; Nuremberg, P.; Horton, J.D.; Cohen, J.C.; Grundy, S.M.; Hobbs, H.H. Prevalence of hepatic steatosis in an urban population in the United States: Impact of ethnicity. Hepatology 2004, 40, 1387–1395. [Google Scholar] [CrossRef] [PubMed]
- Sayiner, M.; Koenig, A.; Henry, L.; Younossi, Z.M. Epidemiology of Nonalcoholic Fatty Liver Disease and Nonalcoholic Steatohepatitis in the United States and the Rest of the World. Clin. Liver Dis. 2016, 20, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Polimeni, L.; Del Ben, M.; Baratta, F.; Perri, L.; Albanese, F.; Pastori, D.; Violi, F.; Angelico, F. Oxidative stress: New insights on the association of non-alcoholic fatty liver disease and atherosclerosis. World J. Hepatol. 2015, 7, 1325–1336. [Google Scholar] [CrossRef] [PubMed]
- Byrne, C.D.; Targher, G. NAFLD: A multisystem disease. J. Hepatol. 2015, 62, S47–S64. [Google Scholar] [CrossRef] [PubMed]
- Dowman, J.K.; Tomlinson, J.W.; Newsome, P.N. Pathogenesis of non-alcoholic fatty liver disease. QJM 2010, 103, 71–83. [Google Scholar] [CrossRef] [PubMed]
- Masarone, M.; Federico, A.; Abenavoli, L.; Loguercio, C.; Persico, M. Non alcoholic fatty liver: Epidemiology and natural history. Rev. Recent Clin. Trials 2014, 9, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Loomba, R.; Sirlin, C.; Schwimmer, J.B.; Lavine, J. Advances in pediatric nonalcoholic fatty liver disease. Hepatology 2009, 50, 1282–1293. [Google Scholar] [CrossRef] [PubMed]
- Angulo, P. Nonalcoholic Fatty Liver Disease. N. Engl. J. Med. 2014, 346, 1221–1231. [Google Scholar] [CrossRef] [PubMed]
- Sumida, Y.; Niki, E.; Naito, Y.; Yoshikawa, T. Involvement of free radicals and oxidative stress in NAFLD/NASH. Free Radic. Res. 2013, 47, 869–880. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Tan, H.-Y.; Wang, N.; Zhang, Z.-J.; Lao, L.; Wong, C.-W.; Feng, Y. The Role of Oxidative Stress and Antioxidants in Liver Diseases. Int. J. Mol. Sci. 2015, 16, 26087–26124. [Google Scholar] [CrossRef] [PubMed]
- Sies, H. Oxidative Stress: Oxidants and Antioxidants. Exp. Phisiol. 1997, 82, 291–295. [Google Scholar] [CrossRef]
- Cusi, K. Role of obesity and lipotoxicity in the development of nonalcoholic steatohepatitis: Pathophysiology and clinical implications. Gastroenterology 2012. [Google Scholar] [CrossRef] [PubMed]
- Ye, P.; Cheah, I.K.; Halliwell, B. A high-fat and cholesterol diet causes fatty liver in guinea pigs. The role of iron and oxidative damage. Free Radic. Res. 2013, 47, 602–613. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Valle, V.; Chavez-Tapia, N.C.; Uribe, M.; Mendez-Sanchez, N. Role of oxidative stress and molecular changes in liver fibrosis: A review. Curr. Med. Chem. 2012, 19, 4850–4860. [Google Scholar] [CrossRef] [PubMed]
- Karadeniz, G.; Acikgoz, S.; Tekin, I.O.; Tascýlar, O.; Gun, B.D.; Cömert, M. Oxidized low-density-lipoprotein accumulation is associated with liver fibrosis in experimental cholestasis. Clinics (Sao Paulo) 2008, 63, 531–540. [Google Scholar] [CrossRef] [PubMed]
- Salomone, F.; Godos, J.; Zelber-Sagi, S. Natural antioxidants for non-alcoholic fatty liver disease: Molecular targets and clinical perspectives. Liver Int. 2015, 36, 5–20. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Wang, C.; Liu, J.; Liu, Z.; Ling, W.; Chen, Y. Greater serum carotenoid levels associated with lower prevalence of nonalcoholic fatty liver disease in Chinese adults. Sci. Rep. 2015. [Google Scholar] [CrossRef] [PubMed]
- Johnson, E.J. The Role of Carotenoids in Human Health. Nutr. Clin. Care 2002, 5, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Rao, A.V.; Rao, L.G. Carotenoids and human health. Pharmacol. Res. 2007, 55, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Latowski, D.; Kuczyńska, P.; Strzałka, K. Xanthophyll cycle—A mechanism protecting plants against oxidative stress. Redox Rep. 2011, 16, 78–90. [Google Scholar] [CrossRef] [PubMed]
- Stahl, W.; Sies, H. Antioxidant activity of carotenoids. Mol. Aspects Med. 2003, 24, 345–351. [Google Scholar] [CrossRef]
- Stahl, W.; Sies, H. Bioactivity and protective effects of natural carotenoids. Biochim. Biophys. Acta 2005, 1740, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Saini, R.K.; Nile, S.H.; Park, S.W. Carotenoids from fruits and vegetables: Chemistry, analysis, occurrence, bioavailability and biological activities. Food Res. Int. 2015, 76, 735–750. [Google Scholar] [CrossRef]
- Maiani, G.; Castón, M.J.P.; Catasta, G.; Toti, E.; Cambrodón, I.G.; Bysted, A.; Granado-Lorencio, F.; Olmedilla-Alonso, B.; Knuthsen, P.; Valoti, M.; et al. Carotenoids: Actual knowledge on food sources, intakes, stability and bioavailability and their protective role in humans. Mol. Nutr. Food Res. 2009, 53, S194–S218. [Google Scholar] [CrossRef] [PubMed]
- Bohn, T. Bioavailability of Non-Provitamin A Carotenoids. Curr. Nutr. Food Sci. 2008, 4, 240–258. [Google Scholar] [CrossRef]
- Castenmiller, J.J.; West, C.E. Bioavailability and bioconversion of carotenoids. Annu. Rev. Nutr. 1998, 18, 19–38. [Google Scholar] [CrossRef] [PubMed]
- Tang, G. Bioconversion of dietary provitamin A carotenoids to vitamin A in humans. Am. J. Clin. Nutr. 2010, 91, 1468–1473. [Google Scholar] [CrossRef] [PubMed]
- Scott, K.J.; Rodriquez-Amaya, D. Pro-vitamin A carotenoid conversion factors: Retinol equivalents—Fact or fiction? Food Chem. 2000, 69, 125–127. [Google Scholar] [CrossRef]
- Murillo, A.G.; Fernandez, M.L. Potential of Dietary Non-Provitamin A Carotenoids in the Prevention and Treatment of Diabetic Microvascular Complications. Adv. Nutr. 2016, 7, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Brazionis, L.; Rowley, K.; Itsiopoulos, C.; O’Dea, K. Plasma carotenoids and diabetic retinopathy. Br. J. Nutr. 2009, 101, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Ruhl, R. Non-pro-vitamin A and pro-vitamin A carotenoids in atopy development. Int. Arch. Allergy Immunol. 2013, 161, 99–115. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Kim, B.; Lee, J. Astaxanthin structure, metabolism, and health benefits. J. Hum. Nutr. Food Sci. 2013, 1, 1–11. [Google Scholar]
- Hussein, G.; Sankawa, U.; Goto, H.; Matsumoto, K.; Watanabe, H. Astaxanthin, a carotenoid with potential in human health and nutrition. J. Nat. Prod. 2006, 69, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Pham, T.X.; Wegner, C.J.; Kim, B.; Ku, C.S.; Park, Y.-K.; Lee, J.-Y. Astaxanthin lowers plasma TAG concentrations and increases hepatic antioxidant gene expression in diet-induced obesity mice. Br. J. Nutr. 2014, 112, 1797–1804. [Google Scholar] [CrossRef] [PubMed]
- Naguib, Y.M. A Antioxidant activities of astaxanthin and related carotenoids. J. Agric. Food Chem. 2000, 48, 1150–1154. [Google Scholar] [CrossRef] [PubMed]
- Ni, Y.; Nagashimada, M.; Zhuge, F.; Zhan, L.; Nagata, N.; Tsutsui, A.; Nakanuma, Y.; Kaneko, S.; Ota, T. Astaxanthin prevents and reverses diet-induced insulin resistance and steatohepatitis in mice: A comparison with vitamin E. Sci. Rep. 2015. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, S.; Goodspeed, L.; Wang, S.; Kim, J.; Zeng, L.; Ioannou, G.N.; Haigh, W.G.; Yeh, M.M.; Kowdley, K.V.; O’Brien, K.D.; et al. Dietary cholesterol exacerbates hepatic steatosis and inflammation in obese LDL receptor-deficient mice. J. Lipid Res. 2011, 52, 1626–1635. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Wu, C.; Kim, J.; Kim, B.; Lee, S.J. Astaxanthin reduces hepatic lipid accumulations in high-fat-fed C57BL/6J mice via activation of peroxisome proliferator-activated receptor (PPAR) alpha and inhibition of PPAR gamma and Akt. J. Nutr. Biochem. 2016, 28, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Hybertson, B.M.; Gao, B.; Bose, S.K.; McCord, J.M. Oxidative stress in health and disease: The therapeutic potential of Nrf2 activation. Mol. Aspects Med. 2011, 32, 234–246. [Google Scholar] [CrossRef] [PubMed]
- Wynn, T.A.; Chawla, A.; Pollard, J.W. Origins and Hallmarks of Macrophages: Development, Homeostasis, and Disease. Nature 2013, 496, 445–455. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.K.; Mantovani, A. Orchestration of metabolism by macrophages. Cell Metab. 2012, 15, 432–437. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Zhao, Q.; Yang, T.; Ding, W.; Zhao, Y. Cellular metabolism and macrophage functional polarization. Int. Rev. Immunol. 2015, 34, 82–100. [Google Scholar] [CrossRef] [PubMed]
- Ni, Y.; Zhuge, F.; Nagashimada, M.; Ota, T. Novel Action of Carotenoids on Non-Alcoholic Fatty Liver Disease: Macrophage Polarization and Liver Homeostasis. Nutrients 2016. [Google Scholar] [CrossRef] [PubMed]
- Szabo, G.; Petrasek, J. Inflammasome activation and function in liver disease. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 387–400. [Google Scholar] [CrossRef] [PubMed]
- Perry, A.; Rasmussen, H.; Johnson, E.J. Xanthophyll (lutein, zeaxanthin) content in fruits, vegetables and corn and egg products. J. Food Compos. Anal. 2009, 22, 9–15. [Google Scholar] [CrossRef]
- Krinsky, N.I.; Johnson, E.J. Carotenoid actions and their relation to health and disease. Mol. Aspects Med. 2005, 26, 459–516. [Google Scholar] [CrossRef] [PubMed]
- Mozaffarieh, M.; Sacu, S.; Wedrich, A. The role of the carotenoids, lutein and zeaxanthin, in protecting against age-related macular degeneration: A review based on controversial evidence. Nutr. J. 2003. [Google Scholar] [CrossRef] [PubMed]
- Roberts, R.L.; Green, J.; Lewis, B. Lutein and zeaxanthin in eye and skin health. Clin. Dermatol. 2009, 27, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Andersen, C.J. Bioactive egg components and inflammation. Nutrients 2015, 7, 7889–7913. [Google Scholar] [CrossRef] [PubMed]
- Landrum, J.T.; Bone, R. A Lutein, zeaxanthin, and the macular pigment. Arch. Biochem. Biophys. 2001, 385, 28–40. [Google Scholar] [CrossRef] [PubMed]
- Krinsky, N.I.; Landrum, J.T.; Bone, R.A. Biologic mechanisms of the protective role of lutein and zeaxanthin in the eye. Annu. Rev. Nutr. 2003, 23, 171–201. [Google Scholar] [CrossRef] [PubMed]
- Zampatti, S.; Ricci, F.; Cusumano, A.; Marsella, L.T.; Novelli, G.; Giardina, E. Review of nutrient actions on age-related macular degeneration. Nutr. Res. 2014, 34, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Kijlstra, A.; Tian, Y.; Kelly, E.R.; Berendschot, T.T. Lutein: More than just a filter for blue light. Prog. Retin. Eye Res. 2012, 31, 303–315. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.E.; Clark, R.M.; Park, Y.; Lee, J.; Fernandez, M.L. Lutein decreases oxidative stress and inflammation in liver and eyes of guinea pigs fed a hypercholesterolemic diet. Nutr. Res. Pract. 2012, 6, 113–119. [Google Scholar] [CrossRef] [PubMed]
- DeOgburn, R.; Leite, J.O.; Ratliff, J.; Volek, J.S.; McGrane, M.M.; Fernandez, M.L. Effects of increased dietary cholesterol with carbohydrate restriction on hepatic lipid metabolism in guinea pigs. Comp. Med. 2012, 62, 109–115. [Google Scholar] [PubMed]
- Nielsen, F.; Mikkelsen, B.B.; Nielsen, J.B.; Andersen, H.R.; Grandjean, P. Plasma malondialdehyde as biomarker for oxidative stress: Reference interval and effects of life-style factors. Clin. Chem. 1997, 43, 1209–1214. [Google Scholar] [PubMed]
- Murillo, A.G.; Aguilar, D.; Norris, G.H.; Dimarco, D.M.; Missimer, A.; Hu, S.; Smyth, J.A.; Gannon, S.; Blesso, C.N.; Luo, Y.; et al. Compared with Powdered Lutein, a Lutein Nanoemulsion Increases Plasma and Liver Lutein, Protects against Hepatic Steatosis, and Affects Lipoprotein Metabolism in Guinea Pigs. J. Nutr. 2016, 146, 1961–1969. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.; Gao, D.; Xiang, X.; Xiong, Y.; Zhu, T.; Liu, L.; Sun, X.; Hao, L. Ameliorative effects of lutein on non-alcoholic fatty liver disease in rats. World J. Gastroenterol. 2015, 21, 8061–8072. [Google Scholar] [PubMed]
- Chamberlain, S.M.; Hall, J.D.; Patel, J.; Lee, J.R.; Marcus, D.M.; Sridhar, S.; Romero, M.J.; Labazi, M.; Caldwell, R.W.; Bartoli, M. Protective effects of the carotenoid zeaxanthin in experimental nonalcoholic steatohepatitis. Dig. Dis. Sci. 2009, 54, 1460–1464. [Google Scholar] [CrossRef] [PubMed]
- Rao, A.V.; Agarwal, S. Role of lycopene as antioxidant carotenoid in the prevention of chronic diseases: A review. Nutr. Res. 1999, 19, 305–323. [Google Scholar] [CrossRef]
- Agarwal, S.; Rao, A.V. Tomato lycopene and its role in human health and chronic diseases. CMAJ 2000, 163, 739–744. [Google Scholar] [PubMed]
- Heber, D.; Lu, Q.-Y. Overview of mechanisms of action of lycopene. Exp. Biol. Med. (Maywood) 2002, 227, 920–923. [Google Scholar] [PubMed]
- Rao, A.V.; Agarwal, S. Role of antioxidant lycopene in cancer and heart disease. J. Am. Coll. Nutr. 2000, 19, 563–569. [Google Scholar] [CrossRef] [PubMed]
- Di Mascio, P.; Kaiser, S.; Sies, H. Lycopene as the most efficient biological carotenoid singlet oxygen quencher. Arch. Biochem. Biophys. 1989, 274, 532–538. [Google Scholar] [CrossRef]
- Stahl, W.; Ale-Agha, N.; Polidori, M.C. Non-antioxidant properties of carotenoids. Biol. Chem. 2002, 383, 553–558. [Google Scholar] [CrossRef] [PubMed]
- Bahcecioglu, I.H.; Kuzu, N.; Metin, K.; Ozercan, I.H.; Ustündag, B.; Sahin, K.; Kucuk, O. Lycopene prevents development of steatohepatitis in experimental nonalcoholic steatohepatitis model induced by high-fat diet. Vet. Med. Int. 2010. [Google Scholar] [CrossRef] [PubMed]
- Ghouri, N.; Preiss, D.; Sattar, N. Liver enzymes, nonalcoholic fatty liver disease, and incident cardiovascular disease: A narrative review and clinical perspective of prospective data. Hepatology 2010, 52, 1156–1161. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ausman, L.M.; Greenberg, A.S.; Russell, R.M.; Wang, X.D. Dietary lycopene and tomato extract supplementations inhibit nonalcoholic steatohepatitis-promoted hepatocarcinogenesis in rats. Int. J. Cancer 2010, 126, 1788–1796. [Google Scholar] [CrossRef] [PubMed]
- Park, D. H.; Shin, J.W.; Park, S.K.; Seo, J.N.; Li, L.; Jang, J.J.; Lee, M.J. Diethylnitrosamine (DEN) induces irreversible hepatocellular carcinogenesis through overexpression of G1/S-phase regulatory proteins in rat. Toxicol. Lett. 2009, 191, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Yonekura, L.; Nagao, A. Intestinal absorption of dietary carotenoids. Mol. Nutr. Food Res. 2007, 51, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Kakuda, Y.; Yeung, D. Antioxidative properties of lycopene and other carotenoids from tomatoes: Synergistic effects. Biofactors 2004, 21, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Piña-Zentella, R.M.; Rosado, J.L.; Gallegos-Corona, M.A.; Madrigal-P↑rez, L.A.; García, O.P.; Ramos-Gomez, M. Lycopene improves diet-mediated recuperation in rat model of nonalcoholic fatty liver disease. J. Med. Food 2016, 19, 607–614. [Google Scholar] [CrossRef] [PubMed]
- Bernal, C.; Martín-Pozuelo, G.; Lozano, A.B.; Sevilla, A.; García-Alonso, J.; Canovas, M.; Periago, M.J. Lipid biomarkers and metabolic effects of lycopene from tomato juice on liver of rats with induced hepatic steatosis. J. Nutr. Biochem. 2013, 24, 1870–1881. [Google Scholar] [CrossRef] [PubMed]
- Fabbrini, E.; Magkos, F. Hepatic steatosis as a marker of metabolic dysfunction. Nutrients 2015, 7, 4995–5019. [Google Scholar] [CrossRef] [PubMed]
- Martín-Pozuelo, G.; Navarro-González, I.; González-Barrio, R.; Santaella, M.; García-Alonso, J.; Hidalgo, N.; Gómez-Gallego, C.; Ros, G.; Periago, M.J. The effect of tomato juice supplementation on biomarkers and gene expression related to lipid metabolism in rats with induced hepatic steatosis. Eur. J. Nutr. 2014, 54, 933–944. [Google Scholar] [CrossRef] [PubMed]
- Angelica, M.D.; Fong, Y. HDL function, dysfunction, and reverse cholesterol transport. Arter. Thromb. Vasc. Biol. 2008, 141, 520–529. [Google Scholar]
- Ahn, J.; Lee, H.; Jung, C.H.; Ha, T. Lycopene inhibits hepatic steatosis via microRNA-21-induced downregulation of fatty acid-binding protein 7 in mice fed a high-fat diet. Mol. Nutr. Food Res. 2012, 56, 1665–1674. [Google Scholar] [CrossRef] [PubMed]
- Santos-Gallego, C.G.; Badimon, J.J.; Rosenson, R.S. Beginning to understand high-density lipoproteins. Endocrinol. Metab. Clin. N. Am. 2014, 43, 913–947. [Google Scholar] [CrossRef] [PubMed]
- Andersen, C.J.; Fernandez, M.L. Dietary approaches to improving atheroprotective HDL functions. Food Funct. 2013, 4, 1304–1313. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Cappello, T.; Wang, L. Emerging role of microRNAs in lipid metabolism. Acta Pharm. Sin. B 2015, 5, 145–150. [Google Scholar] [CrossRef] [PubMed]
- DiMarco, D.M.; Fernandez, M.L. The regulation of reverse cholesterol transport and cellular cholesterol homeostasis by microRNAs. Biology 2015, 4, 494–511. [Google Scholar] [CrossRef] [PubMed]
- Sofi, F.; Casini, A. Mediterranean diet and non-alcoholic fatty liver disease: New therapeutic option around the corner? World J. Gastroenterol. 2014, 20, 7339–7346. [Google Scholar] [CrossRef] [PubMed]
- Abenavoli, L.; Milic, N.; Peta, V.; Alfieri, F.; De Lorenzo, A.; Bellentani, S. Alimentary regimen in non-alcoholic fatty liver disease: Mediterranean diet. World J. Gastroenterol. 2014, 20, 16831–16840. [Google Scholar] [CrossRef] [PubMed]
| Carotenoid | Stage of Liver Disease | Study Model | Dietary Treatment | Dose | Duration of the Intervention | Main Outcomes | Reference |
|---|---|---|---|---|---|---|---|
| Astaxanthin | NASH | Male ob/ob mice and C57BL/6J mice | High-fat, high-cholesterol diet (60% kcal from fat +1.25% cholesterol) | 0.0067 or 0.02% astaxanthin (w/w) | 10 weeks | ↓ Hepatic steatosis ↓ Hepatic lipid uptake and accumulation ↓ Plasma ALT, AST, TG, TC, and NEFA ↓ Hepatic lipid peroxidation ↓ Hepatic lipogenic gene expression ↑ Hepatic insulin sensitivity ↓ Hepatic inflammation and fibrosis | [40] |
| Astaxanthin | NAFLD | Male C57BL/6J mice | High-fat diet (35% w/w) | 0.003, 0.01, or 0.03% astaxanthin (w/w) | 12 weeks | ↓ Plasma ALT and AST (with 0.03%) ↑ Hepatic FA β-oxidation ↓ Hepatic steatosis ↑ Hepatic antioxidant enzyme expression | [38] |
| Lutein | Hepatic steatosis | Male Hartley guinea pigs | Hypercholesterol-emic diet (0.25% w/w) | 0.1% supplemental lutein | 12 weeks | ↓ Hepatic free cholesterol ↓ Hepatic MDA and TNF-α ↓ Hepatic NF-ĸB p65 DNA Binding | [58] |
| Lutein | NAFLD | Male Sprague-Dawley rats | High-fat diet (33% kcal from fat) | 12.5, 25, or 50 mg/kg BW/day | 10 days HFD, then 45 days of HFD + lutein | ↓ Hepatic TC and TG ↑ Serum HDL-cholesterol ↓ Serum ALT ↑ Hepatic insulin sensitivity ↑ Hepatic FA catabolism | [62] |
| Lycopene | NAFLD | Male C57BL/6J mice | High-fat diet (49.29% kcal from fat) | 0.05% lycopene | 8 weeks | ↓ Hepatic lipids, TG, and TC ↑ Hepatic PPARα, CPT1α, LCAD, and ApoA4 expression ↓ Hepatic PPARγ and FASN expression ↓ Hepatic lipogenesis ↑ Hepatic fatty acid β-oxidation ↓ Hepatic FABP7 expression via ↑ miRNA-21 | [81] |
| Lycopene | NASH | Male Sprague-Dawley rats | High-fat diet | 2 or 4 mg/kg BW, given 3× per week | 6 weeks | ↓ Serum ALT (both doses), glucose (with 2 mg/kg) TG (with 4 mg/kg) ↓ Serum MDA ↓ Serum TNF-α ↑ Hepatic glutathione ↓ Hepatic steatosis and inflammation ↓ Hepatic stellate cell activation | [70] |
| Lycopene | NAFLD | Male Sprague-Dawley rats | Hypercholesterol-emic/high-fat diet | 3.15–3.5 mg/day lycopene from tomato juice | 5 weeks | ↓ Plasma TG ↑ Plasma HDL cholesterol ↓ Plasma VCAM ↑ Hepatic amino acids ↓ Urinary isoprostanes ↑ NAD/NADH ratio and redox balance ↑ Hepatic expression of genes related to FA transport, hydrolysis, and β-oxidation | [77,79] |
| Lycopene | NAFLD | Male Sprague-Dawley rats | High-fat diet (71% kcal from fat) | 20 mg/kg BW/day supplemental lycopene | 4 weeks high-fat diet then 4 weeks normal chow diet + lycopene | ↓ Liver weight ↓ Serum LDL-cholesterol ↓ Hepatic TC ↑ Antioxidant enzyme activity ↓ Hepatic steatosis | [76] |
| Lycopene | NASH-promoted hepato-carcinogenesis | Male Sprague-Dawley rats | High-fat diet (71% kcal from fat) | 15 mg/kg BW/day all-trans lycopene supplement or lycopene from tomato extract | 6 weeks | ↓ Cell growth and replication ↓ Precancerous lesions ↓ Inflammatory cytokine expression ↓ Oxidative stress | [72] |
| Zeaxanthin | NASH | Male Mongolian gerbils | Methionine- and choline-deficient diet | 0, 12.5 or 25 mg/kg zeaxanthin | 6 weeks | ↓ Liver fibrosis (at highest dose) ↓ Hepatic lipid hydroperoxides | [63] |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murillo, A.G.; DiMarco, D.M.; Fernandez, M.L. The Potential of Non-Provitamin A Carotenoids for the Prevention and Treatment of Non-Alcoholic Fatty Liver Disease. Biology 2016, 5, 42. https://doi.org/10.3390/biology5040042
Murillo AG, DiMarco DM, Fernandez ML. The Potential of Non-Provitamin A Carotenoids for the Prevention and Treatment of Non-Alcoholic Fatty Liver Disease. Biology. 2016; 5(4):42. https://doi.org/10.3390/biology5040042
Chicago/Turabian StyleMurillo, Ana Gabriela, Diana M. DiMarco, and Maria Luz Fernandez. 2016. "The Potential of Non-Provitamin A Carotenoids for the Prevention and Treatment of Non-Alcoholic Fatty Liver Disease" Biology 5, no. 4: 42. https://doi.org/10.3390/biology5040042
APA StyleMurillo, A. G., DiMarco, D. M., & Fernandez, M. L. (2016). The Potential of Non-Provitamin A Carotenoids for the Prevention and Treatment of Non-Alcoholic Fatty Liver Disease. Biology, 5(4), 42. https://doi.org/10.3390/biology5040042







