Earthworm Species from Diverse Ecological Groups Negatively Affect Enchytraeid Density in a Forest Ecosystem
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Substrates and Mesocosm Setup
2.2. Soil Fauna
2.3. Experimental Conditions
2.4. Enchytraeid Extraction
2.5. Statistical Analyses
3. Results
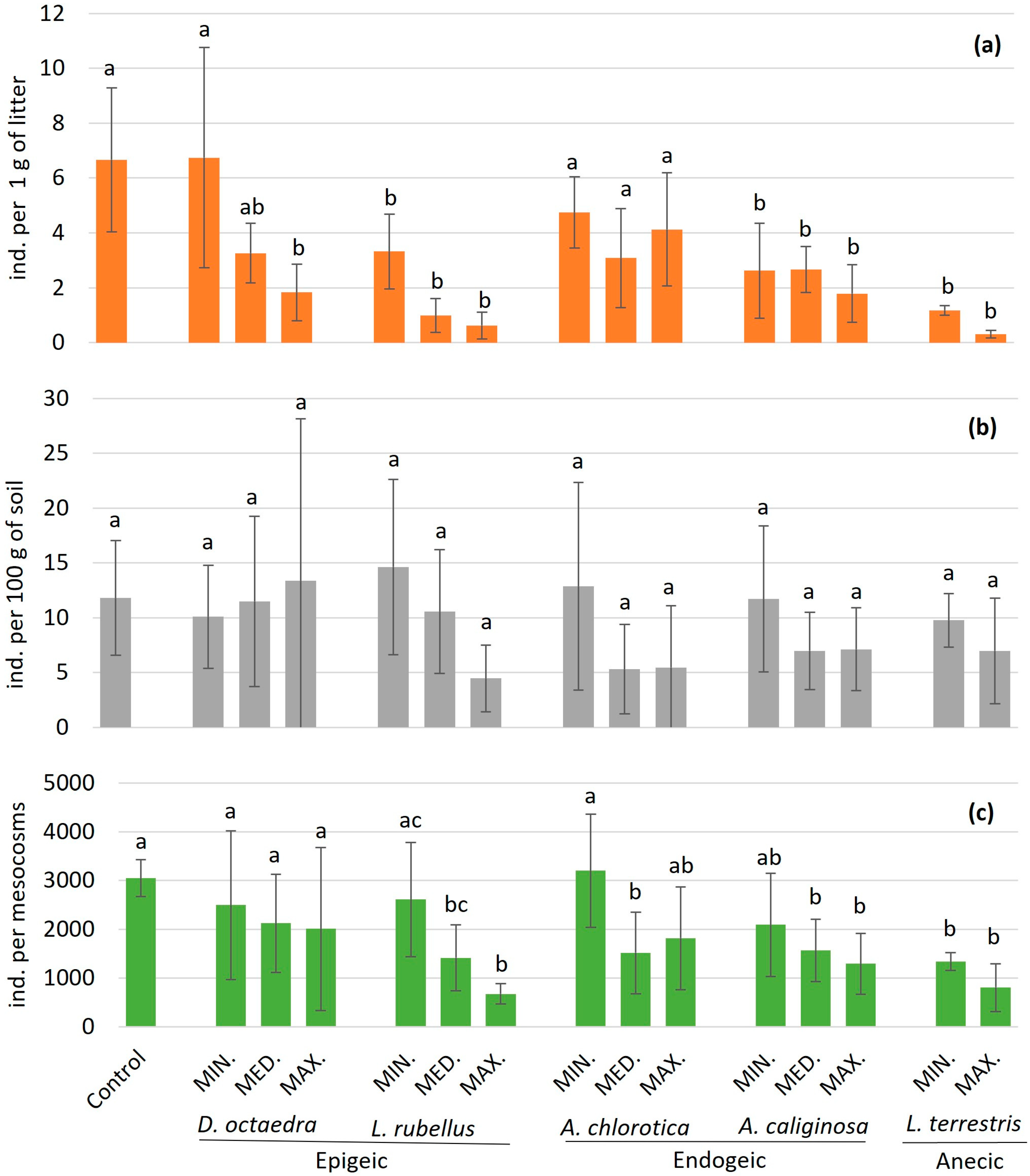
3.1. Effect of Epigeic Earthworm Species
3.2. Effect of Endogeic Earthworm Species
3.3. Effect of Anecic Earthworm Species
4. Discussion
| No. | Earthworm Species | Earthworm Ecological Group | Experimental or Field Data | Direction of Impact | Source |
|---|---|---|---|---|---|
| 1 | Lumbricus terrestris | AN | Seminatural experiment in mesocosms | ↓ | [54] |
| 3 | Lumbricus terrestris | AN | North American deciduous forests | ↑↓ | [21] |
| 4 | Lumbricus terrestris | AN | Agricultural fields in Finland | ↑ | [41] |
| 5 | Aporrectodea caliginosa | EN | Orchards | ↓ | [37] |
| 6 | Aporrectodea caliginosa | EN | Seminatural experiment in mesocosms in deciduous oak–beech forest | ↑ | [35] |
| 7 | Lumbricus rubellus | EP | Laboratory and field experiments on forest soils | ↓ | [53] |
| 8 | Lumbricus rubellus | EP | Peat bog experiment—different earthworm densities | ↓ | [61] |
| 9 | Lumbricus rubellus | EP | Experiment on peat meadows—different earthworm densities | n.s. | [42] |
| 10 | Lumbricus rubellus | EP | Seminatural experiment in mesocosms in deciduous oak–beech forest | ↓ | [35] |
| 11 | Dendrobaena octaedra | EP | Laboratory experiment on soil from a coniferous forest | ↓ | [32] |
| 12 | Dendrobaena octaedra | EP | Seminatural experiment in mesocosms, coniferous spruce forest | ↓ | [52] |
| 13 | Dendrobaena octaedra | EP | Seminatural experiment in mesocosms on soil from spruce and pine forests with the addition of pine and birch litter | ↓ | [62] |
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lee, K.E. Earthworms: Their Ecology and Relationships with Soils and Land Use; Australian Academic Press: Canberra, Australia, 1985; 411p. [Google Scholar]
- Phillips, H.R.P.; Guerra, C.A.; Bartz, M.L.C.; Briones, M.J.I.; Brown, G.; Crowther, T.W.; Ferlian, O.; Gongalsky, K.B.; van den Hoogen, J.; Krebs, J.; et al. Global distribution of earthworm diversity. Science 2019, 366, 480–485. [Google Scholar] [CrossRef] [PubMed]
- Lavelle, P.; Spain, A.V. Soil Ecology; Kluver Academic Publisher: Dordrecht, The Netherlands, 2001; 621p. [Google Scholar] [CrossRef]
- Blouin, M.; Hodson, M.E.; Delgado, E.A.; Baker, G.; Brussaard, L.; Butt, K.R.; Dai, J.; Dendooven, L.; Peres, G.; Tondoh, J.E.; et al. A review of earthworm impact on soil function and ecosystem services. Eur. J. Soil Sci. 2013, 64, 161–182. [Google Scholar] [CrossRef]
- Filser, J.; Faber, J.H.; Tiunov, A.V.; Brussaard, L.; Frouz, J.; De Deyn, G.; Uvarov, A.V.; Berg, M.P.; Lavelle, P.; Loreau, M.; et al. Soil fauna: Key to new carbon models. Soil 2016, 2, 565–582. [Google Scholar] [CrossRef]
- Jones, C.G.; Lawton, J.H.; Shachak, M. Organisms as ecosystem engineers. Oikos 1994, 69, 373–386. [Google Scholar] [CrossRef]
- Jones, C.G.; Lawton, J.H.; Shachak, M. Positive and negative effects of organisms as physical ecosystem engineers. Ecology 1997, 78, 1946–1957. [Google Scholar] [CrossRef]
- Lavelle, P.; Bignell, D.; Lepage, M.; Wolters, V.; Roger, P.; Ineson, P.; Heal, O.W.; Dhillon, S. Soil function in a changing world: The role of invertebrate ecosystem engineers. Eur. J. Soil Biol. 1997, 33, 159–193. [Google Scholar]
- Ammer, S.; Weber, K.; Abs, C.; Ammer, C.; Prietzel, J. Factors influencing distribution and abundance of earthworm communities in pure and converted Scots pine stands. Appl. Soil Ecol. 2006, 33, 10–21. [Google Scholar] [CrossRef]
- Eisenhauer, N.; Partsch, S.; Parkinson, D.; Scheu, S. Invasion of a deciduous forest by earthworms: Changes in soil chemistry; microflora; microarthropods and vegetation. Soil Biol. Biochem. 2007, 39, 1099–1110. [Google Scholar] [CrossRef]
- Bouché, M.B. Strategies Lombriciennes. Ecol. Bull. Stockholm 1977, 25, 122–132. [Google Scholar]
- Edwards, C.A.; Bohlen, P.J. Biology and Ecology of Earthworms; Chapman & Hall: London, UK, 1996; 426p. [Google Scholar]
- Curry, J.P.; Schmidt, O. The feeding ecology of earthworms—A review. Pedobiologia 2007, 50, 463–477. [Google Scholar] [CrossRef]
- Potapov, A.M.; Beaulieu, F.; Birkhofer, K.; Bluhm, S.L.; Degtyarev, M.I.; Devetter, M.; Goncharov, A.A.; Gongalsky, K.B.; Klarner, B.; Korobushkin, D.I.; et al. Feeding habits and multifunctional classification of soil-associated consumers from protists to vertebrates. Biol. Rev. 2022, 26, 1057–1117. [Google Scholar] [CrossRef]
- Cameron, E.K.; Knysh, K.M.; Proctor, H.C.; Bayne, E.M. Influence of two exotic earthworm species with different foraging strategies on abundance and composition of boreal microarthropods. Soil Biol. Biochem. 2013, 57, 334–340. [Google Scholar] [CrossRef]
- Snyder, B.A.; Callaham, M.A., Jr.; Lowe, C.N.; Hendrix, P.F. Earthworm invasion in North Soil Biol. Biochem. America: Food resource competition affects native millipede survival and invasive earthworm reproduction. Soil Biol. Biochem. 2013, 57, 212–216. [Google Scholar] [CrossRef]
- Brown, G.G.; Barois, I.; Lavelle, P. Regulation of soil organic matter and microbial activity in drilosphere and the role of interactions with other edaphic functional domains. Eur. J. Soil Biol. 2000, 36, 177–198. [Google Scholar] [CrossRef]
- Butt, K.R.; Nuutinen, V. The dawn of the dew worm. Biologist 2005, 52, 218–223. [Google Scholar]
- Karaca, A. Biology of Earthworms; Springer: Berlin/Heidelberg, Germany, 2011; 330p. [Google Scholar]
- Stromberger, M.E.; Keith, A.M.; Schmidt, O. Distinct microbial and faunal communities and translocated carbon in Lumbricus terrestris drilospheres. Soil Biol. Biochem. 2012, 46, 155–162. [Google Scholar] [CrossRef]
- Schlaghamerský, J.; Eisenhauer, N.; Frelich, L.E. Earthworm invasion alters enchytraeid community composition and individual biomass in Northern Hardwood Forests of North America. Appl. Soil Ecol. 2014, 83, 159–169. [Google Scholar] [CrossRef]
- Didden, W.A.M. Ecology of terrestrial Enchytraeidae. Pedobiologia 1993, 37, 2–19. [Google Scholar] [CrossRef]
- Benckiser, G. (Ed.) Fauna in Soil Ecosytstem: Recycling Processes, Nutrient Fluxes, and Agricultural Production; Marcel Dekker Inc.: New York, NY, USA, 1997; 414p. [Google Scholar]
- Swift, M.J.; Heal, O.W.; Anderson, J.M. Decomposition in Terrestrial Ecosystems; Blackwell Scientific Publications: Oxford, UK, 1979. [Google Scholar]
- O’Connor, F.B. Enchytraeidae. In Soil Biology; Burges, A., Raw, F., Eds.; Academic Press Inc.: London, UK, 1967; pp. 213–256. [Google Scholar]
- Petersen, H.; Luxton, M. A comparative analysis of soil fauna populations and their role in decomposition processes. Oikos 1982, 39, 287–388. [Google Scholar] [CrossRef]
- Springett, J.A.; Brittain, J.E.; Springett, B.P. Vertical movement of Enchytraeidae (Oligochaeta) in mooorland soils. Oikos 1970, 21, 16–21. [Google Scholar] [CrossRef]
- Maraun, M.; Martens, H.; Migge, S.; Theenhaus, A.; Scheu, S. Adding to “the enigma of soil animals diversity”: Fungal feeders as saphrophagus soil invertebrates prefer similar soil substrates. Eur. J. Soil Biol. 2003, 39, 85–95. [Google Scholar] [CrossRef]
- Hönemann, L.; Nentwig, W. Are survival and reproduction of Enchytraeus albidus (Annelida: Enchytraeidae) at risk by feeding on Bt-maize litter? Eur. J. Soil Biol. 2009, 45, 351–355. [Google Scholar] [CrossRef]
- Didden, A.M.; Frund, H.C.; Graefe, U. Enchytraeidae. In Fauna in Soil Ecosytstem: Recykling Processes, Nutrient Fluxes, and Agricultural Production; Benckiser, G., Ed.; Marcel Dekker Inc.: New York, NY, USA, 1997; pp. 135–172. [Google Scholar]
- Eisenhauer, N. The action of an animal ecosystem engineer: Identification of the main mechanisms of earthworm impacts on soil microarthropods. Pedobiologia 2010, 53, 343–352. [Google Scholar] [CrossRef]
- Hyvönen, R.; Andersson, S.; Clarholm, M.; Persson, T. Effects of lumbricids and enchytraeids on nematodes in limed and unlimited coniferous mor humus. Biol. Fertil. Soils 1994, 17, 201–205. [Google Scholar] [CrossRef]
- Bonkowski, M.; Schaefer, M. Interactions between earthworms and soil protozoa: A trophic component in the soil food web. Soil Biol. Biochem. 1997, 29, 499–502. [Google Scholar] [CrossRef]
- Kristufek, V.; Ravasz, K.; Pizl, V. Changes In den sity of bacteria and microfungi Turing Gut transit in Lumbricus rubellus and Aporectodea caliginosa (Oligohaeta: Lumbricidae). Soil Biol. Biochem. 1992, 24, 1499–1500. [Google Scholar]
- Karaban, K.; Uvarov, A.V. Non-trophic effects of earthworms on enchytraeids: An experimental investigation. Soil Biol. Biochem. 2014, 73, 84–92. [Google Scholar] [CrossRef]
- Lavelle, P.; Spain, A.; Blouin, M.; Brown, G.; Decaëns, D.; Grimaldi, M.; Jiménez, J.J.; McKey, D.; Mathieu, J.; Velasquez, E.; et al. Ecosystem engineers in a self-organized soil: A review of concepts and future research questions. Soil Sci. 2016, 181, 91–109. [Google Scholar] [CrossRef]
- Górny, M. Studies on the relationship between enchytraeids and earthworms. In Soil Biology and Conservation of the Biosphere; Szegi, J., Ed.; Akadémiai Kiadó: Budapest, Hungary, 1984; Volume 2, pp. 769–776. [Google Scholar]
- Makulec, G.; Pilipiuk, I. Influence of plant diversity and Earthworm casts on abudance and species composition of Enchytraeids (Oligochaeta: Enchytraeidae) in a lisymetric experiment. Pol. J. Ecol. 2000, 48, 185–193. [Google Scholar]
- Räty, M.; Huhta, V. Earthworms and pH affect communities of nematodes and enchytraeids in forest soil. Biol. Fertil. Soils. 2003, 38, 52–58. [Google Scholar] [CrossRef]
- Tao, J.; Xu, Y.; Griffiths, B.S.; Hu, F.; Chen, X.; Jiao, J.; Li, H. Earthworms reduce the abundance of nematodes and enchytraeids in a soil mesocosm experimentdespite abundant food resources. Soil Sci. Soc. Am. J. 2011, 75, 1774–1778. [Google Scholar] [CrossRef]
- Nuutinen, V.; Butt, K.R.; Hyväluoma, J.; Ketoja, E.; Mikola, J. Soil faunal and structural responses to the settlement of a semi-sedentary earthworm Lumbricus terrestris in an arable clay field. Soil Biol. Biochem. 2017, 115, 285–296. [Google Scholar] [CrossRef]
- Makulec, G. The role of Lumbricus rubellus Hoffm. in determining biotic and abiotic properties of peat soils. Pol. J. Ecol. 2002, 50, 301–339. [Google Scholar]
- Uvarov, A.V. Inter- and intraspecific interactions in lumbricid earthworms: Their role for earthworm performance and ecosystem functioning. Pedobiologia 2009, 53, 1–27. [Google Scholar] [CrossRef]
- Uvarov, A.V.; Ilieva-Makulec, K.; Karaban, K.; Jakovenko, N.S.; Uchmański, J. Effects of intra- and interspecific interactions in earthworms assemblages: A comparative study. Biol. Bull. 2019, 46, 475–482. [Google Scholar] [CrossRef]
- Golovanova, E.V.; Kniazev, S.Y.; Karaban, K.; Babiy, K.A.; Shekhovtsov, S.V. First short-term study of the relationship between native and invasive earthworms in the zone of soil freezing in Western Siberia—Experiments in Mesocosms. Diversity 2023, 15, 248. [Google Scholar] [CrossRef]
- Dominguez, A.; Bedano, J.C. Earthworm and Enchytraeid Co-occurrence Pattern in Organic and Conventional Farming: Consequences for Ecosystem Engineering. Soil Sci. 2016, 181, 148–156. [Google Scholar] [CrossRef]
- Uvarov, A.V. Density-mediated earthworm effects on soil respiration. Pol. J. Ecol. 2016, 64, 534–546. [Google Scholar] [CrossRef]
- Uvarov, A.V. Density-dependent responses in some common lumbricid species. Pedobiologia 2017, 61, 1–8. [Google Scholar] [CrossRef]
- Kapusta, P.; Sobczyk, Ł.; Rożen, A.; Weiner, J. Species diversity and spatial distribution of enchytraeid communities in forest soils: Effects of habitat characteristics and heavy metal contamination. Appl. Soil Ecol. 2003, 23, 187–198. [Google Scholar] [CrossRef]
- Beylich, A.; Graefe, U. Relationships between microannelid and earthworm activity. Landbauforsch.–VTI Agric. For. Res. 2012, 1–12. [Google Scholar]
- Brown, G.G. How do earthworms affect microfloral and faunal community diversity? Plant Soil 1995, 170, 209–231. [Google Scholar] [CrossRef]
- Huhta, V.; Viberg, K. Competitive interactions between the earthworm Den-drobaena octaedra and the enchytraeid Cognettia sphagnetorum. Pedobiologia 1999, 43, 886–890. [Google Scholar] [CrossRef]
- Haimi, J.; Boucelham, M. Influence of a litter feeding earthworm, Lumbricus rubellus, on soil processes in a simulated coniferous forest floor. Pedobiologia 1991, 35, 247–256. [Google Scholar] [CrossRef]
- Lagerlöf, J.; Lofs-Holmin, A. Relationships between earthworms and soil meso-fauna during decomposition of crop residues. In Soil Fauna and Soil Fertility; Striganova, B.R., Ed.; Nauka: Moscow, Russia, 1987; pp. 377–381. [Google Scholar]
- Craven, D.; Thakur, M.P.; Cameron, E.K.; Frelich, L.E.; Beauséjour, R.; Blair, R.B.; Eisenhauer, N. The unseen invaders: Introduced earthworms as drivers of change in plant communities in North American forests (a meta-analysis). Glob. Change Biol. 2017, 23, 1065–1074. [Google Scholar] [CrossRef] [PubMed]
- Klein, A.; Eisenhauer, N.; Schaefer, I. Invasive lumbricid earthworms in North America—Different life histories but common dispersal? J. Biogeogr. 2020, 47, 674–685. [Google Scholar] [CrossRef]
- Brussaard, L.; Aanen, D.K.; Briones, M.J.I.; Deca¨ens, T.; de Deyn, G.B.; Fayle, T.M.; James, S.W.; Nobre, T. Biogeography and phylogenetic community structureof soil invertebrate ecosystem engineers: Global to local patterns; implications for ecosystem functioning and services and global environmental change impacts. In Soil Ecology Ecosystems Services; Oxford University Press: Oxford, UK, 2012; pp. 201–232. [Google Scholar] [CrossRef]
- Serbource, C.; Sammartino, S.; Cornu, S.; Papillon, J.; Adrien, J.; Pelosi, C. Enchytraeids: Small but important ecosystem engineers. Geoderma 2025, 453, 117150. [Google Scholar] [CrossRef]
- Topoliantz, S.; Ponge, J.-F.; Viaux, P. Earthworm and enchytraeid activity under different arable farming systems; as exemplified by biogenic structures. Plant Soil. 2000, 225, 39–51. [Google Scholar] [CrossRef]
- Uvarov, A.V.; Karaban, K. Do alterations in mesofauna community affect earthworms? Oecologia 2015, 179, 877–887. [Google Scholar] [CrossRef]
- Makulec, G. Mutual relationships between earthworms (Lumbricidae) and enchytraeids (Enchytraeidae). Zesz. Nauk. Akad. Rol. Im. H. Kołłątaja Krak. 1996, 47, 147–154. (In Polish) [Google Scholar]
- Yli-Olli, A.; Huhta, V. Responses of co-occurring populations of Dendrobaena octaedra (Lumbricidae) and Cognettia sphagnetorum (Enchytraeidae) to soil pH, moisture and resource addition. Pedobiologia 2000, 44, 86–95. [Google Scholar] [CrossRef]
| Ecological Group | Earthworm Species | Earthworm Density | ||||||
|---|---|---|---|---|---|---|---|---|
| Control | Minimum | Medium | Maximum | |||||
| MK | m2 | MK | m2 | MK | m2 | |||
| Epigeic | Dendrobaena octaedra | 0 | 5 | 155 | 15 | 465 | 25 | 775 |
| Epigeic | Lumbricus rubellus | 0 | 3 | 96 | 9 | 287 | 15 | 478 |
| Endogeic | Allobophora chlorotica | 0 | 5 | 155 | 15 | 465 | 25 | 775 |
| Endogeic | Aporrectodea caliginosa | 0 | 3 | 96 | 9 | 287 | 15 | 478 |
| Anecic | Lumbricus terrestris | 0 | 2 | 64 | - | 3 | 96 | |
| Earthworm Species | Earthworm Ecological Group | Enchytraeid Density | ||
|---|---|---|---|---|
| Litter Level | Soil Level | Mesocosms (Litter + Soil) | ||
| Lumbricus terrestris | Anecic | ↓ | n.s. | ↓ |
| Aporrectodea caliginosa | Endogeic | ↓ | n.s. | ↓↓ |
| Allobophora chlorotica | Endogeic | n.s. | n.s. | ↓↓ |
| Lumbricus rubellus | Epigeic | ↓ | n.s. | ↓↓ |
| Dendrobaena octaedra | Epigeic | ↓↓ | n.s. | n.s. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karaban, K.; Kaliszewicz, A.; Ilieva-Makulec, K.; Uvarov, A.V. Earthworm Species from Diverse Ecological Groups Negatively Affect Enchytraeid Density in a Forest Ecosystem. Biology 2025, 14, 1283. https://doi.org/10.3390/biology14091283
Karaban K, Kaliszewicz A, Ilieva-Makulec K, Uvarov AV. Earthworm Species from Diverse Ecological Groups Negatively Affect Enchytraeid Density in a Forest Ecosystem. Biology. 2025; 14(9):1283. https://doi.org/10.3390/biology14091283
Chicago/Turabian StyleKaraban, Kamil, Anita Kaliszewicz, Krassimira Ilieva-Makulec, and Alexei V. Uvarov. 2025. "Earthworm Species from Diverse Ecological Groups Negatively Affect Enchytraeid Density in a Forest Ecosystem" Biology 14, no. 9: 1283. https://doi.org/10.3390/biology14091283
APA StyleKaraban, K., Kaliszewicz, A., Ilieva-Makulec, K., & Uvarov, A. V. (2025). Earthworm Species from Diverse Ecological Groups Negatively Affect Enchytraeid Density in a Forest Ecosystem. Biology, 14(9), 1283. https://doi.org/10.3390/biology14091283






