Expression Dynamics of Neurotransmitter System Genes in Early Sea Urchin Embryos: Insights from a Four-Species Comparative Transcriptome Analysis
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Specimen Selection and Transcriptome Data
2.2. Normalization and Thresholding of Transcriptome Data
3. Results
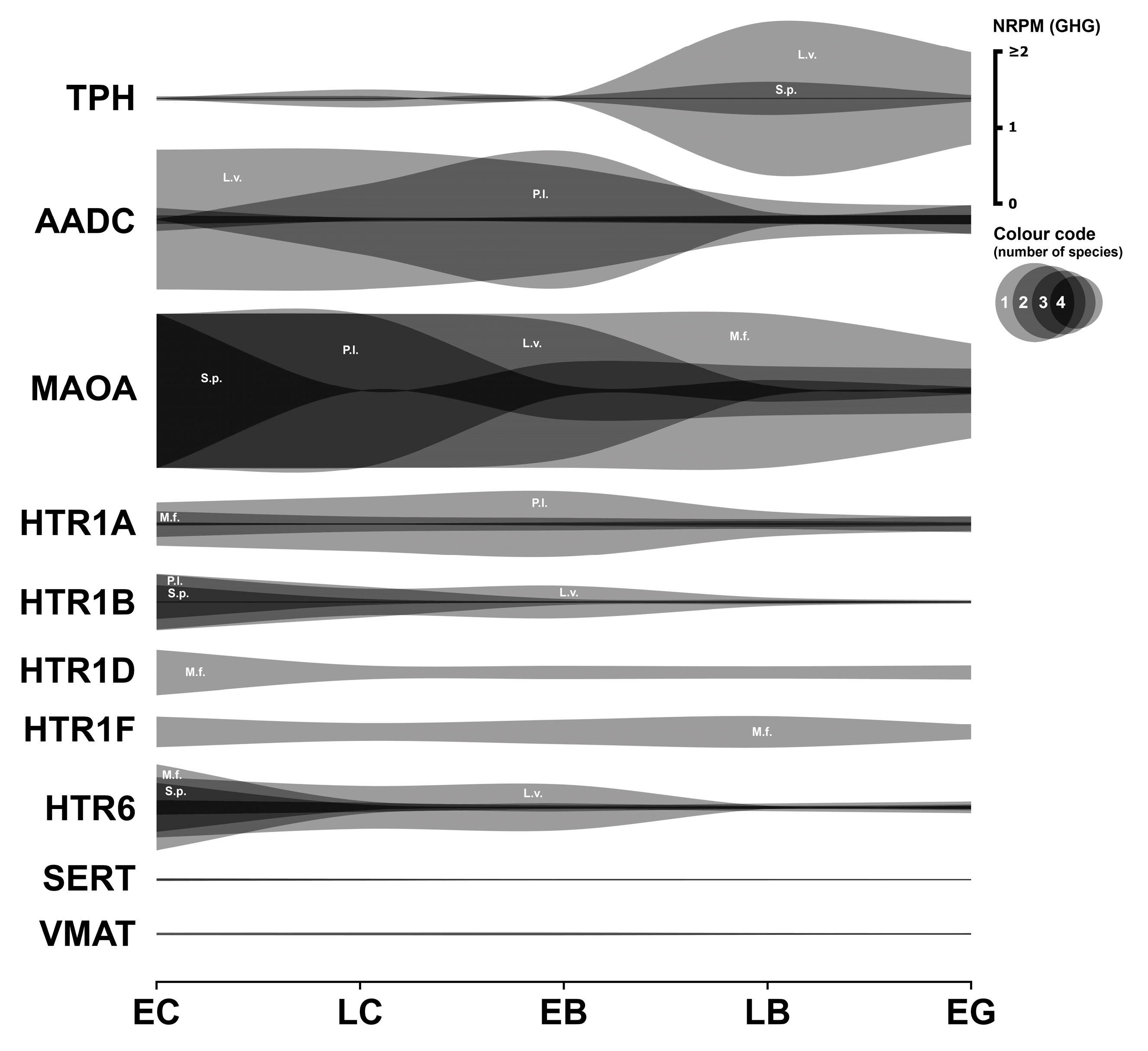
3.1. Serotonergic System
3.1.1. Serotonergic Enzymes
3.1.2. Serotonergic Receptors
3.1.3. Serotonergic Transporters
3.1.4. Summary
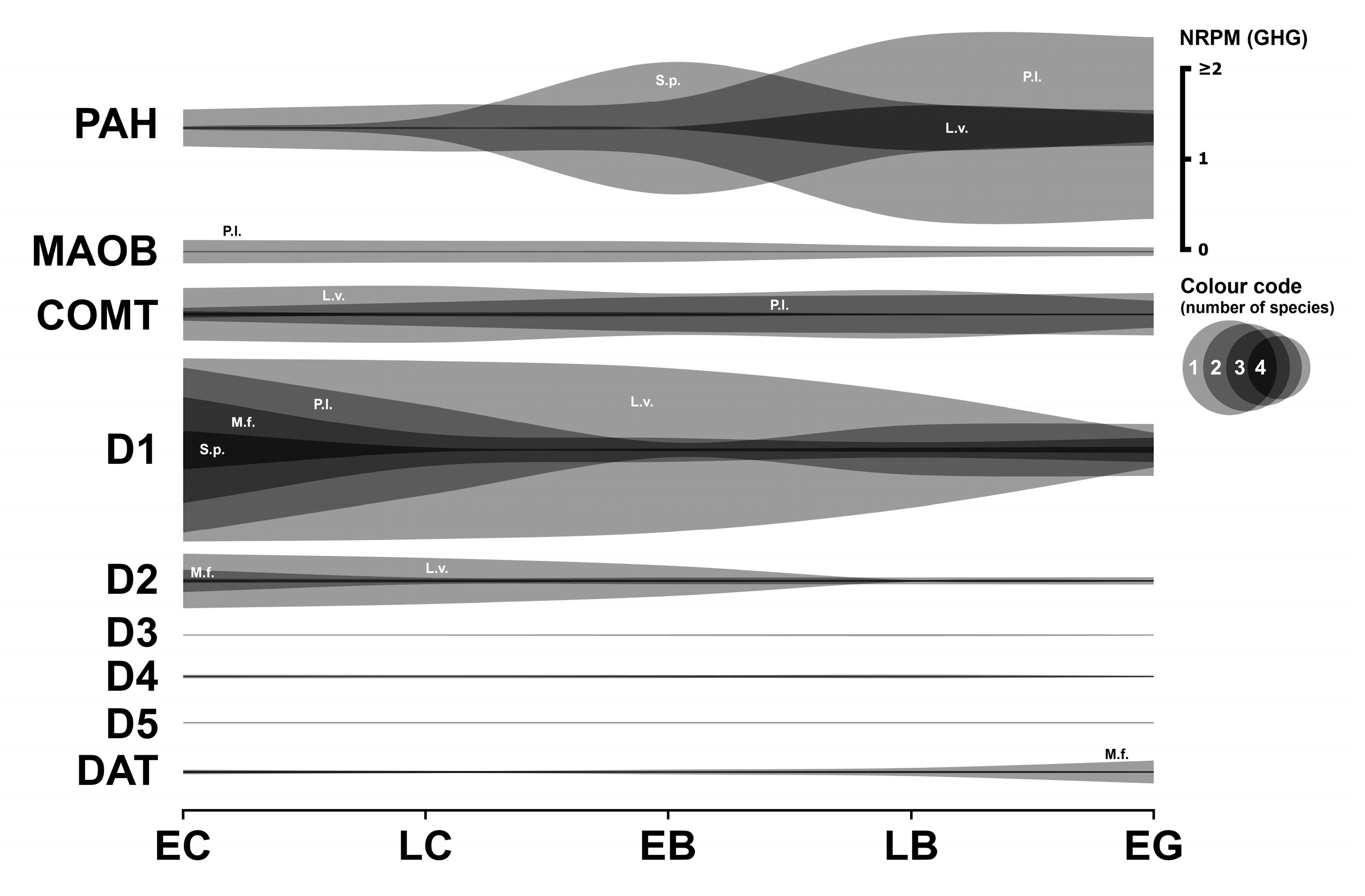
3.2. Dopaminergic System
3.2.1. Dopaminergic Enzymes
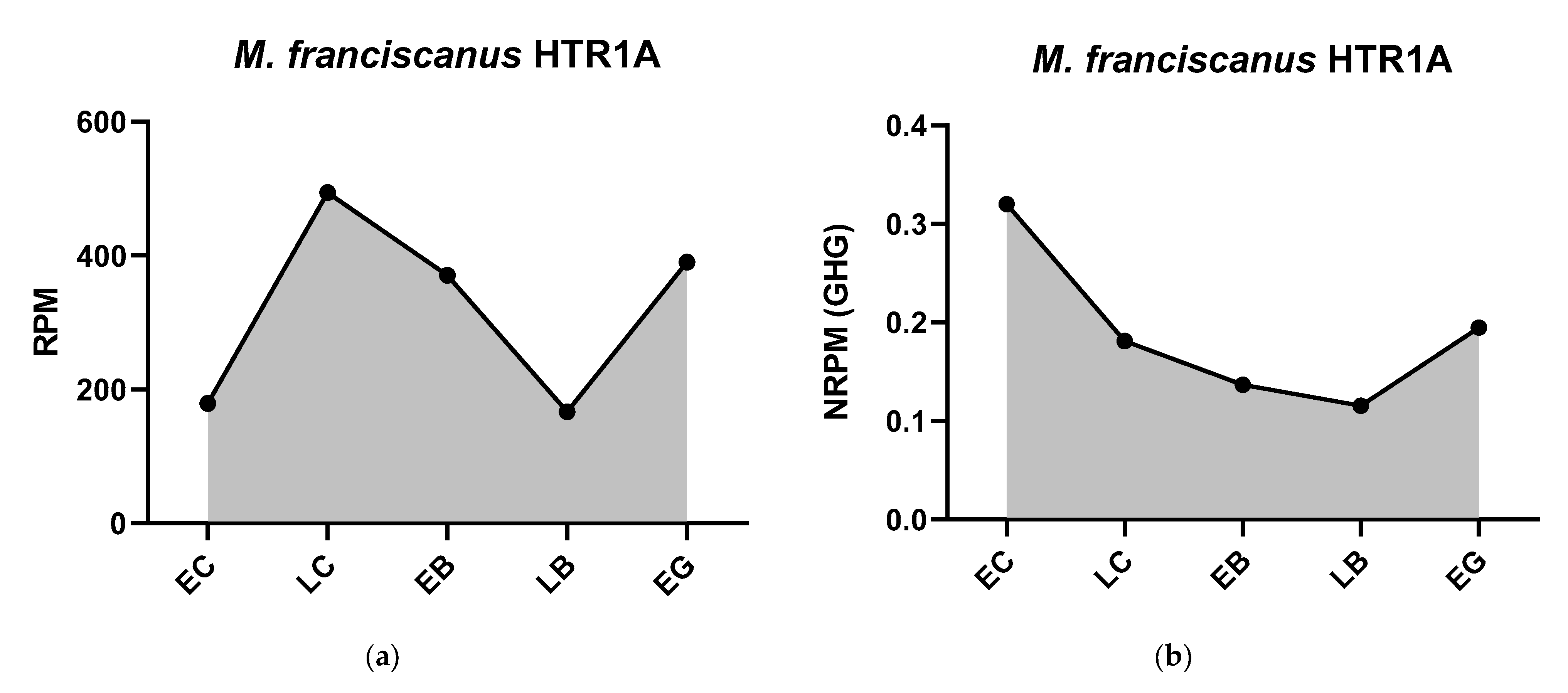
3.2.2. Dopaminergic Receptors
3.2.3. Dopaminergic Transporter
3.2.4. Summary
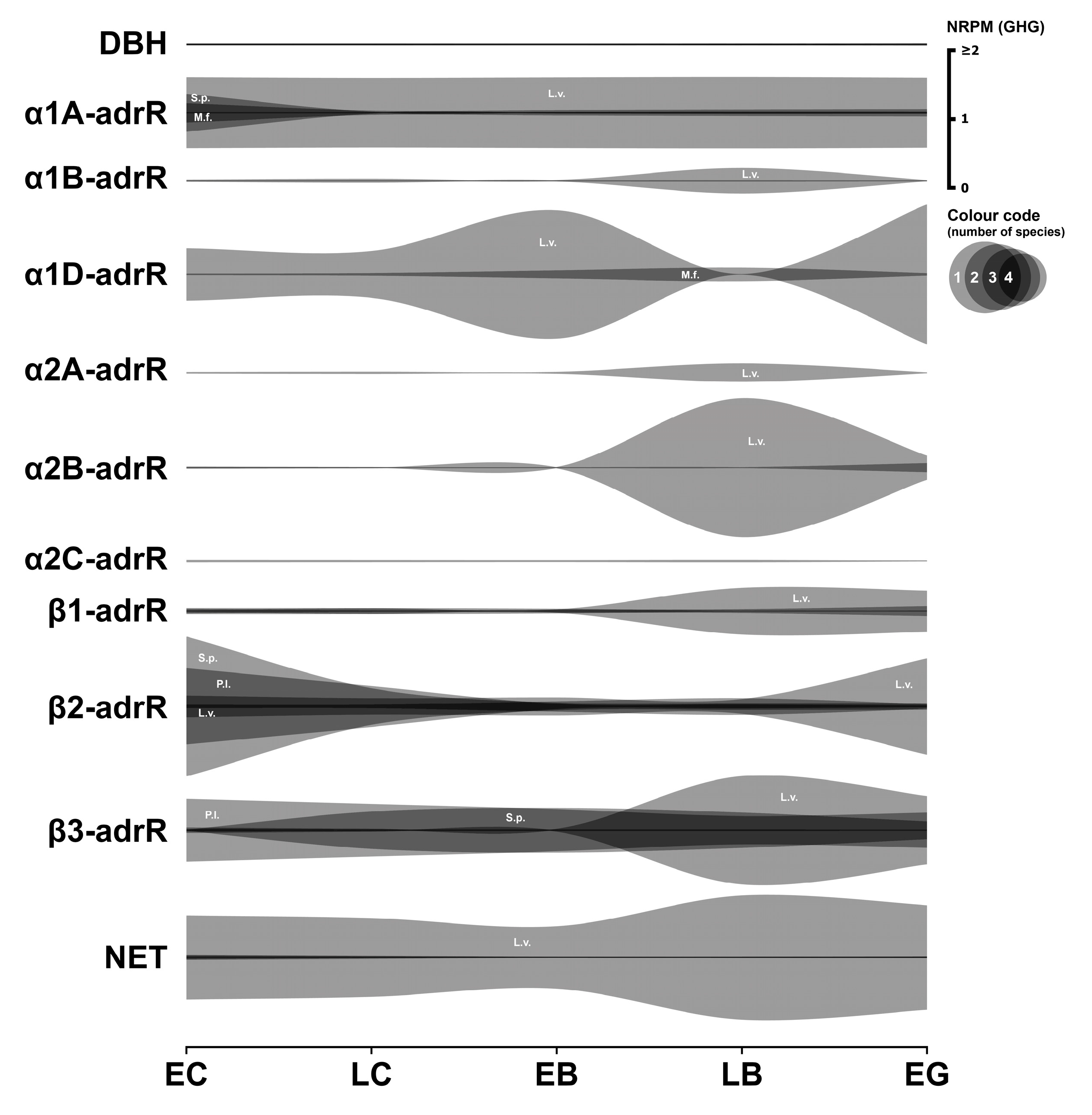
3.3. Adrenergic System
3.3.1. Adrenergic Enzymes
3.3.2. Adrenergic Receptors
3.3.3. Adrenergic Transporter
3.3.4. Summary
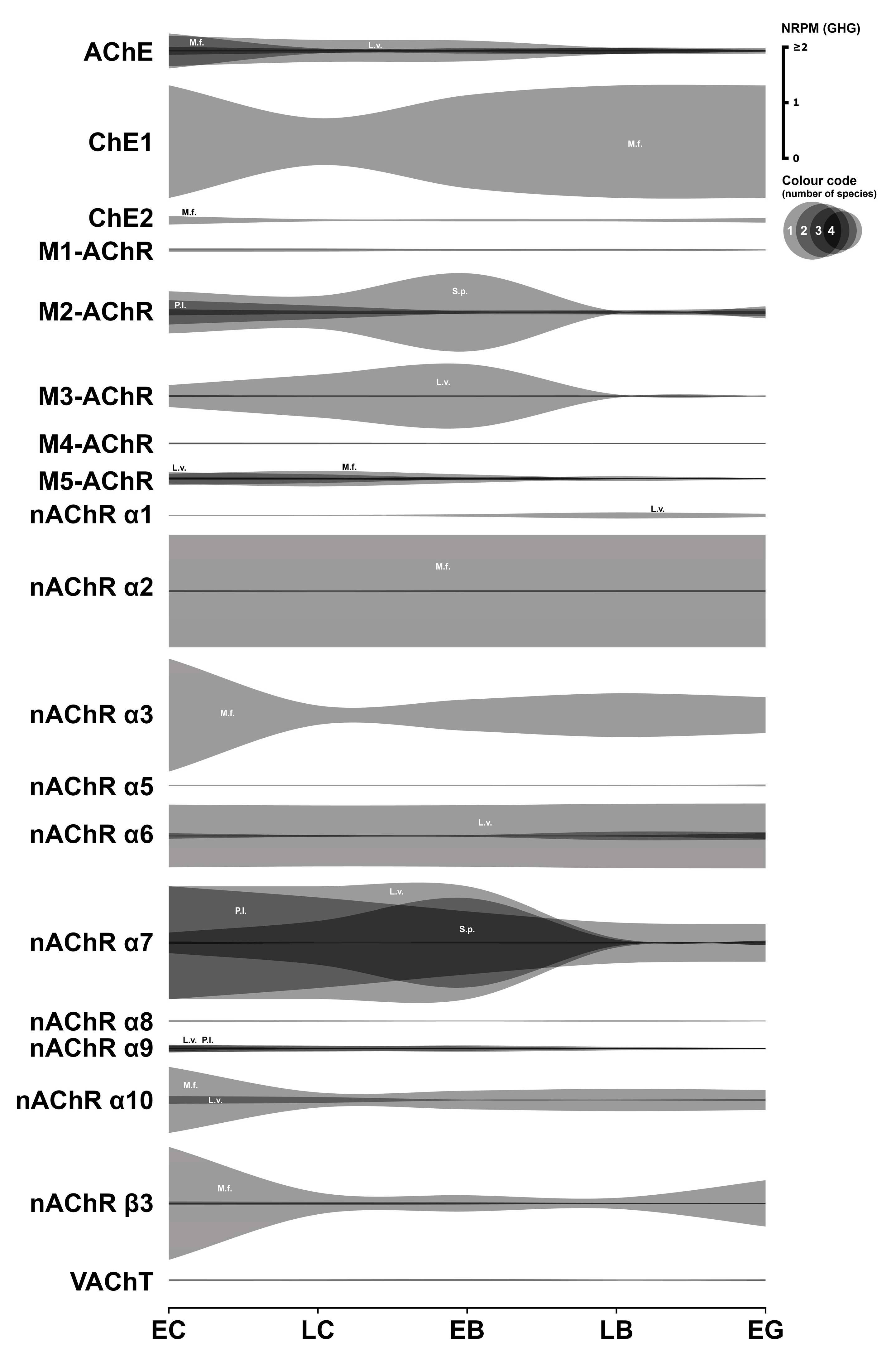
3.4. Cholinergic System
3.4.1. Cholinergic Enzymes
3.4.2. Cholinergic Receptors
3.4.3. Cholinergic Transporter
3.4.4. Summary
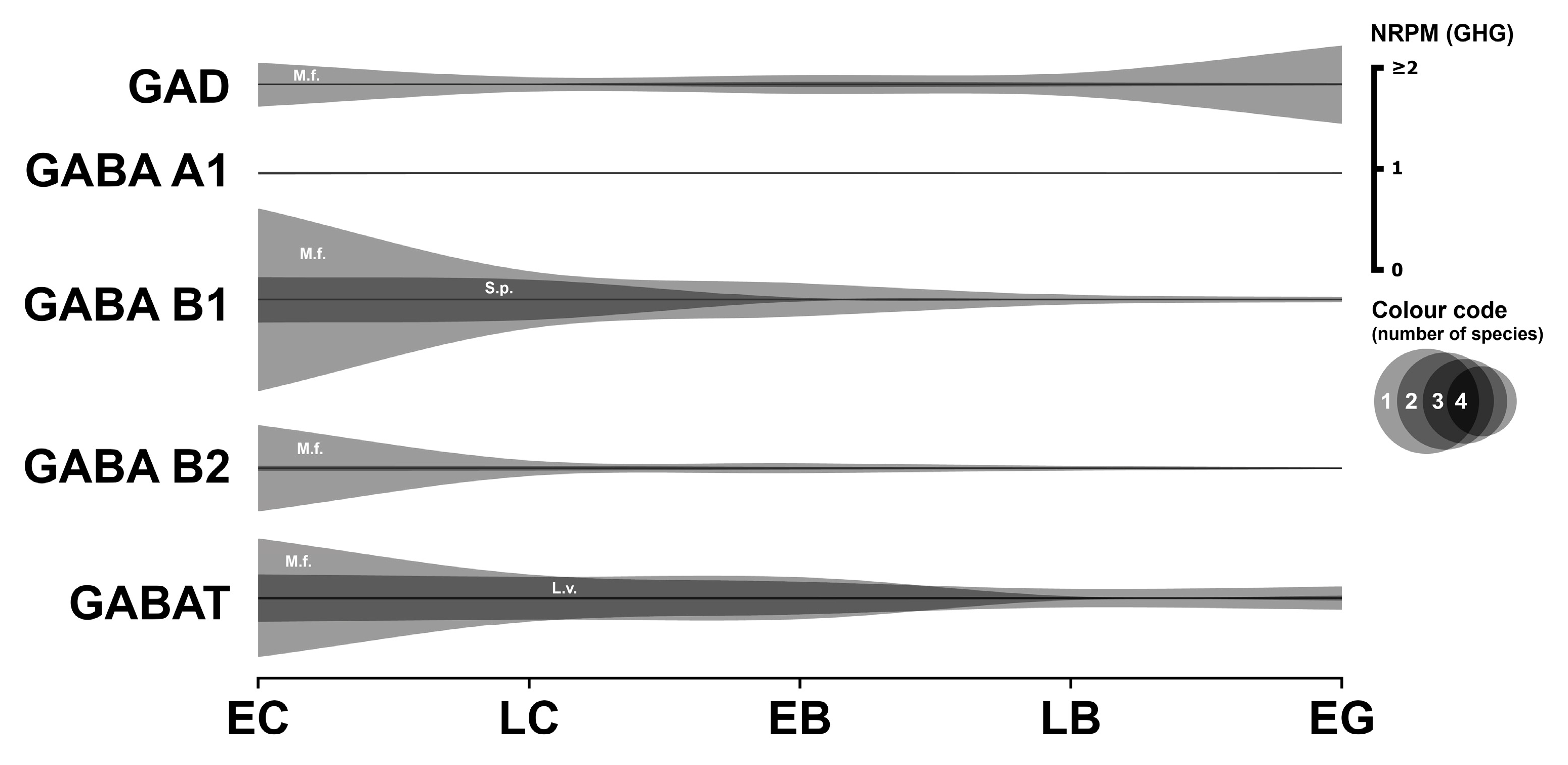
3.5. GABAergic System
3.5.1. GABAergic Enzyme
3.5.2. GABAergic Receptors
3.5.3. GABAergic Transporter
3.5.4. Summary
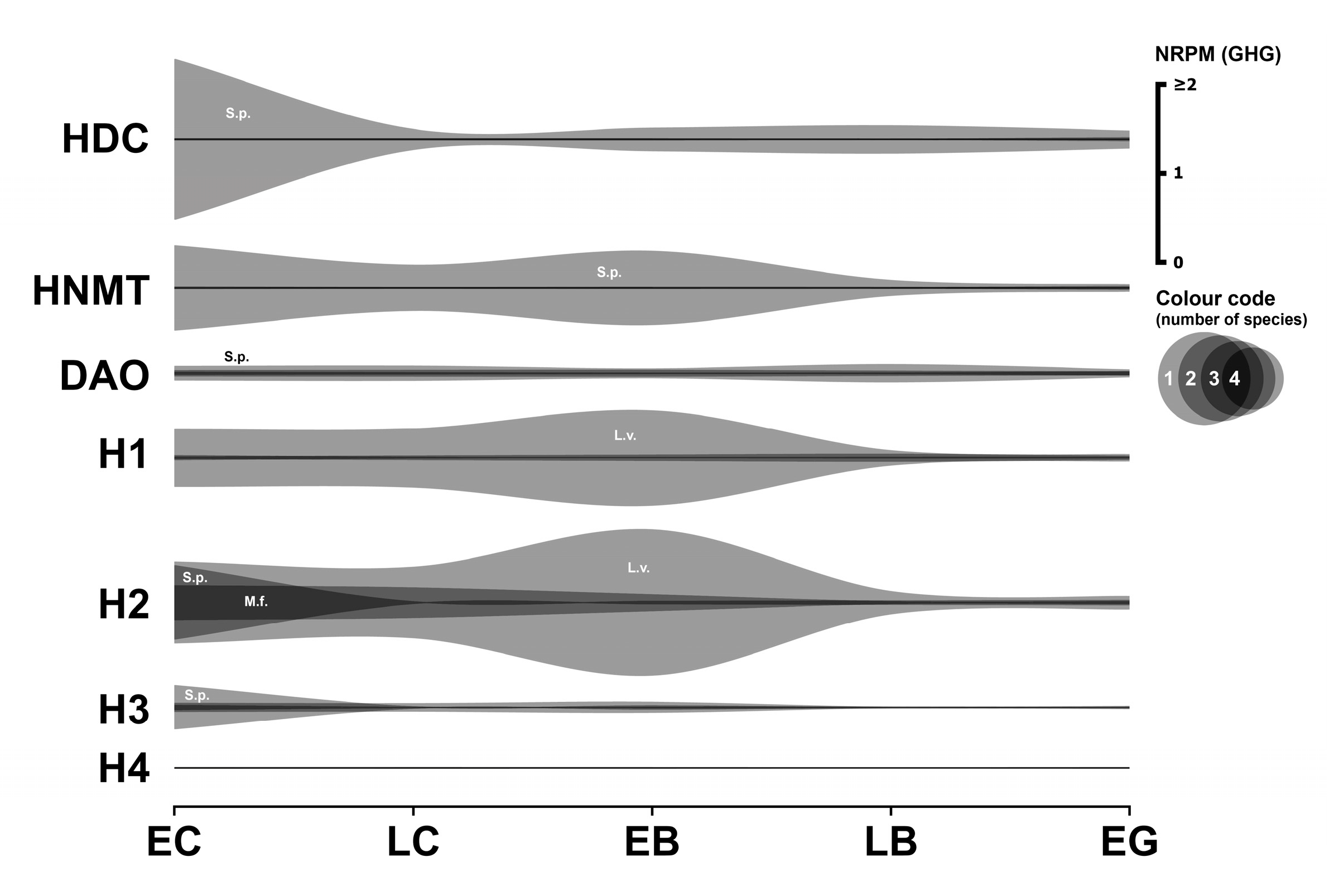
3.6. Histaminergic System
3.6.1. Histaminergic Enzymes
3.6.2. Histaminergic Receptors
3.6.3. Histaminergic Transporter
3.6.4. Summary
3.7. Glutamatergic System
3.7.1. Glutamatergic Receptors
3.7.2. Glutamatergic Transporter
3.7.3. Summary
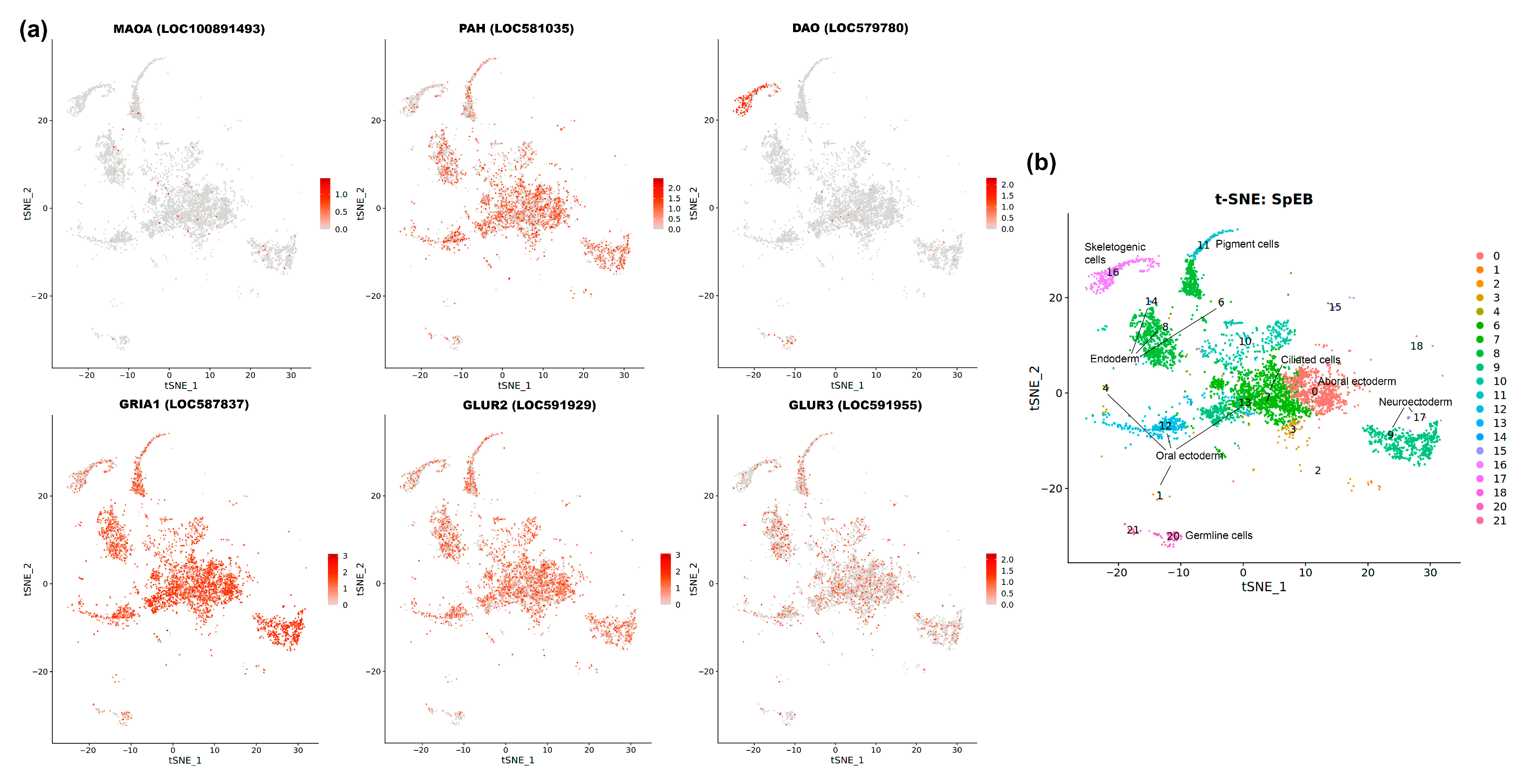
3.8. Spatial Analysis Using Single-Cell Transcriptomic Data
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 5-HT | Serotonin |
| AADC | Aromatic L-amino acid decarboxylase |
| ACh | Acetylcholine |
| AChE | Acetylcholinesterase |
| adrR | Adrenergic receptor |
| AMPA | α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid |
| ChAT | Choline-O-acetyltransferase |
| COMT | Catechol-O-methyltransferase |
| DAO | Diamine oxidase |
| DAT | Dopamine transporter |
| DBH | Dopamine-β-hydroxylase |
| EB | Early blastula |
| EC | Early cleavage |
| EG | Early gastrula |
| FPKM | Fragments Per Kilobase of transcript per Million mapped reads |
| GABA | Gamma-aminobutyric acid |
| GAD | Glutamate decarboxylase |
| GAPDH | Glyceraldehyde-3-phosphate dehydrogenase |
| GHG | Geometric mean of housekeeping genes |
| HDC | Histidine decarboxylase |
| HKG | Housekeeping genes |
| HNMT | Histamine N-methyltransferase |
| HPLC | High-performance liquid chromatography |
| HPRT | Hypoxanthine-guanine phosphoribosyltransferase |
| LB | Late blastula |
| LC | Late cleavage |
| AChR | Acetylcholine receptor |
| MAO | Monoamine oxidase |
| MZT | Maternal-to-zygotic transition |
| NET | Norepinephrine transporter |
| NMDA | N-methyl-D-aspartate |
| NO | Nitric oxide |
| NRPM | Normalized Reads Per Million |
| ODC | Ornithine decarboxylase |
| PAH | Phenylalanine hydroxylase |
| PCR | Polymerase chain reaction |
| RNA-Seq | RNA Sequencing |
| RPM | Reads Per Million |
| RT-PCR | Reverse transcription polymerase chain reaction |
| SERT | Serotonin transporter |
| TH | Tyrosine hydroxylase |
| TPM | Transcripts Per Million |
| TPH | Tryptophan 5-hydroxylase |
| TSA | Transcriptome Shotgun Assembly |
| VAChT | Vesicular acetylcholine transporter |
| VMAT2 | Vesicular monoamine transporter 2 |
References
- Shmukler, Y.B.; Nikishin, D.A. Non-Neuronal Transmitter Systems in Bacteria, Non-Nervous Eukaryotes, and Invertebrate Embryos. Biomolecules 2022, 12, 271. [Google Scholar] [CrossRef] [PubMed]
- Buznikov, G.A.; Sakharova, A.V.; Manukhin, B.N.; Markova, L.N. The Role of Neurohumours in Early Embryogenesis. IV. Fluorometric and Histochemical Study of Serotonin in Cleaving Eggs and Larvae of Sea Urchins. J. Embryol. Exp. Morphol. 1972, 27, 339–351. [Google Scholar] [PubMed]
- Renaud, F.; Parisi, E.; Capasso, A.; De Prisco, P. On the Role of Serotonin and 5-Methoxy-Tryptamine in the Regulation of Cell Division in Sea Urchin Eggs. Dev. Biol. 1983, 98, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Buznikov, G.A. Neurotransmitters in Embryogenesis; Harwood Academic Publishers: Chur, Switzerland, 1990. [Google Scholar]
- Buznikov, G.A. Preneural Transmitters as Regulators of Embryogenesis: Current State of Problem. Russ. J. Dev. Biol. 2007, 38, 213–220. [Google Scholar] [CrossRef]
- Shmukler, Y.B.; Nikishin, D.A. Transmitters in Blastomere Interactions. In Cell Interaction; InTech: Rijeka, Croatia, 2012; pp. 31–66. [Google Scholar]
- Capasso, A.; Parisi, E.; Deprisco, P.; Depetrocellis, B. Catecholamine Secretion and Adenylate Cyclase Activation in Sea Urchin Eggs. Cell Biol. Int. Rep. 1987, 11, 457–463. [Google Scholar] [CrossRef]
- Carginale, V.; Capasso, A.; Madonna, L.; Borrelli, L.; Parisi, E. Adenylate Cyclase from Sea Urchin Eggs Is Positively and Negatively Regulated by D-1 and D-2 Dopamine Receptors. Exp. Cell Res. 1992, 203, 491–494. [Google Scholar] [CrossRef]
- Anitole-Misleh, K.G.; Brown, K.M. Developmental Regulation of Catecholamine Levels during Sea Urchin Embryo Morphogenesis. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2004, 137, 39–50. [Google Scholar] [CrossRef]
- Katow, H.; Suyemitsu, T.; Ooka, S.; Yaguchi, J.; Jin-nai, T.; Kuwahara, I.; Katow, T.; Yaguchi, S.; Abe, H. Development of a Dopaminergic System in Sea Urchin Embryos and Larvae. J. Exp. Biol. 2010, 1, 2808–2819. [Google Scholar] [CrossRef]
- Nikishin, D.A.; Milošević, I.; Gojković, M.; Rakić, L.; Bezuglov, V.V.; Shmukler, Y.B. Expression and Functional Activity of Neurotransmitter System Components in Sea Urchins’ Early Development. Zygote 2016, 24, 206–218. [Google Scholar] [CrossRef]
- Buznikov, G.A.; Grigoriev, N.G. The Effect of Biogenic Monoamines and Their Antagonists on the Cortical Layer in Early Sea Urchin Embryos. J. Evol. Biochem. Physiol. 1990, 26, 614–622. (In Russian) [Google Scholar]
- Numanoi, H. Studies on the Fertilization Substances. IV. Presence of Acetylcholine-Like Substance and Cholinesterase in Echinoderm-Germ Cells during Fertilization. Scient. Pap. Coll. Gen. Educ. Univ. Tokyo 1953, 3, 193–200. [Google Scholar]
- Numanoi, H. Studies on the Fertilization Substances. VI. Formation of Acetylcholine-like Substance in Echinoderm Eggs during Fertilization. Scient. Pap. Coll. Gen. Educ. Univ. Tokyo 1955, 5, 43–54. [Google Scholar]
- Buznikov, G.A.; Rakić, L.; Turpaev, T.M.; Markova, L.N. Sensitivity of Sea Urchin Early Embryos to Antagonists of Acetylcholine and Monoamines. Exp. Cell Res. 1974, 86, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Buznikov, G.A.; Rakich, L. Cholinoreceptors of Early (Preneural) Sea Urchin Embryos. Neurosci. Behav. Physiol. 2000, 30, 53. [Google Scholar] [CrossRef]
- Buznikov, G.A.; Marshak, T.L.; Malchenko, L.A.; Nikitina, L.A.; Shmukler, Y.B.; Buznikov, A.G.; Rakic, L.; Whitaker, M.J. Serotonin and Acetylcholine Modulate the Sensitivity of Early Sea Urchin Embryos to Protein Kinase C Activators. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 1998, 120, 457–462. [Google Scholar] [CrossRef]
- Falugi, C. Localization and Possible Role of Molecules Associated with the Cholinergic System during “Non-Nervous” Developmental Events. Eur. J. Histochem. 1993, 37, 287–294. [Google Scholar]
- Falugi, C.; Lammerding-Koppel, M.; Aluigi, M.G. Sea Urchin Development: An Alternative Model for Mechanistic Understanding of Neurodevelopment and Neurotoxicity. Birth Defects Res. C Embryo Today 2008, 84, 188–203. [Google Scholar] [CrossRef]
- Buznikov, G.A.; Shmukler, Y.B.; Lauder, J.M. From Oocyte to Neuron: Do Neurotransmitters Function in the Same Way Throughout Development? Cell Mol. Neurobiol. 1996, 16, 537–559. [Google Scholar] [CrossRef]
- Shmukler, Y.B.; Nikishin, D.A. On the Intracellular Transmitter Reception. Neurochem. J. 2018, 12, 295–298. [Google Scholar] [CrossRef]
- D’Aniello, E.; Paganos, P.; Anishchenko, E.; D’Aniello, S.; Arnone, M.I. Comparative Neurobiology of Biogenic Amines in Animal Models in Deuterostomes. Front. Ecol. Evol. 2020, 8, 587036. [Google Scholar] [CrossRef]
- Sodergren, E.; Weinstock, G.M.; Davidson, E.H.; Cameron, R.A.; Gibbs, R.A.; Angerer, R.C.; Angerer, L.M.; Arnone, M.I.; Burgess, D.R.; Burke, R.D.; et al. The Genome of the Sea Urchin Strongylocentrotus purpuratus. Science 2006, 314, 941–952. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.M.; Gaitán-Espitia, J.D.; Hofmann, G.E. Transcriptional Profiles of Early Stage Red Sea Urchins (Mesocentrotus franciscanus) Reveal Differential Regulation of Gene Expression across Development. Mar. Genom. 2019, 48, 100692. [Google Scholar] [CrossRef] [PubMed]
- Hogan, J.D.; Keenan, J.L.; Luo, L.; Ibn-Salem, J.; Lamba, A.; Schatzberg, D.; Piacentino, M.L.; Zuch, D.T.; Core, A.B.; Blumberg, C.; et al. The Developmental Transcriptome for Lytechinus variegatus Exhibits Temporally Punctuated Gene Expression Changes. Dev. Biol. 2020, 460, 139–154. [Google Scholar] [CrossRef] [PubMed]
- Gildor, T.; Malik, A.; Sher, N.; Avraham, L.; Ben-Tabou de-Leon, S. Quantitative Developmental Transcriptomes of the Mediterranean Sea Urchin Paracentrotus lividus. Mar. Genom. 2016, 25, 89–94. [Google Scholar] [CrossRef]
- Tu, Q.; Cameron, R.A.; Davidson, E.H. Quantitative Developmental Transcriptomes of the Sea Urchin Strongylocentrotus purpuratus. Dev. Biol. 2014, 385, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Tadros, W.; Lipshitz, H.D. The Maternal-to-Zygotic Transition: A Play in Two Acts. Development 2009, 136, 3033–3042. [Google Scholar] [CrossRef]
- Kipryushina, Y.O.; Yakovlev, K.V. Maternal Control of Early Patterning in Sea Urchin Embryos. Differentiation 2020, 113, 28–37. [Google Scholar] [CrossRef]
- Vandesompele, J.; De Preter, K.; Pattyn, F.; Poppe, B.; Van Roy, N.; De Paepe, A.; Speleman, F. Accurate Normalization of Real-Time Quantitative RT-PCR Data by Geometric Averaging of Multiple Internal Control Genes. Genome Biol. 2002, 3, research0034.1. [Google Scholar] [CrossRef]
- Shmukler, Y.B. A “Micromere Model” of Cell-Cell Interactions in Sea Urchin Early Embryos. Biophysics 2010, 55, 399–405. [Google Scholar] [CrossRef]
- Frolova, V.S.; Nikishina, Y.O.; Shmukler, Y.B.; Nikishin, D.A. Serotonin Signaling in Mouse Preimplantation Development: Insights from Transcriptomic and Structural-Functional Analyses. Int. J. Mol. Sci. 2024, 25, 12954. [Google Scholar] [CrossRef]
- Shmukler, Y.B.; Alyoshina, N.M.; Nikishina, Y.O.; Frolova, V.S.; Nikishin, D.A. All Transmitters within a Single Oocyte: A Transcriptome Analysis of Embryonic Transmitter Systems. Ontogenez 2025, 56, 3–13. [Google Scholar]
- Nikishin, D.A.; Malchenko, L.A.; Milošević, I.; Rakić, L.; Shmukler, Y.B. Effects of Haloperidol and Cyproheptadine on the Cytoskeleton of the Sea Urchin Embryos. Biochem. Suppl. Ser. A Membr. Cell Biol. 2020, 14, 249–254. [Google Scholar] [CrossRef]
- Adams, D.K.; Sewell, M.A.; Angerer, R.C.; Angerer, L.M. Rapid Adaptation to Food Availability by a Dopamine-Mediated Morphogenetic Response. Nat. Commun. 2011, 2, 592. [Google Scholar] [CrossRef]
- Kalachev, A.V. Effect of Dopamine on Early Larvae of Sea Urchins, Mesocentrotus nudus and Strongylocentrotus intermedius. J. Exp. Zool. B Mol. Dev. Evol. 2020, 334, 373–380. [Google Scholar] [CrossRef]
- Katow, H.; Katow, T.; Yoshida, H.; Kiyomoto, M.; Uemura, I. Immunohistochemical and Ultrastructural Properties of the Larval Ciliary Band-Associated Strand in the Sea Urchin Hemicentrotus pulcherrimus. Front. Zool. 2016, 13, 27. [Google Scholar] [CrossRef][Green Version]
- Kalachev, A.V.; Tankovich, A.E. The Dopamine Effect on Sea Urchin Larvae Depends on Their Age. Dev. Growth Differ. 2023, 65, 120–131. [Google Scholar] [CrossRef] [PubMed]
- Obukhova, A.L.; Khabarova, M.Y.; Semenova, M.N.; Starunov, V.V.; Voronezhskaya, E.E.; Ivashkin, E.G. Spontaneous Intersibling Polymorphism in the Development of Dopaminergic Neuroendocrine Cells in Sea Urchin Larvae: Impacts on the Expansion of Marine Benthic Species. Front. Neurosci. 2024, 18, 1348999. [Google Scholar] [CrossRef] [PubMed]
- Aluigi, M.G.; Diaspro, A.; Ramoino, P.; Russo, P.; Falugi, C. The Sea Urchin, Paracentrotus Lividus, as a Model to Investigate the Onset of Molecules Immunologically Related to the α-7 Subunit of Nicotinic Receptors during Embryonic and Larval Development. Curr. Drug Targets 2012, 13, 587–593. [Google Scholar] [CrossRef] [PubMed]
- Schoepfer, R.; Conroy, W.G.; Whiting, P.; Gore, M.; Lindstrom, J. Brain α-Bungarotoxin Binding Protein CDNAs and MAbs Reveal Subtypes of This Branch of the Ligand-Gated Ion Channel Gene Superfamily. Neuron 1990, 5, 35–48. [Google Scholar] [CrossRef]
- Drisdel, R.C.; Green, W.N. Neuronal α-Bungarotoxin Receptors Are A7 Subunit Homomers. J. Neurosci. 2000, 20, 133–139. [Google Scholar] [CrossRef]
- Natarajan, K.; Mukhtasimova, N.; Corradi, J.; Lasala, M.; Bouzat, C.; Sine, S.M. Mechanism of Calcium Potentiation of the A7 Nicotinic Acetylcholine Receptor. J. General. Physiol. 2020, 152, e202012606. [Google Scholar] [CrossRef]
- Angelini, C.; Baccetti, B.; Piomboni, P.; Trombino, S.; Aluigi, M.G.; Stringara, S.; Gallus, L.; Falugi, C. Acetylcholine Synthesis and Possible Functions during Sea Urchin Development. Eur. J. Histochem. 2004, 48, 235–243. [Google Scholar]
- Buznikov, G.A.; Koikov, L.N.; Shmukler, Y.B.; Whitaker, M.J. Nicotinic Antagonists (Piperidines and Quinuclidines) Reduce the Susceptibility of Early Sea Urchin Embryos to Agents Evoking Calcium Shock. General. Pharmacol. Vasc. Syst. 1997, 29, 49–53. [Google Scholar] [CrossRef]
- White, J.H.; Wise, A.; Main, M.J.; Green, A.; Fraser, N.J.; Disney, G.H.; Barnes, A.A.; Emson, P.; Foord, S.M.; Marshall, F.H. Heterodimerization Is Required for the Formation of a Functional GABAB Receptor. Nature 1998, 396, 679–682. [Google Scholar] [CrossRef] [PubMed]
- Katow, H. Mechanisms of the Epithelial-to-Mesenchymal Transition in Sea Urchin Embryos. Tissue Barriers 2015, 3, e1059004. [Google Scholar] [CrossRef] [PubMed]
- Katow, H.; Abe, K.; Katow, T.; Zamani, A.; Abe, H. Development of the γ-Amino Butyric Acid (GABA)-Ergic Signaling System and Its Role in Larval Swimming in Sea Urchin. J. Exp. Biol. 2013, 216, 1704–1716. [Google Scholar] [CrossRef] [PubMed]
- Leguia, M.; Wessel, G.M. The Histamine H1 Receptor Activates the Nitric Oxide Pathway at Fertilization. Mol. Reprod. Dev. 2006, 73, 1550–1563. [Google Scholar] [CrossRef]
- Sutherby, J.; Giardini, J.-L.; Nguyen, J.; Wessel, G.; Leguia, M.; Heyland, A. Histamine Is a Modulator of Metamorphic Competence in Strongylocentrotus purpuratus(Echinodermata: Echinoidea). BMC Dev. Biol. 2012, 12, 14. [Google Scholar] [CrossRef]
- Foster, S.; Oulhen, N.; Wessel, G. A Single Cell RNA Sequencing Resource for Early Sea Urchin Development. Development 2020, 147, dev191528. [Google Scholar] [CrossRef]
- Šindelka, R.; Ferjentsik, Z.; Jonák, J. Developmental Expression Profiles of Xenopus laevis Reference Genes. Dev. Dyn. 2006, 235, 754–758. [Google Scholar] [CrossRef]
- Jiang, T.; Dai, S.; Yi, Y.; Liu, Y.; Zhang, S.; Luo, M.; Wang, H.; Xu, D. The Combination of Hprt and Gapdh Is the Best Compound Reference Genes in the Fetal Rat Hippocampus. Dev. Neurobiol. 2020, 80, 229–238. [Google Scholar] [CrossRef]
- Wang, J.; Garrey, J.; Davis, R.E. Transcription in Pronuclei and One- to Four-Cell Embryos Drives Early Development in a Nematode. Curr. Biol. 2014, 24, 124–133. [Google Scholar] [CrossRef]
- Blythe, S.A.; Cha, S.-W.; Tadjuidje, E.; Heasman, J.; Klein, P.S. β-Catenin Primes Organizer Gene Expression by Recruiting a Histone H3 Arginine 8 Methyltransferase, Prmt2. Dev. Cell 2010, 19, 220–231. [Google Scholar] [CrossRef]
- Giraldez, A.J.; Mishima, Y.; Rihel, J.; Grocock, R.J.; Van Dongen, S.; Inoue, K.; Enright, A.J.; Schier, A.F. Zebrafish MiR-430 Promotes Deadenylation and Clearance of Maternal MRNAs. Science 2006, 312, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Jukam, D.; Shariati, S.A.M.; Skotheim, J.M. Zygotic Genome Activation in Vertebrates. Dev. Cell 2017, 42, 316–332. [Google Scholar] [CrossRef] [PubMed]
- Owens, N.D.L.; Blitz, I.L.; Lane, M.A.; Patrushev, I.; Overton, J.D.; Gilchrist, M.J.; Cho, K.W.Y.; Khokha, M.K. Measuring Absolute RNA Copy Numbers at High Temporal Resolution Reveals Transcriptome Kinetics in Development. Cell Rep. 2016, 14, 632–647. [Google Scholar] [CrossRef] [PubMed]
- Skirkanich, J.; Luxardi, G.; Yang, J.; Kodjabachian, L.; Klein, P.S. An Essential Role for Transcription before the MBT in Xenopus laevis. Dev. Biol. 2011, 357, 478–491. [Google Scholar] [CrossRef]
- Baccetti, B.; Burrini, A.G.; Collodel, G.; Falugi, C.; Moretti, E.; Piomboni, P. Localisation of Two Classes of Acetylcholine Receptor-like Molecules in Sperms of Different Animal Species. Zygote 1995, 3, 207–217. [Google Scholar] [CrossRef]
- Falugi, C.; Prestipino, G. Localization of Putative Nicotinic Cholinoreceptors in the Early Development of Paracentrotus lividus. Cell Mol. Biol. 1989, 35, 147–161. [Google Scholar]
- Ivonnet, P.I.; Chambers, E.L. Nicotinic Acetylcholine Receptors of the Neuronal Type Occur in the Plasma Membrane of Sea Urchin Eggs. Zygote 1997, 5, 277–287. [Google Scholar] [CrossRef]

| EC | LC | EB | LB | EG | |
|---|---|---|---|---|---|
| M. franciscanus | Egg | 7 hpf | ND | 16 hpf | 29 hpf |
| P. lividus | Egg | ND | 12 hpf | 18 hpf | 24 hpf |
| L. variegatus | 1 hpf | 2.5 hpf | 4 hpf | 7 hpf | 12 hpf |
| S. purpuratus | Egg | 10 hpf | 18 hpf | 24 hpf | 30 hpf |
| Species | EC | LC | EB | LB | EG |
|---|---|---|---|---|---|
| M. franciscanus | 559.068 | 2711.161 | ND | 1443.028 | 2004.563 |
| P. lividus | 97.394 | ND | 108.306 | 91.15 | 99.959 |
| L. variegatus | 4056.580 | 5140.630 | 4184.16 | 4166.32 | 7714.20 |
| S. purpuratus | 1388.124 | 5121.778 | 4838.051 | 6287.357 | 7629.709 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shmukler, Y.B.; Alyoshina, N.M.; Nikishina, Y.O.; Nikishin, D.A. Expression Dynamics of Neurotransmitter System Genes in Early Sea Urchin Embryos: Insights from a Four-Species Comparative Transcriptome Analysis. Biology 2025, 14, 1262. https://doi.org/10.3390/biology14091262
Shmukler YB, Alyoshina NM, Nikishina YO, Nikishin DA. Expression Dynamics of Neurotransmitter System Genes in Early Sea Urchin Embryos: Insights from a Four-Species Comparative Transcriptome Analysis. Biology. 2025; 14(9):1262. https://doi.org/10.3390/biology14091262
Chicago/Turabian StyleShmukler, Yuri B., Nina M. Alyoshina, Yulia O. Nikishina, and Denis A. Nikishin. 2025. "Expression Dynamics of Neurotransmitter System Genes in Early Sea Urchin Embryos: Insights from a Four-Species Comparative Transcriptome Analysis" Biology 14, no. 9: 1262. https://doi.org/10.3390/biology14091262
APA StyleShmukler, Y. B., Alyoshina, N. M., Nikishina, Y. O., & Nikishin, D. A. (2025). Expression Dynamics of Neurotransmitter System Genes in Early Sea Urchin Embryos: Insights from a Four-Species Comparative Transcriptome Analysis. Biology, 14(9), 1262. https://doi.org/10.3390/biology14091262







