Cross-Protective Efficacy of Outer Membrane Vesicles (OMVs) Derived from Salmonella enterica Serovar Typhimurium Against Salmonella enterica Serovars Colonization in SPF Chicken
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Statement of Ethics
2.2. Chickens and Bacterial Strains
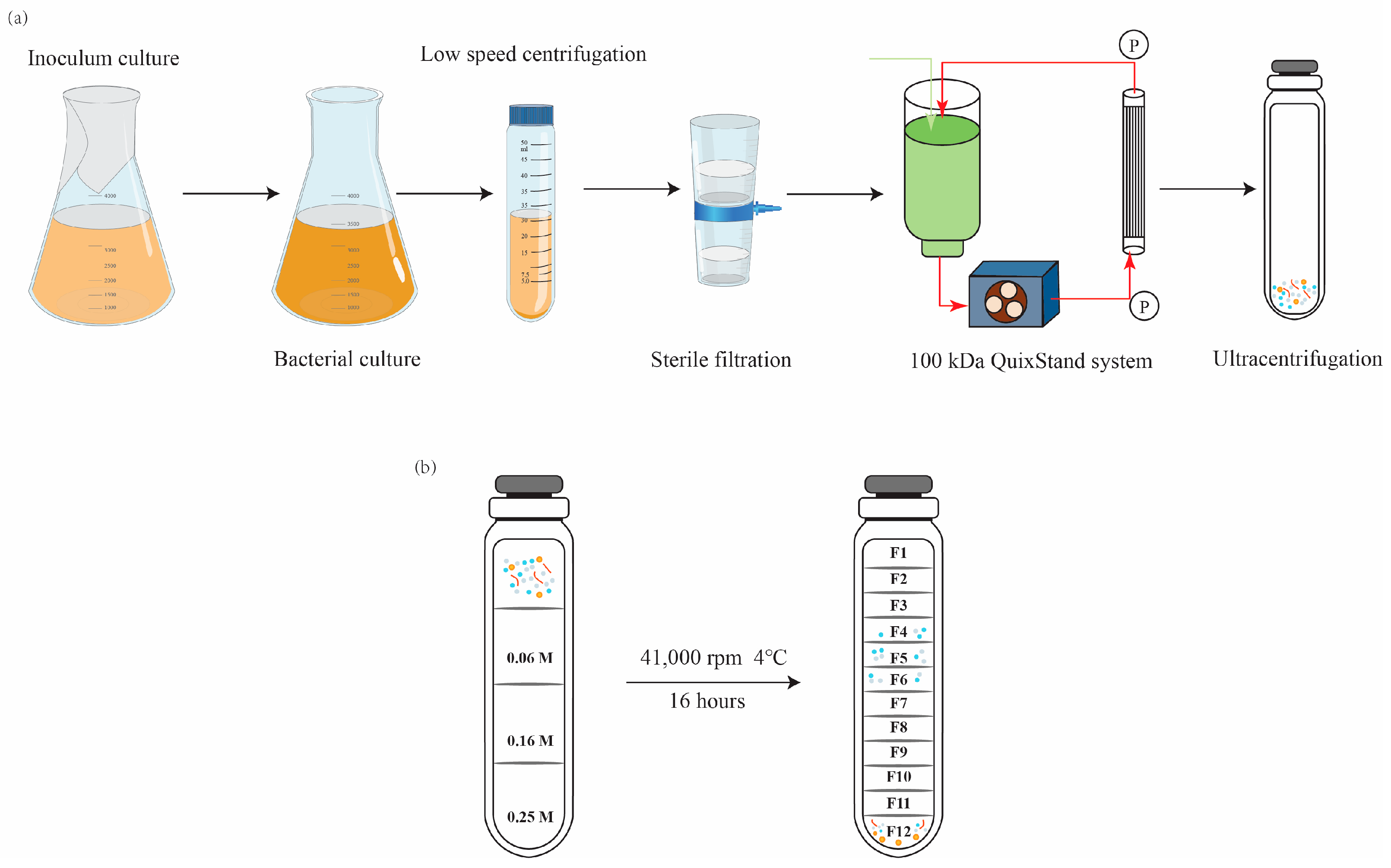
2.3. Isolation and Purification of OMVs from Salmonella Culture Supernatants
2.4. Characterization of OMVs
2.5. Immunization Protocol and Challenge
2.6. Enzyme-Linked Immunosorbent Assay (ELISA)
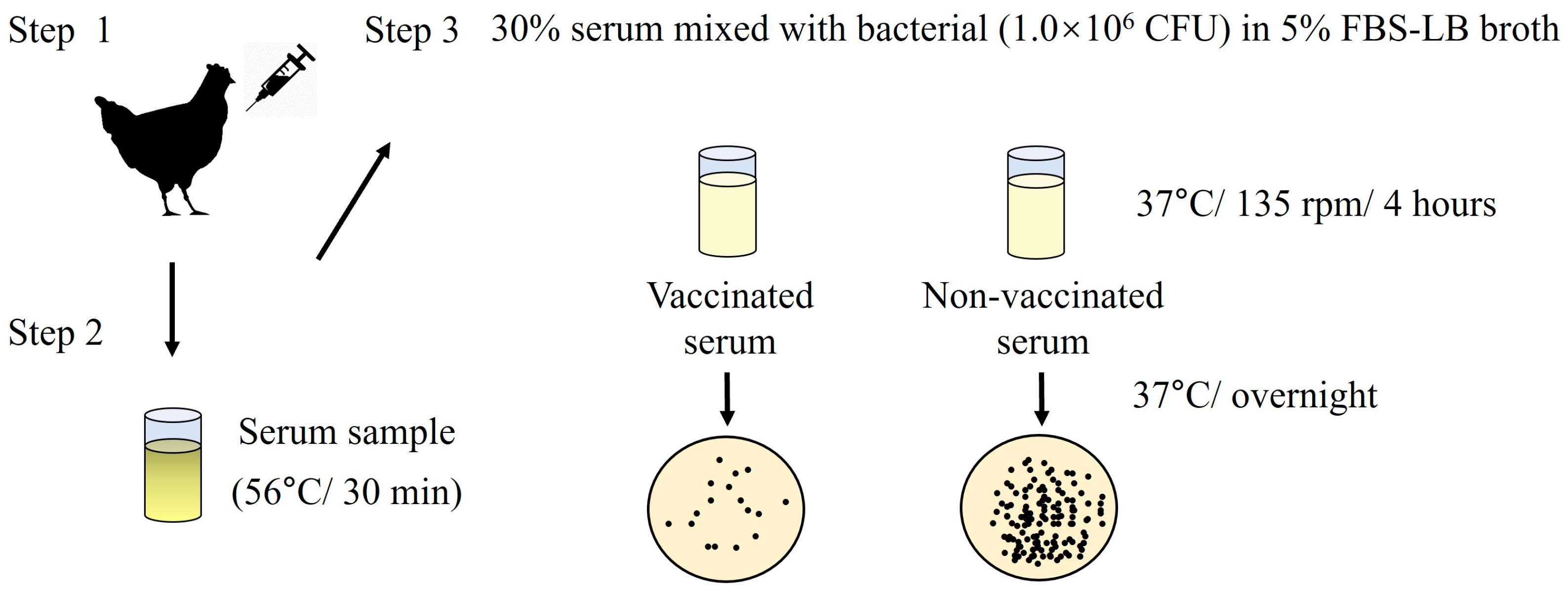
2.7. Serum Bactericidal Activity (SBA) Assay
2.8. Statistical Analysis
3. Results
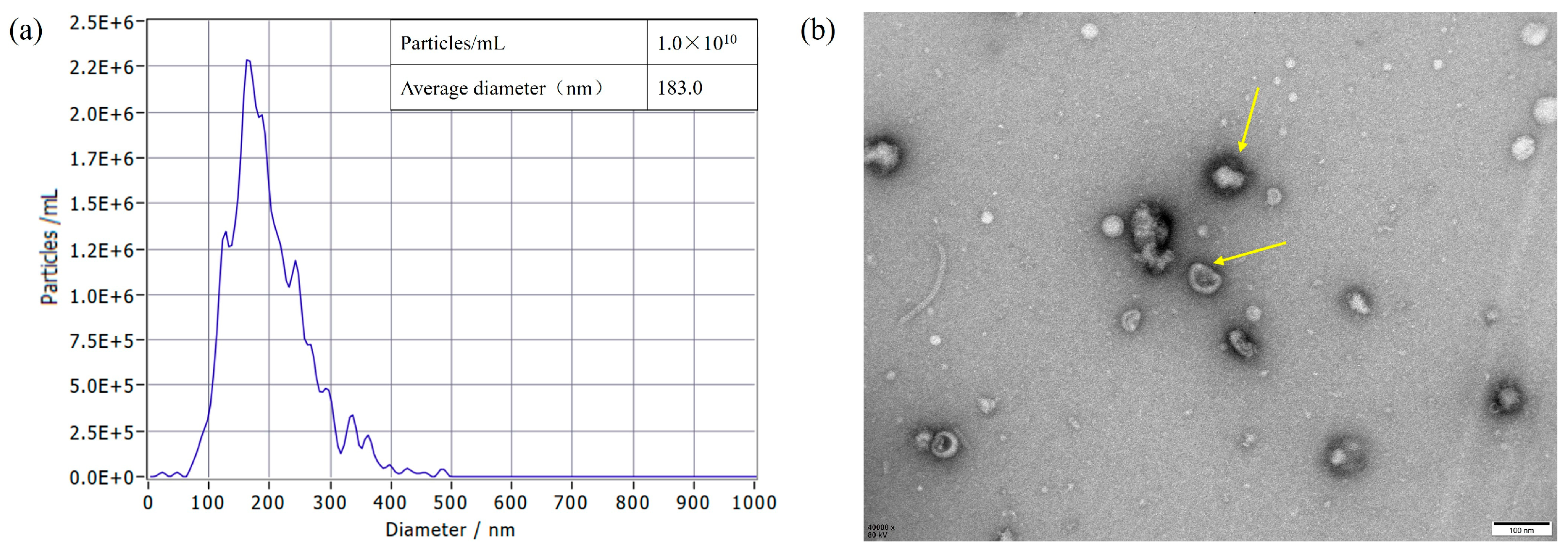
3.1. Isolation and Characterization of S. typhimurium OMVs
3.2. IgG Immune Responses Induced by S. typhimurium OMVs in Mice
3.3. SBA Induced by S. typhimurium in Mice
3.4. Protection Against Virulent S. typhimurium Challenge in Mice
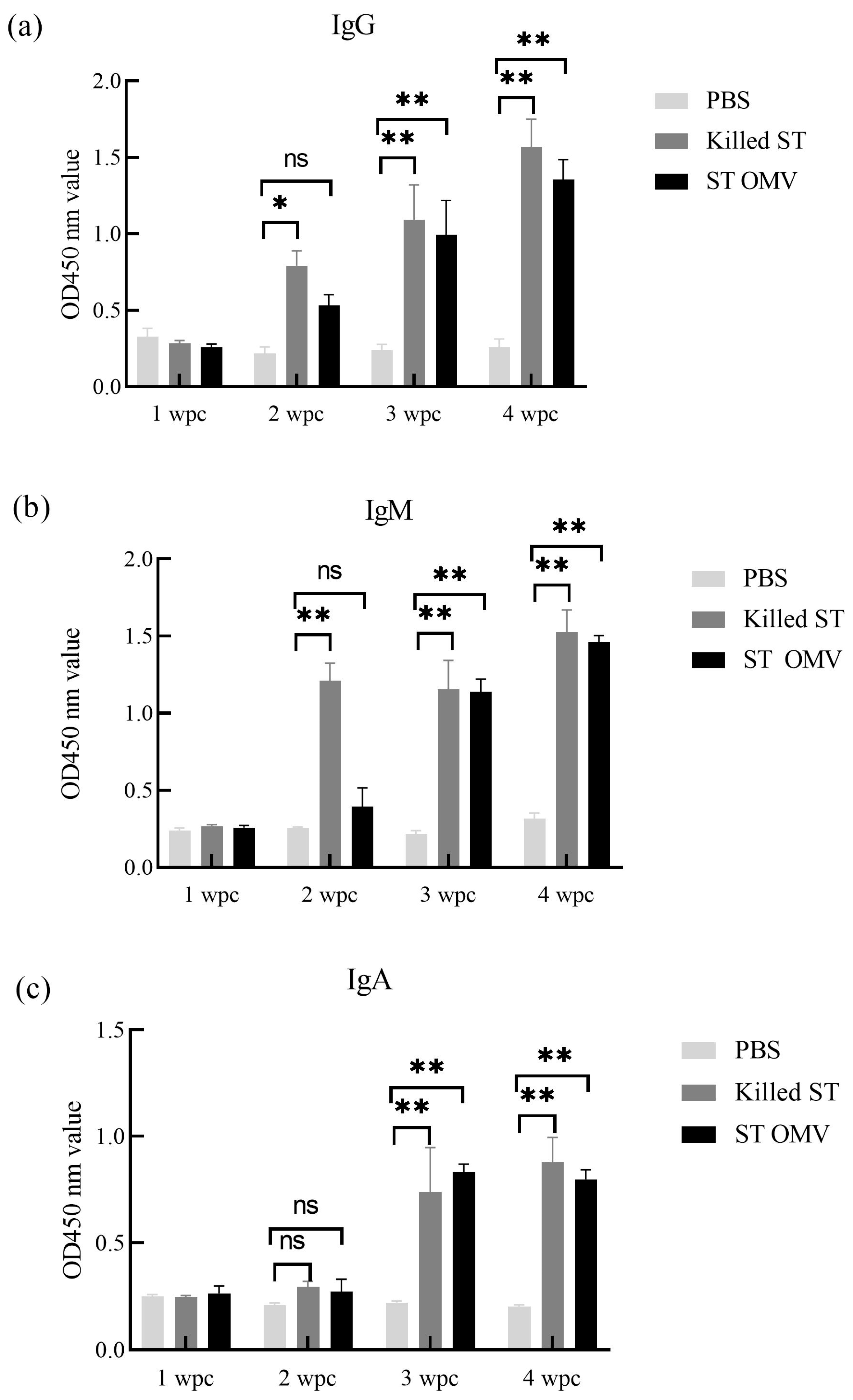
3.5. IgG, IgM, and IgA Immune Responses Induced by S. typhimurium OMVs in Chicken
3.6. Reduction in MDR S. montevideo and S. albany in the Cloacae and Livers of Challenged Chickens After S. typhimurium OMV Vaccination
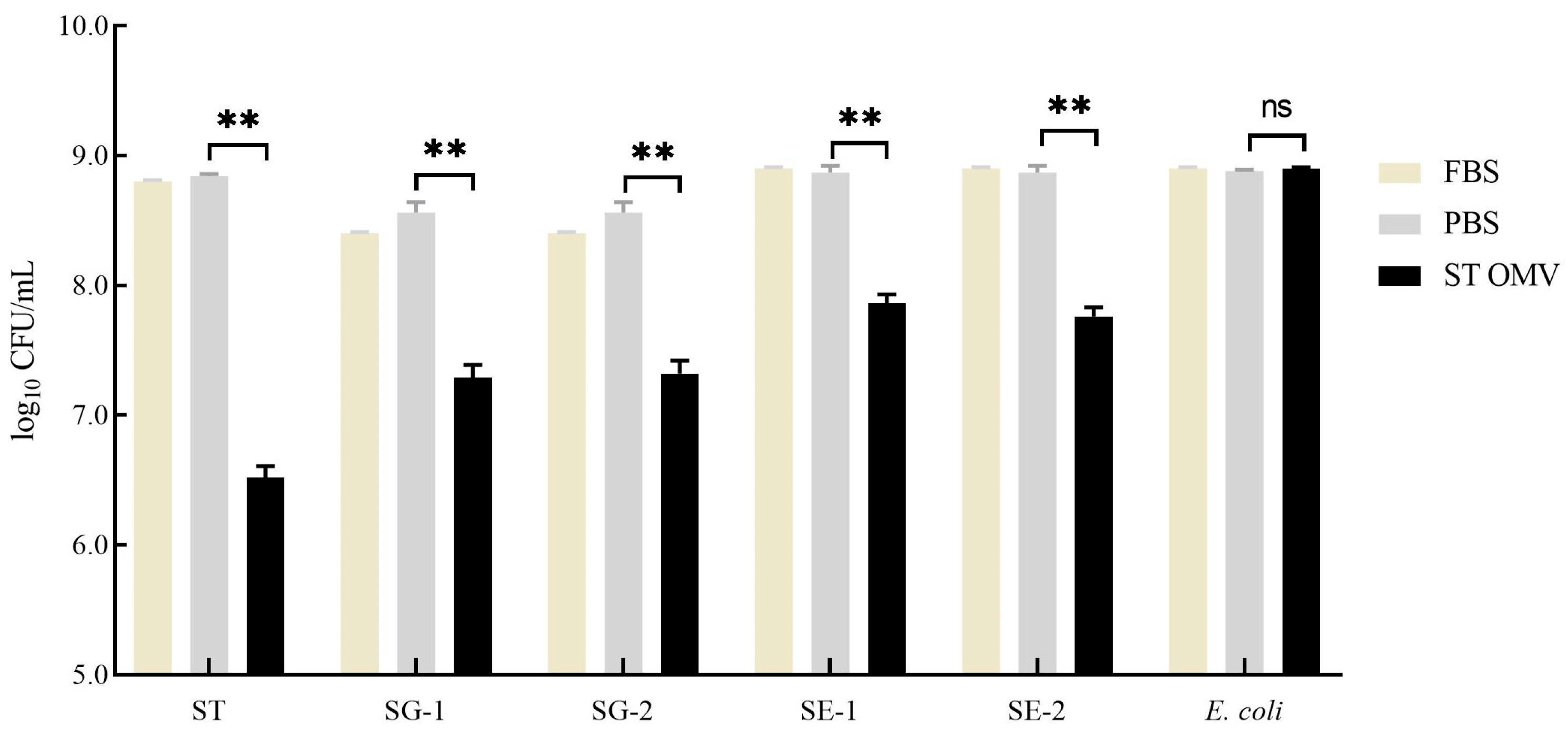
3.7. Bactericidal Ability Against Multiple Strains in Vitro
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Na, S.H.; Moon, D.C.; Kang, H.Y.; Song, H.J.; Kim, S.J.; Choi, J.H.; Yoon, J.W.; Yoon, S.S.; Lim, S.K. Molecular characteristics of extended-spectrum β-lactamase/AmpC-producing Salmonella enterica serovar Virchow isolated from food-producing animals during 2010–2017 in South Korea. Int. J. Food Microbiol. 2020, 322, 108572. [Google Scholar] [CrossRef] [PubMed]
- Singer, R.S. Urinary tract infections attributed to diverse ExPEC strains in food animals: Evidence and data gaps. Front. Microbiol. 2015, 6, 28. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Zhu, T.T.; Liang, Y.; Wei, H.J.; Huang, Z.L.; Liang, L.J.; Zhong, J.H.; Luo, Y.; Lian, X.L.; Zhao, D.H.; et al. Adaptive evolution in asymptomatic host confers MDR Salmonella with enhanced environmental persistence and virulence. Sci. Total Environ. 2024, 908, 168340. [Google Scholar] [CrossRef] [PubMed]
- Yoon, R.H.; Cha, S.Y.; Wei, B.; Roh, J.H.; Seo, H.S.; Oh, J.Y.; Jang, H.K. Prevalence of Salmonella isolates and antimicrobial resistance in poultry meat from South Korea. J. Food Prot. 2014, 77, 1579–1582. [Google Scholar] [CrossRef]
- Wei, B.; Shang, K.; Cha, S.Y.; Zhang, J.F.; Jang, H.K.; Kang, M. Clonal dissemination of Salmonella enterica serovar albany with concurrent resistance to ampicillin, chloramphenicol, streptomycin, sulfisoxazole, tetracycline, and nalidixic acid in broiler chicken in Korea. Poult. Sci. 2021, 100, 101141. [Google Scholar] [CrossRef]
- Lee, H.J.; Youn, S.Y.; Jeong, O.M.; Kim, J.H.; Kim, D.W.; Jeong, J.Y.; Kwon, Y.K.; Kang, M.S. Sequential transmission of Salmonella in the slaughtering process of chicken in Korea. J. Food Sci. 2019, 84, 871–876. [Google Scholar] [CrossRef]
- Animal and Plant Quarantine Agency, South Korea. 2024. Available online: https://www.mfds.go.kr/brd/m_231/view.do?seq=33084&srchFr=&srchTo=&srchWord=&srchTp=&itm_seq_1=0&itm_seq_2=0&multi_itm_seq=0&company_cd=&company_nm=&page=1 (accessed on 25 August 2025).
- Desin, T.S.; Köster, W.; Potter, A.A. Salmonella vaccines in poultry: Past, present and future. Expert Rev. Vaccines 2013, 12, 87–96. [Google Scholar] [CrossRef]
- Beylefeld, A.; Abolnik, C. Salmonella gallinarum strains from outbreaks of fowl typhoid fever in Southern Africa closely related to SG9R vaccines. Front. Vet. Sci. 2023, 10, 1191497. [Google Scholar] [CrossRef]
- Redweik, G.A.J.; Jochum, J.; Mellata, M. Live bacterial prophylactics in modern poultry. Front. Vet. Sci. 2020, 7, 592312. [Google Scholar] [CrossRef]
- Ji, H.J.; Jang, A.Y.; Song, J.Y.; Ahn, K.B.; Han, S.H.; Bang, S.J.; Jung, H.K.; Hur, J.; Seo, H.S. Development of live attenuated Salmonella Typhimurium vaccine strain using radiation mutation enhancement technology (R-MET). Front. Immunol. 2022, 13, 931052. [Google Scholar] [CrossRef]
- Wang, H.; Liang, K.; Kong, Q.; Liu, Q. Immunization with outer membrane vesicles of avian pathogenic Escherichia coli O78 induces protective immunity in chickens. Vet. Microbiol. 2019, 236, 108367. [Google Scholar] [CrossRef]
- Chen, Y.; Jie, K.; Li, B.; Yu, H.; Ruan, H.; Wu, J.; Huang, X.; Liu, Q. Immunization with outer membrane vesicles derived from major outer membrane protein-deficient Salmonella Typhimurium mutants for cross protection against Salmonella Enteritidis and avian pathogenic Escherichia coli O78 infection in chickens. Front. Microbiol. 2020, 11, 588952. [Google Scholar] [CrossRef] [PubMed]
- Macdonald, I.A.; Kuehn, M.J. Stress-induced outer membrane vesicle production by Pseudomonas aeruginosa. J. Bacteriol. 2013, 195, 2971–2981. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Lei, Q.; Zou, X.; Ma, D. The role and mechanisms of gram-negative bacterial outer membrane vesicles in inflammatory diseases. Front. Immunol. 2023, 14, 1157813. [Google Scholar] [CrossRef] [PubMed]
- Schwechheimer, C.; Kuehn, M.J. Outer-membrane vesicles from Gram-negative bacteria: Biogenesis and functions. Nat. Rev. Microbiol. 2015, 13, 605–619. [Google Scholar] [CrossRef]
- Tunheim, G.; Naess, L.M.; Acevedo, R.; Fjeldheim, Å.K.; Bolstad, K.; García, L.; Cardoso, D.; Aase, A.; Zayas, C.; González, H.; et al. Preclinical immunogenicity study of trivalent meningococcal AWX-OMV vaccines for the African meningitis belt. Vaccine 2014, 32, 6631–6638. [Google Scholar] [CrossRef]
- Ellis, T.N.; Leiman, S.A.; Kuehn, M.J. Naturally produced outer membrane vesicles from Pseudomonas aeruginosa elicit a potent innate immune response via combined sensing of both lipopolysaccharide and protein components. Infect. Immun. 2010, 78, 3822–3831. [Google Scholar] [CrossRef]
- Bishop, A.L.; Schild, S.; Patimalla, B.; Klein, B.; Camilli, A. Mucosal immunization with Vibrio cholerae outer membrane vesicles provides maternal protection mediated by antilipopolysaccharide antibodies that inhibit bacterial motility. Infect. Immun. 2010, 78, 4402–4420. [Google Scholar] [CrossRef]
- Pors, S.E.; Pedersen, I.J.; Skjerning, R.B.; Thøfner, I.C.N.; Persson, G.; Bojesen, A.M. Outer membrane vesicles of Gallibacterium anatis induce protective immunity in egg-laying hens. Vet. Microbiol. 2016, 195, 123–127. [Google Scholar] [CrossRef]
- Li, C.; Xue, H.; Du, X.; Nyaruaba, R.; Yang, H.; Wei, H. Outer membrane vesicles generated by an exogenous bacteriophage lysin and protection against Acinetobacter baumannii infection. J. Nanobiotechnol. 2024, 22, 273. [Google Scholar] [CrossRef]
- Muralinath, M.; Kuehn, M.J.; Roland, K.L.; Curtiss, R., 3rd. Immunization with serovar Typhimurium-derived outer membrane vesicles delivering the pneumococcal protein PspA confers protection against challenge with Streptococcus pneumoniae. Infect. Immun. 2011, 79, 887–894. [Google Scholar] [CrossRef]
- Jiang, X.; Chu, C.; Wang, Z.; Gu, J.; Hong, Y.; Li, Q.; Jiao, X. Preclinical evaluation of OMVs as potential vaccine candidates against Salmonella enterica serovar Enteritidis infection. Front. Cell. Infect. Microbiol. 2022, 12, 1037607. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.I.; Kim, M.; Jeon, J.; Han, J.K.; Kim, K.S. Overexpression of MicA induces production of OmpC-enriched outer membrane vesicles that protect against Salmonella challenge. Biochem. Biophys. Res. Commun. 2017, 490, 991–996. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Yi, J.; Liang, K.; Zhang, X.; Liu, Q. Outer membrane vesicles derived from Salmonella Enteritidis protect against the virulent wild-type strain infection in a mouse model. J. Microbiol. Biotechnol. 2017, 27, 1519–1528. [Google Scholar] [CrossRef] [PubMed]
- Abudabos, A.M.; Alyemni, A.H.; Dafalla, Y.M.; Khan, R.U. The effect of phytogenic feed additives to substitute in-feed antibiotics on growth traits and blood biochemical parameters in broiler chicks challenged with Salmonella typhimurium. Environ. Sci. Pollut. Res. Int. 2016, 23, 24151–24157. [Google Scholar] [CrossRef]
- Shang, K.; Wei, B.; Kang, M. Distribution and dissemination of antimicrobial-resistant Salmonella in broiler farms with or without enrofloxacin use. BMC Vet. Res. 2018, 14, 257. [Google Scholar] [CrossRef]
- Shang, K.; Wei, B.; Jang, H.-K.; Kang, M. Phenotypic characteristics and genotypic correlation of antimicrobial resistant (AMR) Salmonella isolates from a poultry slaughterhouse and its downstream retail markets. Food Control 2019, 100, 35–45. [Google Scholar] [CrossRef]
- Zhang, J.F.; Shang, K.; Park, J.Y.; Lee, Y.J.; Choi, Y.R.; Kim, S.W.; Cha, S.Y.; Jang, H.K.; Wei, B.; Kang, M. Antimicrobial resistance and pfge molecular typing of Salmonella enterica serovar Gallinarum isolates from chickens in South Korea from 2013 to 2018. Animals 2021, 12, 83. [Google Scholar] [CrossRef]
- Choi, C.W.; Park, E.C.; Yun, S.H.; Lee, S.Y.; Kim, S.I.; Kim, G.H. Potential usefulness of Streptococcus pneumoniae extracellular membrane vesicles as antibacterial vaccines. J. Immunol. Res. 2017, 2017, 7931982. [Google Scholar] [CrossRef]
- Li, Z.; Niu, L.; Wang, L.; Mei, T.; Shang, W.; Cheng, X.; Li, Y.; Xi, F.; Song, X.; Shao, Y.; et al. Biodistribution of (89)Zr-DFO-labeled avian pathogenic Escherichia coli outer membrane vesicles by PET imaging in chickens. Poult. Sci. 2023, 102, 102364. [Google Scholar] [CrossRef]
- Nieves, W.; Petersen, H.; Judy, B.M.; Blumentritt, C.A.; Russell-Lodrigue, K.; Roy, C.J.; Torres, A.G.; Morici, L.A. A Burkholderia pseudomallei outer membrane vesicle vaccine provides protection against lethal sepsis. Clin. Vaccine Immunol. CVI 2014, 21, 747–754. [Google Scholar] [CrossRef] [PubMed]
- Micoli, F.; Alfini, R.; Di Benedetto, R.; Necchi, F.; Schiavo, F.; Mancini, F.; Carducci, M.; Palmieri, E.; Balocchi, C.; Gasperini, G.; et al. GMMA Is a Versatile Platform to Design Effective Multivalent Combination Vaccines. Vaccines 2020, 8, 540. [Google Scholar] [CrossRef] [PubMed]
- Baliban, S.M.; Lu, Y.J.; Malley, R. Overview of the Nontyphoidal and Paratyphoidal Salmonella Vaccine Pipeline: Current Status and Future Prospects. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2020, 71, S151–S154. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Liu, Q.; Yi, J.; Liang, K.; Liu, T.; Roland, K.L.; Jiang, Y.; Kong, Q. Outer membrane vesicles derived from Salmonella Typhimurium mutants with truncated LPS induce cross-protective immune responses against infection of Salmonella enterica serovars in the mouse model. Int. J. Med. Microbiol. IJMM 2016, 306, 697–706. [Google Scholar] [CrossRef]
- Maiti, S.; Howlader, D.R.; Halder, P.; Bhaumik, U.; Dutta, M.; Dutta, S.; Koley, H. Bivalent non-typhoidal Salmonella outer membrane vesicles immunized mice sera confer passive protection against gastroenteritis in a suckling mice model. Vaccine 2021, 39, 380–393. [Google Scholar] [CrossRef]
- Godlewska, R.; Kuczkowski, M.; Wyszyńska, A.; Klim, J.; Derlatka, K.; Woźniak-Biel, A.; Jagusztyn-Krynicka, E.K. Evaluation of a protective effect of in ovo delivered Campylobacter jejuni OMVs. Appl. Microbiol. Biotechnol. 2016, 100, 8855–8864. [Google Scholar] [CrossRef]
- Kaparakis-Liaskos, M.; Ferrero, R.L. Immune modulation by bacterial outer membrane vesicles. Nat. Rev. Immunol. 2015, 15, 375–387. [Google Scholar] [CrossRef]
- Ehteshaminia, Y.; Golsaz-Shirazi, F.; Amiri, M.M.; Shokri, F. Next-generation pertussis vaccines with special focus on intranasal vaccination. Iran J Immunol 2025, 22. [Google Scholar] [CrossRef]
- Liu, Q.; Li, B.; Lu, J.; Zhang, Y.; Shang, Y.; Li, Y.; Gong, T.; Zhang, C. Recombinant outer membrane vesicles delivering eukaryotic expression plasmid of cytokines act as enhanced adjuvants against Helicobacter pylori infection in mice. Infect. Immun. 2023, 91, e0031323. [Google Scholar] [CrossRef]
- Prior, J.T.; Davitt, C.; Kurtz, J.; Gellings, P.; McLachlan, J.B.; Morici, L.A. Bacterial-derived outer membrane vesicles are potent adjuvants that drive humoral and cellular immune responses. Pharmaceutics 2021, 13, 131. [Google Scholar] [CrossRef]
- Huynh, D.T.; Nolfi, E.; Medfai, L.; van Ulsen, P.; Jong, W.S.P.; Sijts, A.J.A.M.; Luirink, J. Intranasal delivery of Salmonella OMVs decorated with Chlamydia trachomatis antigens induces specific local and systemic immune responses. Hum. Vaccin. Immunother. 2024, 20, 2330768. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Tan, K.; Yuan, J.; Song, K.; Li, R.; Huang, X.; Liu, Q. Flagellin-deficient outer membrane vesicles as adjuvant induce cross-protection of Salmonella Typhimurium outer membrane proteins against infection by heterologous Salmonella serotypes. Int. J. Med. Microbiol. IJMM 2018, 308, 796–802. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Liu, Q.; Yi, J.; Liang, K.; Hu, B.; Zhang, X.; Curtiss, R., 3rd; Kong, Q. Outer membrane vesicles from flagellin-deficient Salmonella enterica serovar Typhimurium induce cross-reactive immunity and provide cross-protection against heterologous Salmonella challenge. Sci. Rep. 2016, 6, 34776. [Google Scholar] [CrossRef] [PubMed]
- Bäumler, A.J.; Hargis, B.M.; Tsolis, R.M. Tracing the origins of Salmonella outbreaks. Science 2000, 287, 50–52. [Google Scholar] [CrossRef]
- Stevenson, T.C.; Cywes-Bentley, C.; Moeller, T.D.; Weyant, K.B.; Putnam, D.; Chang, Y.-F.; Jones, B.D.; Pier, G.B.; DeLisa, M.P. Immunization with outer membrane vesicles displaying conserved surface polysaccharide antigen elicits broadly antimicrobial antibodies. Proc. Natl. Acad. Sci. USA 2018, 115, E3106–E3115. [Google Scholar] [CrossRef]
- Lamarche, M.G.; Dozois, C.M.; Daigle, F.; Caza, M.; Curtiss, R.; Dubreuil, J.D.; Harel, J. Inactivation of the PST system reduces the virulence of an avian pathogenic Escherichia coli O78 strain. Infect. Immun. 2005, 73, 4138–4145. [Google Scholar] [CrossRef]
- Bian, X.; Chen, Y.; Zhang, W.; Liu, X.; Lei, M.; Yuan, H.; Li, M.; Liu, Q.; Kong, Q. Salmonella Typhimurium derived OMV nanoparticle displaying mixed heterologous O-antigens confers immunogenicity and protection against STEC infections in mice. Microb. Cell Fact. 2025, 24, 8. [Google Scholar] [CrossRef]
- Alaniz, R.C.; Deatherage, B.L.; Lara, J.C.; Cookson, B.T. Membrane vesicles are immunogenic facsimiles of Salmonella typhimurium that potently activate dendritic cells, prime B and T cell responses, and stimulate protective immunity in vivo. J. Immunol. 2007, 179, 7692–7701. [Google Scholar] [CrossRef]
- Fiorino, F.; Pettini, E.; Koeberling, O.; Ciabattini, A.; Pozzi, G.; Martin, L.B.; Medaglini, D. Long-Term Anti-Bacterial Immunity against Systemic Infection by Salmonella enterica Serovar Typhimurium Elicited by a GMMA-Based Vaccine. Vaccines 2021, 9, 495. [Google Scholar] [CrossRef]
- Harrell, J.E.; Kurtz, J.R.; Bauer, D.L.; Prior, J.T.; Gellings, P.S.; Morici, L.A.; McLachlan, J.B. An Outer Membrane Vesicle-Adjuvanted Oral Vaccine Protects Against Lethal, Oral Salmonella Infection. Pathogens 2021, 10, 616. [Google Scholar] [CrossRef]
- Jiang, L.; Driedonks, T.A.P.; Jong, W.S.P.; Dhakal, S.; Bart van den Berg van Saparoea, H.; Sitaras, I.; Zhou, R.; Caputo, C.; Littlefield, K.; Lowman, M.; et al. A bacterial extracellular vesicle-based intranasal vaccine against SARS-CoV-2 protects against disease and elicits neutralizing antibodies to wild-type and Delta variants. J. Extracell. Vesicles 2022, 11, e12192. [Google Scholar] [CrossRef]
- Zurita, M.E.; Wilk, M.M.; Carriquiriborde, F.; Bartel, E.; Moreno, G.; Misiak, A.; Mills, K.H.G.; Hozbor, D. A Pertussis Outer Membrane Vesicle-Based Vaccine Induces Lung-Resident Memory CD4 T Cells and Protection Against Bordetella pertussis, Including Pertactin Deficient Strains. Front. Cell. Infect. Microbiol. 2019, 9, 125. [Google Scholar] [CrossRef]
- Rollier, C.S.; Dold, C.; Marsay, L.; Linder, A.; Green, C.A.; Sadarangani, M.; Norheim, G.; Derrick, J.P.; Feavers, I.M.; Maiden, M.C.J.; et al. Human B Cell Responses to Dominant and Subdominant Antigens Induced by a Meningococcal Outer Membrane Vesicle Vaccine in a Phase I Trial. mSphere 2022, 7, e0067421. [Google Scholar] [CrossRef]



| No. | Serotype (Serogroup) | Strain | Antimicrobial Resistance | Source/Host | Purpose of Use | Reference |
|---|---|---|---|---|---|---|
| 1 | Typhimurium (B) | 14028 | Susceptible | NA/Chicken | Challenge | ATCC |
| 2 | Montevideo (C1) | A16-CF-111-L-1 | NAL-NEO-STR-TET | Litter/Chicken | Challenge | [27] |
| 3 | Albany (C2–C3) | A16-CF-360-1S | NAL-SXT | Feces/Chicken | Challenge | [28] |
| 4 | Enteritidis (D) | 13076 | Susceptible | NA/NA | SBA assay | ATCC |
| 5 | Enteritidis (D) | A18-KCI-DEO-1-2S | NAL-AMP-FIS-STR-TET | feces/Chicken | SBA assay | [5] |
| 6 | Gallinarum (D) | 287/91 | Susceptible | Liver/Chicken | SBA assay | NCTC |
| 7 | Gallinarum (D) | A17-DW-005 | STR-FIS-COL-NAL-CIP-GEN | Liver/Chicken | SBA assay | [29] |
| Group | Cloacal Swab | Liver | Cloacal Swab | Liver | ||||
|---|---|---|---|---|---|---|---|---|
| 1 dpc | 3 dpc | 5 dpc | 7 dpc | |||||
| 1 | PBS | 6/6 B | 6/6 | 6/6 | 6/6 | 5/6 C | 2.50 ± 0.64 D | 1.46 ± 1.46 |
| 2 | Killed ST | 4/6 | 5/6 | 3/6 | 2/6 | 2/6 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| 3 | ST OMV | 3/6 | 5/6 | 3/6 | 1/6 | 3/6 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| Group | Cloacal Swab | Liver | Cloacal Swab | Liver | ||||
|---|---|---|---|---|---|---|---|---|
| 1 dpc | 3 dpc | 5 dpc | 7 dpc | |||||
| 1 | PBS + SM challenge | 4/5 B | 4/5 | 3/5 | 1/5 | 2/5 C | 1.36 ± 1.17 D | 0.68 ± 0.83 |
| 2 | ST-OMV + SM challenge | 4/5 | 3/5 | 2/5 | 0/5 | 0/5 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| 3 | PBS + SA challenge | 5/5 | 5/5 | 5/5 | 5/5 | 1/5 | 2.13 ± 0.50 | 0.34 ± 0.68 |
| 4 | ST-OMV + SA challenge | 4/5 | 4/5 | 3/5 | 2/5 | 0/5 | 1.20 ± 1.49 | 0.00 ± 0.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Shang, K.; Choi, Y.-R.; Son, J.-E.; Kim, G.-J.; Zhang, J.-F.; Kim, K.-W.; Jang, H.-K.; Wei, B.; Kang, M. Cross-Protective Efficacy of Outer Membrane Vesicles (OMVs) Derived from Salmonella enterica Serovar Typhimurium Against Salmonella enterica Serovars Colonization in SPF Chicken. Biology 2026, 15, 11. https://doi.org/10.3390/biology15010011
Shang K, Choi Y-R, Son J-E, Kim G-J, Zhang J-F, Kim K-W, Jang H-K, Wei B, Kang M. Cross-Protective Efficacy of Outer Membrane Vesicles (OMVs) Derived from Salmonella enterica Serovar Typhimurium Against Salmonella enterica Serovars Colonization in SPF Chicken. Biology. 2026; 15(1):11. https://doi.org/10.3390/biology15010011
Chicago/Turabian StyleShang, Ke, Yu-Ri Choi, Ji-Eun Son, Gyeong-Jun Kim, Jun-Feng Zhang, Ki-Woong Kim, Hyung-Kwan Jang, Bai Wei, and Min Kang. 2026. "Cross-Protective Efficacy of Outer Membrane Vesicles (OMVs) Derived from Salmonella enterica Serovar Typhimurium Against Salmonella enterica Serovars Colonization in SPF Chicken" Biology 15, no. 1: 11. https://doi.org/10.3390/biology15010011
APA StyleShang, K., Choi, Y.-R., Son, J.-E., Kim, G.-J., Zhang, J.-F., Kim, K.-W., Jang, H.-K., Wei, B., & Kang, M. (2026). Cross-Protective Efficacy of Outer Membrane Vesicles (OMVs) Derived from Salmonella enterica Serovar Typhimurium Against Salmonella enterica Serovars Colonization in SPF Chicken. Biology, 15(1), 11. https://doi.org/10.3390/biology15010011






