1. Introduction
Atherosclerosis remains a leading cause of morbidity and mortality worldwide, caused by the persistent buildup of lipid-rich plaques in arterial walls. An early cellular event in this disease process is the formation of foam cells (FC) from monocytes that have taken up large amounts of oxidized low-density lipoproteins (ox-LDL). This lipid intake, primarily facilitated by scavenger receptors such as CD36 and LOX-1, leads to cholesterol accumulation, which triggers oxidative stress and inflammatory signals that drive plaque growth.
Mitochondria play a crucial role in foam cell formation by regulating cellular energy production and maintaining redox balance. In the mitochondrial electron transport chain (ETC), Complex I (NADH: ubiquinone oxidoreductase) and Complex III (cytochrome bc1 complex) are key components of oxidative phosphorylation (OXPHOS), supporting electron transfer and proton pumping to generate ATP. However, these complexes are also the main sources of mitochondrial reactive oxygen species (ROS), especially superoxide anion (O
2•
−), particularly when their function is impaired or overloaded [
1,
2]. Dysfunction in Complexes I or III can disrupt electron flow, leading to electron leakage and increased ROS production. This process then compromises the mitochondrial membrane potential, reduces ATP output, and triggers cell death pathways, all of which contribute to foam cell formation and the development of atherosclerotic plaque [
3,
4].
Experimental studies have shown that pharmacologic targeting of these mitochondrial complexes can alter disease outcomes. Rotenone, a classical Complex I inhibitor, while toxic at high doses, has paradoxically been found to reduce oxidative stress and vascular smooth muscle cell (VSMC) proliferation in certain disease models [
5,
6]. Likewise, azoxystrobin, an inhibitor of Complex III, can attenuate VSMC activation in response to ox-LDL and has shown partial effectiveness in reducing foam cell formation [
7]. These studies highlight the potential benefits of adjusting mitochondrial activity for the development of a therapeutic strategy for vascular diseases.
The production of ROS by Complex I and III is essential in controlling mitochondrial health and cellular signaling. Under normal conditions, electrons flow through the ETC efficiently, reducing oxygen to water. However, during stress or disease states, electron leakage can happen, especially in Complexes I and III, leading to increased ROS production. While Complex I typically releases superoxide into the mitochondrial matrix, Complex III can do so in both the matrix and the intermembrane space [
1,
6,
7,
8]. This ROS overload damages mitochondrial DNA, proteins, and lipids, impairing the structural integrity of the mitochondria and lowering mitochondrial membrane potential. The resulting dysfunction triggers apoptosis and promotes inflammatory responses that contribute to foam cell formation and plaque vulnerability [
2,
3].
Krüppel-like factor 2 (KLF2) has emerged as a critical regulator of vascular homeostasis and inflammation [
9,
10,
11,
12,
13,
14]. Originally characterized in endothelial cells for its role in mediating shear stress responses, KLF2 is now known to be expressed in RAW264.7 cells, where it exerts a broad range of protective effects. It limits inflammatory expression, enhances cholesterol efflux, and activates antioxidant pathways. Importantly, KLF2 appears to preserve mitochondrial function by stabilizing mitochondrial membrane potential, reducing mitochondrial ROS, and sustaining oxidative metabolism [
15,
16]. In RAW264.7 cells exposed to ox-LDL, KLF2 expression is often suppressed, leading to mitochondrial dysfunction and accelerated foam cell formation.
The activation of KLF2 using a chemical compound, GGTI298 (an inhibitor of geranylgeranyltransferase I) has shown encouraging results in restoring mitochondrial membrane potential and lowering ROS levels [
9,
12,
17]. The fact that similar protective effects are observed with GGTI298 suggests that KLF2 may act, at least in part, by modulating the function of mitochondrial Complexes I and III. This suggests a broader role for KLF2 in regulating mitochondrial superoxide production and bioenergetic stability.
Given the pivotal contribution of mitochondrial dysfunction and oxidative stress to FC development, this study was designed to define the role of KLF2 in modulating these pathological processes in monocytes, as monocytes give rise to FCs after uptaking ox-LDL. Using a monocytic cell line, RAW264.7 cells as a model system, we examined the impact of KLF2 on cellular and mitochondrial ROS generation, mitochondrial membrane potential, and metabolic function. To dissect the regulatory role of KLF2, we employed a chemical, GGTI298, to enhance KLF2 expression and a molecule, GGPP, to suppress expression of KLF2, allowing us to evaluate how shifts in KLF2 activity influence mitochondrial health, function, and lipid accumulation during monocyte differentiation toward FCs. By integrating confocal microscopy, immunocytochemical staining, and Seahorse-based metabolic profiling, our findings highlight KLF2 as a key modulator for maintaining mitochondrial activity towards a healthy condition. Ultimately, this work provides new insight into the mechanisms by which KLF2 may counteract the initiation of FC formation, underscoring its potential as a therapeutic target in atherosclerotic disease.
2. Materials and Methods
2.1. Reagents and Materials
The RAW264.7 cells were obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA, #TIB-71). Oxidized low-density lipoprotein (ox-LDL) (Ann Arbor, MI, USA, #601,181), geranylgeranyl pyrophosphate (GGPP, Ann Arbor, MI, USA, CAS #313263-08-0), and GGTI298 (Ann Arbor, MI, USA, CAS #1217457-86-7) were purchased from Cayman Chemical (Ann Arbor, MI, USA). The fluorescent probe 2′,7′-dichlorodihydrofluorescein diacetate (DCFDA, #4091-99-0) was obtained from Sigma-Aldrich (St. Louis, MO, USA). 4′,6-diamidino-2-phenylindole dihydrochloride (DAPI, #D1306) and TRIzol™ Reagent (#15596026) were procured from Invitrogen (Carlsbad, CA, USA). The High-Capacity RNA-to-cDNA Kit (#4387406) and SYBR™ Green PCR Master Mix (#4309155) were supplied by Applied Biosystems (Foster City, CA, USA). JC-1 Dye (#T3168), used for assessing mitochondrial membrane potential, and MitoSOX™ Red (#M36008), used for detecting mitochondrial superoxide, were purchased from Thermo Fisher Scientific (Waltham, MA, USA). A 4% paraformaldehyde (PFA) solution (#sc-281692) was obtained from Santa Cruz Biotechnology (Dallas, TX, USA). Oil-Red-O stain (catalog #MAK194), 10% neutral buffered formalin (catalog #252549), and isopropanol (CAS #67-63-0) were obtained from Sigma-Aldrich. The Seahorse XFp Cell Mito Stress Test Kit (catalog #103010-100) was purchased from Agilent Technologies.
2.2. Cell Culture and Treatment Conditions
RAW264.7 murine macrophage cells were maintained in Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and 1% penicillin-streptomycin under standard conditions (37 °C, 5% CO2). Before stimulation, cells were serum-starved for 6 h to synchronize metabolic activity. For foam cell differentiation, cells were then incubated with oxidized low-density lipoprotein (Ox-LDL) at the optimized concentration, as determined in preliminary experiments. Where indicated, GGTI298 (20 μM) or GGPP, 10 μM was added at the start of the differentiation process and maintained throughout the culture period (six days) until termination of the experiment. This design ensured continuous modulation of KLF2 activity during foam cell formation. All treatments were performed in parallel with appropriate vehicle controls.
2.3. MTT Assay
Cells were seeded into 96-well plates at a density of 1 × 10
4 cells/well and allowed to attach overnight. Treatments included: (i) untreated control, (ii) RAW264.7 cells treated with GGTI298 (0–100 µM), and (iii) RAW264.7 cells treated with GGPP (0–100 µM). Fresh media containing the appropriate treatments were replaced every 48 h. The duration of treatments was extended up to 6 days. Cell viability was assessed using the MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide] assay (Sigma-Aldrich, St. Louis, MO, USA) as previously described [
18,
19]. At the end of the treatment period (days 1–6), 10 µL of MTT reagent (5 mg/mL) was added to each well and incubated for 4 h at 37 °C. Following incubation, the supernatant was aspirated, and the formazan crystals were dissolved in 100 µL of dimethyl sulfoxide (DMSO; Sigma-Aldrich, St. Louis, MO, USA). Absorbance was measured at 570 nm using a microplate reader (BioTek Synergy H1, Agilent Technologies, Santa Clara, CA, USA). Viability was expressed as a percentage of the untreated control (set as 100%). Experiments were performed in triplicate, and data are presented as mean ± standard deviation (SD).
2.4. Oil Red O Staining for Bright Field and Immunocytochemistry
Oil Red O staining was conducted to assess intracellular lipid accumulation in RAW264.7 cells under both bright field and immunofluorescence microscopes. After various experimental conditions, cells were rinsed gently with phosphate-buffered saline (PBS) and fixed with 10% (
v/
v) neutral buffered formalin for 15 min at room temperature. Following fixation, cells were washed three times with double-distilled water (ddH
2O) and stained for 15 min with freshly prepared Oil Red O working solution, obtained by diluting a 0.5% Oil Red O stock solution (in isopropanol) with ddH
2O at a 3:2 ratio (
v/
v), followed by filtration through a 0.45 μm membrane filter. Post-staining, cells were rinsed three times with ddH
2O to remove background staining. For bright field analysis, stained lipid droplets were imaged under a standard light microscope at 100× magnification. For immunofluorescence, Oil Red O-stained cells were counterstained with DAPI and imaged using a Leica Stellaris 8 STED super-resolution confocal microscope, which is equipped with a 100× oil immersion objective. Fluorescent images were captured using a 510 nm excitation wavelength to detect the intrinsic red fluorescence of Oil Red O, and mean fluorescence intensity (MFI) was quantified using LAS X image analysis software (
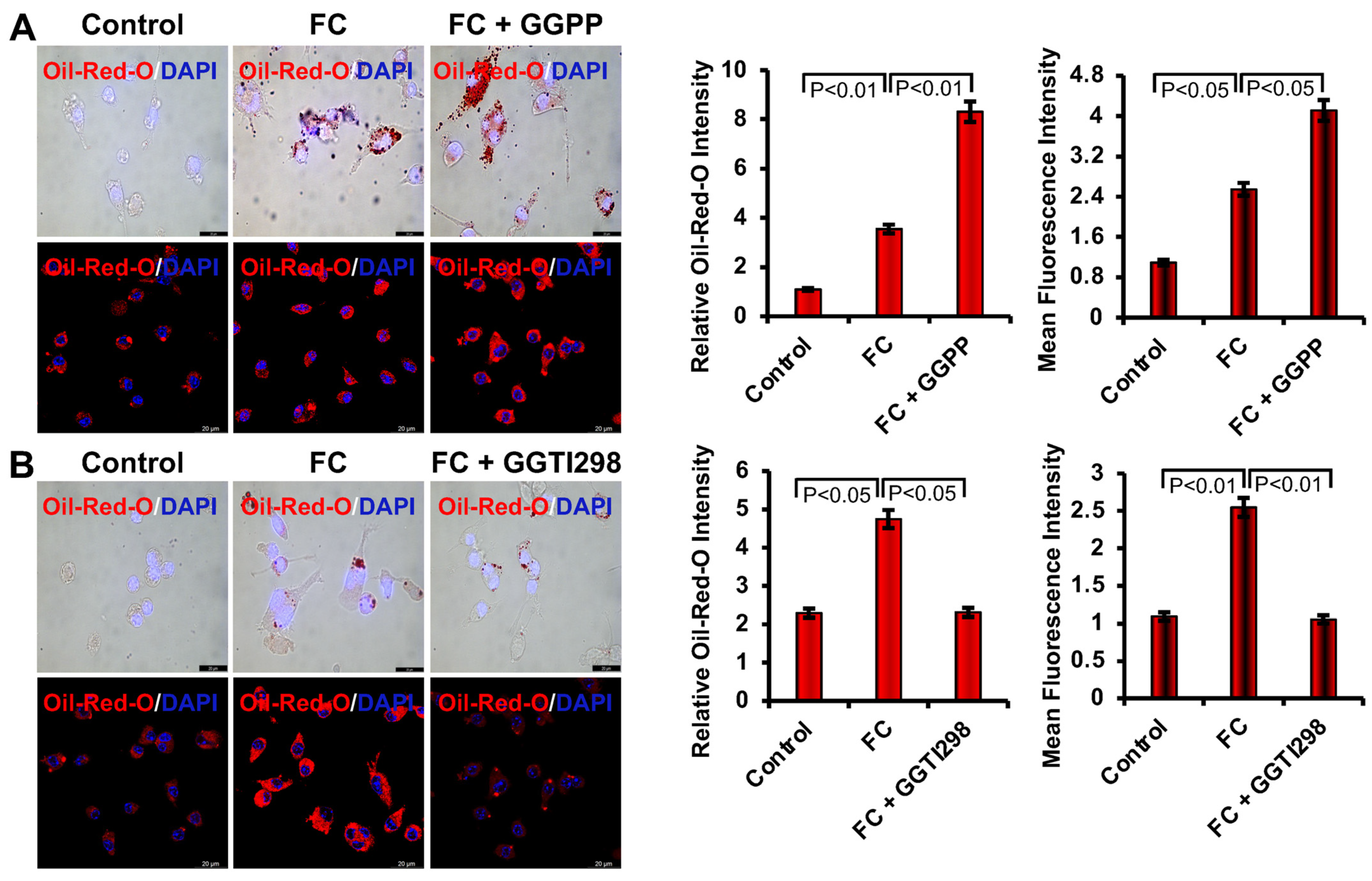
https://www.leica-microsystems.com/products/microscope-software/p/leica-las-x-ls/, accessed on 11 August 2025, Leica Microsystems, Wetzlar, Germany). Oil-Red-O staining was performed after 6 days of ox-LDL treatment to evaluate intracellular lipid accumulation associated with foam cell formation. For quantitative lipid analysis, Oil Red O dye retained within the cells was eluted using 100% isopropanol, and absorbance was measured at 510 nm with a spectrophotometer.
2.5. Evaluation of Mitochondrial Membrane Potential
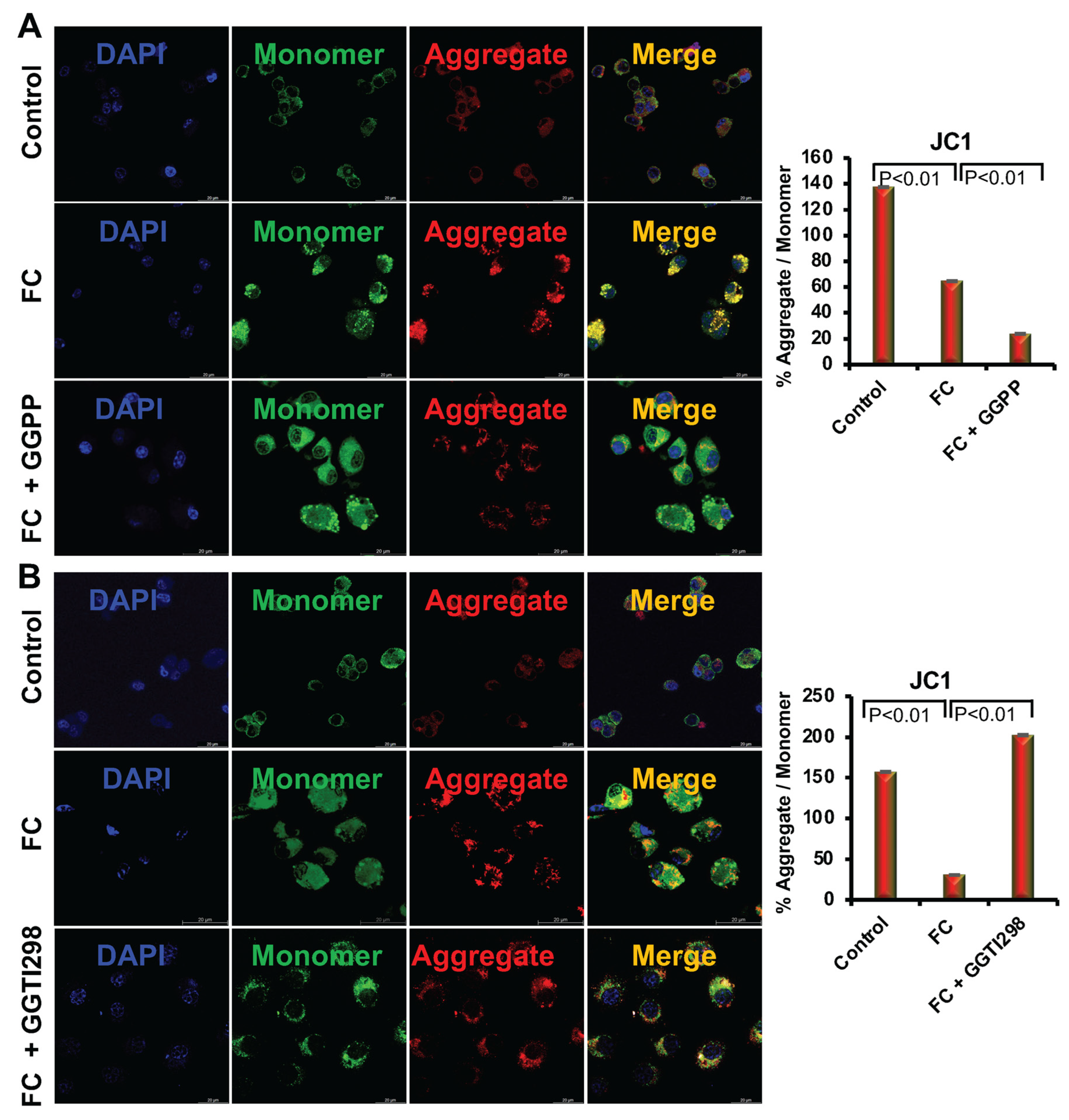
Mitochondrial membrane potential was assessed in cells after staining with JC-1 dye. In brief, RAW264.7 cells (1 × 104 cells/well) were cultured on coverslips for 6 days in the presence of FC differentiation medium, with or without GGTI298 (20 μM) or GGPP (10 μM) in the cell culture. Cells were then rinsed with ice-cold PBS and stained with JC-1 dye (Thermo Fisher Scientific) at 37 °C for 20 min. After thoroughly washing with PBS, cells were mounted with DAPI and imaged using the Leica Stellaris 8 STED super-resolution confocal microscope (100× objective) (Wetzlar, Germany). JC-1 staining was conducted after 6 days of treatment to assess alterations in mitochondrial membrane potential during foam cell differentiation. Image quantification of green (monomeric JC-1) and red (aggregated JC-1) fluorescence was performed using LAS X software (Leica Microsystems, Wetzlar, Germany) to assess mitochondrial polarization. Three biological replicates were analyzed, with five fields imaged per coverslip.
2.6. Assessment of Intracellular Reactive Oxygen Species
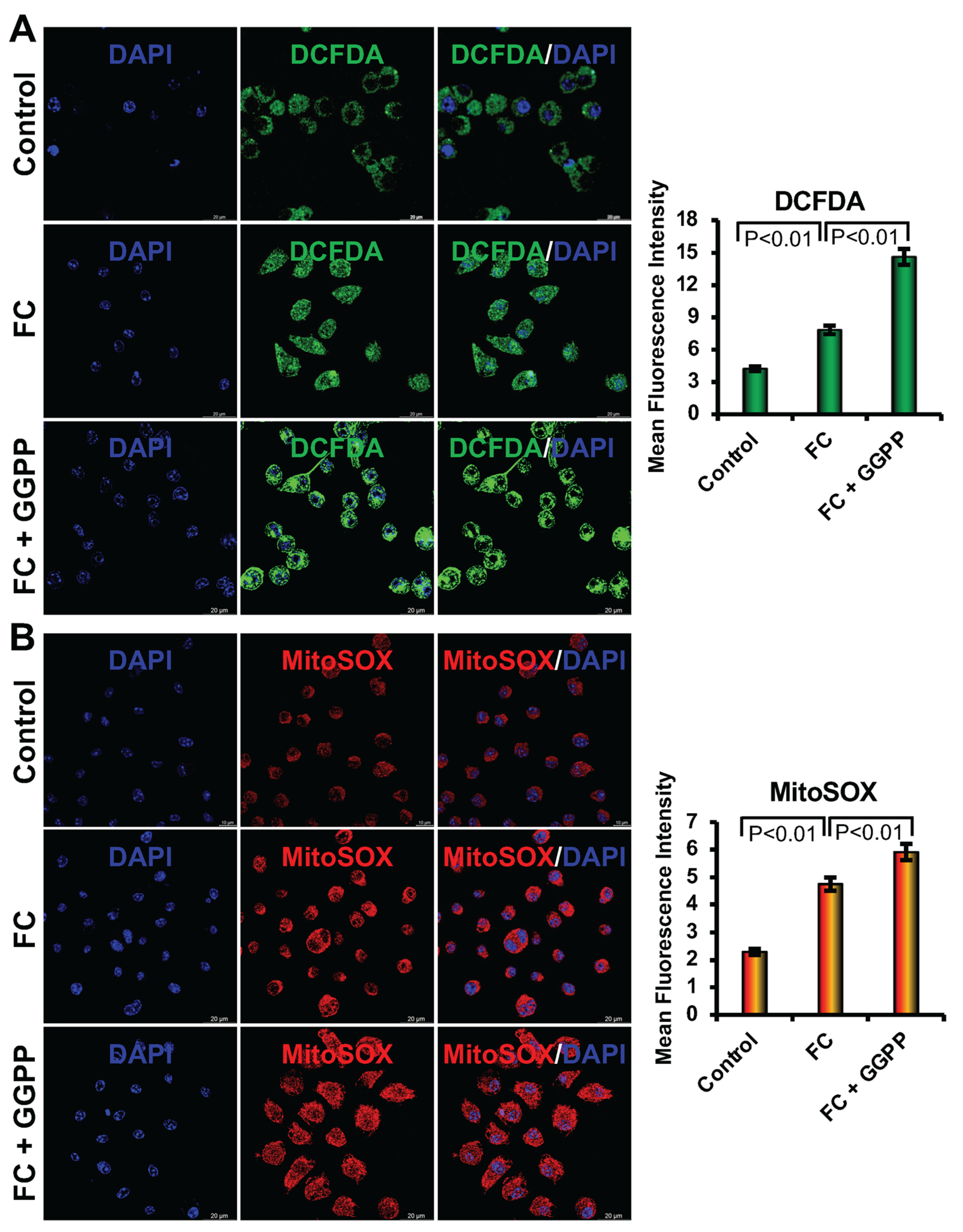
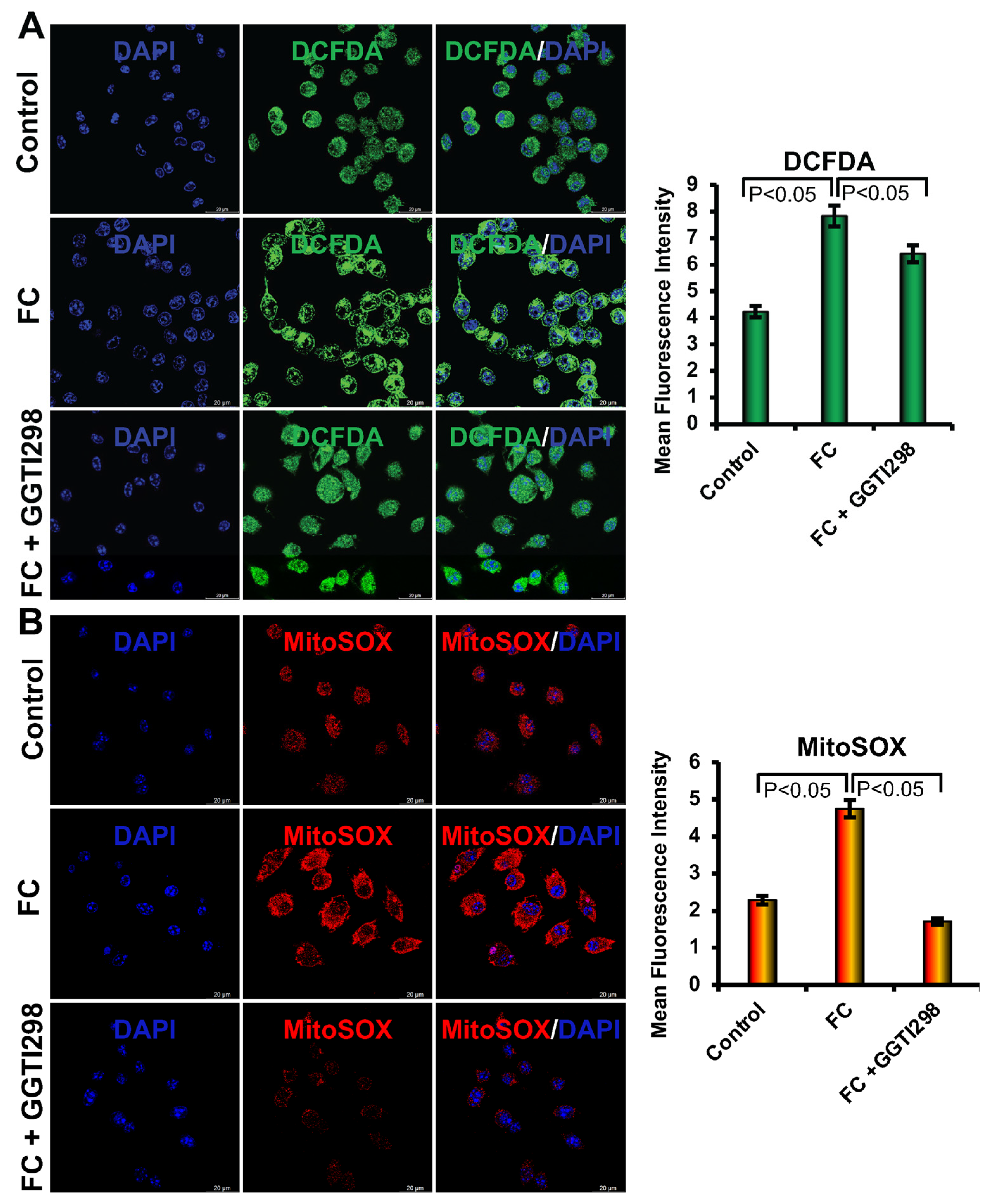
Intracellular reactive oxygen species (ROS) were evaluated after staining cells with the DCFDA (2′,7′-dichlorofluorescin diacetate) dye. In brief, RAW264.7 cells were seeded at a density of 1 × 104 cells/well on sterile glass coverslips and cultured for 6 days in foam cell (FC) differentiation media in the presence or absence of the optimized concentrations of GGTI298 (20 μM) or GGPP (10 μM). Cells were then washed with PBS and incubated with 20 μM DCFDA dye at 37 °C for 30 min. After incubation, coverslips were rinsed thoroughly with PBS and mounted on glass slides using a mounting medium containing DAPI. Fluorescence images were captured using a Leica Stellaris 8 STED super-resolution (Leica Microsystems, Wetzlar, Germany) confocal microscope equipped with a 100× oil immersion objective. Quantification of ROS levels was performed using LAS X image analysis software. Three independent experiments were conducted, and five random fields per coverslip were imaged for quantification.
2.7. Detection of Mitochondrial Superoxide Production
Mitochondrial superoxide levels were measured in cells after staining with MitoSOX™ Red. In brief, RAW264.7 cells (1 × 104 cells/well) were cultured on coverslips under the same differentiation conditions as described above. After 6 days, cells were washed with ice-cold PBS and incubated with a 2 μM concentration of MitoSOX Red at 37 °C for 30 min. Following staining, cells were washed with PBS and mounted on the slide using DAPI. Fluorescent images were acquired using the Leica Stellaris 8 STED confocal microscope with a 100× objective, and image analysis was performed using LAS X software (Leica Microsystems, Wetzlar, Germany). Three independent experiments were performed, and five random fields per sample were analyzed for quantification.
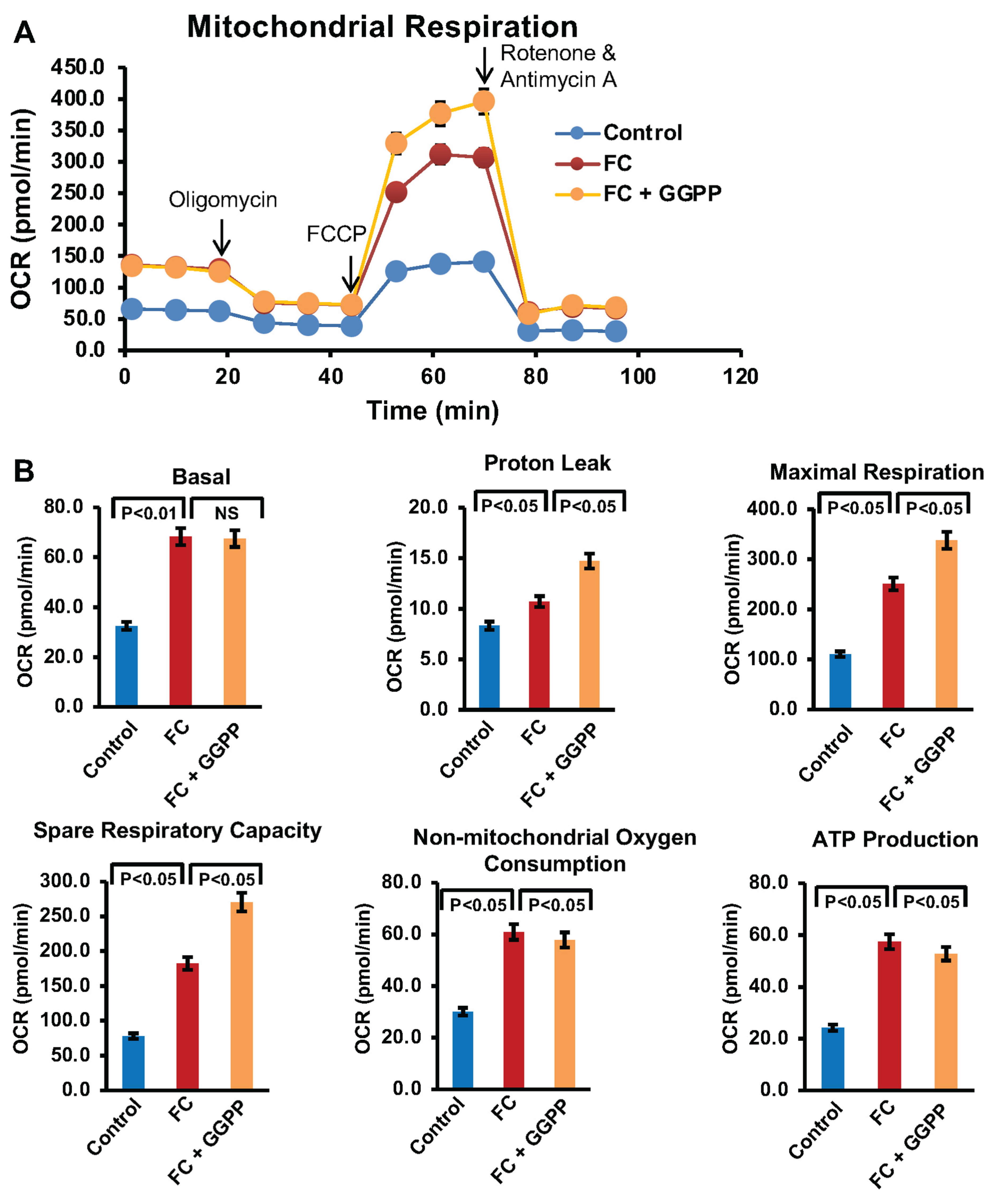
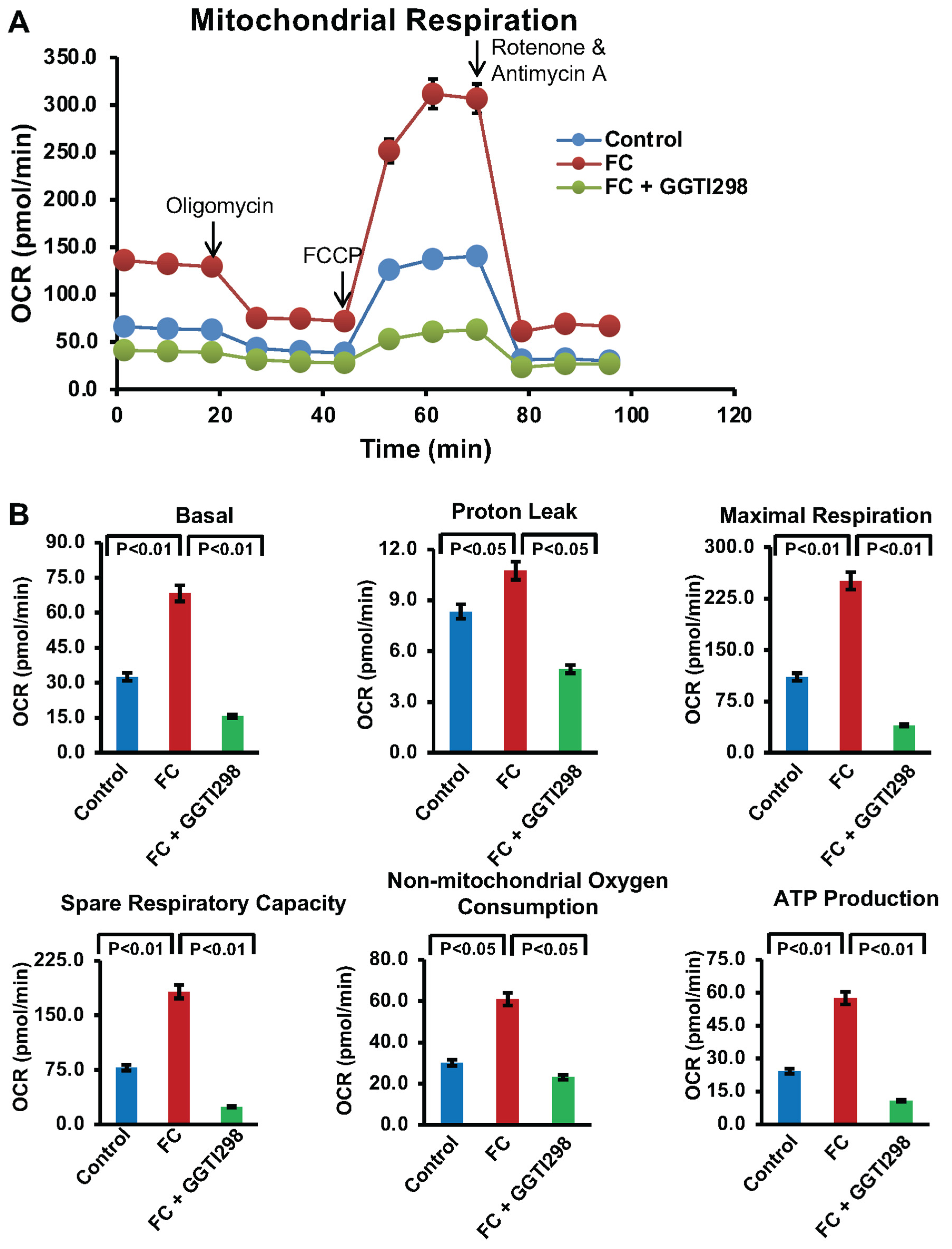
2.8. Mitochondrial Respiration Analysis
To investigate mitochondrial respiratory function, the Seahorse XF Cell Mito Stress Test was performed using an Agilent Seahorse XFe24 extracellular flux analyzer (New York, NY, USA). In brief, RAW264.7 cells (1 × 104 cells/well) were seeded on Seahorse XF24 cell culture microplates and differentiated for 6 days in the presence or absence of GGTI298 (20 μM) or GGPP (10 μM). On the day of the assay, culture medium was replaced with Seahorse XF assay medium (Agilent Technologies) supplemented with 1 mM pyruvate, 2 mM glutamine, and 10 mM glucose, and the cells were incubated at 37 °C in a non-CO2 containing incubator for 1 h. Oxygen consumption rate (OCR) was measured following sequential addition of 1.5 μM oligomycin (ATP synthase inhibitor), 1 μM FCCP (uncoupler), and 2 μM rotenone/antimycin A (complex I/III inhibitors). Data was acquired and analyzed using Seahorse Wave software version 2.6.1 (New York, NY, USA). Four independent replicates were used for each treatment condition, and mitochondrial respiration parameters, including basal respiration, ATP production, maximal respiration, and spare respiratory capacity, were calculated. ECAR was not assessed, as it is not relevant to the mitochondrial pathway being investigated.
2.9. Statistical Analysis
Each experiment was performed at least three times, and each data point was measured in triplicate. Data are presented as mean ± SEM from three independent experiments. Statistical significance was determined using one-way ANOVA followed by Tukey’s post hoc test, with p < 0.05 considered significant.
4. Discussion
In this study, we comprehensively investigated the role of Krüppel-like factor 2 (KLF2) in lipid uptake, mitochondrial health, and regulating mitochondrial function during FC formation, with a specific focus on ox-LDL uptake, mitochondrial dynamics, oxidative stress, and energy metabolism. We have reported earlier that KLF2 plays a critical role in maintaining vascular homeostasis, reducing inflammation, and playing an appropriate role in the cell differentiation process [
9,
10,
11,
12,
16]. However, its role was not investigated elaborately during the FC formation of RAW264.7 cells, as FC formation is the critical, earliest step for the development of plaque formation within the arteries during atherosclerosis pathogenesis. It was shown previously that KLF2 regulates the inflammatory cascade, antioxidant pathways, and mitochondrial functions [
15,
16]. In RAW264.7 cells exposed to ox-LDL, KLF2 expression is often suppressed; however, whether KLF2 plays a critical role in this process we have delineated its role using RAW264.7 cells and loss-of-function and gain-of-function approaches of KLF2 during FC formation. We found that a significant decrease in the KLF2 expression level occurred after the formation of FC, which is consistent with the previous reports [
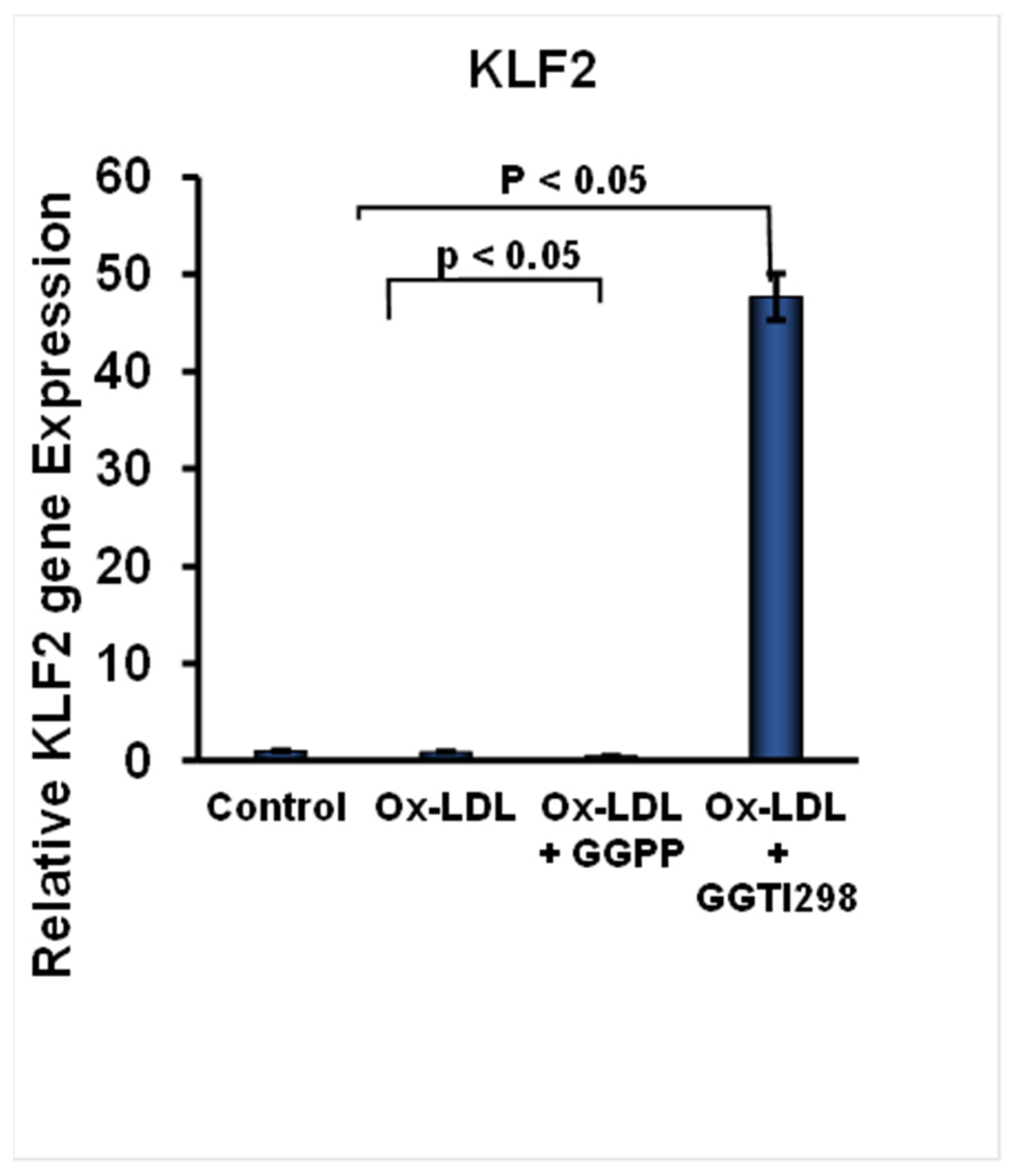
23]. When we tested the involvement of KLF2 in FC formation, we found that this suppression of KLF2 expression was further intensified upon the addition of GGPP. These findings indicate that the presence of GGPP leads to a substantial loss of the expression of KLF2 during FC formation. The loss of KLF2 might contribute to enhanced FC formation and the development of pathogenesis associated with foam cells. To confirm the role of KLF2 in this process, we have added the GGTI298 to the RAW264.7 cells during the induction of FC. There was a significant induction of KLF2 expression above its basal levels during the FC formation of RAW264.7 cells. These results demonstrate that chemical activation of KLF2 is effective in counteracting the suppressive effects of KLF2 expression during FC formation.
We next investigated the role of KLF2 during the foam cell formation through the uptake of ox-LDL and visualized it using light microscopy. We found that Ox-LDL was uptaken by RAW264.7 cells efficiently to become FCs, which is aligned with the previous reports [
24]. However, the lack of KLF2 enhanced the formation of FCs of the monocytes. When we tested the role of KLF2 in this process by adding GGTI298, which caused a substantial reduction in the uptake of ox-LDL by RAW264.7 cells and reduced the formation of FCs. These findings confirmed the regulatory role of KLF2 in the development of FCs from the RAW264.7 cells.
We further observed that when we added ox-LDL to the RAW264.7 cells, there was an increase in both cellular and mitochondrial ROS levels, which are consistent with earlier reports [
1,
2]. This oxidative stress contributes to depolarization of the mitochondrial membrane and impaired ATP synthesis, as indicated by the decreased red/green JC-1 fluorescence ratio. Activation of KLF2 via GGTI298 reversed this trend, significantly reducing ROS accumulation and restoring mitochondrial membrane potential. This aligns with previous reports showing that KLF2 enhances mitochondrial integrity by promoting antioxidant pathways, such as HO-1 and Nrf2 signaling [
16,
25].
Moreover, Seahorse XF analysis provided deeper insight into mitochondrial bioenergetics during FC formation. Ox-LDL treatment markedly increased oxygen consumption rate (OCR), particularly basal respiration and ATP-linked respiration, reflecting the mitochondrial overdrive state associated with high energy demand and oxidative stress. Interestingly, KLF2 activation via GGTI298 mitigated this metabolic shift by significantly lowering OCR values across several parameters, thereby suggesting that KLF2 helps restore metabolic balance and reduces electron leakage, a major source of mtROS from Complexes I and III [
6,
7,
8,
15]. This regulatory effect likely contributes to the preservation of mitochondrial efficiency and the prevention of foam cell transformation.
In contrast, the suppression of KLF2 using GGPP further exacerbated mitochondrial dysfunction. Not only did GGPP-treated cells show higher lipid accumulation and elevated ROS production, but Seahorse analysis also revealed a dramatic increase in OCR beyond the levels observed in foam cells. This hyperactivation suggests a breakdown in respiratory control, possibly due to unregulated activity at Complex I and III, leading to excessive ROS leakage and mitochondrial damage [
1,
3]. These results support the view that KLF2 acts as a key modulator of mitochondrial function, potentially inhibiting or regulating Complex I and III activity to limit mtROS production.
Furthermore, the phenocopying of protective effects by rotenone and azoxystrobin, known inhibitors of Complex I and III, respectively, reinforces the hypothesis that KLF2 functionally intersects with these mitochondrial components. Both inhibitors have been shown to reduce VSMC proliferation and ROS generation [
5], like KLF2 activation, suggesting a mechanistic link. KLF2 may indirectly modulate the redox activity or conformation of these complexes, thereby attenuating the pro-atherogenic effects of mitochondrial overactivation.
Collectively, our findings support the notion that KLF2 exerts a protective role by modulating mitochondrial reactive oxygen species production and maintaining mitochondrial membrane potential, thus preventing the dysfunctional phenotype associated with foam cells. Importantly, this study reveals that KLF2 influences the activities of mitochondrial Complexes I and III, which are central to ROS generation and energy metabolism in RAW264.7 cells undergoing atherogenic transformation. Overall, our findings position KLF2 as a master regulator of mitochondrial homeostasis during FC formation. By dampening mitochondrial hyperactivity, lowering ROS generation, and stabilizing mitochondrial membrane potential, KLF2 curtails the metabolic and inflammatory cascade that promotes foam cell formation, leading to atherosclerotic plaque formation in the crucial arteries. This dual role in lipid metabolism and mitochondrial regulation makes KLF2 a compelling therapeutic target for early intervention in cardiovascular diseases. A limitation of this study is that KLF2 expression was assessed only at the transcript level. Protein-level validation by Western blotting and/or immunocytochemistry was not performed in the present work. Future studies will be directed toward confirming KLF2 protein expression, which will strengthen the mechanistic link between transcriptional regulation and protein function during foam cell formation. We also acknowledge the limitations of our study. Our findings provide a strong proof-of-concept; future studies should aim to validate these key findings in primary cells, such as murine bone marrow-derived macrophages and human monocyte-derived macrophages, to ensure the physiological relevance of the KLF2-mitochondrial axis in atherogenesis.
5. Conclusions
This study highlights the essential role of KLF2 in regulating foam cell formation by maintaining mitochondrial homeostasis, reducing oxidative stress, and limiting lipid uptake in RAW264.7 cells. Loss of KLF2 expression promoted excessive lipid accumulation, mitochondrial depolarization, and elevated ROS generation, all of which contributed to a hyperactive metabolic state that drives foam cell development. In contrast, activation of KLF2 restored mitochondrial function, reduced oxidative stress, and prevented the dysfunctional phenotype characteristic of foam cells.
These findings establish KLF2 as a key modulator of mitochondrial Complex I and III activity, linking lipid metabolism to redox balance during atherogenic stress. By curbing mitochondrial overactivation and stabilizing energy production, KLF2 protects against the early events of atherosclerosis. Taken together, our results underscore the therapeutic potential of targeting KLF2 pathways to slow or prevent cardiovascular disease progression. Future validation in primary murine and human macrophages will be critical for translating these insights into clinically relevant strategies.