Comparative Study of Hirudins and Encoding Genes in Hirudo nipponia and Hirudo tianjinensis
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sequence Extraction
2.3. Variant Site Statistics
2.4. Gene Expression Analysis
2.5. Protein Biochemical Property Analysis
2.6. Protein Three-Dimensional Structure Analysis
2.7. Pichia Pastoris Eukaryotic Expression
2.8. Antithrombin Activity Assay
3. Results
3.1. Intraspecific Variation in the Hirudin Genes and Their Proteins
3.2. Gene Expression
3.3. Protein Biochemical Property
3.4. Protein Three-Dimensional Structure
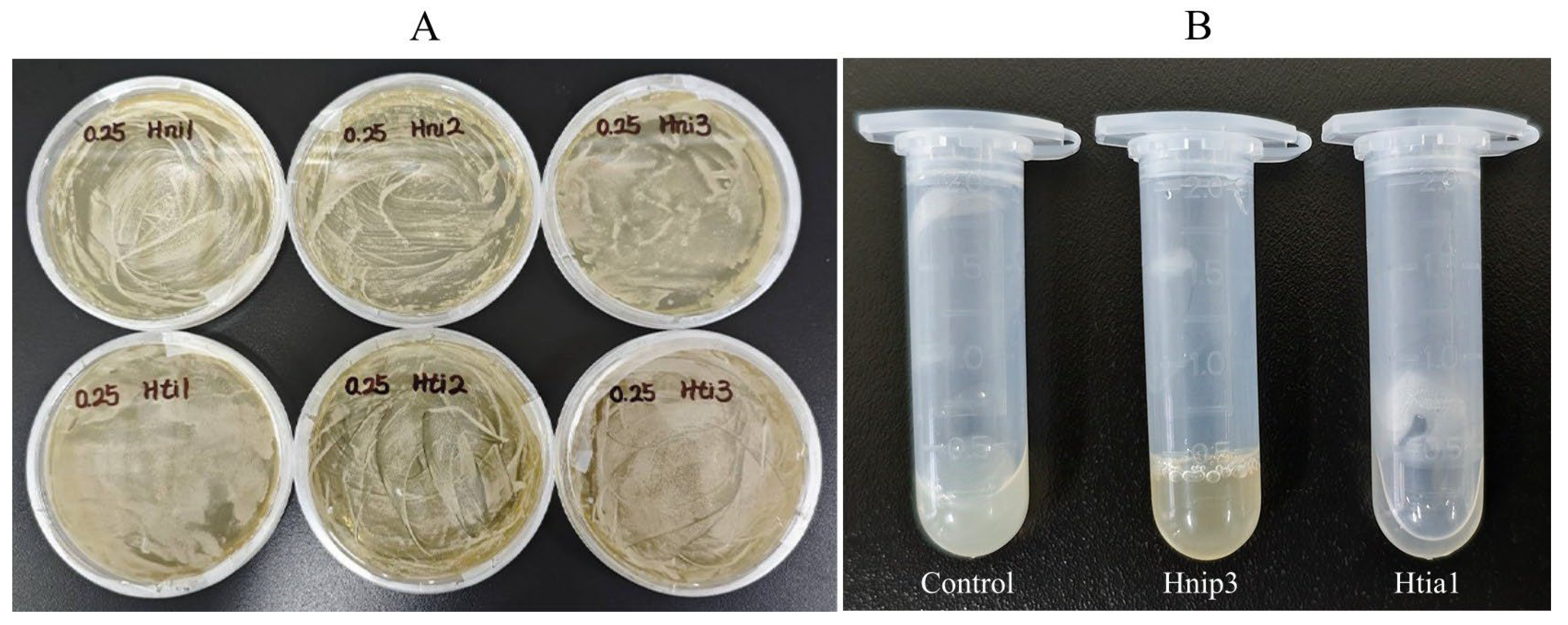
3.5. Antithrombin Activity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CDS | coding sequence |
| VS | number of variable sites |
| HN | number of haplotypes |
| WD | Watterson’s Theta diversity |
| TPM | transcripts per million |
| PSL | protein subcellular localization |
| pI | isoelectric point |
| II | instability index |
| GRAVY | grand average of hydropathicity |
References
- WHO. The Top 10 Causes of Death; WHO: Geneva, Switzerland, 2020. [Google Scholar]
- Mensah, G.A.; Fuster, V.; Murray, C.J.L.; Roth, G.A. Global burden of cardiovascular diseases and risks, 1990–2022. J. Am. Coll. Cardiol. 2023, 82, 2350–2473. [Google Scholar] [CrossRef]
- DeLoughery, T.G. Hemostasis and Thrombosis; Springer Nature Switzerland AG: Cham, Switzerland, 2019. [Google Scholar]
- Hildebrandt, J.P.; Lemke, S. Small bite, large impact-saliva and salivary molecules in the medicinal leech, Hirudo medicinalis. Naturwissenschaften 2011, 98, 995–1008. [Google Scholar] [CrossRef]
- Müller, C.; Haase, M.; Lemke, S.; Hildebrandt, J.P. Hirudins and hirudin-like factors in Hirudinidae: Implications for function and phylogenetic relationships. Parasitol. Res. 2017, 116, 313–325. [Google Scholar] [CrossRef]
- Zheng, J.; Wang, X.; Feng, T.; Rehman, S.U.; Yan, X.; Shan, H.; Ma, X.; Zhou, W.; Xu, W.; Lu, L.; et al. Molecular mechanisms underlying hematophagia revealed by comparative analyses of leech genomes. Gigascience 2022, 12, giad023. [Google Scholar] [CrossRef] [PubMed]
- Müller, C.; Wang, Z.; Hamann, M.; Sponholz, D.; Hildebrandt, J.P. Life without blood: Molecular and functional analysis of hirudins and hirudin-like factors of the Asian non-hematophagous leech Whitmania pigra. J. Thromb. Haemost. 2022, 20, 1808–1817. [Google Scholar] [CrossRef] [PubMed]
- Fritsma, G.A. Monitoring the direct thrombin inhibitors. Clin. Lab. Sci. 2013, 26, 54–57. [Google Scholar] [CrossRef] [PubMed]
- Müller, C.; Lukas, P.; Böhmert, M.; Hildebrandt, J.P. Hirudin or hirudin-like factor—That is the question: Insights from the analyses of natural and synthetic HLF variants. FEBS Lett. 2020, 594, 841–850. [Google Scholar] [CrossRef]
- Al-Amer, O.M. The role of thrombin in haemostasis. Blood Coagul. Fibrinolysis 2022, 33, 145–148. [Google Scholar] [CrossRef]
- Chen, J.; Xie, X.; Zhang, H.; Li, G.; Yin, Y.; Cao, X.; Gao, Y.; Li, Y.; Zhang, Y.; Peng, F.; et al. Pharmacological activities and mechanisms of hirudin and its derivatives—A review. Front. Pharmacol. 2021, 12, 660757. [Google Scholar]
- Mousa, R.; Hidmi, T.; Pomyalov, S.; Lansky, S.; Khouri, L.; Shalev, D.E.; Shoham, G.; Metanis, N. Diselenide crosslinks for enhanced and simplified oxidative protein folding. Commun. Chem. 2021, 4, 30. [Google Scholar] [CrossRef]
- Kvist, S.; Manzano-Marín, A.; de Carle, D.; Trontelj, P.; Siddall, M.E. Draft genome of the European medicinal leech Hirudo medicinalis (Annelida, Clitellata, Hirudiniformes) with emphasis on anticoagulants. Sci. Rep. 2020, 10, 9885. [Google Scholar] [CrossRef]
- Zhang, J.; Lan, N. Hirudin variants production by genetic engineered microbial factory. Biotechnol. Genet. Eng. Rev. 2018, 34, 261–280. [Google Scholar] [CrossRef] [PubMed]
- Michalsen, A.; Roth, M.; Dobos, G. Medicinal Leech Therapy; Georg Thieme Verlag: Stuttgart, Germany, 2007. [Google Scholar]
- Dong, H.; Ren, J.X.; Wang, J.J.; Ding, L.S.; Zhao, J.J.; Liu, S.Y.; Gao, H.M. Chinese medicinal leech: Ethnopharmacology, phytochemistry, and pharmacological activities. J. Evid.-Based Complement. Altern. Med. 2016, 2016, 7895935. [Google Scholar] [CrossRef] [PubMed]
- Kvist, S.; Oceguera-Figueroa, A.; Siddall, M.E.; Erséus, C. Barcoding, types and the Hirudo files: Using information content to critically evaluate the identity of DNA barcodes. Mitochondrial DNA 2010, 21, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Saglam, N.; Saunders, R.; Lang, S.A.; Shain, D.H. A new species of Hirudo (Annelida: Hirudinidae): Historical biogeography of Eurasian medicinal leeches. BMC Zool. 2016, 1, 5. [Google Scholar] [CrossRef]
- Yang, T. Fauna Sinica (Annelida Hirudinea); Science Press: Beijing, China, 1996. [Google Scholar]
- Commission, C.P. Pharmacopoeia of the People’s Republic of China; Medicine Science and Technology Press: Beijing, China, 2020. [Google Scholar]
- Cheng, B.; Liu, F.; Guo, Q.; Lu, Y.; Shi, H.; Ding, A.; Xu, C. Identification and characterization of hirudin-HN, a new thrombin inhibitor, from the salivary glands of Hirudo nipponia. PeerJ 2019, 7, e7716. [Google Scholar] [CrossRef]
- Yu, M.; Su, S.; Zhou, M.; Cao, M.; Feng, X. Identification of suspected species of Hirudo nipponia based on COI gene sequence. J. Southwest Univ. (Nat. Sci. Ed.) 2022, 44, 74–80. [Google Scholar] [CrossRef]
- Zhao, F.; Huang, Z.; He, B.; Liu, K.; Li, J.; Liu, Z.; Lin, G. Comparative genomics of two Asian medicinal leeches Hirudo nipponia and Hirudo tianjinensis: With emphasis on antithrombotic genes and their corresponding proteins. Int. J. Biol. Macromol. 2024, 270, 132278. [Google Scholar] [CrossRef]
- Dodt, J.; Müller, H.-P.; Seemüller, U.; Chang, J.-Y. The complete amino acid sequence of hirudin, a thrombin specific inhibitor: Application of colour carboxymethylation. FEBS Lett. 1984, 165, 180–184. [Google Scholar] [CrossRef]
- Ahmed, R.B.; Abilov, A.; Müller, C. Diversity of hirudin and hirudin-like factor genes in the North-African medicinal leech, Hirudo troctina. Parasitol. Res. 2024, 123, 382. [Google Scholar] [CrossRef]
- Liu, Z.; Zhao, F.; Huang, Z.; He, B.; Liu, K.; Shi, F.; Zhao, Z.; Lin, G. A chromosome-level genome assembly of the non-hematophagous leech Whitmania pigra (Whitman 1884): Identification and expression analysis of antithrombotic genes. Genes 2024, 15, 164. [Google Scholar] [CrossRef] [PubMed]
- Müller, C.; Mescke, K.; Liebig, S.; Mahfoud, H.; Lemke, S.; Hildebrandt, J.P. More than just one: Multiplicity of Hirudins and Hirudin-like Factors in the Medicinal Leech, Hirudo medicinalis. Mol. Genet. Genom. 2016, 291, 227–240. [Google Scholar] [CrossRef] [PubMed]
- Nowak, G.; Schrör, K. Hirudin--the long and stony way from an anticoagulant peptide in the saliva of medicinal leech to a recombinant drug and beyond. A historical piece. Thromb. Haemost. 2007, 98, 116–119. [Google Scholar] [CrossRef] [PubMed]
- Vecchioni, L.; Bellucci, D.; Novaga, R.; Faraone, F.P.; Utevsky, S.; Marrone, F. Further evidence of the southern Mediterranean medicinal leech Hirudo verbana (Annelida, Hirudinea) feeding on fish, with a review of the use of fish hosts by Hirudo spp. Limnetica 2025, 44, 101–111. [Google Scholar] [CrossRef]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef]
- Li, D.; Liu, C.M.; Luo, R.; Sadakane, K.; Lam, T.W. MEGAHIT: An ultra-fast single-node solution for large and complex metagenomics assembly via succinct de Bruijn graph. Bioinformatics 2015, 31, 1674–1676. [Google Scholar] [CrossRef]
- Grabherr, M.G.; Haas, B.J.; Yassour, M.; Levin, J.Z.; Thompson, D.A.; Amit, I.; Adiconis, X.; Fan, L.; Raychowdhury, R.; Zeng, Q.; et al. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat. Biotechnol. 2011, 29, 644–652. [Google Scholar] [CrossRef]
- Seppey, M.; Manni, M.; Zdobnov, E.M. BUSCO: Assessing genome assembly and annotation completeness. Methods Mol. Biol. 2019, 1962, 227–245. [Google Scholar] [CrossRef]
- Camacho, C.; Coulouris, G.; Avagyan, V.; Ma, N.; Papadopoulos, J.; Bealer, K.; Madden, T.L. BLAST+: Architecture and applications. BMC Bioinform. 2009, 10, 421. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Rozas, J.; Ferrer-Mata, A.; Sánchez-DelBarrio, J.C.; Guirao-Rico, S.; Librado, P.; Ramos-Onsins, S.E.; Sánchez-Gracia, A. DnaSP 6: DNA sequence polymorphism analysis of large data sets. Mol. Biol. Evol. 2017, 34, 3299–3302. [Google Scholar] [CrossRef]
- Xia, X. DAMBE5: A comprehensive software package for data analysis in molecular biology and evolution. Mol. Biol. Evol. 2013, 30, 1720–1728. [Google Scholar] [CrossRef]
- Patro, R.; Duggal, G.; Love, M.I.; Irizarry, R.A.; Kingsford, C. Salmon provides fast and bias-aware quantification of transcript expression. Nat. Methods 2017, 14, 417–419. [Google Scholar] [CrossRef]
- Janson, G.; Paiardini, A. PyMod 3: A complete suite for structural bioinformatics in PyMOL. Bioinformatics 2021, 37, 1471–1472. [Google Scholar] [CrossRef]
- Schrödinger, L.L.C. PyMOL, Version 3.0. 2020. Available online: https://pymol.org/ (accessed on 26 April 2025).
- Li, Z.; Jaroszewski, L.; Iyer, M.; Sedova, M.; Godzik, A. FATCAT 2.0: Towards a better understanding of the structural diversity of proteins. Nucleic Acids Res. 2020, 48, W60–W64. [Google Scholar] [CrossRef]
- Vitali, J.; Martin, P.D.; Malkowski, M.G.; Robertson, W.D.; Lazar, J.B.; Winant, R.C.; Johnson, P.H.; Edwards, B.F. The structure of a complex of bovine alpha-thrombin and recombinant hirudin at 2.8-A resolution. J. Biol. Chem. 1992, 267, 17670–17678. [Google Scholar] [CrossRef]
- Sig, A.K.; Guney, M.; Uskudar Guclu, A.; Ozmen, E. Medicinal leech therapy—An overall perspective. Integr. Med. Res. 2017, 6, 337–343. [Google Scholar] [CrossRef]
- Wang, H.; Meng, F.M.; Jin, S.J.; Gao, J.W.; Tong, X.R.; Liu, Z.C. A new species of medicinal leech in the genus Hirudo Linnaeus, 1758 (Hirudiniformes, Hirudinidae) from Tianjin City, China. Zookeys 2022, 1095, 83–96. [Google Scholar] [CrossRef] [PubMed]
- Lewin, T.D.; Liao, I.J.; Luo, Y.J. Annelid Comparative Genomics and the Evolution of Massive Lineage-Specific Genome Rearrangement in Bilaterians. Mol. Biol. Evol. 2024, 41, msae172. [Google Scholar] [CrossRef] [PubMed]
- Montinari, M.R.; Minelli, S. From ancient leech to direct thrombin inhibitors and beyond: New from old. Biomed. Pharmacother. 2022, 149, 112878. [Google Scholar] [CrossRef]
- Zhao, F.; Tang, L.; He, B.; Liu, Z.; Wu, Q.; Huang, Z.; Lin, G. Intraspecific variation in the hirudin gene family of the Asian buffalo leech (Hirudinaria manillensis). J. Jinggangshan Univ. (Nat. Sci.) 2024, 45, 38–45. [Google Scholar] [CrossRef]
- Sánchez-Montes, G.; Ariño, A.H.; Vizmanos, J.L.; Wang, J.; Martínez-Solano, Í. Effects of Sample Size Full Sibs on Genetic Diversity Characterization: A Case Study of Three Syntopic Iberian Pond-Breeding Amphibians. J. Hered. 2017, 108, 535–543. [Google Scholar] [CrossRef]
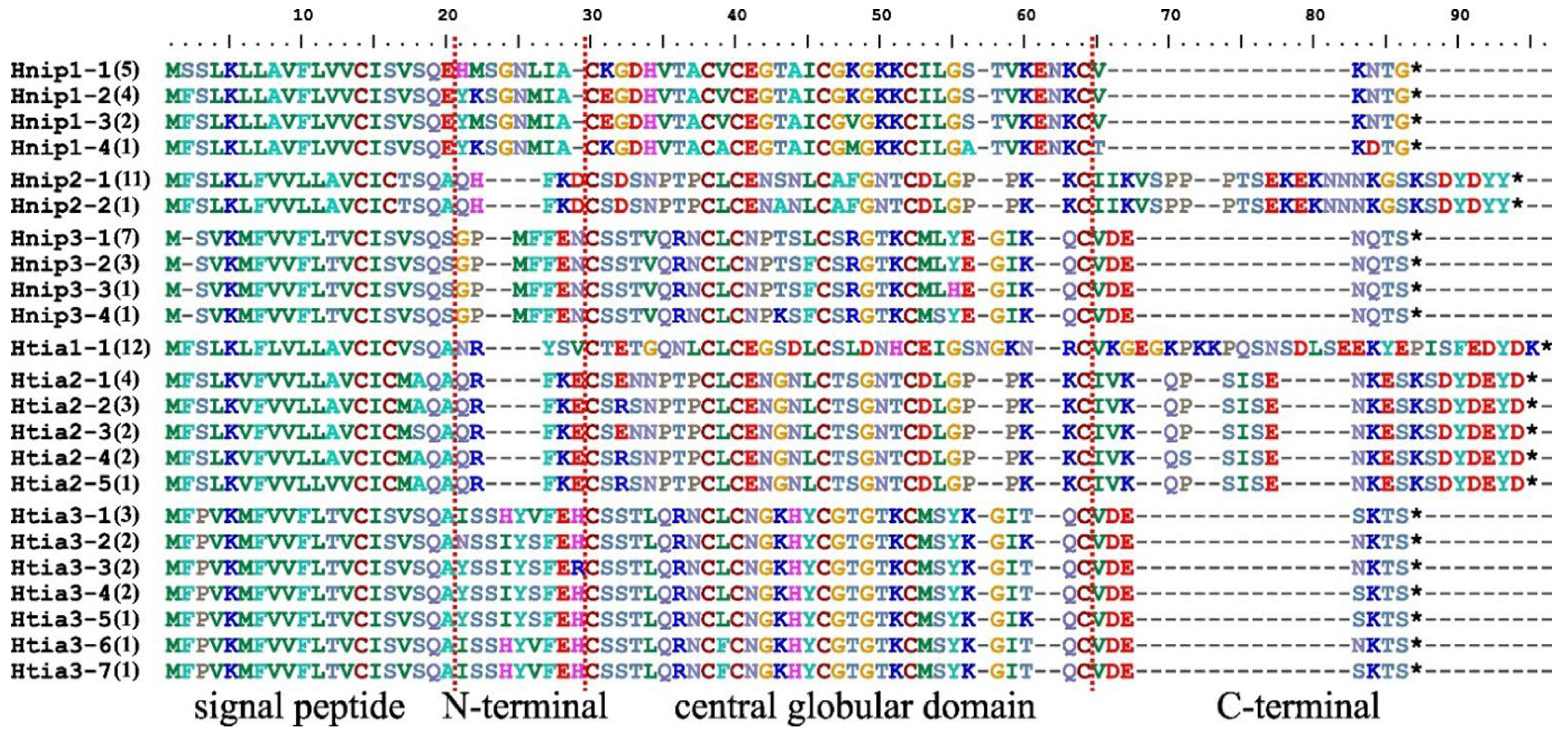

| Hirudin CDS | Length of Sequence | Coding Sequence | Protein Sequence | ||||
|---|---|---|---|---|---|---|---|
| VS | HN | WD | VS | HN | WD | ||
| Hnip1 | 204 | 13 | 4 | 0.03476 | 10 | 4 | 0.08021 |
| Hnip2 | 252 | 2 | 2 | 0.00794 | 1 | 2 | 0.01190 |
| Hnip3 | 198 | 4 | 4 | 0.01054 | 4 | 4 | 0.03162 |
| Htia1 | 270 | 0 | 1 | 0.00000 | 0 | 1 | 0.00000 |
| Htia2 | 234 | 8 | 6 | 0.01390 | 5 | 5 | 0.02857 |
| Htia3 | 207 | 15 | 7 | 0.02958 | 7 | 7 | 0.04141 |
| Total | 1365 | 42 | 24 | — | 27 | 23 | — |
| Factor | PSL (Reliability) | pI | II | GRAVY |
|---|---|---|---|---|
| Hnip1 | Extracellular (3.686) | 8.87 | 26.28 | −0.036 |
| Hnip2 | Extracellular (4.243) | 6.72 | 35.27 | −1.070 |
| Hnip3 | Extracellular (3.165) | 6.21 | 16.54 | −0.437 |
| Htia1 | Extracellular (4.250) | 4.84 | 39.29 | −1.113 |
| Htia2 | Extracellular (4.142) | 5.22 | 65.38 | −1.135 |
| Htia3 | Extracellular (3.390) | 7.79 | 20.78 | −0.390 |
| Protein | Structural Comparison | Docking Score | ||
|---|---|---|---|---|
| p-Value | RMSD | Bovine Thrombin | Human Thrombin | |
| Hnip1 | 1.44 × 10−12 | 0.22 | 899.209 | 933.955 |
| Hnip2 | 1.25 × 10−5 | 3.37 | 922.550 | 2089.823 |
| Hnip3 | 1.56 × 10−12 | 0.36 | 2136.436 | 1754.866 |
| Htia1 | 5.83 × 10−10 | 1.47 | 1609.944 | 1728.753 |
| Htia2 | 3.80 × 10−8 | 2.07 | 1661.418 | 824.945 |
| Htia3 | 2.00 × 10−12 | 0.39 | 1241.655 | 1059.792 |
| Hirudin | Antithrombin Activity (ATU/mg) | |||
|---|---|---|---|---|
| Repeat 1 | Repeat 2 | Repeat 3 | Mean ± SD | |
| Hnip1 | 212.766 | 222.222 | 227.273 | 220.754 ±7.364 a |
| Hnip2 | 66.225 | 66.667 | 67.568 | 66.820 ± 0.684 b |
| Hnip3 | 0.000 | 0.000 | 0.000 | 0.000 ± 0.000 c |
| Htia1 | 16.892 | 17.123 | 16.667 | 16.894 ± 0.228 A |
| Htia2 | 7.267 | 7.184 | 7.353 | 7.268 ± 0.085 B |
| Htia3 | 0.000 | 0.000 | 0.000 | 0.000 ± 0.000 C |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, J.; Liu, Z.; Yu, Y.; Wang, A.; Huang, Z.; Tang, L.; Zhao, F.; Lin, G. Comparative Study of Hirudins and Encoding Genes in Hirudo nipponia and Hirudo tianjinensis. Biology 2025, 14, 1250. https://doi.org/10.3390/biology14091250
Yin J, Liu Z, Yu Y, Wang A, Huang Z, Tang L, Zhao F, Lin G. Comparative Study of Hirudins and Encoding Genes in Hirudo nipponia and Hirudo tianjinensis. Biology. 2025; 14(9):1250. https://doi.org/10.3390/biology14091250
Chicago/Turabian StyleYin, Jingjing, Zichao Liu, Yunfei Yu, Anping Wang, Zuhao Huang, Lizhou Tang, Fang Zhao, and Gonghua Lin. 2025. "Comparative Study of Hirudins and Encoding Genes in Hirudo nipponia and Hirudo tianjinensis" Biology 14, no. 9: 1250. https://doi.org/10.3390/biology14091250
APA StyleYin, J., Liu, Z., Yu, Y., Wang, A., Huang, Z., Tang, L., Zhao, F., & Lin, G. (2025). Comparative Study of Hirudins and Encoding Genes in Hirudo nipponia and Hirudo tianjinensis. Biology, 14(9), 1250. https://doi.org/10.3390/biology14091250






