Investigation of the Physiological and Antioxidant Properties of the Medicinal and Endemic Hypericum bilgehan-bilgilii Species Under Different Cultivation Methods
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Obtaining Plant Material
2.1.1. Wild Plants
2.1.2. Grown Plants in Peat in the Laboratory Environment (In Vivo in Lab)
2.1.3. In Vitro Culture Formation
2.2. Conducted Physiological Analyses
2.2.1. Photosynthetic Pigment Content (PPC)
2.2.2. Total Protein (TP) Quantities
2.2.3. Proline Amounts (PA)
2.2.4. Malondialdehyde Content (MDA)
2.2.5. Total Phenolic Content (TPC)
2.2.6. Determination of Phenylalanine Ammonium Lyase (PAL) Activity
2.3. Identification of Antioxidant Quantities and Activity
2.3.1. Superoxide Dismutase (SOD) Enzyme Activity
2.3.2. Catalase (CAT) Enzyme Activity
2.3.3. 2,2-Diphenyl-1-Picryl Hydrazyl (DPPH) Radical Scavenging Activity
2.3.4. Determination of Carotenoid Amount
2.4. Statistical Analysis (SA)
3. Results
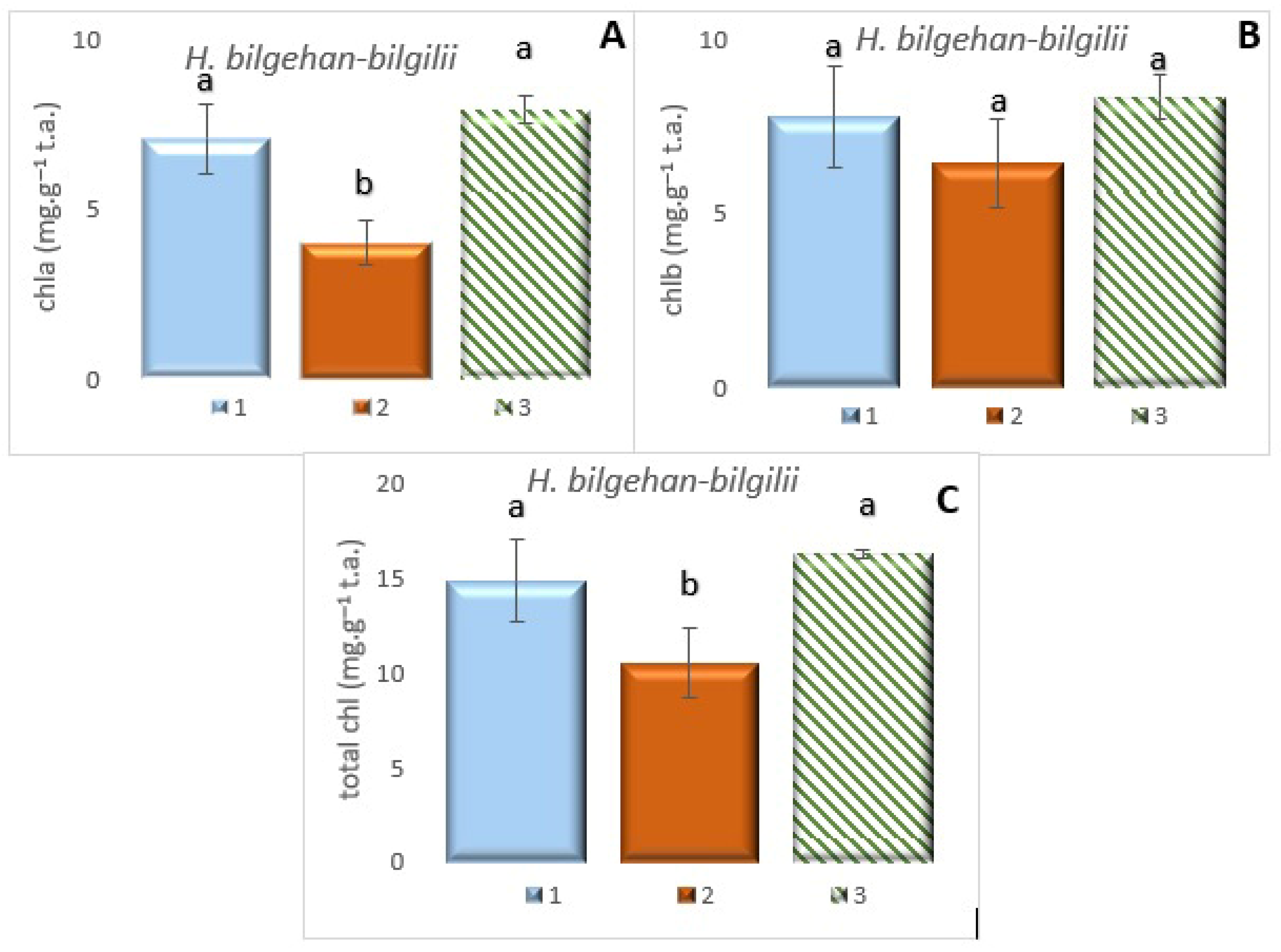
3.1. Quantification of Photosynthetic Pigment Content
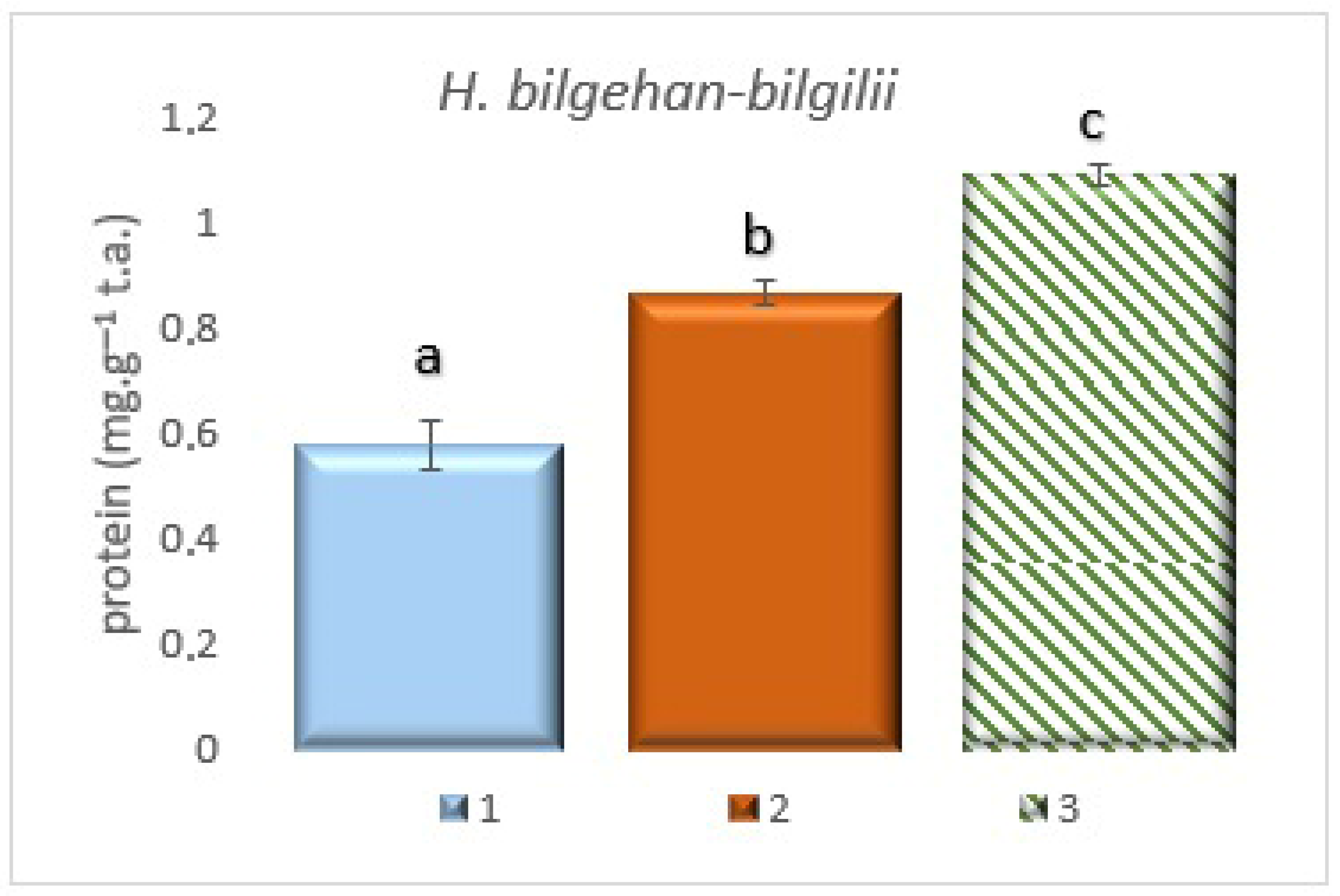
3.2. Identification of Total Protein Quantities
3.3. Proline Amount Determination
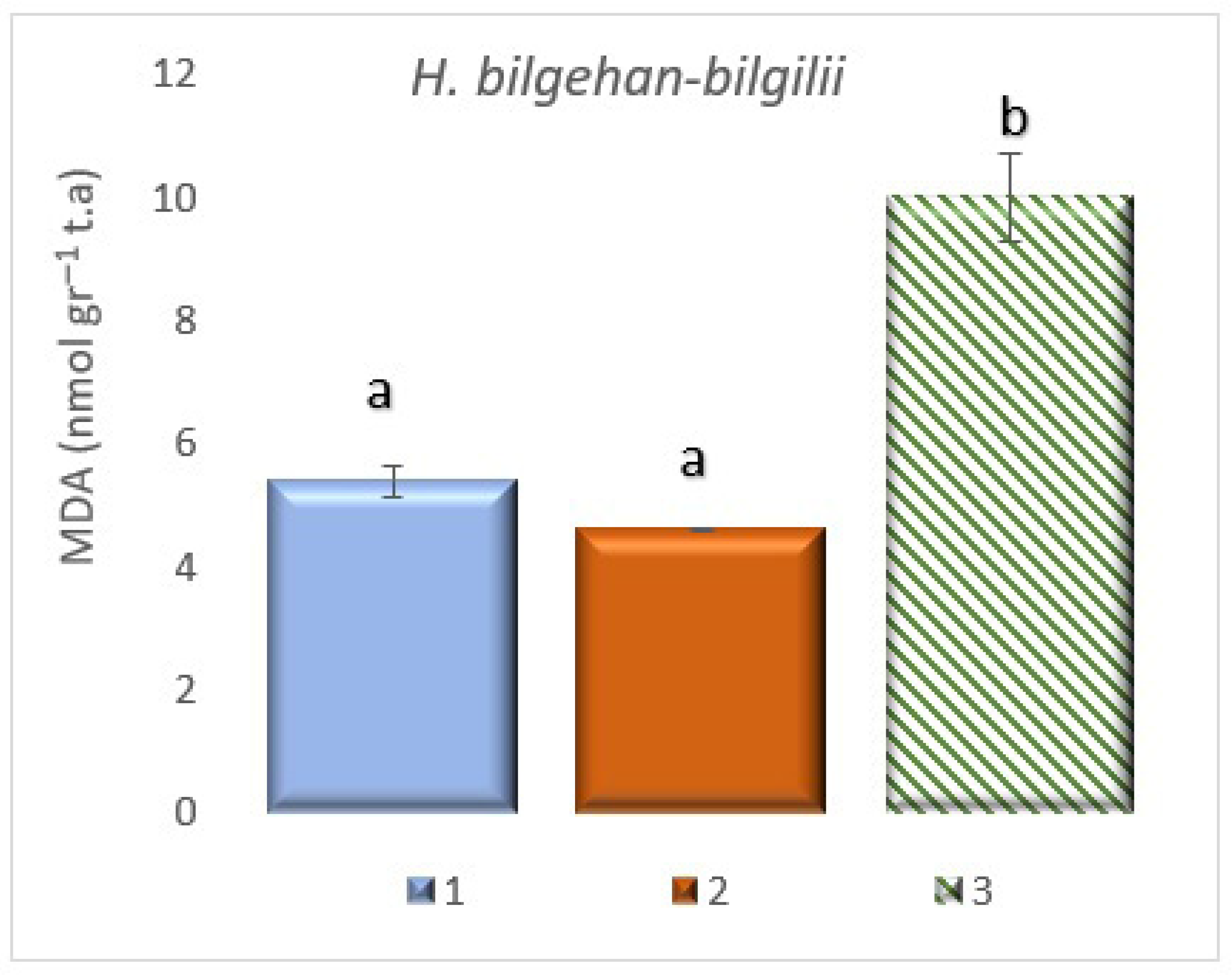
3.4. Detection of Malondialdehyde (MDA) Contents
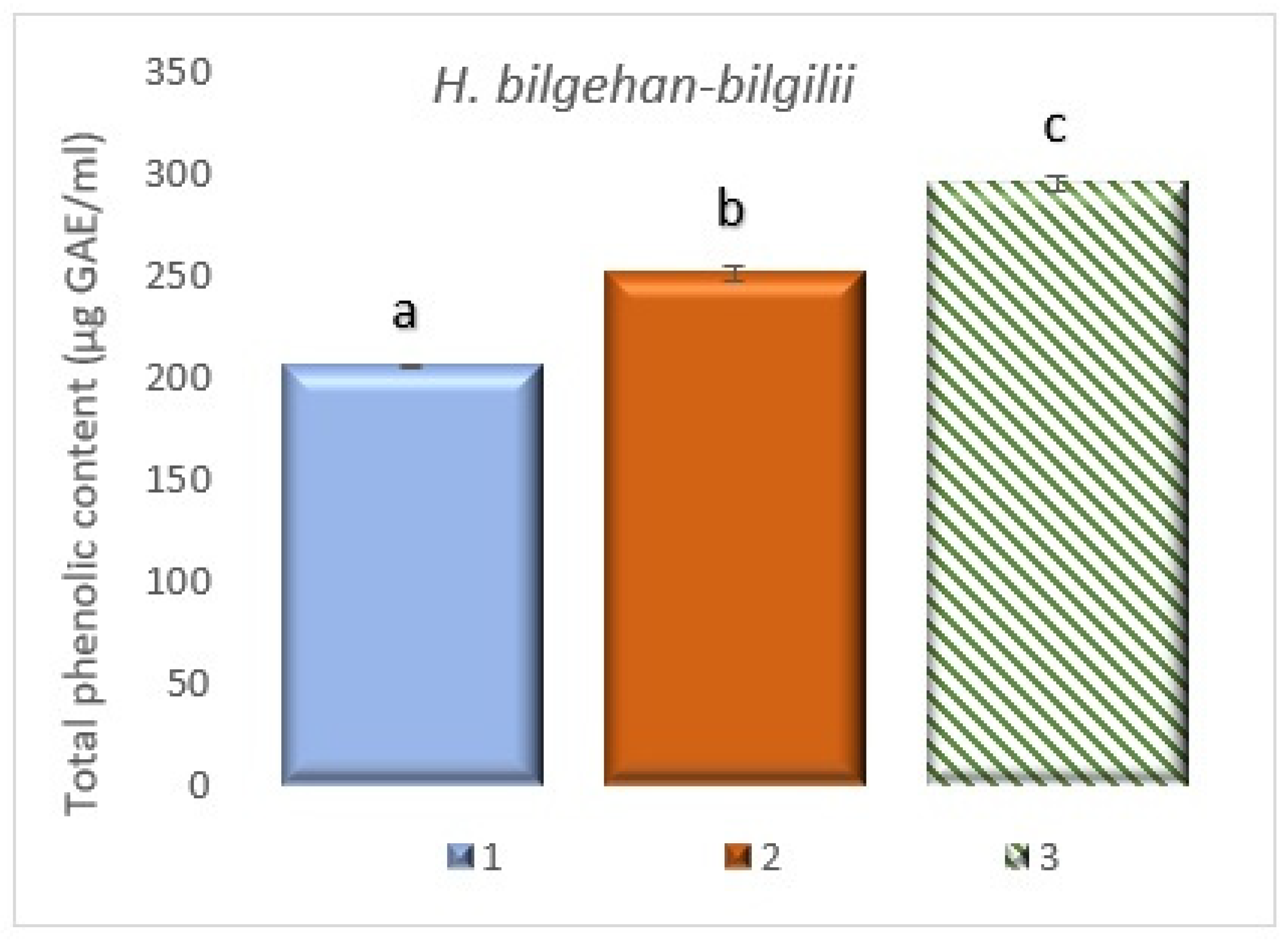
3.5. Quantification of Total Phenolic Content
3.6. The Determination of Phenylalanine Ammonia Lyase (PAL) Activity
3.7. Antioxidant Content and Activity Determination
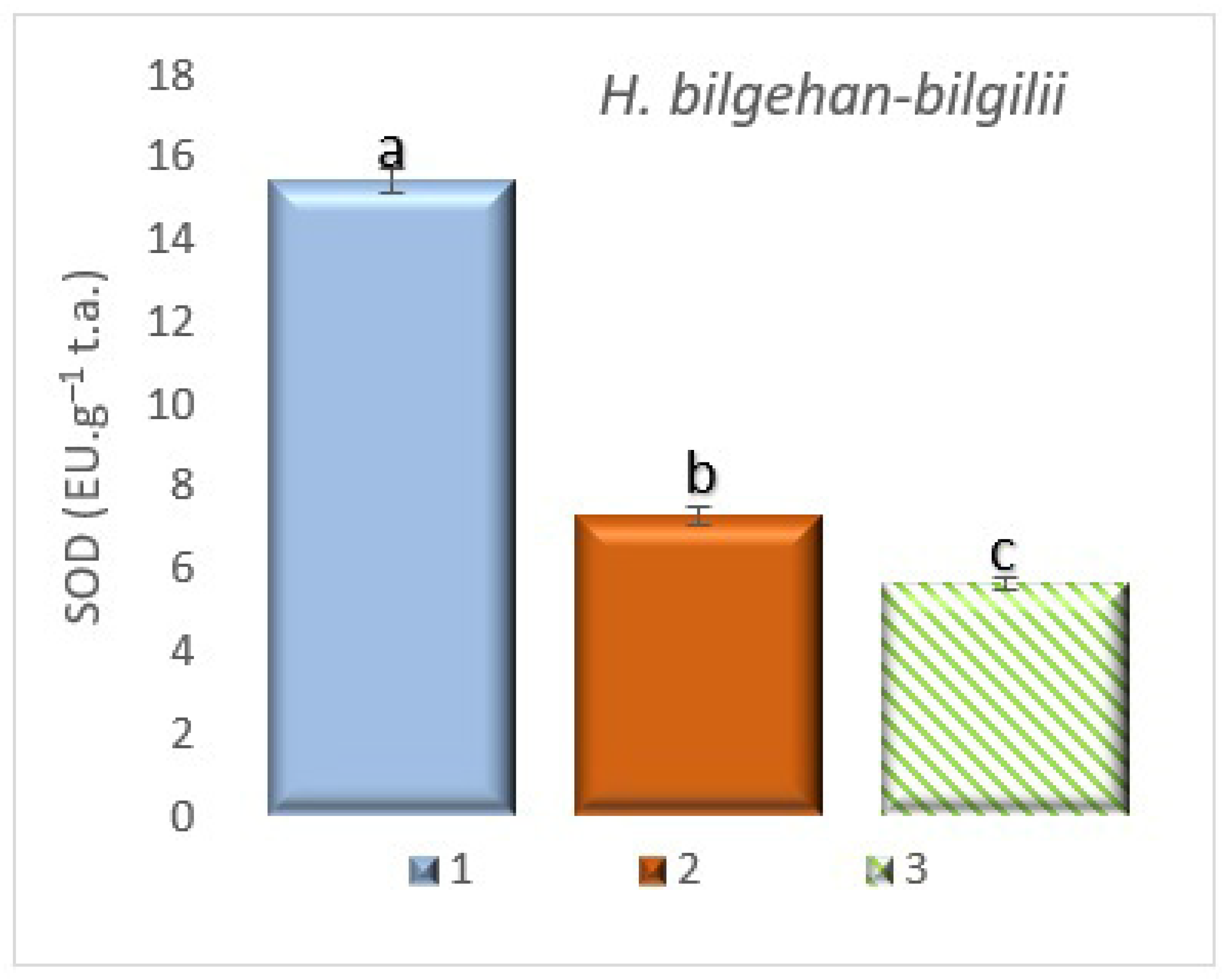
3.7.1. The Detection of Superoxide Dismutase (SOD) Enzyme Activity
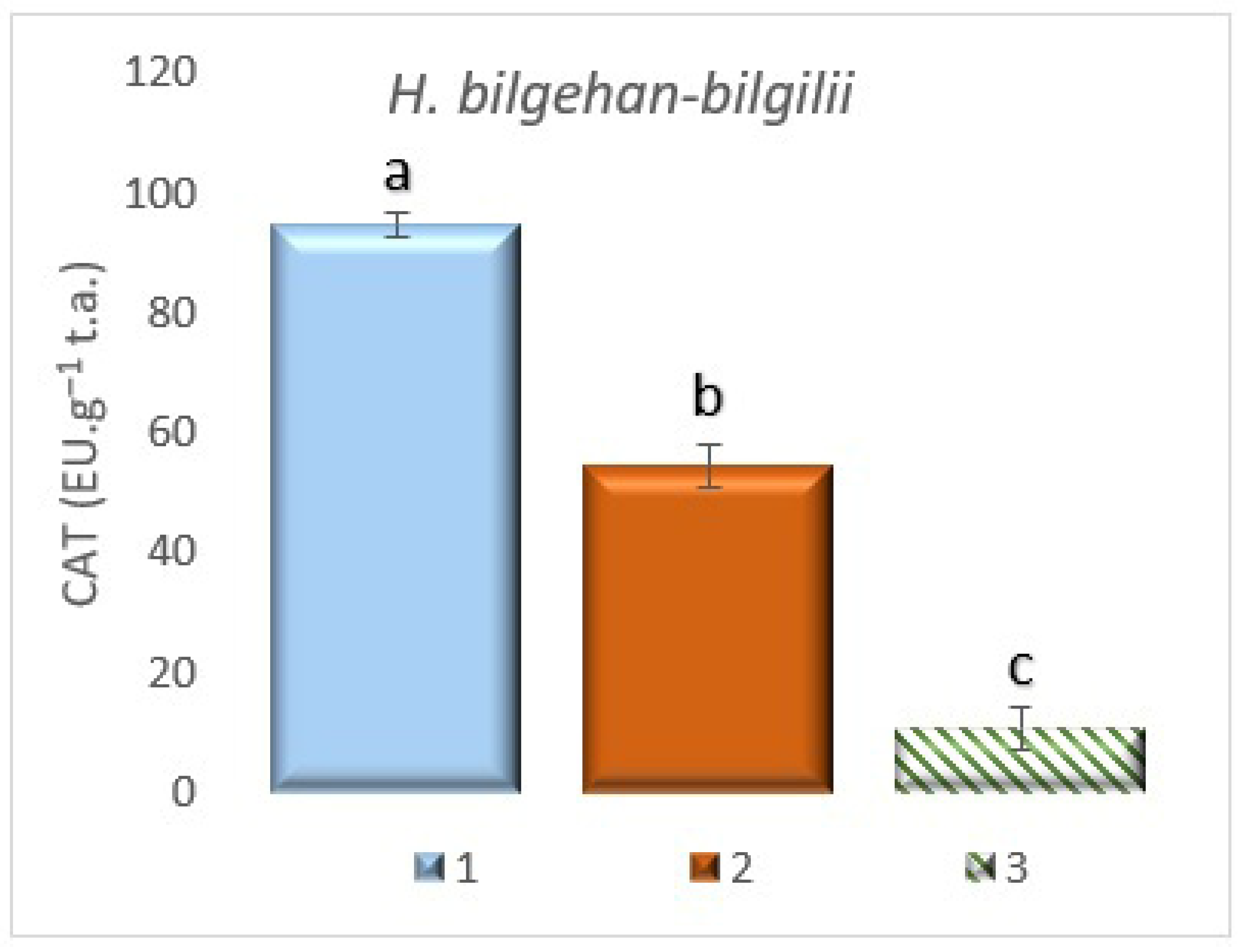
3.7.2. The Determination of Catalase (CAT) Enzyme Activity
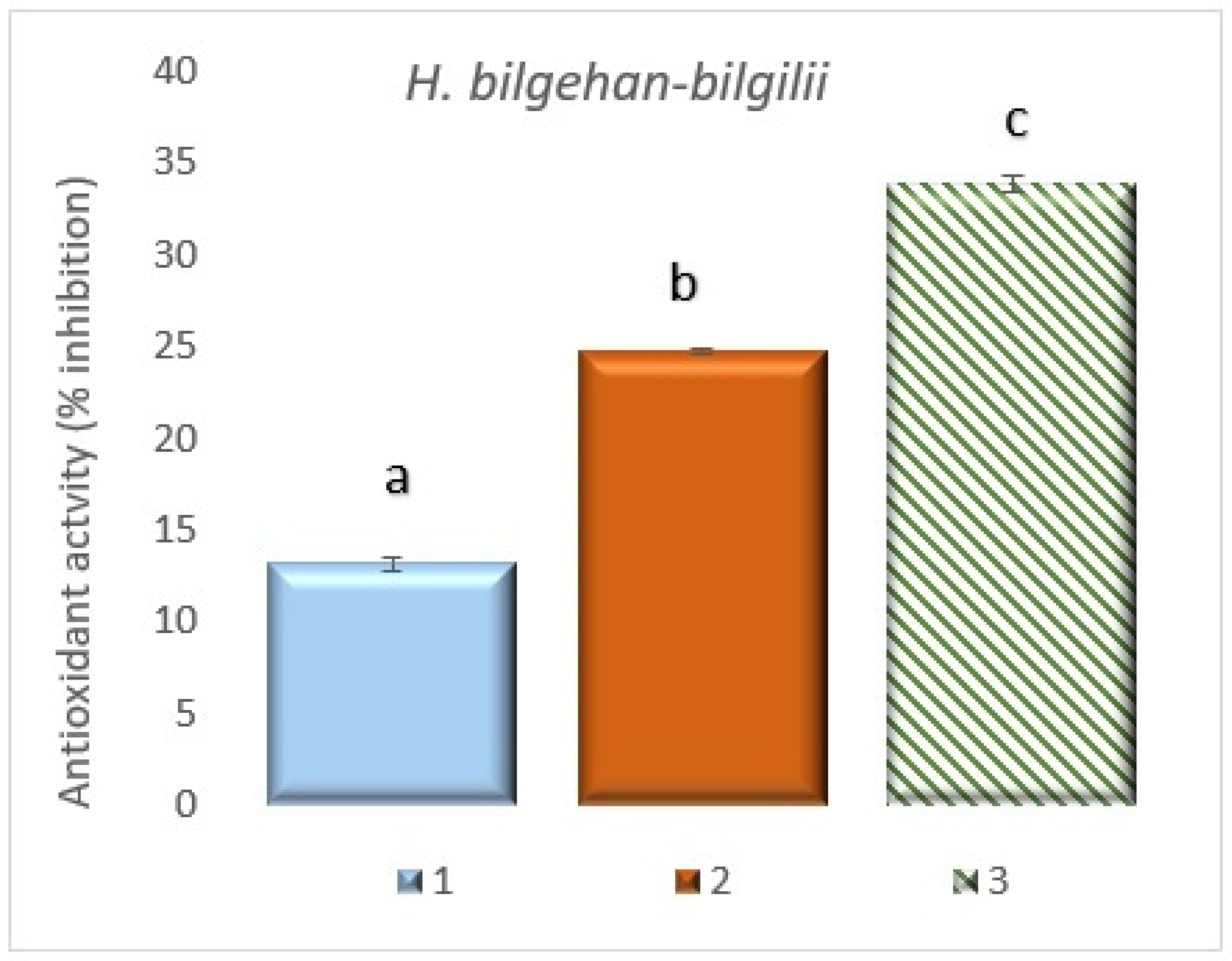
3.7.3. Quantification of 2,2-Diphenyl-1-picrylhydrazyl (DPPH) Radical Scavenging Activity
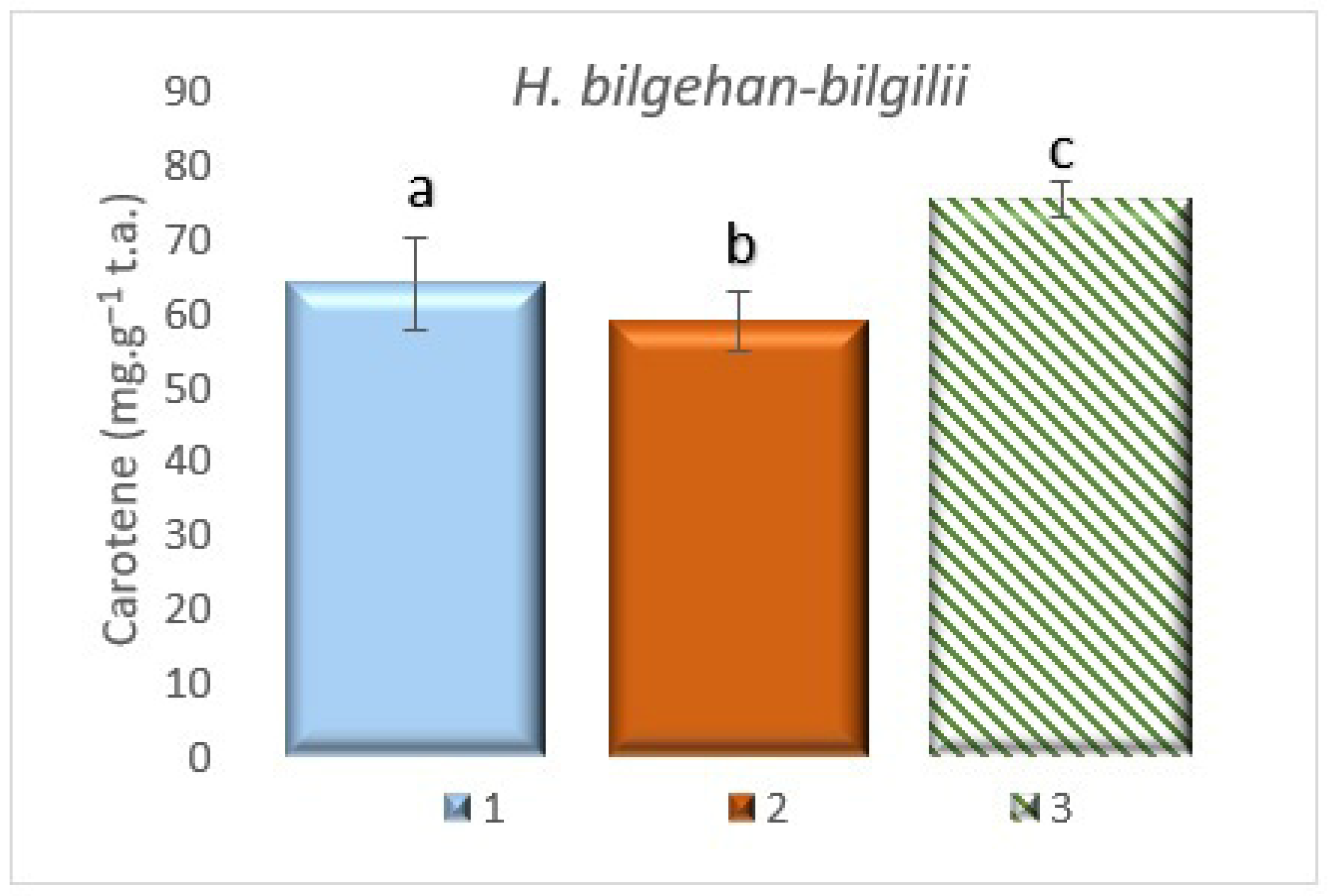
3.7.4. Determining the Amount of Carotenoids
4. Discussion
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| GA | Gibberellic acid |
| 2,4-D | Dichlorophenoxy acetic acid |
| BAP | Benzyl amino purine |
| BSA | Bovine serum albumin |
| TP | Total protein |
| MDA | Malondialdehyde content |
| TPC | Total phenolic content |
| PAL | Phenylalanine ammonium lyase |
| SOD | Superoxide dismutase |
| CAT | Catalase |
| DPPH | 2,2-Diphenyl-1-picryl hydrazyl |
| SA | Statistical analysis |
References
- Başköse, İ.; Savran, A. A new species from southern Anatolia (Dedegöl Mountain series-Çürük Mountain) in Turkey: Hypericum bilgehan-bilgilii (Hypericaceae). Phytotaxa 2018, 374, 110–118. [Google Scholar] [CrossRef]
- Fırat, M.; Eroğlu, H. Hypericum celikaensis (Hypericaceae), a new species from southeastern Anatolia (Adıyaman–Turkey). Candollea 2023, 78, 79–87. [Google Scholar] [CrossRef]
- Ma, W.; Ren, F.-C.; Wang, X.-R.; Li, N. Three new xanthones and other anti-inflammatory components from the aerial parts of Hypericum beanii. Arch. Pharmacal Res. 2025, 48, 89–101. [Google Scholar] [CrossRef] [PubMed]
- Robson, N.K. Studies in the genus Hypericum L. (Hypericaceae) 5 (2). Sections 17. Hirtella to 19. Coridium. Phytotaxa 2010, 4, 127–258. [Google Scholar] [CrossRef]
- Turker, H.; Unal, B.T. Development of a micropropagation protocol for endangered Hypericum bilgehan-bilgilii başkose & savran (Hypericaceae) species, local endemic to Turkey. Pak. J. Bot. 2022, 54, 1089–1095. [Google Scholar]
- Turker, H.; Unal, B.T. In Vitro Callus Induction and Plant Regeneration of Endemic and Threatened Hypericum bilgehan-bilgilii with Various Plant Growth Regulators. Biol. Bull. 2024, 51, S90–S99. [Google Scholar] [CrossRef]
- Duman, H.; Çakır-Dindar, E.G. Hypericum alacamdaglariense (Hypericaceae), a new species from Turkey. Phytotaxa 2020, 470, 176–185. [Google Scholar] [CrossRef]
- Caldeira, G.I.; Gouveia, L.P.; Serrano, R.; Silva, O.D. Hypericum genus as a natural source for biologically active compounds. Plants 2022, 11, 2509. [Google Scholar] [CrossRef]
- Ersoy, E.; Ozkan, E.E.; Boga, M.; Mat, A. Evaluation of in vitro biological activities of three Hypericum species (H. calycinum, H. confertum, and H. perforatum) from Turkey. S. Afr. J. Bot. 2020, 130, 141–147. [Google Scholar] [CrossRef]
- Unal, B.T.; Turker, H. Phytohormones used in the ex situ and in vitro conservation of Hypericum spp. In Phytohormones and Stress Responsive Secondary Metabolites; Elsevier: Amsterdam, The Netherlands, 2023; pp. 27–34. [Google Scholar]
- Unal, B.T.; Turker, H.; Ozturk, M. Ex-situ conservation of medicinal and aromatic plants using in vitro techniques. In Plants as Medicine and Aromatics; CRC Press: Boca Raton, FL, USA, 2023; pp. 13–22. [Google Scholar]
- Fobofou, S.A.T.; Franke, K.; Sanna, G.; Porzel, A.; Bullita, E.; La Colla, P.; Wessjohann, L.A. Isolation and anticancer, anthelminthic, and antiviral (HIV) activity of acylphloroglucinols, and regioselective synthesis of empetrifranzinans from Hypericum roeperianum. Bioorganic Med. Chem. 2015, 23, 6327–6334. [Google Scholar] [CrossRef]
- Mirmalek, S.A.; Azizi, M.A.; Jangholi, E.; Yadollah-Damavandi, S.; Javidi, M.A.; Parsa, Y.; Parsa, T.; Salimi-Tabatabaee, S.A.; Ghasemzadeh Kolagar, H.; Alizadeh-Navaei, R. Cytotoxic and apoptogenic effect of hypericin, the bioactive component of Hypericum perforatum on the MCF-7 human breast cancer cell line. Cancer Cell Int. 2015, 16, 3. [Google Scholar] [CrossRef] [PubMed]
- Zhai, X.-J.; Chen, F.; Chen, C.; Zhu, C.-R.; Lu, Y.-N. LC–MS/MS based studies on the anti-depressant effect of hypericin in the chronic unpredictable mild stress rat model. J. Ethnopharmacol. 2015, 169, 363–369. [Google Scholar] [CrossRef]
- Zorzetto, C.; Sánchez-Mateo, C.C.; Rabanal, R.M.; Lupidi, G.; Petrelli, D.; Vitali, L.A.; Bramucci, M.; Quassinti, L.; Caprioli, G.; Papa, F. Phytochemical analysis and in vitro biological activity of three Hypericum species from the Canary Islands (Hypericum reflexum, Hypericum canariense and Hypericum grandifolium). Fitoterapia 2015, 100, 95–109. [Google Scholar] [CrossRef]
- Saddiqe, Z.; Naeem, I.; Maimoona, A. A review of the antibacterial activity of Hypericum perforatum L. J. Ethnopharmacol. 2010, 131, 511–521. [Google Scholar] [CrossRef]
- El-Chaghaby, G.A.; Mohammed, F.S.; Rashad, S.; Uysal, I.; Koçer, O.; Lekesiz, Ö.; Doğan, M.; Şabik, A.E.; Sevindik, M. Genus Hypericum: General properties, chemical contents and biological activities. Egypt. J. Bot. 2024, 64, 1–26. [Google Scholar] [CrossRef]
- Kakouri, E.; Trigas, P.; Daferera, D.; Skotti, E.; Tarantilis, P.A.; Kanakis, C. Chemical characterization and antioxidant activity of nine Hypericum species from Greece. Antioxidants 2023, 12, 899. [Google Scholar] [CrossRef]
- Kızıl, G.; Kızıl, M.; Yavuz, M.; Emen, S.; Hakimoğlu, F. Antioxidant Activities of Ethanol Extracts of Hypericum triquetrifolium. and Hypericum scabroides. Pharm. Biol. 2008, 46, 231–242. [Google Scholar] [CrossRef]
- Orčić, D.Z.; Mimica-Dukić, N.M.; Francišković, M.M.; Petrović, S.S.; Jovin, E.Đ. Antioxidant activity relationship of phenolic compounds in Hypericum perforatum L. Chem. Cent. J. 2011, 5, 34. [Google Scholar] [CrossRef] [PubMed]
- Öztürk, N.; Tunçel, M.; Potoğlu-Erkara, İ. Phenolic compounds and antioxidant activities of some Hypericum species: A comparative study with H. perforatum. Pharm. Biol. 2009, 47, 120–127. [Google Scholar] [CrossRef]
- Zheleva-Dimitrova, D.; Nedialkov, P.; Kitanov, G. Radical scavenging and antioxidant activities of methanolic extracts from Hypericum species growing in Bulgaria. Pharmacogn. Mag. 2010, 6, 74. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Witham, F.H.; Feung, C.-S.; Hamilton, R.H. Metabolism of 2, 4-dichlorophenoxyacetic acid by soybean cotyledon callus tissue cultures. J. Agric. Food Chem. 1971, 19, 475–479. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Bates, L.S.; Waldren, R.; Teare, I. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Zhou, W.; Leul, M. Uniconazole-induced tolerance of rape plants to heat stress in relation to changes in hormonal levels, enzyme activities and lipid peroxidation. Plant Growth Regul. 1999, 27, 99–104. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Pascholati, S.; Nicholson, R.; Butler, L. Phenylalanine ammonia-lyase activity and anthocyanin accumulation in wounded maize mesocotyls. J. Phytopathol. 1986, 115, 165–172. [Google Scholar] [CrossRef]
- Beauchamp, C.; Fridovich, I. Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef] [PubMed]
- Bergmeyer, H.U. Methoden der Enzymatischen Analyse. Band 1; Walter de Gruyter GmbH & Co KG: Berlin, Germany, 1970. [Google Scholar]
- Brand-Williams, W.; Cuvelier, M.-E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Tukey, J.W. Causation, Regression, and Path Anaysis. Stat. Math. Biol. 1954, 35–66. [Google Scholar]
- Yaman, C.; Onlu, S.; Ahmed, H.; Erenler, R. Comparison of phytochemicals and antioxidant capacity of Hypericum perforatum; Wild plant parts and in vitro samples. J. Anim. Plant Sci.-JAPS 2022, 32, 596–603. [Google Scholar] [CrossRef]
- Bulda, O.; Rassadina, V.; Alekseichuk, H.; Laman, N. Spectrophotometric measurement of carotenes, xanthophylls, and chlorophylls in extracts from plant seeds. Russ. J. Plant Physiol. 2008, 55, 544–551. [Google Scholar] [CrossRef]
- Asadian, G.; Rahnavard, A.; Pourshamsian, K.; Ghorbanpour, M.; Taghavi, M. Study of variation of biochemical components in Hypericum perforatum L. grown in north of Iran. J. Res. Agric. Sci. 2011, 7, 27–36. [Google Scholar]
- Briskin, D.P.; Gawienowski, M.C. Differential effects of light and nitrogen on production of hypericins and leaf glands in Hypericum perforatum. Plant Physiol. Biochem. 2001, 39, 1075–1081. [Google Scholar] [CrossRef]
- Khan, S.R.; Rose, R.; Haase, D.L.; Sabin, T.E. Effects of shade on morphology, chlorophyll concentration, and chlorophyll fluorescence of four Pacific Northwest conifer species. New For. 2000, 19, 171–186. [Google Scholar] [CrossRef]
- Fan, Y.; Wang, Y.; Tan, R.; Zhang, Z. Seasonal and sexual variety of Ginkgo flavonol glycosides in the leaves of Ginkgo biloba L. Zhongguo Zhong Yao Za Zhi = Zhongguo Zhongyao Zazhi = China J. Chin. Mater. Medica 1998, 23, 267–269, 319. [Google Scholar]
- Kefeli, V.I.; Kalevitch, M.V.; Borsari, B. Phenolic cycle in plants and environment. J. Cell Mol. Biol. 2003, 2, 13–18. [Google Scholar]
- Xie, B.-D.; Wang, H.-T. Effects of light spectrum and photoperiod on contents of flavonoid and terpene in leaves of Ginkgo biloba L. J. Nanjing For. Univ. 2016, 30, 51. [Google Scholar]
- Yaman, C. Phytochemicals and antioxidant capacity of wild growing and in vitro Hypericum heterophyllum. Rom. Biotechnol. Lett. 2020, 25, 2111–2117. [Google Scholar] [CrossRef]
- Ghavamaldin, A.; Aptin, R.; Khalil, P.; Mansour, G.; Mariamalsadat, T. Study of variation of biochemical components in Hypericum perforatum L. growm in north of Iran. J. Med. Plants Res. 2012, 6, 366–372. [Google Scholar]
- Southwell, I.A.; Bourke, C.A. Seasonal variation in hypericin content of Hypericum perforatum L. (St. John’s wort). Phytochemistry 2001, 56, 437–441. [Google Scholar] [CrossRef]
- Ashraf, M.A.; Riaz, M.; Arif, M.S.; Rasheed, R.; Iqbal, M.; Hussain, I.; Mubarik, M.S. The role of non-enzymatic antioxidants in improving abiotic stress tolerance in plants. In Plant Tolerance to Environmental Stress; CRC Press: Boca Raton, FL, USA, 2019; pp. 129–144. [Google Scholar]
- Jan, R.; Asaf, S.; Numan, M.; Lubna; Kim, K.-M. Plant secondary metabolite biosynthesis and transcriptional regulation in response to biotic and abiotic stress conditions. Agronomy 2021, 11, 968. [Google Scholar] [CrossRef]
- Turker, H.; Beydilli, S.; Unal, B.T.; Unal, D. Exogenous proline-mediated stress tolerance in plants. In Exogenous Priming and Engineering of Plant Metabolic and Regulatory Genes; Elsevier: Amsterdam, The Netherlands, 2025; pp. 157–164. [Google Scholar]
- Bruňáková, K.; Bálintová, M.; Petijová, L.; Čellárová, E. Does phenotyping of Hypericum secondary metabolism reveal a tolerance to biotic/abiotic stressors? Front. Plant Sci. 2022, 13, 1042375. [Google Scholar] [CrossRef]
- Bruňáková, K.; Zámečník, J.; Urbanová, M.; Čellárová, E. Dehydration status of ABA-treated and cold-acclimated Hypericum perforatum L. shoot tips subjected to cryopreservation. Thermochim. Acta 2011, 525, 62–70. [Google Scholar] [CrossRef]
- Danova, K.; Nikolova-Damianova, B.; Denev, R.; Dimitrov, D. Influence of vitamins on polyphenolic content, morphological development, and stress response in shoot cultures of Hypericum spp. Plant Cell Tissue Organ Cult. (PCTOC) 2012, 110, 383–393. [Google Scholar]
- Danova, K.; Nikolova-Damianova, B.; Denev, R.; Markovska, Y. Impact of pre-culture on short-and long-term in vitro recovery of the biosynthetic potential and enzymatic and non-enzymatic antioxidant defense of Hypericum rumeliacum Boiss. after cryostorage. Plant Growth Regul. 2012, 68, 447–457. [Google Scholar] [CrossRef]
- Skyba, M.; Urbanová, M.; Kapchina-Toteva, V.; Košuth, J.; Harding, K.; Čellárová, E. Physiological, biochemical and molecular characteristics of cryopreserved Hypericum perforatum L. shoot tips. CryoLetters 2010, 31, 249–260. [Google Scholar]
- Szabados, L.; Savouré, A. Proline: A multifunctional amino acid. Trends Plant Sci. 2010, 15, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Sudipta, K.; Kumara Swamy, M.; Balasubramanya, S.; Anuradha, M. Assessment of genetic fidelity, antioxidant enzyme activity and proline content of micropropagated and field grown plants of Leptadenia reticulata (wight & arn.)—An endangered medicinal plant. Plant Cell Biotechnol. Mol. Biol. 2014, 15, 127–135. [Google Scholar]
- Benson, E.E. Special symposium: In vitro plant recalcitrance do free radicals have a role in plant tissue culture recalcitrance? Vitr. Cell. Dev. Biol. -Plant 2000, 36, 163–170. [Google Scholar] [CrossRef]
- Tusevski, O.; Todorovska, M.; Todorovska, I.; Petreska Stanoeva, J.; Gadzovska Simic, S. Production of Phenylpropanoids, Naphthodianthrones and Antioxidant Status of Hypericum perforatum L. Transgenic Shoots. Horticulturae 2024, 10, 59. [Google Scholar] [CrossRef]
- Ozden, M. Secondary metabolite production in callus cultures of Vitis vinifera: Influence of genotype and sucrose concentration in the medium on antioxidant activity. Acta Physiol. Plant. 2024, 46, 6. [Google Scholar] [CrossRef]
- Ahmed, S.I.; Hayat, M.Q.; Tahir, M.; Mansoor, Q.; Ismail, M.; Keck, K.; Bates, R.B. Pharmacologically active flavonoids from the anticancer, antioxidant and antimicrobial extracts of Cassia angustifolia Vahl. BMC Complement. Altern. Med. 2016, 16, 460. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Pandey, A.K. Chemistry and biological activities of flavonoids: An overview. Sci. World J. 2013, 2013, 162750. [Google Scholar] [CrossRef]
- Tungmunnithum, D.; Thongboonyou, A.; Pholboon, A.; Yangsabai, A. Flavonoids and other phenolic compounds from medicinal plants for pharmaceutical and medical aspects: An overview. Medicines 2018, 5, 93. [Google Scholar] [CrossRef]
- Ullah, A.; Munir, S.; Badshah, S.L.; Khan, N.; Ghani, L.; Poulson, B.G.; Emwas, A.-H.; Jaremko, M. Important flavonoids and their role as a therapeutic agent. Molecules 2020, 25, 5243. [Google Scholar] [CrossRef]
- Bernardi, A.P.M.; de Matos Nunes, J.; Marchioro, M.K.; Rosa, L.M.G.; von Poser, G.L.; Rech, S.B. Phenolic compounds profiles during ex vitro acclimatization of micropropagated Hypericum polyanthemum. Plant Physiol. Biochem. 2008, 46, 694–700. [Google Scholar] [CrossRef]
- Önlü, Ş.; Yaman, C.; Kurtul, E.; Önlü, H.; Bahadir-Acikara, Ö.; Tusevski, O.; Simic, S.G.; Özcan, S. Production of elicitor-induced phytochemicals in callus and shoot cultures of Hypericum heterophyllum. S. Afr. J. Bot. 2025, 177, 295–304. [Google Scholar] [CrossRef]
- Pinhatti, A.V.; de Matos Nunes, J.; Maurmann, N.; Rosa, L.M.G.; von Poser, G.L.; Rech, S.B. Phenolic compounds accumulation in Hypericum ternum propagated in vitro and during plant development acclimatization. Acta Physiol. Plant. 2010, 32, 675–681. [Google Scholar] [CrossRef]
- Azeez, H.A.; Ibrahim, K.M. Hypericum triquetrifolium callus cultures a potential source of phenolics and flavonoids. JZS-Part A Spec. Issue 2014, 16, 381–388. [Google Scholar] [CrossRef]
- Boga, M.; Ersoy, E.; Ozkan, E.E.; Cinar, E.; Kara, E.M.; Canturk, Y.Y.; Zengin, G. Volatile and phenolic profiling of a traditional medicinal plant, Hypericum empetrifolium with in vitro biological activities. J. Ethnopharmacol. 2021, 272, 113933. [Google Scholar] [CrossRef]
- Danova, K.; Čellárová, E.; Macková, A.; Daxnerová, Z.; Kapchina-Toteva, V. In vitro culture of Hypericum rumeliacum Boiss. and production of phenolics and flavonoids. Vitr. Cell. Dev. Biol. -Plant 2010, 46, 422–429. [Google Scholar] [CrossRef]
- Makarova, K.; Sajkowska-Kozielewicz, J.J.; Zawada, K.; Olchowik-Grabarek, E.; Ciach, M.A.; Gogolewski, K.; Dobros, N.; Ciechowicz, P.; Freichels, H.; Gambin, A. Harvest time affects antioxidant capacity, total polyphenol and flavonoid content of Polish St John’s wort’s (Hypericum perforatum L.) flowers. Sci. Rep. 2021, 11, 3989. [Google Scholar] [CrossRef] [PubMed]
- Napoli, E.; Siracusa, L.; Ruberto, G.; Carrubba, A.; Lazzara, S.; Speciale, A.; Cimino, F.; Saija, A.; Cristani, M. Phytochemical profiles, phototoxic and antioxidant properties of eleven Hypericum species–A comparative study. Phytochemistry 2018, 152, 162–173. [Google Scholar] [CrossRef] [PubMed]
- Shabani, A.; Karapandzova, M.; Karanfilova, I.C.; Stefkov, G.; Crcarevska, M.S.; Kulevanova, S. Distribution of total phenols, flavonoids and hypericin in different plant organs of wild-growing St. John’s-wort (Hypericum perforatum L., Hypericaceae) from North Macedonia. Maced. Pharm. Bull. 2019, 65, 83–89. [Google Scholar] [CrossRef]
- Tusevski, O.; Todorovska, M.; Spasenoski, M.; Simić, S.G. Antioxidant activity and phenolic compounds in Hypericum perforatum L. wildgrowing plants collected in the Republic of Macedonia. Biol. Nyssana 2019, 10, 159. [Google Scholar]
- Saensouk, S.; Benjamin, S.; Chumroenphat, T.; Saensouk, P. In Vitro Propagation, Evaluation of Antioxidant Activities, and Phytochemical Profiling of Wild and In Vitro-Cultured Plants of Curcuma larsenii Makino & Jenjitikul—A Rare Plant Species in Thailand. Horticulturae 2024, 10, 1181. [Google Scholar]
- Saensouk, S.; Sonthongphithak, P.; Chumroenphat, T.; Muangsan, N.; Souladeth, P.; Saensouk, P. In Vitro Multiplication, Antioxidant Activity, and Phytochemical Profiling of Wild and In Vitro-Cultured Plants of Kaempferia larsenii Sirirugsa—A Rare Plant Species in Thailand. Horticulturae 2025, 11, 281. [Google Scholar] [CrossRef]
- Nag, S.; Kumaria, S. In vitro propagation of medicinally threatened orchid Vanda coerulea: An improved method for the production of phytochemicals, antioxidants and phenylalanine ammonia lyase activity. J. Pharmacogn. Phytochem. 2018, 7, 2973–2982. [Google Scholar]
- Tang, W.; Newton, R.J. Increase of polyphenol oxidase and decrease of polyamines correlate with tissue browning in Virginia pine (Pinus virginiana Mill.). Plant Sci. 2004, 167, 621–628. [Google Scholar] [CrossRef]
- Tusevski, O.; Gadzovska Simic, S. Non-enzymatic and enzymatic antioxidant responses of Hypericum perforatum L. Hairy roots upon photooxidative stress. Horticulturae 2023, 9, 581. [Google Scholar] [CrossRef]
- Kong, J.-Q. Phenylalanine ammonia-lyase, a key component used for phenylpropanoids production by metabolic engineering. RSC Adv. 2015, 5, 62587–62603. [Google Scholar] [CrossRef]
- Eray, N.; Dalar, A.; Turker, M. The effects of abiotic stressors and signal molecules on phenolic composition and antioxidant activities of in vitro regenerated Hypericum perforatum (St. John’s Wort). S. Afr. J. Bot. 2020, 133, 253–263. [Google Scholar] [CrossRef]
- Gadzovska Simic, S.; Tusevski, O.; Maury, S.; Delaunay, A.; Joseph, C.; Hagège, D. Effects of polysaccharide elicitors on secondary metabolite production and antioxidant response in Hypericum perforatum L. shoot cultures. Sci. World J. 2014, 2014, 609649. [Google Scholar] [CrossRef] [PubMed]
- Gadzovska Simic, S.; Tusevski, O.; Maury, S.; Hano, C.; Delaunay, A.; Chabbert, B.; Lamblin, F.; Lainé, E.; Joseph, C.; Hagège, D. Fungal elicitor-mediated enhancement in phenylpropanoid and naphtodianthrone contents of Hypericum perforatum L. cell cultures. Plant Cell Tissue Organ Cult. (PCTOC) 2015, 122, 213–226. [Google Scholar] [CrossRef]
- Klejdus, B.; Kováčik, J.; Babula, P. PAL inhibitor evokes different responses in two Hypericum species. Plant Physiol. Biochem. 2013, 63, 82–88. [Google Scholar] [CrossRef]
- Wang, J.; Qian, J.; Yao, L.; Lu, Y. Enhanced production of flavonoids by methyl jasmonate elicitation in cell suspension culture of Hypericum perforatum. Bioresour. Bioprocess. 2015, 2, 5. [Google Scholar] [CrossRef]
- Talukder, P.; Talapatra, S.; Ghoshal, N.; Sen Raychaudhuri, S. Antioxidant activity and high-performance liquid chromatographic analysis of phenolic compounds during in vitro callus culture of Plantago ovata Forsk. and effect of exogenous additives on accumulation of phenolic compounds. J. Sci. Food Agric. 2016, 96, 232–244. [Google Scholar] [CrossRef]
- Turker, H.; Unal, B.T. Bryophytes as the potential source of antioxidant. Anatol. Bryol. 2020, 6, 129–137. [Google Scholar] [CrossRef]
- Treneva, G.; Markovska, Y.; Wolfram, E.; Danova, K. Effect of plant growth regulators on growth patterns and enzymatic antioxidant activities in Hypericum calycinum shoot cultures. Bulg. J. Agric. Sci. 2014, 20, 46–50. [Google Scholar]
- Mir, M.Y.; Kamili, A.N.; Hassan, Q.P.; Rafi, S.; Parray, J.A.; Jan, S. In vitro regeneration and free radical scavenging assay of Hypericum perforatum L. Natl. Acad. Sci. Lett. USA 2019, 42, 161–167. [Google Scholar] [CrossRef]
- Kwiecień, I.; Miceli, N.; Kędzia, E.; Cavò, E.; Taviano, M.F.; Beerhues, L.; Ekiert, H. Different types of Hypericum perforatum cvs. (Elixir, Helos, Topas) in vitro cultures: A rich source of bioactive metabolites and biological activities of biomass extracts. Molecules 2023, 28, 2376. [Google Scholar] [CrossRef]
- Barış, D.; Kızıl, M.; Aytekin, Ç.; Kızıl, G.; Yavuz, M.; Çeken, B.; Ertekin, A.S. In vitro antimicrobial and antioxidant activity of ethanol extract of three Hypericum and three Achillea species from Turkey. Int. J. Food Prop. 2011, 14, 339–355. [Google Scholar] [CrossRef]
- Dall’Acqua, S.; Ak, G.; Sinan, K.I.; Elbasan, F.; Ferrarese, I.; Sut, S.; Yıldıztugay, E.; Peron, G.; Schievano, E.; Nancy Picot-Allain, M.C. Hypericum triquetrifolium and H. neurocalycinum as sources of antioxidants and multi-target bioactive compounds: A comprehensive characterization combining in vitro bioassays and integrated NMR and LC-MS characterization by using a multivariate approach. Front. Pharmacol. 2021, 12, 660735. [Google Scholar] [CrossRef]
- Ozkan, E.E.; Ozden, T.Y.; Ozsoy, N.; Mat, A. Evaluation of chemical composition, antioxidant and anti-acetylcholinesterase activities of Hypericum neurocalycinum and Hypericum malatyanum. S. Afr. J. Bot. 2018, 114, 104–110. [Google Scholar] [CrossRef]
- Seyrekoğlu, F.; Temiz, H. Effect of Extraction Conditions on the Phenolic Content and DPPH Radical Scavenging Activity of Hypericum perforatum L. Turk. J. Agric. -Food Sci. Technol. 2020, 8, 226–229. [Google Scholar]
- Juan, M.; Rivero, R.M.; Romero, L.; Ruiz, J.M. Evaluation of some nutritional and biochemical indicators in selecting salt-resistant tomato cultivars. Environ. Exp. Bot. 2005, 54, 193–201. [Google Scholar] [CrossRef]
- Koyro, H.-W. Effect of salinity on growth, photosynthesis, water relations and solute composition of the potential cash crop halophyte Plantago coronopus (L.). Environ. Exp. Bot. 2006, 56, 136–146. [Google Scholar] [CrossRef]
- Georgieva, E.; Petrova, D.; Yordanova, Z.; Kapchina-Toteva, V.; Cellarova, E.; Chaneva, G. Influence of cryopreservation on the antioxidative activity of in vitro cultivated Hypericum species. Biotechnol. Biotechnol. Equip. 2014, 28, 863–870. [Google Scholar] [CrossRef] [PubMed]
- Zobayed, S.; Murch, S.; Rupasinghe, H.; Saxena, P. In vitro production and chemical characterization of St. John’s wort (Hypericum perforatum L. cv ‘New Stem’). Plant Sci. 2004, 166, 333–340. [Google Scholar] [CrossRef]
- Rahman, M.M.; Rahaman, M.S.; Islam, M.R.; Rahman, F.; Mithi, F.M.; Alqahtani, T.; Almikhlafi, M.A.; Alghamdi, S.Q.; Alruwaili, A.S.; Hossain, M.S. Role of phenolic compounds in human disease: Current knowledge and future prospects. Molecules 2021, 27, 233. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Chiu, W.K.; Nagashima, Y.; Taguchi, T. Application of antioxidative Maillard reaction products from histidine and glucose to sardine products. Nippon Suisan Gakkaishi 1988, 54, 1409–1414. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Turker, H. Investigation of the Physiological and Antioxidant Properties of the Medicinal and Endemic Hypericum bilgehan-bilgilii Species Under Different Cultivation Methods. Biology 2025, 14, 1302. https://doi.org/10.3390/biology14091302
Turker H. Investigation of the Physiological and Antioxidant Properties of the Medicinal and Endemic Hypericum bilgehan-bilgilii Species Under Different Cultivation Methods. Biology. 2025; 14(9):1302. https://doi.org/10.3390/biology14091302
Chicago/Turabian StyleTurker, Huseyin. 2025. "Investigation of the Physiological and Antioxidant Properties of the Medicinal and Endemic Hypericum bilgehan-bilgilii Species Under Different Cultivation Methods" Biology 14, no. 9: 1302. https://doi.org/10.3390/biology14091302
APA StyleTurker, H. (2025). Investigation of the Physiological and Antioxidant Properties of the Medicinal and Endemic Hypericum bilgehan-bilgilii Species Under Different Cultivation Methods. Biology, 14(9), 1302. https://doi.org/10.3390/biology14091302






