HSP60 and SARS-CoV-2: Les Liaisons Dangereuses
Simple Summary
Abstract
1. Introduction
2. Pathogenesis of COVID-19 and the Role of Molecular Mimicry
3. Hsp60, Immune Response, and Inflammation
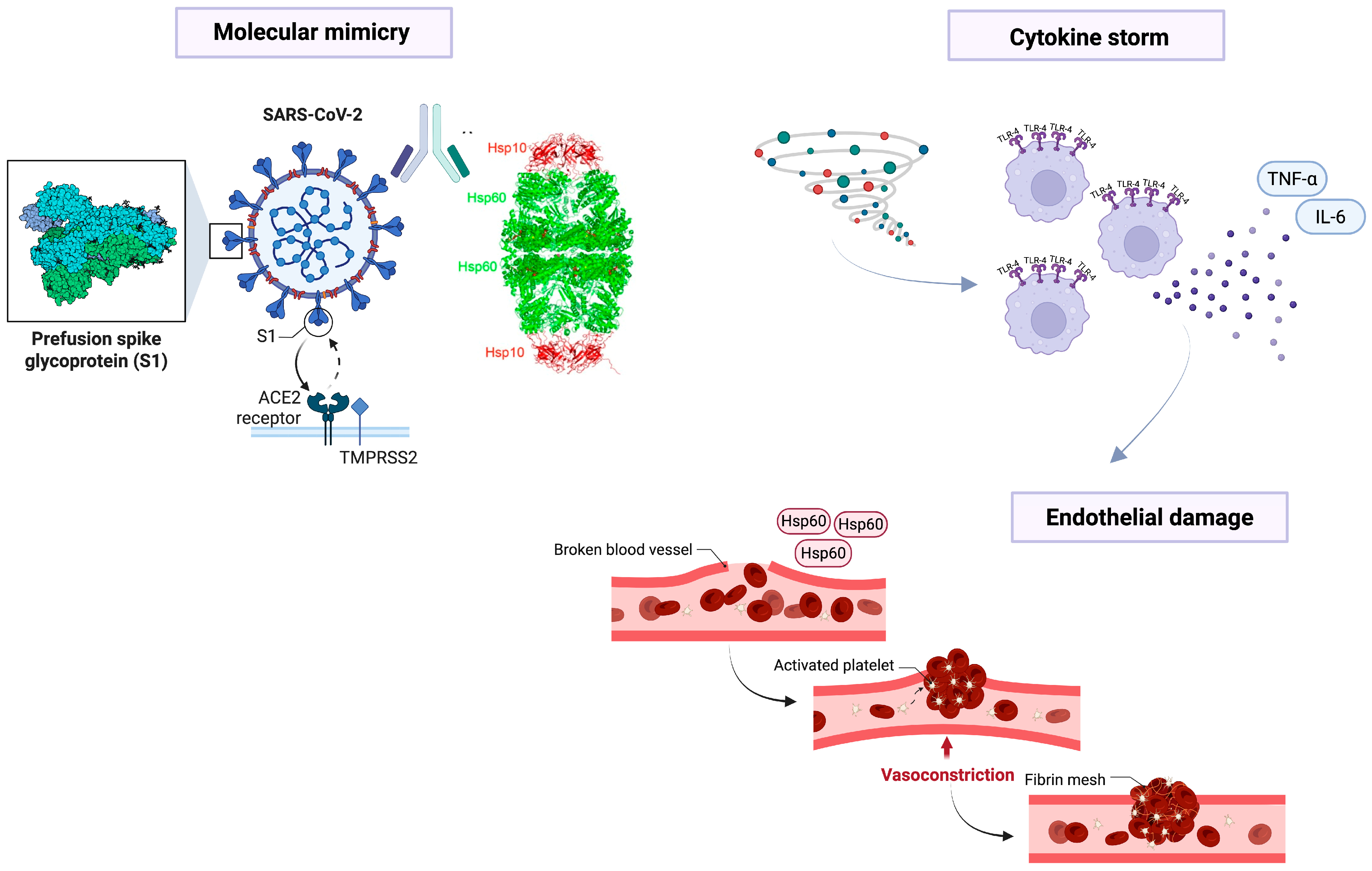
4. Hsp60 and Autoimmunity During COVID-19
5. Hsp60 and Endothelial Damage
6. Hsp60 as a Biomarker or a Therapeutic Target
7. Hsp60 and Vaccine Preparation
8. The Other Side of the Coin: The Anti-Tumoral Immunity
9. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Macario, A.J.L.; Conway de Macario, E. Sick chaperones, cellular stress, and disease. N. Engl. J. Med. 2005, 353, 1489–1501. [Google Scholar] [CrossRef]
- Cappello, F.; Conway de Macario, E.; Marino Gammazza, A.; Bonaventura, G.; Carini, F.; Czarnecka, A.M.; Farina, F.; Zummo, G.; Macario, A.J.L. Hsp60 and human aging: Les liaisons dangereuses. Front. Biosci. Landmark Ed. 2013, 18, 626–637. [Google Scholar] [CrossRef]
- Caruso Bavisotto, C.; Alberti, G.; Vitale, A.M.; Paladino, L.; Campanella, C.; Rappa, F.; Gorska, M.; Conway de Macario, E.; Cappello, F.; Macario, A.J.L.; et al. Hsp60 Post-translational Modifications: Functional and Pathological Consequences. Front. Mol. Biosci. 2020, 7, 95. [Google Scholar] [CrossRef]
- Campanella, C.; Bucchieri, F.; Ardizzone, N.M.; Marino Gammazza, A.; Montalbano, A.; Ribbene, A.; Di Felice, V.; Bellafiore, M.; David, S.; Rappa, F.; et al. Upon oxidative stress, the antiapoptotic Hsp60/procaspase-3 complex persists in mucoepidermoid carcinoma cells. Eur. J. Histochem. 2008, 52, 221–228. [Google Scholar] [CrossRef]
- Merendino, A.M.; Bucchieri, F.; Campanella, C.; Marcianò, V.; Ribbene, A.; David, S.; Zummo, G.; Burgio, G.; Corona, D.F.V.; Conway de Macario, E.; et al. Hsp60 is actively secreted by human tumor cells. PLoS ONE 2010, 5, e9247. [Google Scholar] [CrossRef]
- Cappello, F.; Conway de Macario, E.; Di Felice, V.; Zummo, G.; Macario, A.J.L. Chlamydia trachomatis infection and anti-Hsp60 immunity: The two sides of the coin. PLoS Pathog. 2009, 5, e1000552. [Google Scholar] [CrossRef]
- Cappello, F.; Conway de Macario, E.; Marasà, L.; Zummo, G.; Macario, A.J.L. Hsp60 expression, new locations, functions and perspectives for cancer diagnosis and therapy. Cancer Biol. Ther. 2008, 7, 801–809. [Google Scholar] [CrossRef]
- Marino Gammazza, A.; Légaré, S.; Lo Bosco, G.; Fucarino, A.; Angileri, F.; Conway de Macario, E.; Macario, A.J.; Cappello, F. Human molecular chaperones share with SARS-CoV-2 antigenic epitopes potentially capable of eliciting autoimmunity against endothelial cells: Possible role of molecular mimicry in COVID-19. Cell Stress Chaperones 2020, 25, 737–741. [Google Scholar] [CrossRef]
- Barone, R.; Marino Gammazza, A.; Paladino, L.; Pitruzzella, A.; Spinoso, G.; Salerno, M.; Sessa, F.; Pomara, C.; Cappello, F.; Rappa, F. Morphological Alterations and Stress Protein Variations in Lung Biopsies Obtained from Autopsies of COVID-19 Subjects. Cells 2021, 10, 3136. [Google Scholar] [CrossRef]
- Li, C.; He, Q.; Qian, H.; Liu, J. Overview of the pathogenesis of COVID-19 (Review). Exp. Ther. Med. 2021, 22, 1011. [Google Scholar] [CrossRef]
- Bourgonje, A.R.; Abdulle, A.E.; Timens, W.; Hillebrands, J.-L.; Navis, G.J.; Gordijn, S.J.; Bolling, M.C.; Dijkstra, G.; Voors, A.A.; Osterhaus, A.D.; et al. Angiotensin-converting enzyme 2 (ACE2), SARS-CoV-2 and the pathophysiology of coronavirus disease 2019 (COVID-19). J. Pathol. 2020, 251, 228–248. [Google Scholar] [CrossRef]
- Jackson, C.B.; Farzan, M.; Chen, B.; Choe, H. Mechanisms of SARS-CoV-2 entry into cells. Nat. Rev. Mol. Cell Biol. 2022, 23, 3–20. [Google Scholar] [CrossRef]
- Vahabi, M.; Ghazanfari, T.; Sepehrnia, S. Molecular mimicry, hyperactive immune system, and SARS-COV-2 are three prerequisites of the autoimmune disease triangle following COVID-19 infection. Int. Immunopharmacol. 2022, 112, 109183. [Google Scholar] [CrossRef]
- Nunez-Castilla, J.; Stebliankin, V.; Baral, P.; Balbin, C.A.; Sobhan, M.; Cickovski, T.; Mondal, A.M.; Narasimhan, G.; Chapagain, P.; Mathee, K.; et al. Potential Autoimmunity Resulting from Molecular Mimicry between SARS-CoV-2 Spike and Human Proteins. Viruses 2022, 14, 1415. [Google Scholar] [CrossRef]
- Cappello, F. COVID-19 and molecular mimicry: The Columbus’ egg? J. Clin. Neurosci. 2020, 77, 246. [Google Scholar] [CrossRef]
- Churilov, L.P.; Normatov, M.G.; Utekhin, V.J. Molecular Mimicry between SARS-CoV-2 and Human Endocrinocytes: A Prerequisite of Post-COVID-19 Endocrine Autoimmunity? Pathophysiology 2022, 29, 486–494. [Google Scholar] [CrossRef]
- Lucchese, G.; Flöel, A. SARS-CoV-2 and Guillain-Barré syndrome: Molecular mimicry with human heat shock proteins as potential pathogenic mechanism. Cell Stress Chaperones 2020, 25, 731–735. [Google Scholar] [CrossRef]
- Ruiz-Vázquez, E.; de Castro, P. “2-6-11” motif in heat shock protein 60 and central nervous system antigens: A preliminary study in multiple sclerosis patients. J. Physiol. Biochem. 2003, 59, 1–9. [Google Scholar] [CrossRef]
- D’Anna, S.E.; Vitale, A.M.; D’Amico, G.; Caruso Bavisotto, C.; Ambrosino, P.; Cappello, F.; Maniscalco, M.; Marino Gammazza, A. Autoimmunity against Nucleus Ambiguous Is Putatively Possible in Both Long-COVID-19 and Vaccinated Subjects: Scientific Evidence and Working Hypothesis. Biology 2024, 13, 359. [Google Scholar] [CrossRef]
- Kovačić, D.; Jotanović, J.; Laković, J. The possible role of molecular mimicry in SARS-CoV-2-mediated autoimmunity: An immunobiochemical basis. J. Med. Sci. 2021, 90, e560. [Google Scholar] [CrossRef]
- von Herrath, M.G.; Fujinami, R.S.; Whitton, J.L. Microorganisms and autoimmunity: Making the barren field fertile? Nat. Rev. Microbiol. 2003, 1, 151–157. [Google Scholar] [CrossRef]
- Nazerian, Y.; Ghasemi, M.; Yassaghi, Y.; Nazerian, A.; Hashemi, S.M. Role of SARS-CoV-2-induced cytokine storm in multi-organ failure: Molecular pathways and potential therapeutic options. Int. Immunopharmacol. 2022, 113 Pt B, 109428. [Google Scholar] [CrossRef]
- Grundtman, C.; Kreutmayer, S.B.; Almanzar, G.; Wick, M.C.; Wick, G. Heat shock protein 60 and immune inflammatory responses in atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 960–968. [Google Scholar] [CrossRef]
- Cohen, I.R. Autoantibody repertoires, natural biomarkers, and system controllers. Trends Immunol. 2013, 34, 620–625. [Google Scholar] [CrossRef]
- Sangiorgi, C.; Vallese, D.; Gnemmi, I.; Bucchieri, F.; Balbi, B.; Brun, P.; Leone, A.; Giordano, A.; Conway de Macario, E.; Macario, A.J.; et al. HSP60 activity on human bronchial epithelial cells. Int. J. Immunopathol. Pharmacol. 2017, 30, 333–340. [Google Scholar] [CrossRef]
- Shirsath, K.; Joshi, A.; Vohra, A.; Devkar, R. HSP60 knockdown exerts differential response in endothelial cells and monocyte derived macrophages during atherogenic transformation. Sci. Rep. 2021, 11, 1086. [Google Scholar] [CrossRef]
- Singh, M.K.; Shin, Y.; Han, S.; Ha, J.; Tiwari, P.K.; Kim, S.S.; Kang, I. Molecular Chaperonin HSP60: Current Understanding and Future Prospects. Int. J. Mol. Sci. 2024, 25, 5483. [Google Scholar] [CrossRef]
- Mehta, P.; McAuley, D.F.; Brown, M.; Sanchez, E.; Tattersall, R.S.; Manson, J.J. COVID-19: Consider cytokine storm syndromes and immunosuppression. Lancet 2020, 395, 1033–1034. [Google Scholar] [CrossRef]
- Vallese, D.; Ricciardolo, F.L.M.; Gnemmi, I.; Casolari, P.; Brun, P.; Sorbello, V.; Capelli, A.; Cappello, F.; Cavallesco, G.N.; Papi, A.; et al. Phospho-p38 MAPK expression in COPD patients and asthmatics and in challenged bronchial epithelium. Respiration 2015, 89, 329–342. [Google Scholar] [CrossRef]
- Di Stefano, A.; Ricciardolo, F.L.M.; Caramori, G.; Adcock, I.M.; Chung, K.F.; Barnes, P.J.; Brun, P.; Leonardi, A.; Andò, F.; Vallese, D.; et al. Bronchial inflammation and bacterial load in stable COPD is associated with TLR4 overexpression. Eur. Respir. J. 2017, 49, 1602006. [Google Scholar] [CrossRef]
- Leonardi, A.; Tarricone, E.; Corrao, S.; Alaibac, M.; Corso, A.J.; Zavan, B.; Venier, P.; Conway de Macario, E.; Macario, A.J.L.; Di Stefano, A.; et al. Chaperone patterns in vernal keratoconjunctivitis are distinctive of cell and Hsp type and are modified by inflammatory stimuli. Allergy 2016, 71, 403–411. [Google Scholar] [CrossRef]
- Martinus, R.D.; Goldsbury, J. Endothelial TNF-α induction by Hsp60 secreted from THP-1 monocytes exposed to hyperglycaemic conditions. Cell Stress Chaperones 2018, 23, 519–525. [Google Scholar] [CrossRef]
- Cappello, F.; Caramori, G.; Campanella, C.; Vicari, C.; Gnemmi, I.; Zanini, A.; Spanevello, A.; Capelli, A.; La Rocca, G.; Anzalone, R.; et al. Convergent Sets of Data from In Vivo and In Vitro Methods Point to an Active Role of Hsp60 in Chronic Obstructive Pulmonary Disease Pathogenesis. PLoS ONE 2011, 6, e28200. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, L.; Hagiwara, N.; Knowlton, A.A. Regulation of heat shock protein 60 and 72 expression in the failing heart. J. Mol. Cell. Cardiol. 2010, 48, 360–366. [Google Scholar] [CrossRef]
- Tabeta, K.; Yamazaki, K.; Hotokezaka, H.; Yoshie, H.; Hara, K. Elevated humoral immune response to heat shock protein 60 (hsp60) family in periodontitis patients. Clin. Exp. Immunol. 2000, 120, 285–293. [Google Scholar] [CrossRef]
- Meziane, F.Z.; Dali-Sahi, M.; Dennouni-Medjati, N.; Boulenouar, H.; Kachekouche, Y.; Benslama, Y.; Harek, Y. Molecular mimicry between varicella, measles virus and Hsp60 in type 1 diabetes associated HLA-DR3/DR4 molecules. Diabetes Metab. Syndr. 2020, 14, 1783–1789. [Google Scholar] [CrossRef]
- Gutman, E.G.; Fernandes, R.A.; Raposo-Vedovi, J.V.; Salvio, A.L.; Duarte, L.A.; Tardim, C.F.; Costa, V.G.C.; Pereira, V.C.S.R.; Bahia, P.R.V.; da Silva, M.M.; et al. Molecular Mimicry between SARS-CoV-2 Proteins and Human Self-Antigens Related with Autoimmune Central Nervous System (CNS) Disorders. Microorganisms 2023, 11, 2902. [Google Scholar] [CrossRef]
- Angileri, F.; Légaré, S.; Marino Gammazza, A.; Conway de Macario, E.; Macario, A.J.L.; Cappello, F. Is molecular mimicry the culprit in the autoimmune haemolytic anaemia affecting patients with COVID-19? Br. J. Haematol. 2020, 190, e92–e93. [Google Scholar] [CrossRef]
- Wick, G.; Jakic, B.; Buszko, M.; Wick, M.C.; Grundtman, C. The role of heat shock proteins in atherosclerosis. Nat. Rev. Cardiol. 2014, 11, 516–529. [Google Scholar] [CrossRef]
- Knoflach, M.; Bernhard, D.; Wick, G. Anti-HSP60 immunity is already associated with atherosclerosis early in life. Ann. N. Y. Acad. Sci. 2005, 1051, 323–331. [Google Scholar] [CrossRef]
- Zanin, L.; Saraceno, G.; Panciani, P.P.; Renisi, G.; Signorini, L.; Migliorati, K.; Fontanella, M.M. SARS-CoV-2 can induce brain and spine demyelinating lesions. Acta Neurochir. 2020, 162, 1491–1494. [Google Scholar] [CrossRef]
- Zhao, H.; Shen, D.; Zhou, H.; Liu, J.; Chen, S. Guillain-Barré syndrome associated with SARS-CoV-2 infection: Causality or coincidence? Lancet Neurol. 2020, 19, 383–384. [Google Scholar] [CrossRef]
- Monsalve, D.M.; Acosta-Ampudia, Y.; Acosta, N.G.; Celis-Andrade, M.; Şahin, A.; Yilmaz, A.M.; Shoenfeld, Y.; Ramírez-Santana, C. NETosis: A key player in autoimmunity, COVID-19, and long COVID. J. Transl. Autoimmun. 2025, 10, 100280. [Google Scholar] [CrossRef]
- Jakovac, H. COVID-19 and hypertension: Is the HSP60 culprit for the severe course and worse outcome? Am. J. Physiol. Heart Circ. Physiol. 2020, 319, H793–H796. [Google Scholar] [CrossRef]
- Hernandez-Cedeño, M.; Venegas-Rodriguez, R.; Peña-Ruiz, R.; Bequet-Romero, M.; Santana-Sanchez, R.; Penton-Arias, E.; Martinez-Donato, G.; Guillén-Nieto, G.; Dominguez-Horta, M.D.C. CIGB-258, a peptide derived from human heat-shock protein 60, decreases hyperinflammation in COVID-19 patients. Cell Stress Chaperones 2021, 26, 515–525. [Google Scholar] [CrossRef]
- Mantej, J.; Bednarek, M.; Sitko, K.; Świętoń, M.; Tukaj, S. Autoantibodies to heat shock protein 60, 70, and 90 are not altered in the anti-SARS-CoV-2 IgG-seropositive humans without or with mild symptoms. Cell Stress Chaperones 2021, 26, 735–740. [Google Scholar] [CrossRef]
- Saha, A.; Ahmed, S. The Link Between Heat Shock Proteins, Renin-Angiotensin System, and the Coagulation Cascade in the Pathogenesis of the Coronavirus-19 Disease. Adv. Exp. Med. Biol. 2023, 1409, 161–171. [Google Scholar] [CrossRef]
- Marino Gammazza, A.; Légaré, S.; Lo Bosco, G.; Fucarino, A.; Angileri, F.; Oliveri, M.; Cappello, F. Molecular mimicry in the post-COVID-19 signs and symptoms of neurovegetative disorders? Lancet Microbe 2021, 2, e94. [Google Scholar] [CrossRef]
- Joshi, P.; Garg, S.; Mani, S.; Shoaib, R.; Jakhar, K.; Almuqdadi, H.T.A.; Sonar, S.; Marothia, M.; Behl, A.; Biswas, S.; et al. Targeting host inducible-heat shock protein 70 with PES-Cl is a promising antiviral strategy against SARS-CoV-2 infection and pathogenesis. Int. J. Biol. Macromol. 2024, 279 Pt 1, 135069. [Google Scholar] [CrossRef]
- Zangeneh, Z.; Khamisipour, G. Elevated HSP70 and HSP90 as Predictive Markers of Immune Activation and Lung Injury in SARS-COV-2 Disease. Iran. J. Immunol. IJI 2023, 20, 368–373. [Google Scholar] [CrossRef]
- Cappello, F.; Gammazza, A.M.; Dieli, F.; Macario, E.; Macario, A.J. Does SARS-CoV-2 Trigger Stress-InducedAutoimmunity by Molecular Mimicry? A Hypothesis. J. Clin. Med. 2020, 9, 2038. [Google Scholar] [CrossRef] [PubMed]
- Wick, C. Tolerization against atherosclerosis using heat shock protein 60. Cell Stress Chaperones 2016, 21, 201–211. [Google Scholar] [CrossRef]
- Liu, L.; Wang, Y.; Cao, Z.-Y.; Wang, M.-M.; Liu, X.-M.; Gao, T.; Hu, Q.-K.; Yuan, W.-J.; Lin, L. Up-regulated TLR4 in cardiomyocytes exacerbates heart failure after long-term myocardial infarction. J. Cell. Mol. Med. 2015, 19, 2728–2740. [Google Scholar] [CrossRef] [PubMed]
- Kanduc, D.; Shoenfeld, Y. Molecular mimicry between SARS-CoV-2 spike glycoprotein and mammalian proteomes: Implications for the vaccine. Immunol. Res. 2020, 68, 310–313. [Google Scholar] [CrossRef]
- Krishnan-Sivadoss, I.; Mijares-Rojas, I.A.; Villarreal-Leal, R.A.; Torre-Amione, G.; Knowlton, A.A.; Guerrero-Beltrán, C.E. Heat shock protein 60 and cardiovascular diseases: An intricate love-hate story. Med. Res. Rev. 2021, 41, 29–71. [Google Scholar] [CrossRef]
- Varga, Z.; Flammer, A.J.; Steiger, P.; Haberecker, M.; Andermatt, R.; Zinkernagel, A.S.; Mehra, M.R.; Schuepbach, R.A.; Ruschitzka, F.; Moch, H. Endothelial cell infection and endotheliitis in COVID-19. Lancet Lond. Engl. 2020, 395, 1417–1418. [Google Scholar] [CrossRef]
- Ackermann, M.; Verleden, S.E.; Kuehnel, M.; Haverich, A.; Welte, T.; Laenger, F.; Vanstapel, A.; Werlein, C.; Stark, H.; Tzankov, A.; et al. Pulmonary Vascular Endothelialitis, Thrombosis, and Angiogenesis in Covid-19. N. Engl. J. Med. 2020, 383, 120–128. [Google Scholar] [CrossRef]
- Monteil, V.; Kwon, H.; Prado, P.; Hagelkrüys, A.; Wimmer, R.A.; Stahl, M.; Leopoldi, A.; Garreta, E.; Hurtado Del Pozo, C.; Prosper, F.; et al. Inhibition of SARS-CoV-2 Infections in Engineered Human Tissues Using Clinical-Grade Soluble Human ACE2. Cell 2020, 181, 905–913.e7. [Google Scholar] [CrossRef]
- Ohashi, K.; Burkart, V.; Flohé, S.; Kolb, H. Cutting edge: Heat shock protein 60 is a putative endogenous ligand of the toll-like receptor-4 complex. J. Immunol. 2000, 164, 558–561. [Google Scholar] [CrossRef]
- Kol, A.; Lichtman, A.H.; Finberg, R.W.; Libby, P.; Kurt-Jones, E.A. Cutting edge: Heat shock protein (HSP) 60 activates the innate immune response: CD14 is an essential receptor for HSP60 activation of mononuclear cells. J. Immunol. 2000, 164, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-L.; Wang, Y.-L.; Zheng, J.; Zhang, Y.; Zhang, X.-F. Inhibiting expression of HSP60 and TLR4 attenuates paraquat-induced microglial inflammation. Chem. Biol. Interact. 2019, 299, 179–185. [Google Scholar] [CrossRef]
- Swaroop, S.; Sengupta, N.; Suryawanshi, A.R.; Adlakha, Y.K.; Basu, A. HSP60 plays a regulatory role in IL-1β-induced microglial inflammation via TLR4-p38 MAPK axis. J. Neuroinflamm. 2016, 13, 27. [Google Scholar] [CrossRef] [PubMed]
- Ragone, C.; Mauriello, A.; Cavalluzzo, B.; Cavalcanti, E.; Russo, L.; Manolio, C.; Mangano, S.; Cembrola, B.; Tagliamonte, M.; Buonaguro, L. Molecular mimicry of SARS-COV-2 antigens as a possible natural anti-cancer preventive immunization. Front. Immunol. 2024, 15, 1398002. [Google Scholar] [CrossRef] [PubMed]
- Burgio, S.; Conway de Macario, E.; Macario, A.J.; Cappello, F. SARS-CoV-2 in patients with cancer: Possible role of mimicry of human molecules by viral proteins and the resulting anti-cancer immunity. Cell Stress Chaperones 2021, 26, 611–616. [Google Scholar] [CrossRef] [PubMed]
- Cappello, F.; Burgio, S.; Conway de Macario, E.; Macario, A.J.L. Unexpected tumor reduction in metastatic colorectal cancer patients during SARS-Cov-2 infection: Effect of ACE-2 expression on tumor cells or molecular mimicry phenomena? Two not mutually exclusive hypotheses. Ther. Adv. Med. Oncol. 2021, 13, 17588359211027825. [Google Scholar] [CrossRef]
- Ottaiano, A.; Scala, S.; D’Alterio, C.; Trotta, A.; Bello, A.; Rea, G.; Picone, C.; Santorsola, M.; Petrillo, A.; Nasti, G. Unexpected tumor reduction in metastatic colorectal cancer patients during SARS-Cov-2 infection. Ther. Adv. Med. Oncol. 2021, 13, 17588359211011455. [Google Scholar] [CrossRef]
- Campanella, C.; Rappa, F.; Sciumè, C.; Marino Gammazza, A.; Barone, R.; Bucchieri, F.; David, S.; Curcurù, G.; Caruso Bavisotto, C.; Pitruzzella, A.; et al. Heat shock protein 60 levels in tissue and circulating exosomes in human large bowel cancer before and after ablative surgery. Cancer 2015, 121, 3230–3239. [Google Scholar] [CrossRef]
| Pathogenetic Mechanisms | Role of Hsp60 in COVID-19 | Mechanistic Context | References |
|---|---|---|---|
| Inflammation and immune response | Extracellular Hsp60 acts as a ligand for TLR-4, activating NF-κB and promoting pro-inflammatory cytokine release (IL-6, TNF-α). It can modulate macrophage activation and contribute to cytokine storm. | Drives hyperinflammation and multi-organ failure in acute COVID-19; may perpetuate chronic inflammation in long-COVID. | [25,26,28,29,30,31,32,60] 11 September 2025 20:41:00 |
| Autoimmunity | Overexpression of Hsp60 under stress leads to its presentation on the cell surface and recognition by autoreactive lymphocytes. Shared epitopes between Hsp60 and SARS-CoV-2 proteins promote molecular mimicry. | Triggers autoimmune diseases such as Guillain-Barré syndrome, CNS demyelination, vasculitis, neuropathies, and autoimmune thyroiditis. | [8,9,10,11,12,13,14,15,16,17,18,19,20,21,37,38,39,40,41,42,43,44,45,46] |
| Endothelial damage | Hsp60 is upregulated in endothelial cells under oxidative stress and SARS-CoV-2 infection. Its interaction with TLR-4 amplifies inflammatory cascades and adhesion molecule expression. | Contributes to endothelial dysfunction, microvascular injury, thromboembolic complications, and systemic vascular damage. | [9,26,32,55,56,57,58,59] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carista, A.; Gratie, M.I.; Cappello, F.; Burgio, S. HSP60 and SARS-CoV-2: Les Liaisons Dangereuses. Biology 2025, 14, 1281. https://doi.org/10.3390/biology14091281
Carista A, Gratie MI, Cappello F, Burgio S. HSP60 and SARS-CoV-2: Les Liaisons Dangereuses. Biology. 2025; 14(9):1281. https://doi.org/10.3390/biology14091281
Chicago/Turabian StyleCarista, Adelaide, Melania Ionelia Gratie, Francesco Cappello, and Stefano Burgio. 2025. "HSP60 and SARS-CoV-2: Les Liaisons Dangereuses" Biology 14, no. 9: 1281. https://doi.org/10.3390/biology14091281
APA StyleCarista, A., Gratie, M. I., Cappello, F., & Burgio, S. (2025). HSP60 and SARS-CoV-2: Les Liaisons Dangereuses. Biology, 14(9), 1281. https://doi.org/10.3390/biology14091281









