Abstract
Heterochrony is an enabling concept in evolution theory that metaphorically captures the mechanism of biologic change due to mechanisms of growth and development. The spatio-temporal patterns of morphogenesis are determined by cell-to-cell signaling mediated by specific soluble growth factors and their cognate receptors on nearby cells of different germline origins. Subsequently, down-stream production of second messengers generates patterns of form and function. Environmental upheavals such as Romer’s hypothesized drying up of bodies of water globally caused the vertebrate water-land transition. That transition caused physiologic stress, modifying cell-cell signaling to generate terrestrial adaptations of the skeleton, lung, skin, kidney and brain. These tissue-specific remodeling events occurred as a result of the duplication of the Parathyroid Hormone-related Protein Receptor (PTHrPR) gene, expressed in mesodermal fibroblasts in close proximity to ubiquitously expressed endodermal PTHrP, amplifying this signaling pathway. Examples of how and why PTHrPR amplification affected the ontogeny, phylogeny, physiology and pathophysiology of the lung are used to substantiate and further our understanding through insights to the heterochronic mechanisms of evolution, such as the fish swim bladder evolving into the vertebrate lung, interrelated by such functional homologies as surfactant and mechanotransduction. Instead of the conventional description of this phenomenon, lung evolution can now be understood as adaptive changes in the cellular-molecular signaling mechanisms underlying its ontogeny and phylogeny.
1. Introduction
Heterochrony is an enabling concept in evolution theory because it captures the image of dynamic biologic change diachronically across space and time. The term heterochrony (see Table 1 below) was first used by Haeckel in his explanation of the Biogenetic Law [1]. De Beer subsequently used Heterochrony to denote differences in the ontogenies of related taxa [2]. The latter comparative definition is the one in principal use [3]. More recent interest in the question of Heterchrony has arisen because of the integration of developmental biology into evolution theory, or EvoDevo [4]. But this initiative has failed to incorporate the highly mechanistic nature of contemporary Developmental Biology [5] beginning in the late 1970s with the discovery of soluble growth factors and their complementary receptors residing on neighboring cell-types, determining the patterns of morphogenesis [6]. The associations of growth factors with the process of evolution are sometimes described [7], but the significance of the growth factor receptors, down-stream signals and subsequent effects on morphogenesis are never specifically addressed—yet this is a microcosm of what evolution constitutes, if only we would perceive it appropriately [8,9,10]. The only reasonable explanation for such gross oversights is that Cell Biology itself has been overarched by the evolutionists, largely due to an incident of history [11]. Haeckel and Spemann were unable to provide scientific evidence for their theories of the Biogenetic Law, and for the “Organizer”, respectively, so the evolutionists turned to the geneticists to advance their agenda, rejecting the embryologists in the process. As a result, the nominal mechanism of evolution is constituted by genetic mutation and natural selection [12]. If this were indeed the case, then there would be no need to delineate or utilize the principles of cellular-molecular morphogenesis, yet therein lie the fundamental principles of form and function [13]. The following is in service to functionally integrating our contemporary knowledge of the cellular-molecular mechanisms of development with the role of heterochrony in evolutionary biology.

Table 1.
Background and significance for the reinterpretation of heterochrony.
|
|
|
|
|
|
|
|
|
|
2. Normal Embryologic Development, or “Monochrony”, in Contrast to Heterochrony
The spatio-temporal patterns of vertebrate embryogenesis are determined by the elaboration of cell-specific growth factors signaling to their cell surface G-Protein Coupled Receptors are neighboring cells of differing germline origins to form patterns of growth and differentiation, from the zygote [15] to the offspring [16]. This is an iterative process by which the zygote divides, giving rise to the animal and vegetal poles, the blastula, gastrula, and so on [17] during embryogenesis, followed by fetal growth and differentiation [18] to generate the offspring. All of these processes are mediated by growth factor-receptor signaling mechanisms that form the tissues and organs of the body. If the environmental conditions remain unchanged, this process would simply be recapitulated from one life cycle to the next. But the environment is in perpetual flux [19]—climate, topography, seasons, food abundance, competition with other organisms—so organisms must be able to adapt in order to survive using the mechanism we refer to as evolution [20]. This is particularly apparent when environmental conditions are physiologically stressful, since the adaptive changes are both discernable and measurable [21]. Classic examples are the consequences of the five mass extinctions [22], and the transition of plants and animals from water to land [23], brought about by carbon dioxide causing an atmospheric Green House Effect that dried up water sources globally [24]. During that period there were several genetic adaptations that profoundly affected vertebrate physiology, allowing for successful adaptation to terrestrial life [10]. By focusing on those events both ontogenetically and phylogenetically [10], we can envision how heterochrony facilitated vertebrate evolution.
The terrestrial forms of the vertebrate lung, kidney, bone, skin and brain all evolved during the water-land transition in adaptation to terrestrial life. The epitome of the mechanisms underlying these phenotypic changes is the duplication of the Parathyroid Hormone-related Protein Receptor (PTHrPR) [25], expressed in all of these organs by the mesoderm [26] in close proximity to ubiquitous epithelial PTHrP production. The duplication of the PTHrPR gene amplified signal transduction for PTHrP signaling from the endodermal epithelium to the mesodermal fibroblast [27]. In the case of the lung, it facilitated the formation of alveoli [28]; in the kidney, PTHrP signaling amplification generated glomeruli [29]; in bone, increased PTHrP amplification allowed for the five documented phenotypic changes in the skeleton that compensated for the increased effect of gravity on the skeleton relative to buoyancy in water [30]; in the skin, PTHrP fostered barrier formation by skin cells for prevention of water and electrolyte loss [31]; the brain is thought to have evolved from the skin [32], and it has a number of molecular traits that are derivative of the latter at the molecular level [9] that would have facilitated its evolution for land adaptation.
3. Lung Evolution as Ontogeny and Phylogeny
In hindsight, it is obvious that land vertebrates had to evolve lungs in order to adapt to air breathing, yet phylogenetically the fish organ of gas exchange—the gill—is an analog of the lung, not the functionally ancestral homolog—counterintuitively, the fish swim bladder, which facilitates buoyancy, is actually the functional homolog of the lung [33]. This example epitomizes the value of a cellular-molecular ontogenetic-phylogenetic approach to evolution. The swim bladder is an adaptation to gravity that utilizes atmospheric gas to inflate or deflate the bladder, aided by the secretion of cholesterol into the air space by the gas gland epithelium. The swim bladder expresses both Cholesterol, the most primitive lung lipid component of surfactant [34], and Surfactant Protein A [35], a host defense peptide that originated phylogenetically from the gut.
The lung has adapted to atmospheric oxygen phylogenetically and ontogenetically by reducing the surface area of the gas exchange unit, increasing the ratio of the gas-exchange surface area to the blood volume for increased oxygenation. Concomitant ontogenetic and phylogenetic increases in the biological activity of lung surfactant secreted into the alveolar space prevented the alveolar collapse that would otherwise have been caused by the increase in surface tension resulting from the decreased surface area (surface tension being inversely related to surface area by the Law of Laplace). The stretch-regulated mechanism of alveolar surfactant production [10] is functionally homologous with the swim bladder, both of which are gravity-sensing mechanisms [30]. By focusing on the developmental/homeostatic cell-cell interactions that have evolved from the gas-exchange unit of fish (swim bladder) to that of land vertebrates to form the alveoli [36], one can see how they were selected for the thinning of the alveolar wall and the anti-atelectatic function of the surfactant system [37].
4. The Lipofibroblast as a “Rosetta Stone” for Lung Evolution
The alveolar lipofibroblast (LIF) is a molecular Rosetta Stone [38], “translating” the developmental changes across vertebrate species into the evolution of the lung. The physiologic relevance of the LIF to alveolar growth, differentiation, homeostasis and repair has revealed such evolutionary homologies as (1) the peroxisome [39], which is thought to have evolved in response to the otherwise pathologic effects of Endoplasmic Reticulum stress in unicellular organisms; (2) Neutral Lipid Trafficking (Figure 1) [40], encompassing facilitated lipid uptake in defense against hyperoxia [41] mediated by Adipocyte Differentiation Related Protein (ADRP) storage [42], and release under the control of Prostaglandin E2 [43], referring all the way back to the biosynthesis and insertion of cholesterol into the eukaryotic cell membrane [8]; and (3) the secretion of the fat cell hormone leptin [44] to regulate surfactant production by the Alveolar Type II Cell (ATII), coming full circle from the antioxidant property of the LIF [41]. For orientation of these cellular-molecular evolutionary properties to the lung, the pathways for ontogeny, phylogeny and evolution of the LIF-ATII interactions are depicted in Figure 2.
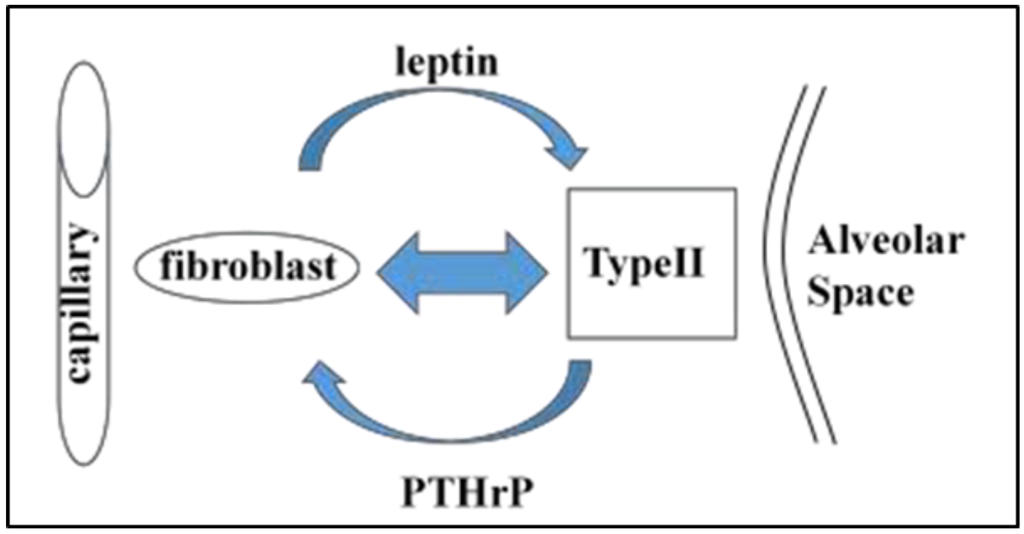
Figure 1.
Active recruitment of neutral lipid from lipofibroblasts by alveolar Type II cells. Neutral lipids stored in lipofibroblasts are actively “trafficked” to alveolar Type II cells by means of Adipocyte Differentiation Related Protein (ADRP), regulated by Parathyroid Hormone-related Protein (PTHrP) produced by Type II cells. The Type II cells secrete Prostaglandin E2, stimulating the secretion of the neutral lipids, and the uptake of the neutral lipid by the Type II cells is regulated by leptin produced by the lipofibroblasts. Each of these steps is coordinately stretch-regulated to increase surfactant phospholipid synthesis by the Type II cell. The net result is surfactant phospholipid production integrated with the distension of the alveolar wall during breathing.
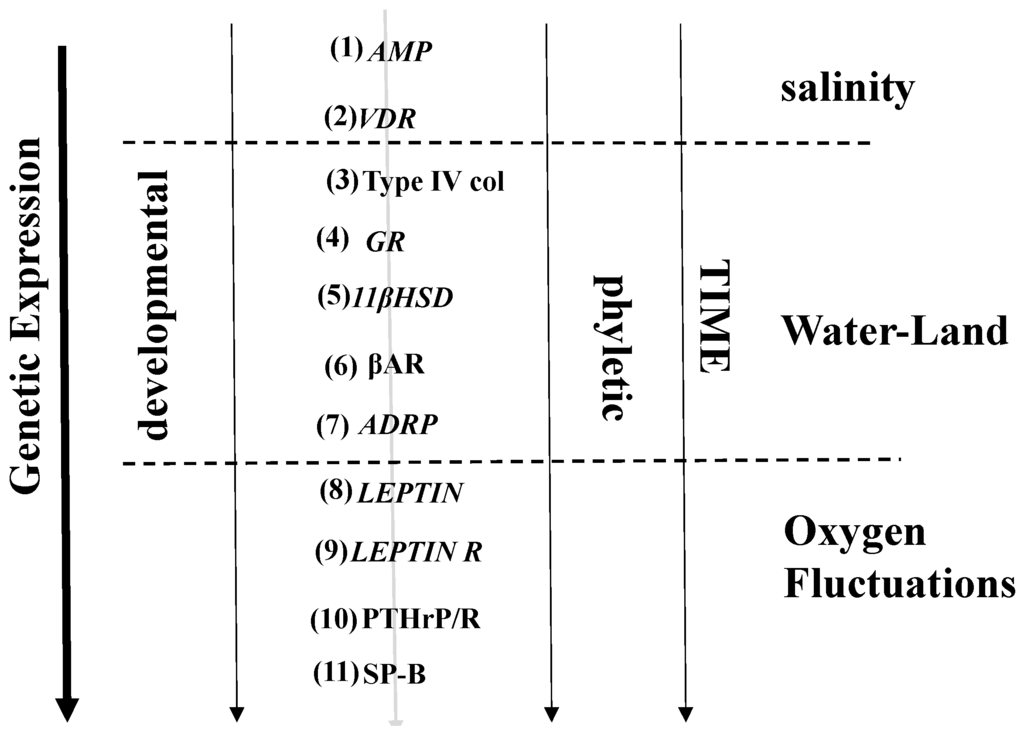
Figure 2.
Pathways for the developmental and phyletic evolution of lipofibroblast-Type II cell interactions. Extrinsic selection pressures are shown in italics; intrinsic selection pressures are shown in bold. (1) AMPs = Antimicrobial Peptides; (2) VDR = Vitamin D Receptor; (3) Type IV col = Type IV collagen ; (4) GR = Glucocorticoid Receptor; (5) 11βHSD = 11beta Hydroxysteroid Dehydrogenase; (6) βAR = beta Adrenergic Receptor; (7) ADRP = Adipocyte Differentiation Related Protein; (8) Leptin = Leptin; (9) Leptin R = Leptin Receptor; (10) PTHrP = Parathyroid Hormone-related Protein; (11) SP-B = Surfactant Protein-B. These changes in genetic expression were sequentially brought about by such environmental factors as salinity, water-land transition and fluctuations in atmospheric oxygen tension over the last 500 million years.
LIFs in the alveolar wall of the rat lung were first described by Hitchcock et al. [45], and extensively documented in rodent [46,47,48] and more recently in human lung [49]. However, their functional relevance to the alveolus was not determined for two more decades, though their cytoprotective nature was suggested earlier by the comparative lung physiologic studies of Frank et al. [50], who showed the association between the LIFs and their putative role in antioxidant protection. These physiologic studies were paralleled by biochemical studies of triglyceride metabolism conducted by Mostello et al. [51].
The breakthrough in understanding the functional nature of these cells in lung alveolar physiology came with the co-culture of LIFs containing radiolabeled triglyceride and naive ATIIs, resulting in rapid passage of the tagged triglyceride from the LIF to the ATII, and their subsequent robust, enriched incorporation into surfactant phospholipid [52], termed Neutral Lipid Trafficking. Experimentally, it was observed that LIFs could readily take up triglyceride and store it in a stable form; furthermore, this process was under hormonal control by glucocorticoids, pointing to its regulated nature. Moreover, the presence of neutral lipid droplets in the LIFs protected them against oxidant injury [41], providing a function for these cells for the first time. It was subsequently determined that the uptake, storage and transit of the neutral lipids was actively mediated by ADRP, one of the proteins that mediate the trafficking and storage of neutral lipids throughout the body [53].
As mentioned above, during the course of these studies, it was empirically discovered that ATIIs could not absorb triglycerides (TGs) [52], whereas LIFs could not release them. That led to the discovery that Prostaglandin E2 (PGE2), secreted by ATIIs [43], specifically causes PGE2 receptor-mediated release of TGs by LIFs, and leptin produced by the LIFs facilitates the uptake of TGs by binding to its cell surface receptors on ATIIs [44].
The climax of these coordinated cell-cell interactions mediating and facilitating the production of lung surfactant was the discovery that Neutral Lipid Trafficking is stretch-regulated, providing key insights into both the cellular-molecular basis for the mechanism of alveolar ventilation-perfusion matching [54], and to the evolutionary history of the lung [54]. Initially, it was discovered that PTHrP is necessary for the formation of alveoli during lung morphogenesis [27], and that PTHrP secreted by the ATIIs stimulates LIF development, including TG uptake [55] and leptin secretion [44], providing evidence for the earliest epithelial signal to the mesenchyme during the course of the cell-cell interactions that mediate alveolar development. That, combined with the observation that PTHrP mRNA expression by ATIIs is stretch-regulated [56] led to the broader physiologic insight that the PTHrP Receptor (PTHrPR), leptin and the leptin receptor are all stretch-regulated signals, coordinating the physical distension of the alveolar wall with the “on-demand” up-regulation of surfactant production [40], promoting lung development in utero [57], and preventing alveolar atelectasis during air breathing [54]. The elucidation of this cellular-molecular mechanism for ventilation-perfusion matching is the first such evidence for the cellular-molecular basis for a physiologic property ever to be determined.
The ancestral relationship between the effect of stretch on PTHrP expression and microgravity was subsequently shown empirically. ATIIs were subjected to 0 × g conditions using a Rotating Wall Vessel Bioreactor [30]. The amount of PTHrP mRNA decreased over the first 8–12 h of microgravitational exposure, reaching a new stable baseline; when the cells were returned to unit gravity, the amount of PTHrP mRNA returned to its pre-microgravity exposure level. The deep significance of this relationship between alveolar regulation, mechanotransduction and lung development was revealed by study of the effect of microgravity on yeast [58]. Exposure to 0 × g caused loss of polarity and budding. The former is a reflection of the inability to mediate calcium flux [59], and the latter reflects the inability to reproduce [60]. These ancient physiologic properties refer all the way back to the unicellular state, when cholesterol facilitated eukaryotic evolution from prokaryotes, promoting metabolism, respiration and locomotion, the basic characteristics of vertebrate evolution [61].
These insights led to the realization that the endodermal and mesodermal components of the alveolar wall evolved over evolutionary time to generate their structural-functional properties through cell-cell interactions [8,36,62]. In support of that process, the PTHrPR duplicated during the vertebrate water-land transition some 300 mya (Million years ago) [25], amplifying the PTHrP signaling pathway in the lung, skin and bone. The causal nature of this interrelationship is evidenced by the deletion of PTHrP in developing mice, causing failure of lung, skin and bone development [27]. PTHrP is also expressed in the developing swim bladder along with many other genes expressed in lung [33], establishing the functional homology between these organs. Moreover, the gas gland epithelial cells that line the swim bladder secrete cholesterol, the most primitive lung surfactant, preventing the walls of the bladder from sticking to one another. That functional homology relates ancestrally all the way back to the advent of cholesterol in the cell membranes of evolving unicellular eukaryotes from prokaryotes, thinning the phospholipid bilayer, facilitating oxygenation, metabolism and locomotion, the fundamental properties of vertebrate physiologic evolution [61]. Cholesterol was subsequently coopted for the formation of lipid rafts, the structural site for cell-surface receptors, and much later were the substrate for steroid hormones and vitamin D, key elements of the endocrine system.
5. Physical Stress and Heterochrony—The Role of Gravity
Gravitational force is the oldest, constant, unidirectional force on Earth. As such, it affects biologic systems through mechanotransduction to affect cellular physiology. The fundamental nature of this effector is reflected by experiments in which yeast were exposed to microgravity, showing phenotypic effects on polarity and budding [58]. The former effect is a reflection of the role of gravity in calcium flux [59], the latter reflecting the effect of gravity on reproduction [60]. Experiments in our laboratory have similarly shown effects of microgravity on PTHrP expression in lung and bone cells in vitro [29], which were corroborated by assaying for PTHrP mRNA in the bones of rats flown in deep space for two weeks on NASA STS-58 [30]. The mRNA levels were significantly lower in the weight-bearing bones (tibia, femur) than in the non-weight bearing skull bones [30], consistent with the theory of mechanical effects on bone remodeling.
Affecting such fundamental adaptive mechanotransductive mechanisms has produced heterochronic changes over the history of organisms.
6. Physiologic Stress—The Role of Hypoxia
Oxygen has profoundly affected the evolution of terrestrial organisms, fueling their metabolism, causing increased growth [62] and differentiation [8]. This effect is most apparent over the course of the last 500 million years, during the Phanerozoic eon, oxygen rising and falling between 15% and 35% [63]. The increases have driven the growth of large insects and animals [64], whereas the subsequent decreases have profoundly affected visceral evolution since hypoxia is the most potent physiologic stressor. The residual of those insults is seen in the concerted evolution of the neuroendocrine [65], endocrine [66] and respiratory systems [8] of land vertebrates, hypothetically generating homeothermy/endothermy [10].
As added evidence for the interrelationship between physiologic stress and the co-evolution of the neuroendocrine and respiratory systems, lung surfactant has evolved to optimize surface tension reducing activity during the transition from poikilotherms to endotherms [66]. Initially, the stress of periodic hypoxia during land vertebrate evolution stimulated the hypothalamic-pituitary adrenal axis, increasing catecholamine secretion from the adrenal medulla [67]. Catecholamines stimulate surfactant secretion by the alveoli, making the alveoli more distensible, transiently relieving the hypoxic constraint on the evolving lung. Over time, the increased distension of the alveoli stimulates PTHrP secretion from the ATIIs, promoting alveolarization [27], providing a long-term solution for adaptation to air breathing [68]. In tandem, catecholamines stimulate fatty acid secretion from peripheral fat stores, increasing metabolism and body heat. The phospholipid composition of surfactant in the alveoli of land vertebrates has evolved through progressive increases in the percentage of dipalmitoylphosphatidylcholine (DPPC), which is 300% more bioactive at 37 °C than it is at 25 °C due to its increased phase transition temperature [66], offering a mechanistic explanation for the positive selection for DPPC [69]. In support of the causal effect of the ambient atmospheric temperature on lung surfactant phospholipid composition, Lau and Keogh [70] had shown such an interrelationship experimentally in MAP turtles. Moreover, hibernation, in association with decreased catecholamine production, demonstrates the opposite effect on the phosphatidylcholine content of lung surfactant [71], lending credence to the environmental effect on lung surfactant composition and surface tension reducing activity.
And since all of these properties are the net result of changes in cell-cell signaling mechanisms for structure and function, they can be characterized as heterochronies.
7. Chronic Lung Disease as “Reverse” Heterochrony
Many chronic diseases such as emphysema and Bronchopulmonary Dysplasia are characterized by structural simplification [72], atavistically reverting back to an earlier stage in their ontogeny and phylogeny. In the lung, this relationship has been well delineated by the recognition of the transdifferentiation of mesodermal fibroblasts from an adipocyte-like lipofibroblast to a myofibroblast, reversing their direction both developmentally [73] and phylogenetically [62]. The cause of this loss of differentiation is due to the breakdown in communication between the epithelial and mesenchymal components of the alveolar wall that fostered the growth and differentiation of the alveoli during development [7].
Similarly, modification of these cell-cell communications was responsible for the evolution of the lung from the swim bladder [10] phylogenetically, and ultimately in evolutionary adaptation to air breathing [12]. Thus, the heterochronic principle can be seen during the course of the reorientation of the cell-cell signaling mechanisms in the tissues that evolved to adapt to a novel environment [9,10]. As evidence of the causal nature of this mechanism, Peroxisome Proliferator Activated Receptor gamma (PPARγ) agonists [74] can prevent this loss of differentiated structure and function because it acts on the pathway that originally evolved to protect the lung against oxidant injury [41], referring all the way back in vertebrate phylogeny to a more general adaptation to oxidant injury by mesodermal cells [75].
PPARγ regulates peroxisome formation [76], and as such refers to the stage in eukaryote evolution when rising levels of oxygen caused Endoplasmic Reticulum Stress in unicellular organisms, resulting in calcium leaking into the cytoplasm, threatening to congeal nucleotides, proteins and lipids alike [77]. The evolutionary epistatic balancing mechanism was the Peroxisome [39], which utilizes lipids to buffer calcium dyshomeostasis. Therefore, the antecedents of the heterochronic redistribution of genetic expression can be seen in this pathobiologic model of loss and gain of homeostasis as the essence of evolution.
8. Goodpasture’s Syndrome as Waterproofing
Goodpasture’s Syndrome exhibits a similar evolutionary etiology. The disease state is due to the formation of autoantibodies against an isoform of Type IV collagen, namely Alpha 3(IV)NC1. It first appears phylogenetically in fish, and is omnipresent in amphibians, reptiles, mammals and birds. Evolutionarily, it is more hydrophobic than other Type IV collagen isoforms, preventing water loss across lung and kidney epithelial barriers in terrestrial vertebrates. Its appearance in land animals was probably due to this adaptive property.
Bearing in mind that the extracellular matrix is generated by cell-cell interactions, this isoform of Type IV collagen would have been the result of a heterochronic process.
9. Conclusions
The perspective expressed in this paper is that heterochrony is not a random mutational event, but instead is like Jacob’s “tinkering” mechanism [78], reallocating biologic properties for novel uses by “rewiring” cell-cell signaling mechanisms as the source of novel phenotypic change. Mutations can occur within a specific biologic context, resulting in change consistent with the prevailing physical constraint when “deciphered” by the biologic cell-cell signaling. This has been true right from the inception of life itself, the micelle providing a protected environment for the evolution of catalysis, negentropy, chemiosmosis and homeostasis in order to cope with the vicissitudes of perpetual environmental change [79]. The key to understanding this process is in focusing on the communication between the organism and its environment, internalizing and compartmentalizing toxic substances (oxygen, ions, heavy metals) that would otherwise have destroyed it, forming physiologic systems in the process [8]. The subsequent formation of multicellular organisms was predicated on cell-cell communication for further adaptation, but always returning to the unicellular state, perhaps because it is the unicellular state that is the primary level of selection [10].
By seeing the process of evolution as communication, novel insights are gained that would otherwise remain tautologies and dogma [80]. Kuhn [14] defined a paradigm shift as a change in the language. The shift from heterochrony as a descriptive change in timing to alterations in developmental-homeostatic mechanisms changes the language. This is analogous to the paradigm shift in our understanding of gravity from Newton’s Law of Gravity that described the process to Einstein’s explanation of gravity as the distortion of space-time.
Acknowledgments
I wish to thank William B. Miller for reviewing this manuscript. John S. Torday has been supported by NIH Grant HL055268.
Conflicts of Interest
The author declare no conflict of interest.
References
- Gilbert, S.F. Ernst haeckel and the biogenetic law. In Developmental Biology; Sinauer Associates: Sunderland, MA, USA, 2006. [Google Scholar]
- Gould, S.J. Ontogeny and Phylogeny; Harvard University Press: Cambridge, MA, USA, 1977. [Google Scholar]
- Smith, K.K. Time’s arrow: Heterochrony and the evolution of development. Int. J. Dev. Biol. 2003, 47, 613–621. [Google Scholar] [PubMed]
- Gilbert, S.F.; Opitz, J.M.; Raff, R.A. Resynthesizing evolutionary and developmental biology. Dev. Biol. 1996, 173, 357–372. [Google Scholar] [CrossRef] [PubMed]
- De Robertis, E.M.; Larraín, J.; Oelgeschläger, M.; Wessely, O. The establishment of Spemann’s organizer and patterning of the vertebrate embryo. Nat. Rev. Genet. 2000, 1, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Danesh, S.M.; Villasenor, A.; Chong, D.; Soukup, C.; Cleaver, O. BMP and BMP receptor expression during murine organogenesis. Gene Expr. Patterns 2009, 9, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Croce, J.C.; McClay, D.R. Evolution of the Wnt pathways. Methods Mol. Biol. 2008, 469, 3–18. [Google Scholar] [PubMed]
- Torday, J.S.; Rehan, V.K. Evolutionary Biology, Cell-Cell Communication and Complex Disease; Wiley: Hoboken, NJ, USA, 2012. [Google Scholar]
- Torday, J.S. Evolutionary biology redux. Perspect. Biol. Med. 2013, 56, 455–484. [Google Scholar] [CrossRef] [PubMed]
- Torday, J.S. A central theory of biology. Med. Hypotheses 2015, 85, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Smocovitis, V.B. Unifying Biology; Princeton University Press: Princeton, NJ, USA, 1996. [Google Scholar]
- Gould, S.J. The Structure of Evolutionary Theory; Harvard University Press: Cambridge, MA, USA, 2002. [Google Scholar]
- Thisse, B.; Thisse, C. Formation of the vertebrate embryo: Moving beyond the Spemann organizer. Semin. Cell Dev. Biol. 2015, 42, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, T.S. The Structure of Scientific Revolutions; University of Chicago Press: Chicago, IL, USA, 1962. [Google Scholar]
- Saiz, N.; Plusa, B. Early cell fate decisions in the mouse embryo. Reproduction 2013, 145, R65–R80. [Google Scholar] [CrossRef] [PubMed]
- Hubaud, A.; Pourquié, O. Signalling dynamics in vertebrate segmentation. Nat. Rev. Mol. Cell Biol. 2014, 15, 709–721. [Google Scholar] [CrossRef] [PubMed]
- De Paepe, C.; Krivega, M.; Cauffman, G.; Geens, M.; van de Velde, H. Totipotency and lineage segregation in the human embryo. Mol. Hum. Reprod. 2014, 20, 599–618. [Google Scholar] [CrossRef] [PubMed]
- Fowden, A.L.; Forhead, A.J. Glucocorticoids as regulatory signals during intrauterine development. Exp. Physiol. 2015, 100, 1477–1487. [Google Scholar] [CrossRef] [PubMed]
- Taylor, S.A.; Larson, E.L.; Harrison, R.G. Hybrid zones: Windows on climate change. Trends Ecol. Evol. 2015, 30, 398–406. [Google Scholar] [CrossRef] [PubMed]
- Wollstein, A.; Stephan, W. Inferring positive selection in humans from genomic data. Investig. Genet. 2015. [Google Scholar] [CrossRef] [PubMed]
- Shubin, N.H.; Daeschler, E.B.; Jenkins, F.A., Jr. Pelvic girdle and fin of Tiktaalik roseae. Proc. Natl. Acad. Sci. USA 2014, 111, 893–899. [Google Scholar] [CrossRef] [PubMed]
- Davies, T.J. Losing history: How extinctions prune features from the tree of life. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2015. [Google Scholar] [CrossRef] [PubMed]
- Ward, P.; Labandeira, C.; Laurin, M.; Berner, R.A. Confirmation of Romer’s Gap as a low oxygen interval constraining the timing of initial arthropod and vertebrate terrestrialization. Proc. Natl. Acad. Sci. USA 2006, 103, 16818–16822. [Google Scholar] [CrossRef] [PubMed]
- Romer, A.S. The Vertebrate Story; University of Chicago Press: Chicago, IL, USA, 1949. [Google Scholar]
- Pinheiro, P.L.; Cardoso, J.C.; Power, D.M.; Canário, A.V. Functional characterization and evolution of PTH/PTHrP receptors: Insights from the chicken. BMC Evol. Biol. 2012. [Google Scholar] [CrossRef] [PubMed]
- Verheijen, M.H.; Defize, L.H. Signals governing extraembryonic endoderm formation in the mouse: Involvement of the type 1 parathyroid hormone-related peptide (PTHrP) receptor, p21Ras and cell adhesion molecules. Int. J. Dev. Biol. 1999, 43, 711–721. [Google Scholar] [PubMed]
- Rubin, L.P.; Kifor, O.; Hua, J.; Brown, E.M.; Torday, J.S. Parathyroid hormone (PTH) and PTH-related protein stimulate surfactant phospholipid synthesis in rat fetal lung, apparently by a mesenchymal-epithelial mechanism. Biochim. Biophys. Acta 1994, 1223, 91–100. [Google Scholar] [CrossRef]
- Rubin, L.P.; Kovacs, C.S.; de Paepe, M.E.; Tsai, S.W.; Torday, J.S.; Kronenberg, H.M. Arrested pulmonary alveolar cytodifferentiation and defective surfactant synthesis in mice missing the gene for parathyroid hormone-related protein. Dev. Dyn. 2004, 230, 278–289. [Google Scholar] [CrossRef] [PubMed]
- Hochane, M.; Raison, D.; Coquard, C.; Imhoff, O.; Massfelder, T.; Moulin, B.; Helwig, J.J.; Barthelmebs, M. Parathyroid hormone-related protein is a mitogenic and a survival factor of mesangial cells from male mice: Role of intracrine and paracrine pathways. Endocrinology 2013, 154, 853–864. [Google Scholar] [CrossRef] [PubMed]
- Torday, J.S. Parathyroid hormone-related protein is a gravisensor in lung and bone cell biology. Adv. Space Res. 2003, 32, 1569–1576. [Google Scholar] [CrossRef]
- Foley, J.; Longely, B.J.; Wysolmerski, J.J.; Dreyer, B.E.; Broadus, A.E.; Philbrick, W.M. PTHrP regulates epidermal differentiation in adult mice. J. Investig. Dermatol. 1998, 111, 1122–1128. [Google Scholar] [CrossRef] [PubMed]
- Holland, N.D. Early central nervous system evolution: An era of skin brains? Nat. Rev. Neurosci. 2003, 4, 617–627. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Wang, Z.; Collins, J.E.; Andrews, R.M.; Stemple, D.; Gong, Z. Comparative transcriptome analyses indicate molecular homology of zebrafish swimbladder and mammalian lung. PLoS ONE 2011, 6, e24019. [Google Scholar] [CrossRef] [PubMed]
- Daniels, C.B.; Orgeig, S.; Sullivan, L.C.; Ling, N.; Bennett, M.B.; Schürch, S.; Val, A.L.; Brauner, C.J. The origin and evolution of the surfactant system in fish: Insights into the evolution of lungs and swim bladders. Physiol. Biochem. Zool. 2004, 77, 732–749. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, L.C.; Daniels, C.B.; Phillips, I.D.; Orgeig, S.; Whitsett, J.A. Conservation of surfactant protein A: Evidence for a single origin for vertebrate pulmonary surfactant. J. Mol. Evol. 1998, 46, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Torday, J.S.; Rehan, V.K. The evolutionary continuum from lung development to homeostasis and repair. Am. J. Physiol. Lung Cell. Mol. Physiol. 2007, 292, L608–L611. [Google Scholar] [CrossRef] [PubMed]
- Torday, J.S.; Rehan, V.K. The evolution of cell communication: The road not taken. Cell Commun. Insights 2009, 2, 17–25. [Google Scholar] [PubMed]
- Torday, J.S.; Rehan, V.K. On the evolution of the Lipofibroblast. Exp. Cell Res. 2015, in press. [Google Scholar] [CrossRef] [PubMed]
- De Duve, C. Evolution of the peroxisome. Ann. N. Y. Acad. Sci. 1969, 168, 369–381. [Google Scholar] [CrossRef] [PubMed]
- Torday, J.S.; Rehan, V.K. Developmental cell/molecular biologic approach to the etiology and treatment of bronchopulmonary dysplasia. Pediatr. Res. 2007, 62, 2–7. [Google Scholar] [CrossRef] [PubMed]
- Torday, J.S.; Torday, D.P.; Gutnick, J.; Qin, J.; Rehan, V. Biologic role of fetal lung fibroblast triglycerides as antioxidants. Pediatr. Res. 2001, 49, 843–849. [Google Scholar] [CrossRef] [PubMed]
- Schultz, C.J.; Torres, E.; Londos, C.; Torday, J.S. Role of adipocyte differentiation-related protein in surfactant phospholipid synthesis by type II cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 2002, 283, L288–L296. [Google Scholar] [CrossRef] [PubMed]
- Torday, J.S.; Sun, H.; Qin, J. Prostaglandin E2 integrates the effects of fluid distension and glucocorticoid on lung maturation. Am. J. Physiol. 1998, 274, L106–L111. [Google Scholar] [PubMed]
- Torday, J.S.; Sun, H.; Wang, L.; Torres, E.; Sunday, M.E.; Rubin, L.P. Leptin mediates the parathyroid hormone-related protein paracrine stimulation of fetal lung maturation. Am. J. Physiol. Lung Cell. Mol. Physiol. 2002, 282, L405–L410. [Google Scholar] [PubMed]
- O’Hare, K.H.; Sheridan, M.N. Electron microscopic observations on the morphogenesis of the albino rat lung, with special reference to pulmonary epithelial cells. Am. J. Anat. 1970, 127, 181–206. [Google Scholar] [CrossRef] [PubMed]
- Vaccaro, C.; Brody, J.S. Ultrastructure of developing alveoli. I. The role of the interstitial fibroblast. Anat. Rec. 1978, 192, 467–480. [Google Scholar] [CrossRef] [PubMed]
- Maksvytis, H.J.; Niles, R.M.; Simanovsky, L.; Minassian, I.A.; Richardson, L.L.; Hamosh, M.; Hamosh, P.; Brody, J.S. In vitro characteristics of the lipid-filled interstitial cell associated with postnatal lung growth: Evidence for fibroblast heterogeneity. J. Cell Physiol. 1984, 118, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, N.B.; Grant, M.M.; Brody, J.S. The lipid interstitial cell of the pulmonary alveolus. Age and species differences. Am. Rev. Respir. Dis. 1985, 132, 1307–1312. [Google Scholar] [PubMed]
- Rehan, V.K.; Sugano, S.; Wang, Y.; Santos, J.; Romero, S.; Dasgupta, C.; Keane, M.P.; Stahlman, M.T.; Torday, J.S. Evidence for the presence of lipofibroblasts in human lung. Exp. Lung Res. 2006, 32, 379–393. [Google Scholar] [CrossRef] [PubMed]
- Frank, L.; Bucher, J.R.; Roberts, R.J. Oxygen toxicity in neonatal and adult animals of various species. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 1978, 45, 699–704. [Google Scholar] [PubMed]
- Mostello, D.J.; Hamosh, M.; Hamosh, P. Effect of dexamethasone on lipoprotein lipase activity of fetal rat lung. Biol. Neonate 1981, 40, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Torday, J.; Hua, J.; Slavin, R. Metabolism and fate of neutral lipids of fetal lung fibroblast origin. Biochim. Biophys. Acta 1995, 1254, 198–206. [Google Scholar] [CrossRef]
- Londos, C.; Sztalryd, C.; Tansey, J.T.; Kimmel, A.R. Role of PAT proteins in lipid metabolism. Biochimie 2005, 87, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Torday, J.; Rehan, V. Neutral lipid trafficking regulates alveolar type II cell surfactant phospholipid and surfactant protein expression. Exp. Lung Res. 2011, 37, 376–386. [Google Scholar] [CrossRef] [PubMed]
- Torday, J.S.; Sanchez-Esteban, J.; Rubin, L.P. Paracrine mediators of mechanotransduction in lung development. Am. J. Med. Sci. 1998, 316, 205–208. [Google Scholar] [PubMed]
- Sanchez-Esteban, J.; Cicchiello, L.A.; Wang, Y.; Tsai, S.W.; Williams, L.K.; Torday, J.S.; Rubin, L.P. Mechanical stretch promotes alveolar epithelial type II cell differentiation. J. Appl. Physiol. 2001, 91, 589–595. [Google Scholar] [PubMed]
- Torday, J.S.; Rehan, V.K. Up-regulation of fetal rat lung parathyroid hormone-related protein gene regulatory network down-regulates the Sonic Hedgehog/Wnt/betacatenin gene regulatory network. Pediatr. Res. 2006, 60, 382–388. [Google Scholar] [CrossRef] [PubMed]
- Purevdorj-Gage, B.; Sheehan, K.B.; Hyman, L.E. Effects of low-shear modeled microgravity on cell function, gene expression, and phenotype in Saccharomyces cerevisiae. Appl. Environ. Microbiol. 2006, 72, 4569–4575. [Google Scholar] [CrossRef] [PubMed]
- Stein, M.; Wandinger-Ness, A.; Roitbak, T. Altered trafficking and epithelial cell polarity in disease. Trends Cell Biol. 2002, 12, 374–381. [Google Scholar] [CrossRef]
- Knop, M. Yeast cell morphology and sexual reproduction—A short overview and some considerations. C. R. Biol. 2011, 334, 599–606. [Google Scholar] [CrossRef] [PubMed]
- Perry, S.F.; Carrier, D.R. The coupled evolution of breathing and locomotion as a game of leapfrog. Physiol. Biochem. Zool. 2006, 79, 997–999. [Google Scholar] [CrossRef] [PubMed]
- Payne, J.L.; Boyer, A.G.; Brown, J.H.; Finnegan, S.; Kowalewski, M.; Krause, R.A., Jr.; Lyons, S.K.; McClain, C.R.; McShea, D.W.; Novack-Gottshall, P.M.; et al. Two-phase increase in the maximum size of life over 3.5 billion years reflects biological innovation and environmental opportunity. Proc. Nat. Acad. Sci. USA 2009, 106, 24–27. [Google Scholar] [CrossRef] [PubMed]
- Berner, R.A.; Vandenbrooks, J.M.; Ward, P.D. Evolution. Oxyg. Evol. Sci. 2007, 316, 557–558. [Google Scholar]
- Graham, J.B.; Dudley, R.; Aguilar, N.M.; Gans, C. Implications of the later Palaeozoic oxygen pulse for physiology and evolution. Nature 1995, 375, 117–120. [Google Scholar] [CrossRef]
- Mamillapalli, R.; Wysolmerski, J. The calcium-sensing receptor couples to Galpha(s) and regulates PTHrP and ACTH secretion in pituitary cells. J. Endocrinol. 2010, 204, 287–297. [Google Scholar] [CrossRef] [PubMed]
- Suri, L.N.; McCaig, L.; Picardi, M.V.; Ospina, O.L.; Veldhuizen, R.A.; Staples, J.F.; Possmayer, F.; Yao, L.J.; Perez-Gil, J.; Orgeig, S. Adaptation to low body temperature influences pulmonary surfactant composition thereby increasing fluidity while maintaining appropriately ordered membrane structure and surface activity. Biochim. Biophys. Acta 2012, 1818, 1581–1589. [Google Scholar] [CrossRef] [PubMed]
- Perlman, R.L.; Chalfie, M. Catecholamine release from the adrenal medulla. Clin. Endocrinol. Metab. 1977, 6, 551–576. [Google Scholar] [CrossRef]
- Maina, J.N. Structure, function and evolution of the gas exchangers: Comparative perspectives. J Anat. 2002, 201, 281–304. [Google Scholar] [CrossRef] [PubMed]
- Daniels, C.B.; Lopatko, O.V.; Orgeig, S. Evolution of surface activity related functions of vertebrate pulmonary surfactant. Clin. Exp. Pharmacol. Physiol. 1998, 25, 716–721. [Google Scholar] [CrossRef] [PubMed]
- Lau, M.J.; Keough, K.M. Lipid composition of lung and lung lavage fluid from map turtles (Malaclemys geographica) maintained at different environmental temperatures. Can. J. Biochem. 1981, 59, 208–219. [Google Scholar] [CrossRef] [PubMed]
- Lopatko, O.V.; Orgeig, S.; Palmer, D.; Schürch, S.; Daniels, C.B. Alterations in pulmonary surfactant after rapid arousal from torpor in the marsupial Sminthopsis crassicaudata. J. Appl. Physiol. 1999, 86, 1959–1970. [Google Scholar] [PubMed]
- Voelkel, N.F.; MacNee, W. Chronic Obstructive Lung Diseases 2; BC Decker Inc.: Hamilton, ON, Canada, 2008. [Google Scholar]
- McGowan, S.E.; Torday, J.S. The pulmonary lipofibroblast (lipid interstitial cell) and its contributions to alveolar development. Annu. Rev. Physiol. 1997, 59, 43–62. [Google Scholar] [CrossRef] [PubMed]
- Cerny, L.; Torday, J.S.; Rehan, V.K. Prevention and treatment of bronchopulmonary dysplasia: Contemporary status and future outlook. Lung 2008, 186, 75–89. [Google Scholar] [CrossRef] [PubMed]
- Csete, M.; Walikonis, J.; Slawny, N.; Wei, Y.; Korsnes, S.; Doyle, J.C.; Wold, B. Oxygen-mediated regulation of skeletal muscle satellite cell proliferation and adipogenesis in culture. J. Cell Physiol. 2001, 189, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Tontonoz, P.; Hu, E.; Spiegelman, B.M. Regulation of adipocyte gene expression and differentiation by peroxisome proliferator activated receptor gamma. Curr. Opin. Genet. Dev. 1995, 5, 571–576. [Google Scholar] [CrossRef]
- Case, R.M.; Eisner, D.; Gurney, A.; Jones, O.; Muallem, S.; Verkhratsky, A. Evolution of calcium homeostasis: From birth of the first cell to an omnipresent signaling system. Cell Calcium 2007, 42, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Jacob, F. Evolution and tinkering. Science 1977, 196, 1161–1166. [Google Scholar] [CrossRef] [PubMed]
- Deamer, D.W. Polycyclic aromatic hydrocarbons: Primitive pigment systems in the prebiotic environment. Adv. Space Res. 1992, 12, 183–189. [Google Scholar] [CrossRef]
- Roux, E. The concept of function in modern physiology. J. Physiol. 2014, 592, 2245–2249. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the author; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).