How Glutamate Is Managed by the Blood–Brain Barrier
Abstract
:1. Introduction
2. Glutamate in the Brain and the Circulation
3. Glutamate in ECF Cannot Be Permitted to Increase
4. Facilitative and Active Transport Systems for Glutamate in the BBB
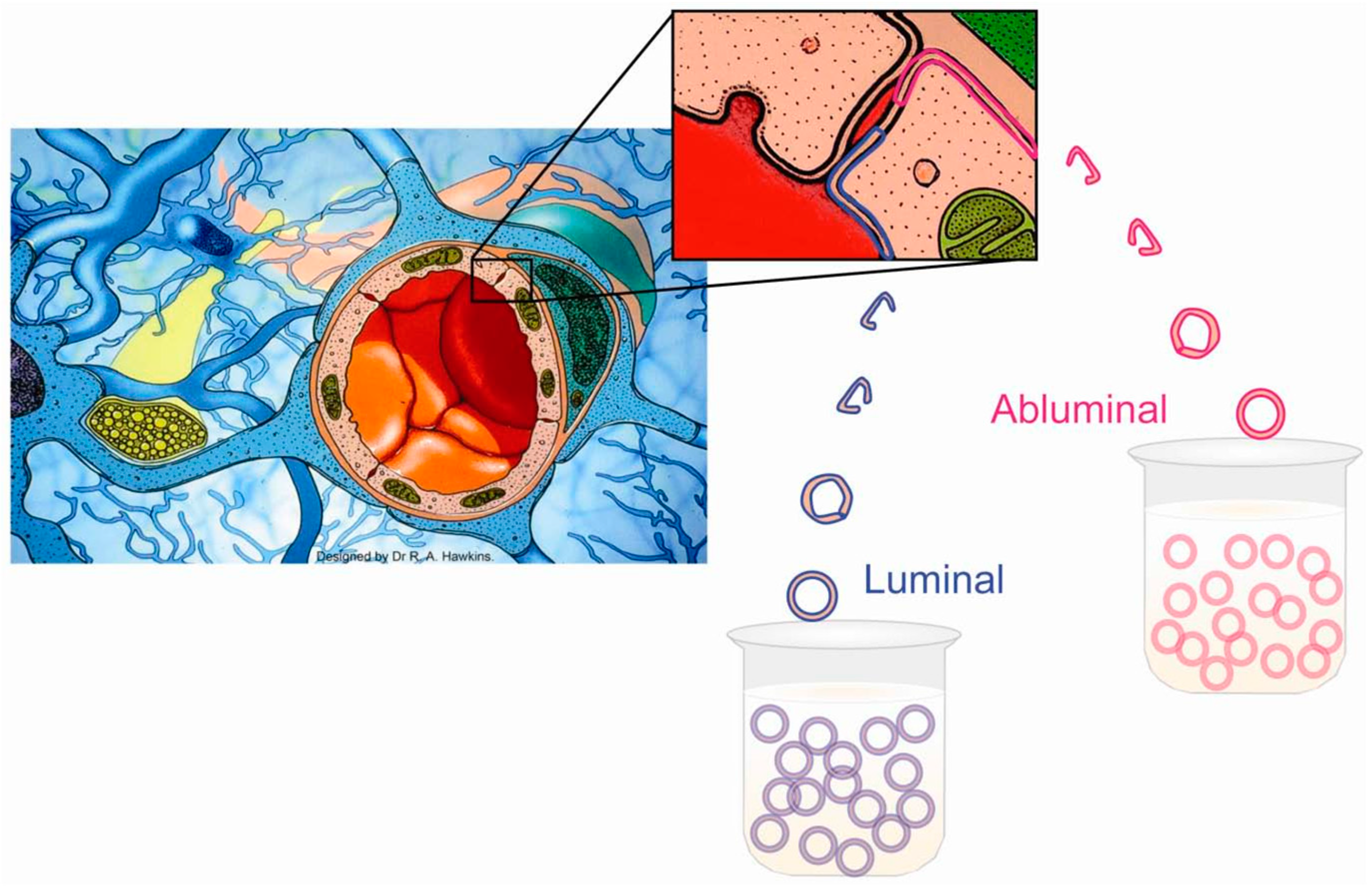
5. Studying Each Side of the BBB Separately
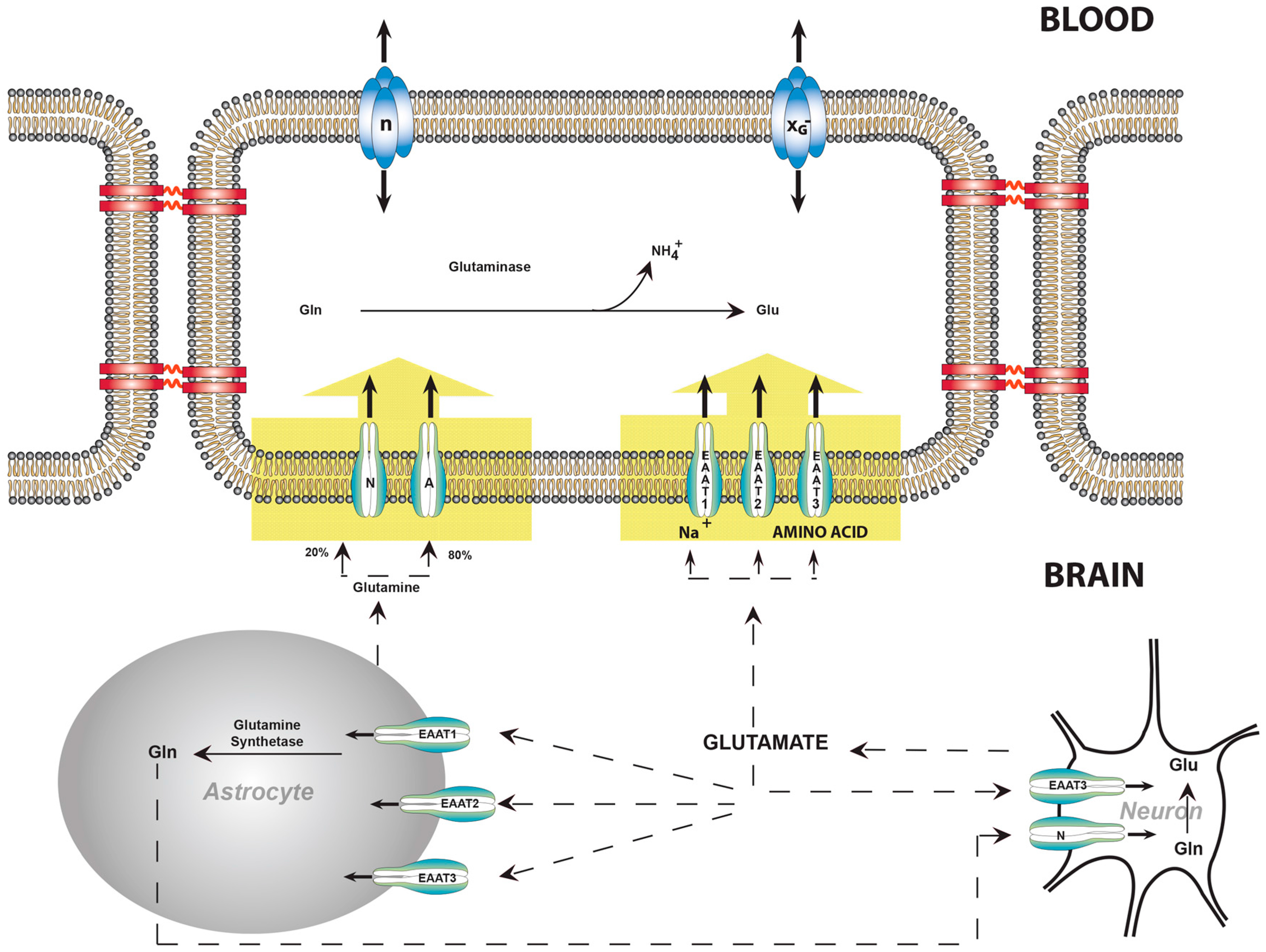
6. Facilitative Transport of Glutamate Is Restricted to Luminal Membranes
7. Active Transport Systems of the BBB Can Expel Glutamate from the ECF
8. Balance of Glutamate, Glutamine, and Ammonia
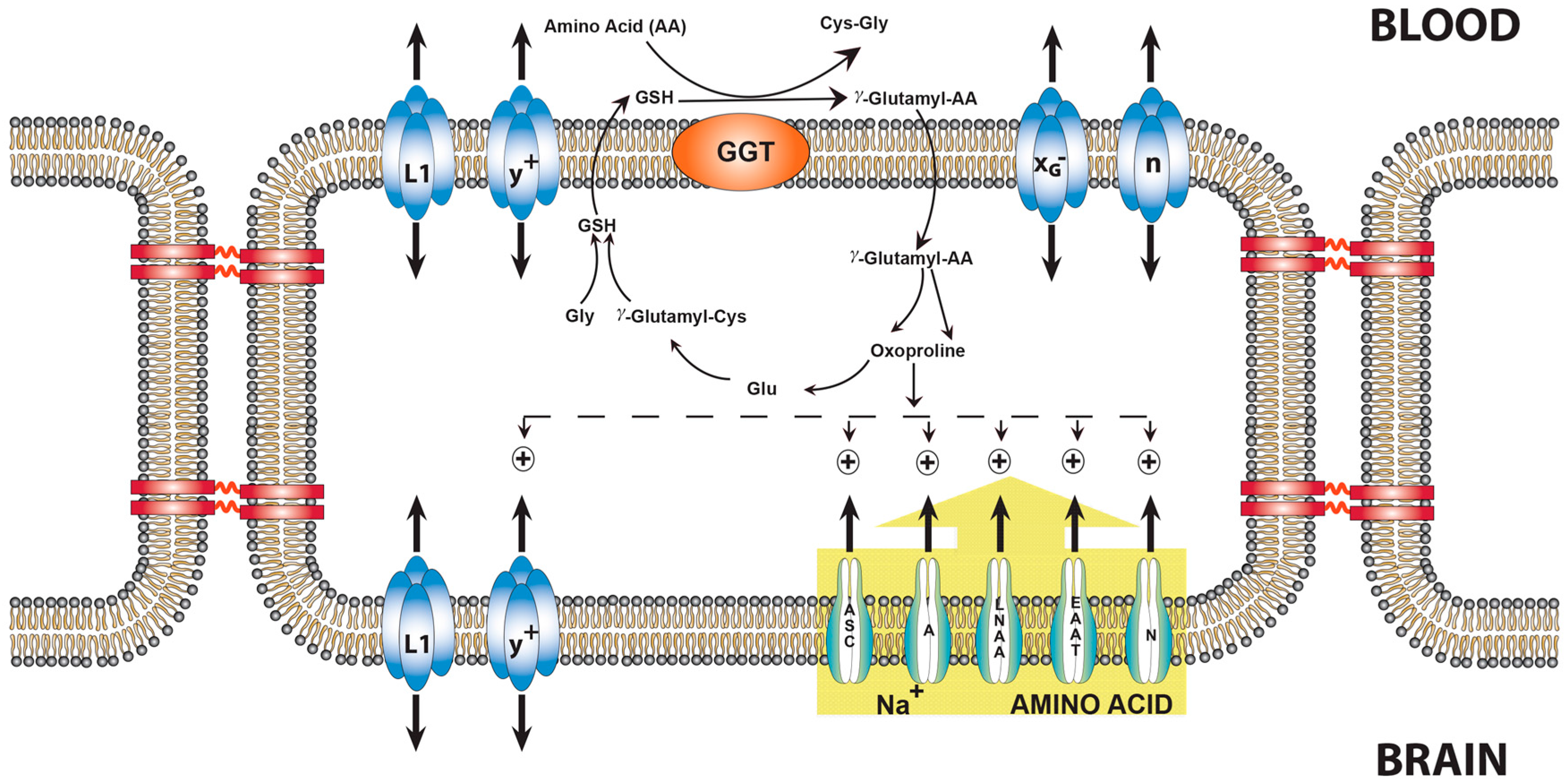
9. Transport of Amino Acids across the BBB and the Role of Oxoproline
10. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zhao, Z.; Nelson, A.R.; Betsholtz, C.; Zlokovic, B.V. Establishment and Dysfunction of the Blood–Brain Barrier. Cell 2015, 163, 1064–1078. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, R.A.; Mans, A.M. Intermediary metabolism of carbohydrates and other fuels. In Handbook of Neurochemistry; Lajtha, A., Ed.; Plenum Publishing Corp.: New York, NY, USA, 1983; Volume 3, pp. 259–294. [Google Scholar]
- Tossman, U.; Jonsson, G.; Ungerstedt, U. Regional distribution and extracellular levels of amino acids in rat central nervous system. Acta Physiol. Scand. 1986, 127, 533–545. [Google Scholar] [CrossRef] [PubMed]
- Meldrum, B.S. Glutamate as aneurotransmitter in the brain: Review of physiology and pathology. J. Nutr. 2000, 130, 1007S–1015S. [Google Scholar] [PubMed]
- Price, M.T.; Olney, J.W.; Lowry, O.H.; Buchsbaum, S. Uptake of exogenous glutamate and aspartate by circumventricular organs but not other regions of brain. J. Neurochem. 1981, 36, 1774–1780. [Google Scholar] [CrossRef] [PubMed]
- Olney, J.W.; Sharpe, L.G. Brain lesions in an infant rhesus monkey treated with monosodium glutamate. Science 1969, 166, 386–388. [Google Scholar] [CrossRef] [PubMed]
- Castillo, J.; Davalos, A.; Naveiro, J.; Noya, M. Neuroexcitatory amino acids and their relation to infarct size and neurological deficit in ischemic stroke. Stroke 1996, 27, 1060–1065. [Google Scholar] [CrossRef] [PubMed]
- Martin, R.L.; Lloyd, H.G.; Cowan, A.I. The early events of oxygen and glucose deprivation: Setting the scene for neuronal death? Trends Neurosci. 1994, 17, 251–257. [Google Scholar] [CrossRef]
- Rothstein, J.D.; Dykes-Hoberg, M.; Pardo, C.A.; Bristol, L.A.; Jin, L.; Kuncl, R.W.; Kanai, Y.; Hediger, M.A.; Wang, Y.; Schielke, J.P.; et al. Knockout of glutamate transporters reveals a major role for astroglial transport in excitotoxicity and clearance of glutamate. Neuron 1996, 16, 675–686. [Google Scholar] [CrossRef]
- Benveniste, H.; Drejer, J.; Schousboe, A.; Diemer, N.H. Elevations of the extracellular concentrations of glutamate and aspartate in rat hippocampus during transient cerebral ischemia monitored by intracerebral microdialysis. J. Neurochem. 1984, 43, 1369–1374. [Google Scholar] [CrossRef] [PubMed]
- Oldendorf, W.H. Measurement of brain uptake of radiolabeled substances using a tritiated water internal standard. Brain Res. 1970, 24, 372–376. [Google Scholar] [CrossRef]
- Oldendorf, W.H. Uptake of radiolabeled essential amino acids by brain following arterial injection. Proc. Soc. Exp. Biol. Med. 1971, 136, 385–386. [Google Scholar] [CrossRef] [PubMed]
- Oldendorf, W.H. Brain uptake of radiolabeled amino acids, amines, and hexoses after arterial injection. Am. J. Physiol. 1971, 221, 1629–1639. [Google Scholar] [PubMed]
- Drewes, L.R.; Conway, W.P.; Gilboe, D.D. Net amino acid transport between plasma and erythrocytes and perfused dog brain. Am. J. Physiol. 1977, 233, E320–E325. [Google Scholar] [PubMed]
- Hawkins, R.A.; DeJoseph, M.R.; Hawkins, P.A. Regional brain glutamate transport in rats at normal and raised concentrations of circulating glutamate. Cell Tissue Res. 1995, 281, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Viña, J.R.; DeJoseph, M.R.; Hawkins, P.A.; Hawkins, R.A. Penetration of glutamate into brain of 7-day-old rats. Metab. Brain Dis. 1997, 12, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Sánchez del Pino, M.M.; Hawkins, R.A.; Peterson, D.R. Neutral amino acid transport by the blood–brain barrier: Membrane vesicle studies. J. Biol. Chem. 1992, 267, 25951–25957. [Google Scholar] [PubMed]
- Hoshi, Y.; Uchida, Y.; Tachikawa, M.; Inoue, T.; Ohtsuki, S.; Terasaki, T. Quantitative atlas of blood–brain barrier transporters, receptors, and tight junction proteins in rats and common marmoset. J. Pharm. Sci. 2013, 102, 3343–3355. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, R.A.; Peterson, D.R.; Viña, J.R. The complementary membranes forming the blood–brain barrier. IUBMB Life 2002, 54, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.J.; Hawkins, R.A.; Viña, J.R.; Peterson, D.R. Glutamine transport by the blood–brain barrier: A possible mechanism for nitrogen removal. Am. J. Physiol. 1998, 274, C1101–C1107. [Google Scholar] [PubMed]
- Christensen, H.N. Developments in amino acid transport, illustrated for the blood–brain barrier. Biochem. Pharmacol. 1979, 28, 1989–1992. [Google Scholar] [CrossRef]
- Sershen, H.; Lajtha, A. Capillary transport of amino acids in the developing brain. Exp. Neurol. 1976, 53, 465–474. [Google Scholar] [CrossRef]
- Smith, Q.R.; Stoll, J. Blood–brain barrier amino acid transport. In Introduction to the Blood–Brain Barrier: Methodology, Biology, and Pathology; Pardridge, W.M., Ed.; Cambridge University Press: Cambridge, UK, 1998; pp. 188–197. [Google Scholar]
- Oldendorf, W.H.; Szabo, J. Amino acid assignment to one of three blood–brain barrier amino acid carriers. Am. J. Physiol. 1976, 230, 94–98. [Google Scholar] [PubMed]
- Benrabh, H.; Lefauconnier, J.M. Glutamate is transported across the rat blood–brain barrier by a sodium-independent system. Neurosci. Lett. 1996, 210, 9–12. [Google Scholar] [CrossRef]
- Smith, Q.R. Transport of glutamate and other amino acids at the blood–brain barrier. J. Nutr. 2000, 130, 1016S–1022S. [Google Scholar] [PubMed]
- Fahlke, C.; Kortzak, D.; Machtens, J.P. Molecular physiology of EAAT anion channels. Pflugers Arch. 2016, 468, 491–502. [Google Scholar] [CrossRef] [PubMed]
- Rothstein, J.D.; Martin, L.; Levey, A.I.; Dykes-Hoberg, M.; Jin, L.; Wu, D.; Nash, N.; Kuncl, R.W. Localization of neuronal and glial glutamate transporters. Neuron 1994, 13, 713–725. [Google Scholar] [CrossRef]
- Attwell, D. Brain uptake of glutamate: Food for thought. J. Nutr. 2000, 130, 1023S–1025S. [Google Scholar] [PubMed]
- Lehre, K.P.; Levy, L.M.; Ottersen, O.P.; Storm-Mathisen, J.; Danbolt, N.C. Differential expression of two glial glutamate transporters in the rat brain: Quantitative and immunocytochemical observations. J. Neurosci. 1995, 15, 1835–1853. [Google Scholar] [PubMed]
- Miralles, V.J.; Martínez-López, I.; Zaragozá, R.; Borrás, E.; García, C.; Pallardó, F.V.; Viña, J.R. Na+ dependent glutamate transporters (EAAT1, EAAT2, and EAAT3) in primary astrocyte cultures: Effect of oxidative stress. Brain Res. 2001, 922, 21–29. [Google Scholar] [CrossRef]
- O’Kane, R.L.; Martinez-Lopez, I.; DeJoseph, M.R.; Viña, J.R.; Hawkins, R.A. Na(+)-dependent glutamate transporters (EAAT1, EAAT2, and EAAT3) of the blood–brain barrier. A mechanism for glutamate removal. J. Biol. Chem. 1999, 274, 31891–31895. [Google Scholar] [PubMed]
- Hawkins, R.A.; Viña, J.R.; Mokashi, A.; Peterson, D.R.; Okane, R.O.; Simpson, I.A.; Dejoseph, M.R.; Rasgado-Flores, H. Synergism between the Two Membranes of the Blood–brain Barrier: Glucose and Amino Acid Transport. Am. J. Neurosci. Res. 2013, 1, 1–25. [Google Scholar]
- Cohen-Kashi-Malina, K.; Cooper, I.; Teichberg, V.I. Mechanisms of glutamate efflux at the blood–brain barrier: Involvement of glial cells. J. Cereb. Blood Flow Metab. 2012, 32, 177–189. [Google Scholar] [CrossRef] [PubMed]
- Arriza, J.L.; Fairman, W.A.; Wadiche, J.I.; Murdoch, G.H.; Kavanaugh, M.P.; Amara, S.G. Functional comparisons of three glutamate transporter subtypes cloned from human motor cortex. J. Neurosci. 1994, 14, 5559–5569. [Google Scholar] [PubMed]
- Danbolt, N.C.; Furness, D.N.; Zhou, Y. Neuronal vs. glial glutamate uptake: Resolving the conundrum. Neurochem. Int. 2016, 98, 29–45. [Google Scholar] [PubMed]
- Martinez-Hernandez, A.; Bell, K.P.; Norenberg, M.D. Glutamine synthetase: Glial localization in brain. Science 1977, 195, 1356–1358. [Google Scholar] [CrossRef] [PubMed]
- Cooper, A.J.; McDonald, J.M.; Gelbard, A.S.; Gledhill, R.F.; Duffy, T.E. The metabolic fate of 13N-labeled ammonia in rat brain. J. BiolChem. 1979, 254, 4982–4992. [Google Scholar]
- Cooper, A.J.; Plum, F. Biochemistry and physiology of brain ammonia. Physiol. Rev. 1987, 67, 440–519. [Google Scholar] [PubMed]
- Meister, A. Transport and metabolism of glutathione and gamma-glutamyl amino acids. Biochem. Soc. Trans. 1983, 11, 793–794. [Google Scholar] [CrossRef]
- Meister, A.; Anderson, M.E. Glutathione. Annu. Rev. Biochem. 1983, 52, 711–760. [Google Scholar] [CrossRef] [PubMed]
- Curto, K.A.; Sweeney, W.E.; Avner, E.D.; Piesco, N.P.; Curthoys, N.P. Immunocytochemical localization of gamma-glutamyltranspeptidase during fetal development of mouse kidney. J. Histochem. Cytochem. 1988, 36, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Viña, J.R.; Palacin, M.; Puertes, I.R.; Hernandez, R.; Viña, J. Role of the g-glutamyl cycle in the regulation of amino acid translocation. Am. J. Physiol. 1989, 257, E916–E922. [Google Scholar] [PubMed]
- Garvey, T.Q.; Hyman, P.E.; Isselbacher, K.J. Gamma-glutamyltranspeptidase of rat intestine: Localization and possible role in amino acid transport. Gastroenterology 1976, 71, 778–785. [Google Scholar] [PubMed]
- Anderson, M.E.; Underwood, M.; Bridges, R.J.; Meister, A. Glutathione metabolism at the blood-cerebrospinal fluid barrier. FASEB J. 1989, 3, 2527–2531. [Google Scholar] [PubMed]
- Orlowski, M.; Sessa, G.; Green, J.P. Gamma-glutamyltranspeptidase in brain capillaries: Possible site of a blood–brain barrier for amino acids. Science 1974, 184, 66–68. [Google Scholar] [CrossRef] [PubMed]
- Orlowski, M.; Meister, A. The g-glutamyl cycle: A possible transport system for amino acids. Proc. Natl. Acad. Sci. USA. 1970, 67, 1248–1255. [Google Scholar] [CrossRef] [PubMed]
- Meister, A. On the enzymology of amino acid transport. Science 1973, 180, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.J.; Hawkins, R.A.; Peterson, D.R.; Viña, J.R. Role of oxoproline in the regulation of neutral amino acid transport across the blood–brain barrier. J. Biol. Chem. 1996, 271, 19129–19133. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hawkins, R.A.; Viña, J.R. How Glutamate Is Managed by the Blood–Brain Barrier. Biology 2016, 5, 37. https://doi.org/10.3390/biology5040037
Hawkins RA, Viña JR. How Glutamate Is Managed by the Blood–Brain Barrier. Biology. 2016; 5(4):37. https://doi.org/10.3390/biology5040037
Chicago/Turabian StyleHawkins, Richard A., and Juan R. Viña. 2016. "How Glutamate Is Managed by the Blood–Brain Barrier" Biology 5, no. 4: 37. https://doi.org/10.3390/biology5040037
APA StyleHawkins, R. A., & Viña, J. R. (2016). How Glutamate Is Managed by the Blood–Brain Barrier. Biology, 5(4), 37. https://doi.org/10.3390/biology5040037



