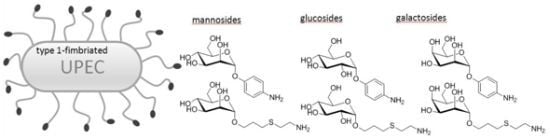
Effect of Aminophenyl and Aminothiahexyl α-D-Glycosides of the Manno-, Gluco-, and Galacto-Series on Type 1 Fimbriae-Mediated Adhesion of Escherichia coli
Abstract
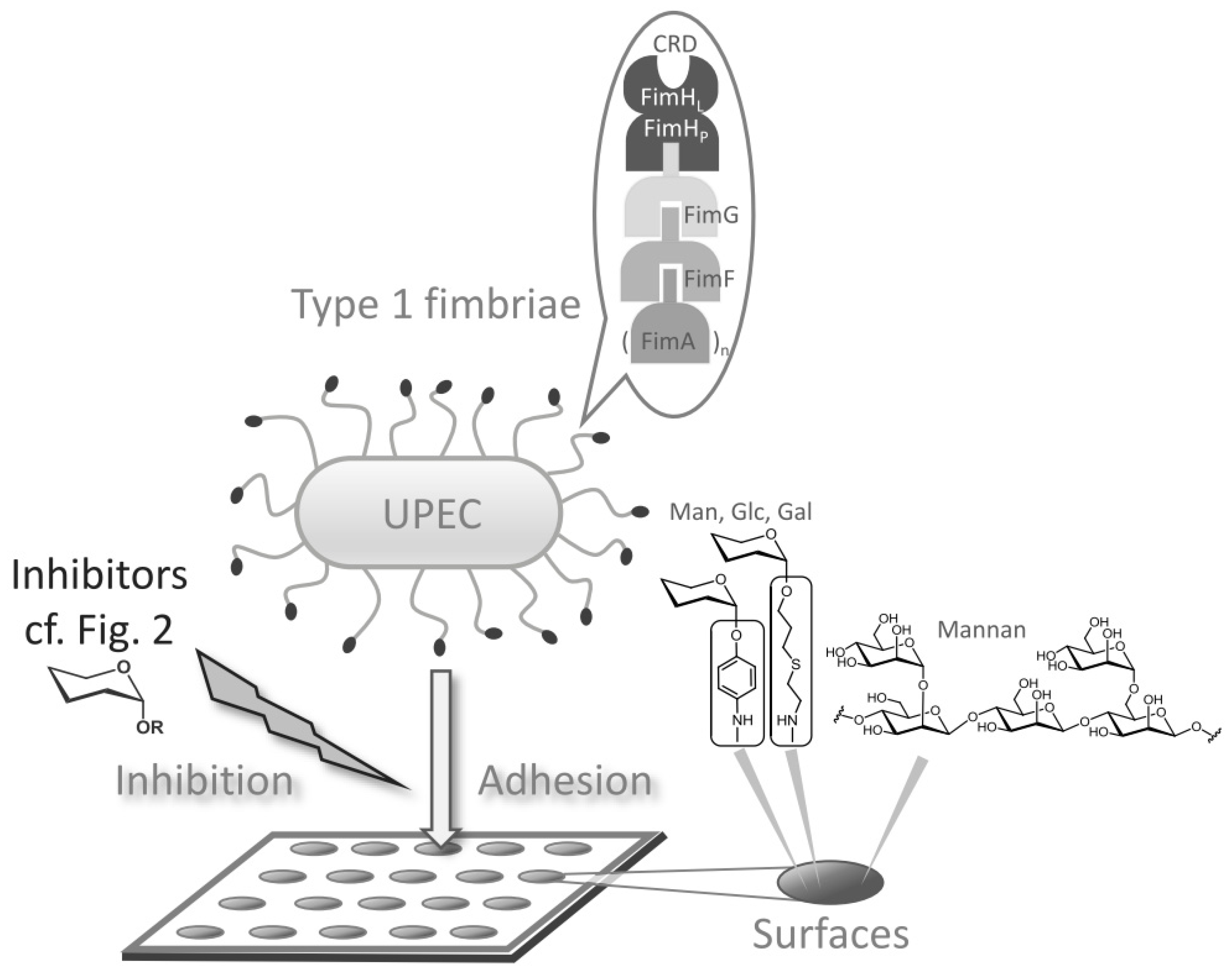
:1. Introduction
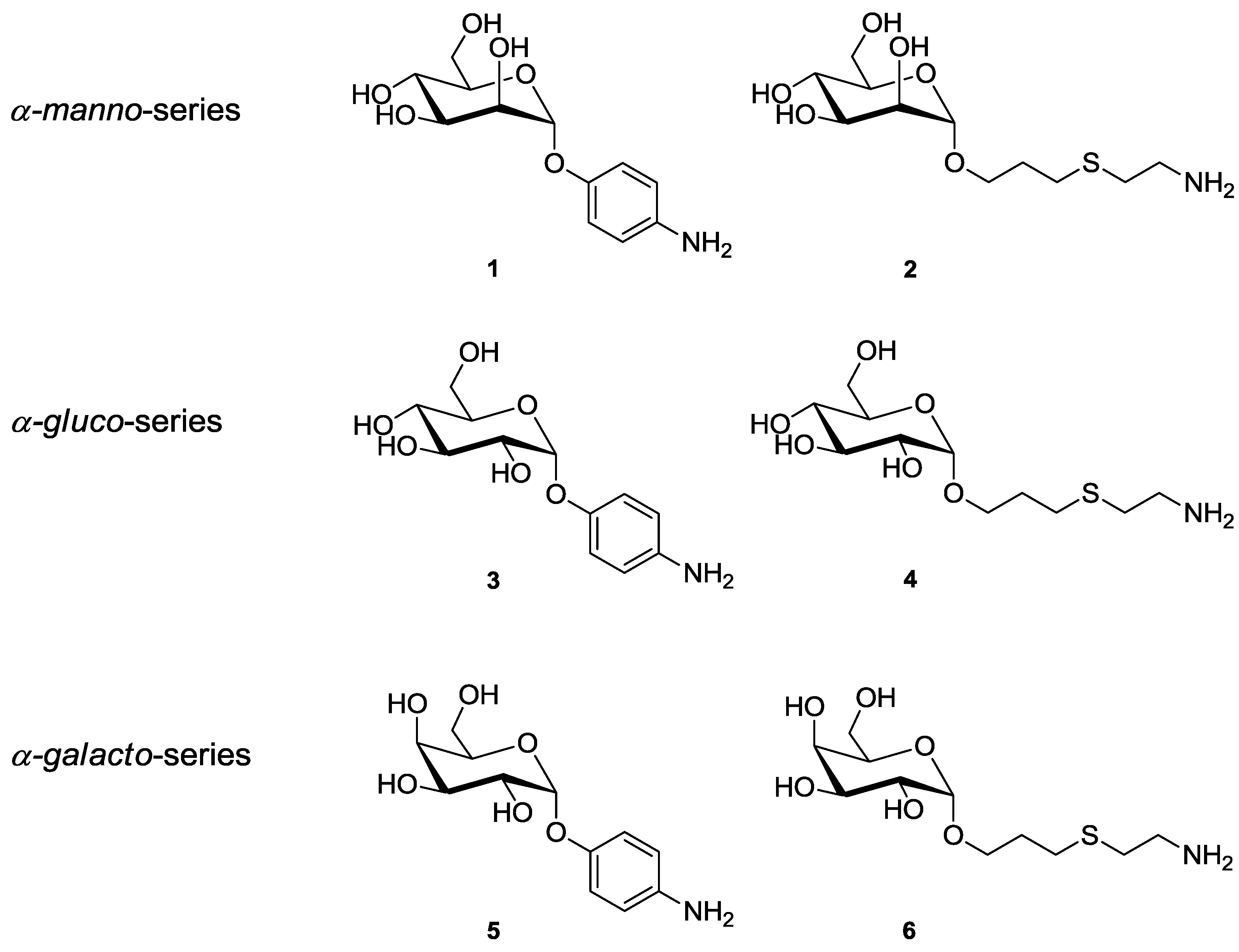
2. Experimental Section
2.1. Glycoside Synthesis
2.2. Media and Buffer Solutions
2.3. Bacterial Strain, Growth Conditions
2.4. Mannan Coating of Microtiter Plates, Covalent Functionalization
2.5. Covalent Functionalization of Microtiter Plates: Glycoarray Preparation
2.6. Binding Assay with GFP-Tagged E. coli
2.7. Adhesion-Inhibition Assay with GFP-Tagged E. coli
2.8. Preincubation-Inhibition-Adhesion Assay with GFP-Tagged E. coli
2.9. Bacterial Growth Tests
3. Results
3.1. Binding of GFP-Tagged E. coli to Glycoarrays on Polystyrene Microtiter Plates
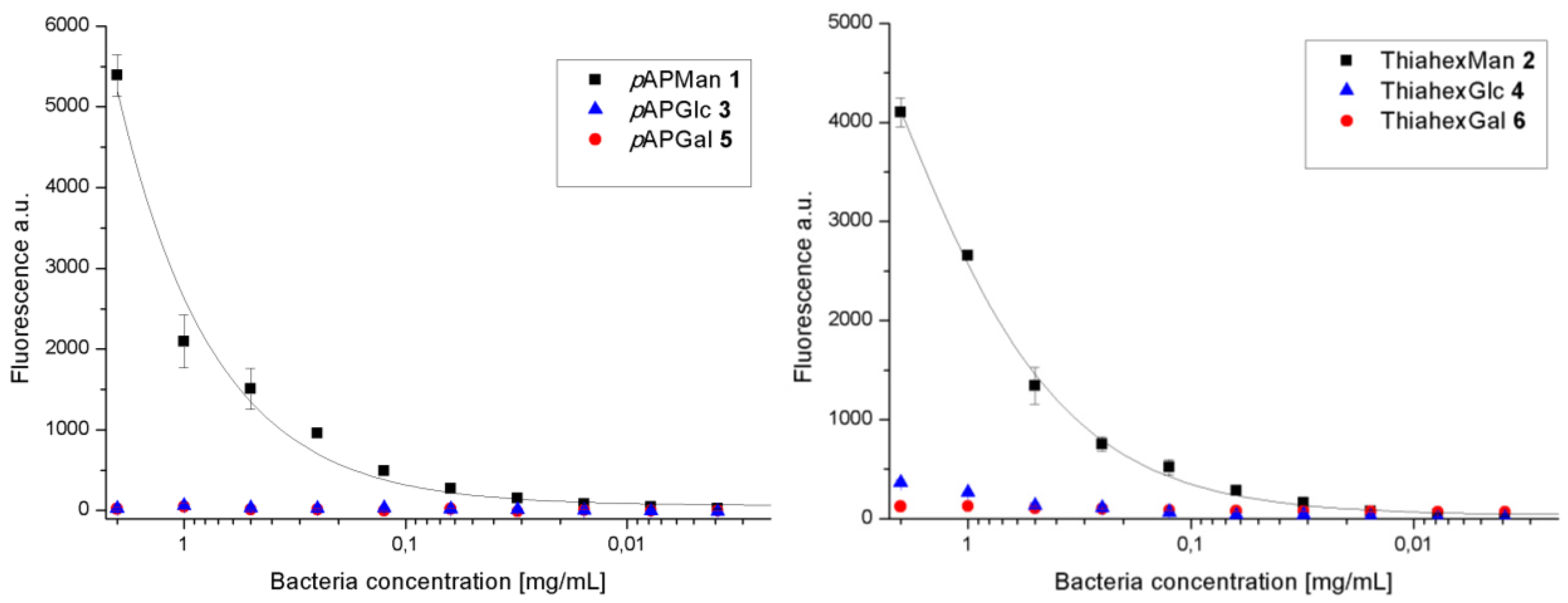
3.2. Adhesion-Inhibition Assay with GFP-Tagged E. coli

3.3. Preincubation-Inhibition-Adhesion Assay with GFP-Tagged E. coli
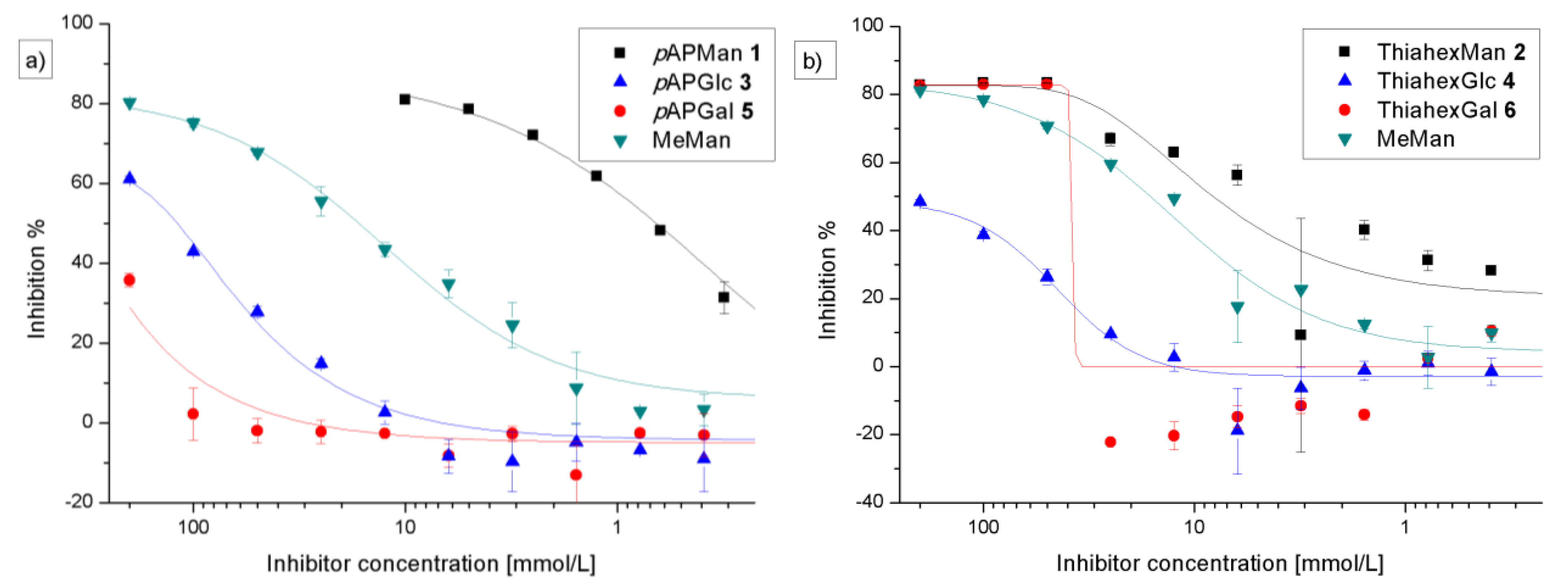
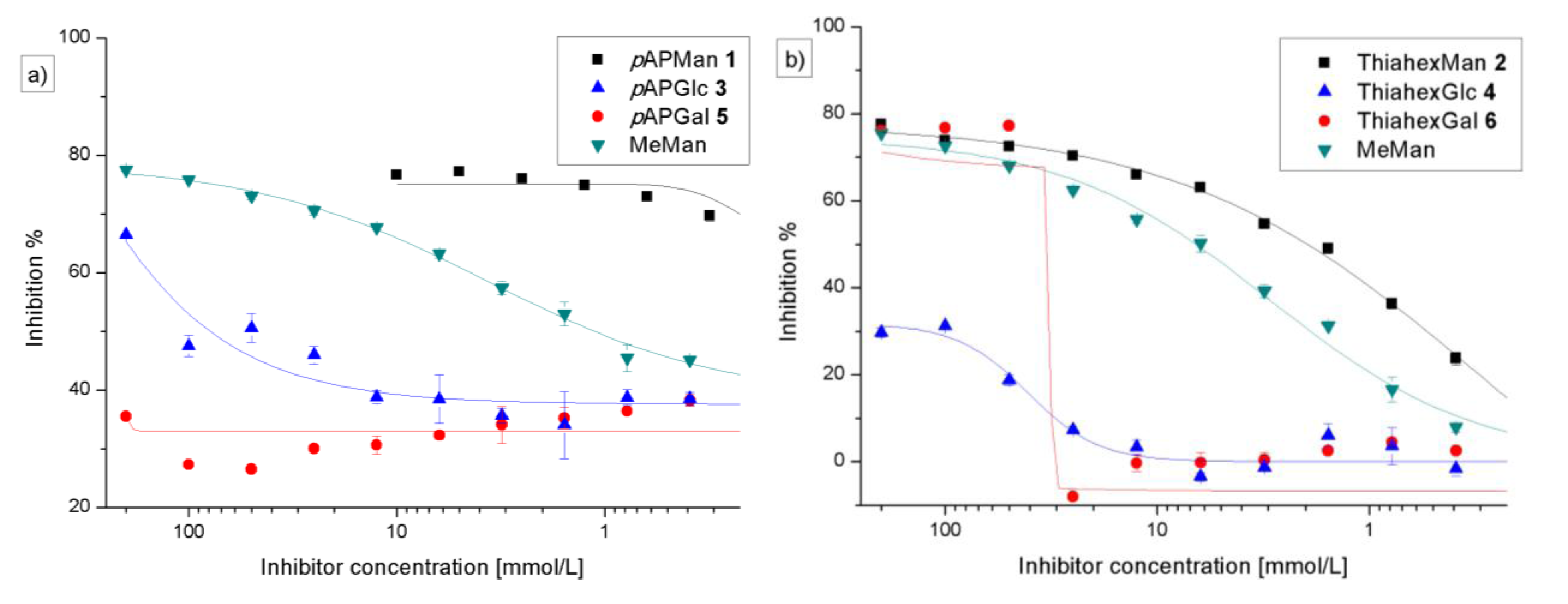
| Adhesion-inhibition assay | Preincubation-inhibition-adhesion assay | |||||
|---|---|---|---|---|---|---|
| Glycoside | IC50 [mm] (SD) | RIP#(SD) | total inhibition % | IC50 [mm] (SD) | RIP#(SD) | total inhibition % |
| 1 (pAPMan) | 0.62 (0.21) | 27.8 (1.65) | 83 | 0.035 (0.02) | 30.2 (7.8) | 76 |
| 2 (ThiahexMan) | 5.28 (2.47) | 2.41 (0.37) | 80 | 1.19 (0.79) | 3.46 (0.12) | 78 |
| 3 (pAPGlc) | 122 (1.99) | 0.135 (0.01) | 60 | 51.35 (27) | 0.023 (0.01) | 63 |
| 4 (ThiahexGlc) | − | − | 45 | − | − | 46 |
| 5 (pAPGal) | − | − | 32 | − | − | 49 |
| 6 (ThiahexGal) | − | − | 77 | − | − | 75 |
3.4. Bacterial Growth Tests
| pAPMan 1 | pAPGlc 3 | pAPGal 5 | ThiahexMan 2 | ThiahexGlc 4 | ThiahexGal 6 | |
| 200 mM | + | + | + | − | − | − |
| 100 mM | + | + | + | − | − | − |
| 50 mM | + | + | + | − | + | − |
| 25 mM | + | + | + | − | + | + |
| 12.5 mM | n.t. | n.t. | n.t. | + | n.t. | n.t. |
4. Discussion and Conclusion
Acknowledgments
Conflicts of Interest
References and Notes
- Brumbaugh, A.R.; Mobley, H.L. Preventing urinary tract infection: Progress towards an effective Escherichia coli vaccine. Expert Rev. Vaccines 2012, 11, 663–676. [Google Scholar] [CrossRef]
- Melican, K.; Sandoval, R.M.; Kader, A.; Josefsson, L.; Tanner, G.A.; Molitoris, B.A.; Richter-Dahlfors, A. Uropathogenic Escherichia coli P and type 1 fimbriae act in synergy in a living host to facilitate renal colonization leading to nephron obstruction. PLoS Pathog. 2011, 7, e1001298. [Google Scholar] [CrossRef]
- Klemm, P.; Schembri, M.A. Bacterial adhesins: Function and structure. Int. J. Med. Microbiol. 2000, 290, 27–35. [Google Scholar] [CrossRef]
- Ohlsen, K.; Oelschlaeger, T.A.; Hacker, J.; Khan, A.S. Carbohydrate receptors of bacterial adhesins: Implications and reflections. Top. Curr. Chem. 2009, 288, 109–120. [Google Scholar]
- Kau, A.L.; Hunstad, D.A.; Hultgren, S.J. Interaction of uropathogenic Escherichia coli with host uroepithelium. Curr. Opin. Microbiol. 2005, 8, 54–59. [Google Scholar]
- Kim, K.S. Current concepts on the pathogenesis of Escherichia coli meningitis: Implications for therapy and prevention. Curr. Opin. Infect. Dis. 2012, 25, 273–280. [Google Scholar] [CrossRef]
- Chassaing, B.; Darfeuille-Michaud, A. The σE pathway is involved in biofilm formation by Crohn’s disease-associated adherent Escherichia coli. J. Bacteriol. 2013, 195, 76–84. [Google Scholar] [CrossRef]
- Ofek, I.; Beachey, E.H. Mannose Binding and Epithelial Cell Adherence of Escherichia coli. Infect. Immun. 1978, 22, 247–254. [Google Scholar]
- Firon, N.; Ofek, I.; Sharon, N. Carbohydrate specificity of the surface lectins of Escherichia coli, Klebsiella pneumoniae, and Salmonella typhimurium. Carbohydr. Res. 1983, 120, 235–249. [Google Scholar] [CrossRef]
- Firon, N.; Ashkenazi, S.; Mirelman, D.; Ofek, I.; Sharon, N. Aromatic alpha-glycosides of mannose are powerful inhibitors of the adherence of type 1 fimbriated Escherichia coli to yeast and intestinal epithelial cells. Infect. Immun. 1987, 55, 472–476. [Google Scholar]
- Neeser, J.R.; Koellreutter, B.; Wuersch, P. Oligomannoside-type glycopeptides inhibiting adhesion of Escherichia coli strains mediated by type 1 pili: Preparation of potent inhibitors from plant glycoproteins. Infect. Immun. 1986, 52, 428–436. [Google Scholar]
- Sharon, N. Bacterial lectins, cell-cell recognition and infectious disease. FEBS Lett. 1987, 217, 145–157. [Google Scholar] [CrossRef]
- Crocker, P.R.; Feizi, T. Carbohydrate recognition systems: Functional triads in cell-cell interactions. Curr. Opin. Struct. Biol. 1996, 6, 679–691. [Google Scholar] [CrossRef]
- Lindhorst, T.K.; Kieburg, C.; Krallmann-Wenzel, U. Inhibition of the type 1 fimbriae-mediated adhesion of Escherichia coli to erythrocytes by multiantennary α-mannosyl clusters: The effect of multivalency. Glycoconj. J. 1998, 15, 605–613. [Google Scholar] [CrossRef]
- Nagahori, N.; Lee, R.T.; Nishimura, S.-I.; Pagé, D.; Roy, R.; Lee, Y.C. Inhibition of Adhesion of Type 1 Fimbriated Escherichia coli to Highly Mannosylated Ligands. Chem. Bio. Chem. 2002, 3, 836–844. [Google Scholar] [CrossRef]
- Dubber, M.; Sperling, O.; Lindhorst, T.K. Oligomannoside mimetics by glycosylation of ‘octopus glycosides’ and their investigation as inhibitors of type 1 fimbriated-mediated adhesion of Escherichia coli. Org. Biomol. Chem. 2006, 4, 3901–3912. [Google Scholar] [CrossRef]
- Touaibia, M.; Wellens, A.; Shiao, T.C.; Wang, Q.; Sirois, S.; Bouckaert, J.; Roy, R. Mannosylated G(0) dendrimers with nanomolar affinities to Escherichia coli FimH. Chem. Med. Chem. 2007, 2, 1190–1201. [Google Scholar]
- Heidecke, C.; Lindhorst, T.K. Iterative Synthesis of Spacered Glycodendrons as Oligomannoside Mimetics and Evaluation of Their Antiadhesive Properties. Chem. Eur. J. 2007, 13, 9056–9067. [Google Scholar] [CrossRef]
- Touaibia, M.; Roy, R. Glycodendrimers as anti-adhesion drugs against type 1 fimbriated E. coli uropathogenic infections. Mini Rev. Med. Chem. 2007, 7, 1270–1283. [Google Scholar] [CrossRef]
- Pieters, R.J. Intervention with Bacterial Adhesion by Multivalent Carbohydrates. Med. Res. Rev. 2007, 27, 796–816. [Google Scholar] [CrossRef]
- Gouin, S.G.; Wellens, A.; Bouckaert, J.; Kovensky, J. Synthetic multimeric heptyl mannosides as potent antiadhesives of uropathogenic Escherichia coli. Chem. Med. Chem. 2009, 4, 749–755. [Google Scholar]
- Chabre, Y.M.; Roy, R. Design and creativity in synthesis of multivalent neoglycoconjugates. Adv. Carbohydr. Chem. Biochem. 2010, 63, 165–393. [Google Scholar] [CrossRef]
- Pieters, R.J. Carbohydrate-Mediated Bacterial Adhesion. Adv. Exp. Med. Biol. 2011, 715, 227–240. [Google Scholar] [CrossRef]
- Papadopoulos, A.; Chieh Shiao, T.; Roy, R. Diazo Transfer and Click Chemistry in the Solid Phase Syntheses of Lysine-Based Glycodendrimers as Antagonists against Escherichia coli FimH. Mol. Pharmaceutics 2012, 9, 394–403. [Google Scholar] [CrossRef]
- Bouckaert, J.; Berglund, J.; Schembri, M.A.; De Genst, E.; Cools, L.; Wuhrer, M.; Hung, C.-S.; Pinkner, J.; Slättegård, R.; Zavialov, A.; et al. Receptor binding studies disclose a novel class of high-affinity inhibitors of the Escherichia coli FimH adhesion. Mol. Microbiol. 2005, 55, 441–455. [Google Scholar]
- Klein, T.; Abgottspon, D.; Wittwer, M.; Rabbani, S.; Herold, J.; Jiang, X.; Kleeb, S.; Lüthi, C.; Scharenberg, M.; Bezencon, J.; et al. FimH antagonist for the oral treatment of urinary tract infections: From design and synthesis to in vitro and in vivo evaluation. J. Med. Chem. 2010, 53, 8627–8641. [Google Scholar] [CrossRef]
- Grabosch, C.; Hartmann, M.; Schmidt-Lassen, J.; Lindhorst, T.K. Squaric Acid Monoamide Mannosides as Ligands for the Bacterial Lectin FimH: Covalent inhibition or not? Chem. Bio. Chem. 2011, 12, 1066–1074. [Google Scholar] [CrossRef]
- Zuilhof, H.; Pinkner, J.S.; Ford, B.; Chorell, E.; Crowley, J.M.; Cusumano, C.K.; Campbell, S.; Henderson, J.P.; Hultgren, S.J.; Janetka, J.W. Lead Optimization Studies on FimH Antagonists: Discovery of potent and orally bioavailable ortho-substituted biphenyl mannosides. J. Med. Chem. 2012, 55, 3945–3959. [Google Scholar]
- Jiang, X.; Abgottspon, D.; Kleeb, S.; Rabbani, S.; Scharenberg, M.; Wittwer, M.; Haug, M.; Schwardt, O.; Ernst, B. Anti-adhesion therapy for urinary tract infections—A balanced PK/PD-profile proved to be key for success. J. Med. Chem. 2012, 55, 4700–4713. [Google Scholar] [CrossRef]
- Abgottspon, D.; Ernst, B. In vivo Evaluation of FimH antagonists—A novel class of antimicrobials for the treatment of urinary tract infection. Chimia 2012, 66, 166–169. [Google Scholar] [CrossRef]
- Hartmann, M.; Lindhorst, T.K. The bacterial lectin FimH, a target for drug discovery—Carbohydrate inhibitors of type 1 fimbriae-mediated bacterial adhesion. Eur. J. Org. Chem. 2011. [Google Scholar] [CrossRef]
- Bernardi, A.; Jiménez-Barbero, J.; Casnati, A.; de Castro, C.; Darbre, T.; Fieschi, F.; Finne, J.; Funken, H.; Jaeger, K.E.; Lahmann, M.; et al. Multivalent glycoconjugates as anti-pathogenic agents. Chem. Soc. Rev. 2013, 42, 4709–4727. [Google Scholar] [CrossRef]
- Waksman, G.; Hultgren, S.J. Structural biology of the chaperone-usher pathway of pilus biogenesis. Nat. Rev. Microbiol. 2009, 7, 765–774. [Google Scholar] [CrossRef]
- Hung, C.-S.; Bouckaert, J.; Hung, D.; Pinkner, J.; Widberg, C.; DeFusco, A.; Auguste, C.G.; Strouse, R.; Langermann, S.; Waksman, G.; et al. Structure basis of tropism of Escherichia coli to the bladder during urinary tract infection. Mol. Microbiol. 2002, 44, 903–915. [Google Scholar] [CrossRef]
- Wellens, A.; Garofalo, C.; Nguyen, H.; van Gerven, N.; Slättegard, R.; Hernalsteens, J.P.; Wyns, L.; Oscarson, S.; de Greve, H.; Hultgren, S.; et al. Intervening with urinary tract infections using anti-adhesives based on the crystal structure of the FimH-oligomannose-3 complex. PLoS One 2008, 3, e2040. [Google Scholar] [CrossRef]
- Knight, S.D.; Bouckaert, J. Structure, function, and assembly of type 1 fimbriae. Top. Curr. Chem. 2009, 288, 67–107. [Google Scholar] [CrossRef]
- Wellens, A.; Lahmann, M.; Touaibia, M.; Vaucher, J.; Oscarson, S.; Roy, R.; Remaut, H.; Bouckaert, J. The tyrosine gate as a potential entropic lever in the receptor-binding site of the bacterial adhesin FimH. Biochemistry 2012, 51, 4790–4799. [Google Scholar] [CrossRef]
- Sperling, O.; Fuchs, A.; Lindhorst, T.K. Evaluation of the carbohydrate recognition domain of the bacterial adhesion FimH: Design, synthesis and binding properties of mannoside ligands. Org. Biomol. Chem. 2006, 4, 3913–1922. [Google Scholar] [CrossRef]
- Ernst, B.; Magnani, J.L. From carbohydrate leads to glycomimetic drugs. Nat. Rev. Drug Discov. 2009, 8, 661–677. [Google Scholar] [CrossRef]
- Ofek, I.; Hasty, D.L.; Sharon, N. Anti-adhesion therapy of bacterial diseases: Prospects and problems. FEMS Immunol. Med. Microbiol. 2003, 38, 181–191. [Google Scholar] [CrossRef]
- Sharon, N. Carbohydrates as future anti-adhesion drugs for infectious disease. Biochim. Biophys. Acta. 2006, 1760, 527–537. [Google Scholar]
- Totsika, M.; Kostakioti, M.; Hannan, T.J.; Upton, M.; Beatson, S.A.; Janetka, J.W.; Hultgren, S.J.; Schembri, M.A. A FimH inhibitor prevents acute bladder infection and treats chronic cystitis caused by multidrug-resistant uropathogenic Escherichia coli ST131. J. Infect. Dis. 2013. [Google Scholar] [CrossRef]
- Thomas, W. Catch bonds in adhesion. Annu. Rev. Biomed. Eng. 2008, 10, 39–57. [Google Scholar]
- Le Trong, I.; Aprikian, P.; Kidd, B.A.; Forero-Shelton, M.; Tchesnokova, V.; Rajagopal, P.; Rodgriguez, V.; Interlandi, G.; Klevit, R.; Vogel, V.; et al. Structural basis for mechanical force regulation of the adhesin FimH via finger trap-like beta sheet twisting. Cell 2010, 141, 645–655. [Google Scholar] [CrossRef]
- Ambrosi, M.; Cameron, N.R.; Davis, B.G. Lectins: Tools for the molecular understanding of the glycocode. Org. Biomol. Chem. 2005, 3, 1593–1608. [Google Scholar] [CrossRef]
- Hartmann, M.; Horst, A.K.; Klemm, P.; Lindhorst, T.K. A kit for the investigation of live Escherichia coli cell adhesion to glycosylated surfaces. Chem. Commun. 2010, 46, 330–332. [Google Scholar] [CrossRef]
- Reisner, A.; Haagensen, J.A.J.; Schembri, M.A.; Zechner, E.L.; Molin, S. Development and maturation of Escherichia coli K-12 biofilms. Mol. Microbiol. 2003, 48, 933–946. [Google Scholar] [CrossRef]
- Turnbull, J.E.; Field, R.A. Emerging glycomics technologies. Nat. Chem. Biol. 2007, 3, 74–77. [Google Scholar] [CrossRef]
- Horlacher, T.; Seeberger, P.H. Carbohydrate arrays as tools for research and diagnostics. Chem. Soc. Rev. 2008, 37, 1414–1422. [Google Scholar] [CrossRef]
- Liu, Y.; Palma, A.S.; Feizi, T. Carbohydrate microarrays: Key developments in glycobiology. Biol. Chem. 2009, 390, 647–656. [Google Scholar]
- Grabosch, C.; Kolbe, K.; Lindhorst, T.K. Glycoarrays by a new tandem noncovalent-covalent modification of polystyrene microtiter plates and their interrogation with live cells. Chem. Bio. Chem. 2012, 13, 1874–1879. [Google Scholar] [CrossRef]
- Wehner, J.W.; Weissenborn, M.J.; Hartmann, M.; Gray, C.J.; Šardzík, R.; Eyers, C.E.; Flitsch, S.L.; Lindhorst, T.K. Dual purpose S-trityl-linkers for glycoarray fabrication on both polystyrene and gold. Org. Biomol. Chem. 2012, 10, 8919–8926. [Google Scholar] [CrossRef]
- Narla, S.N.; Sun, X.-L. Orientated glyco-macroligand formation based on site-specific immobilization of O-cyanate chain-end functionalized glycopolymer. Org. Biomol. Chem. 2011, 9, 845–850. [Google Scholar] [CrossRef]
- Larsen, K.; Thygesen, M.B.; Guillaumie, F.; Willats, W.G.T.; Jensen, K.J. Solid-phase chemical tools for glycobiology. Carbohydr. Res. 2006, 341, 1209–1234. [Google Scholar] [CrossRef]
- Siebold, C.; Flükiger, K.; Beutler, R.; Erni, B. Carbohydrate transporters of the bacterial phosphoenolpyruvate: Sugar phosphotransferase systems (PTS). FEBS Lett. 2001, 504, 104–111. [Google Scholar] [CrossRef]
- Hartmann, M.; Papavlassopoulos, H.; Chandrasekaran, V.; Grabosch, C.; Beiroth, F.; Lindhorst, T.K.; Röhl, C. Activity and cyctotoxicity of five synthetic mannosides as inhibitors of bacterial adhesion. FEBS Lett. 2012, 586, 1459–1465. [Google Scholar] [CrossRef]
- Sato, M.; Takemura, M.; Atarashi, S.; Higashi, K.; Nagahara, T.; Furukawa, M. Modification of the cysteamine side chain of thienamycin. J. Antibiot. 1987, 9, 1292–1302. [Google Scholar]
- Tew, G.N.; Liu, D.; Chen, B.; Doerksen, R.J.; Kaplan, J.; Carroll, P.J.; Klein, M.K.; DeGrado, W.F. De novo design of biomimetic antimicrobial polymers. PNAS 2002, 99, 5110–5114. [Google Scholar]
- Choi, J.; Kim, J.; Kim, K.; Yang, S.-T.; Kim, J.-I.; Jon, S. A rationally designed macrocyclic cavitand that kills bacteria with high efficacy and good selectivity. Chem. Commun. 2007. [Google Scholar] [CrossRef]
- Koteswara Rao, V.; Janardhan Rao, A.; Subba Reddy, S.; Naga Raju, C.; Visweswara Rao, P.; Ghosh, S.K. Synthesis, spectral characterization and biological evaluation of phosphorylated derivatives of galanthamine. Eur. J. Med. Chem. 2010, 45, 203–209. [Google Scholar] [CrossRef]
- Dondoni, A.; Massi, A.; Nanni, P.; Roda, A. A new ligation strategy for peptide and protein glycosylation: Photoinduced thiol-ene coupling. Chem. Eur. J. 2009, 15, 11444–11449. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Fessele, C.; Lindhorst, T.K. Effect of Aminophenyl and Aminothiahexyl α-D-Glycosides of the Manno-, Gluco-, and Galacto-Series on Type 1 Fimbriae-Mediated Adhesion of Escherichia coli. Biology 2013, 2, 1135-1149. https://doi.org/10.3390/biology2031135
Fessele C, Lindhorst TK. Effect of Aminophenyl and Aminothiahexyl α-D-Glycosides of the Manno-, Gluco-, and Galacto-Series on Type 1 Fimbriae-Mediated Adhesion of Escherichia coli. Biology. 2013; 2(3):1135-1149. https://doi.org/10.3390/biology2031135
Chicago/Turabian StyleFessele, Claudia, and Thisbe K. Lindhorst. 2013. "Effect of Aminophenyl and Aminothiahexyl α-D-Glycosides of the Manno-, Gluco-, and Galacto-Series on Type 1 Fimbriae-Mediated Adhesion of Escherichia coli" Biology 2, no. 3: 1135-1149. https://doi.org/10.3390/biology2031135
APA StyleFessele, C., & Lindhorst, T. K. (2013). Effect of Aminophenyl and Aminothiahexyl α-D-Glycosides of the Manno-, Gluco-, and Galacto-Series on Type 1 Fimbriae-Mediated Adhesion of Escherichia coli. Biology, 2(3), 1135-1149. https://doi.org/10.3390/biology2031135