Molecular and Functional Characterization of Neuropeptide F Receptor in Pomacea canaliculata: Roles in Feeding and Digestion and Communication with the Insulin Pathway
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Snail Collection and Husbandry
2.2. Experimental Design and Sampling
2.2.1. Spatiotemporal Expression Profiling of PcNPFR
2.2.2. dsNPFR Knockdown Treatment
2.2.3. Restoration Treatment by Injection of Truncated Form of NPF (trNPF)
2.2.4. Sampling Setup
2.3. Bioinformatics Analysis
2.4. Diet Intake and Behavioral Examination
2.5. Determination of Digestive Enzyme Activities
2.6. Total RNA Isolation
2.7. cDNA Synthesis and qRT-PCR Analysis
2.8. dsRNA and trNPF In Vitro Synthesis and In Vivo Injection
2.9. Protein Extraction and Western Blotting
2.10. Statistics
3. Result
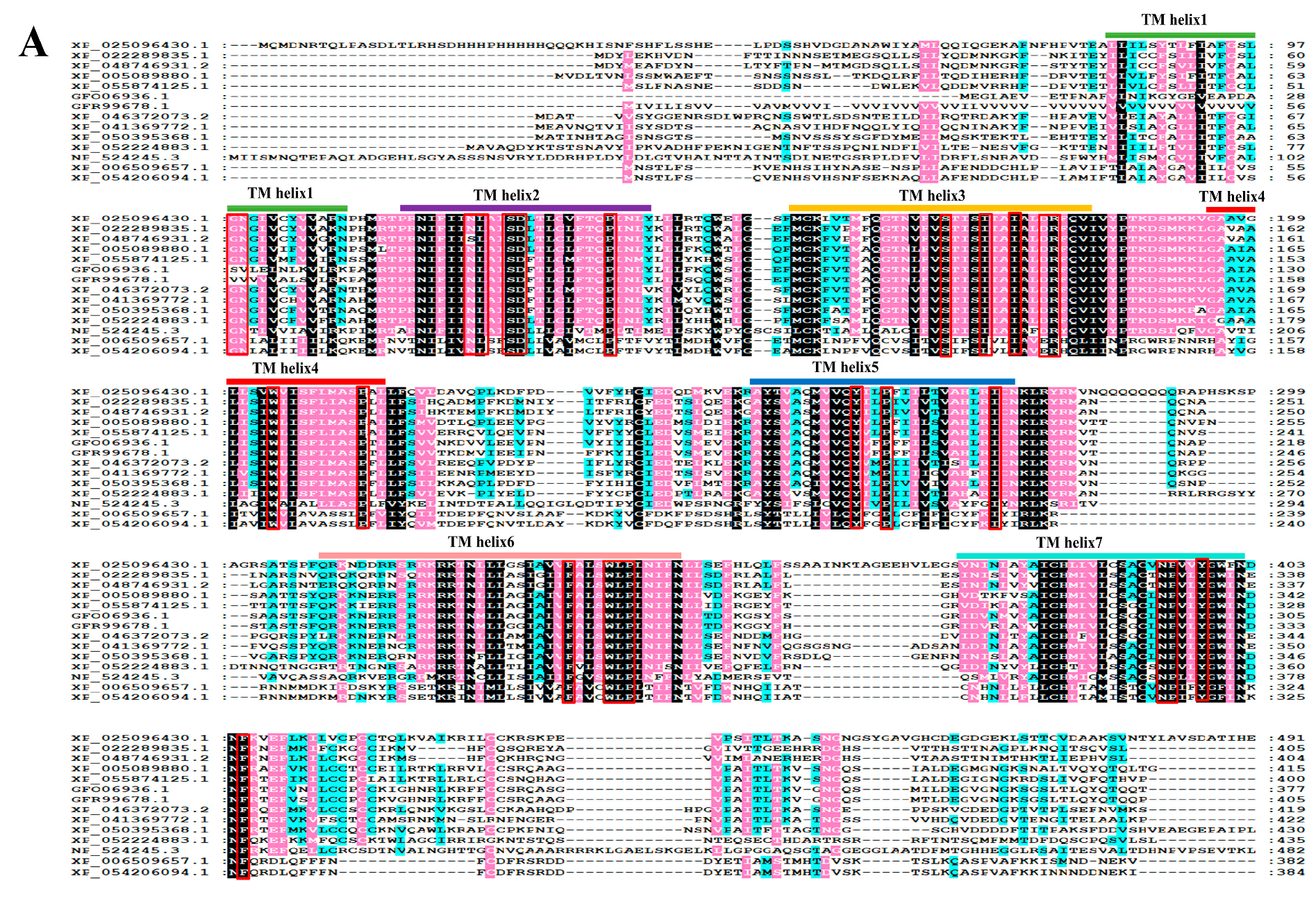
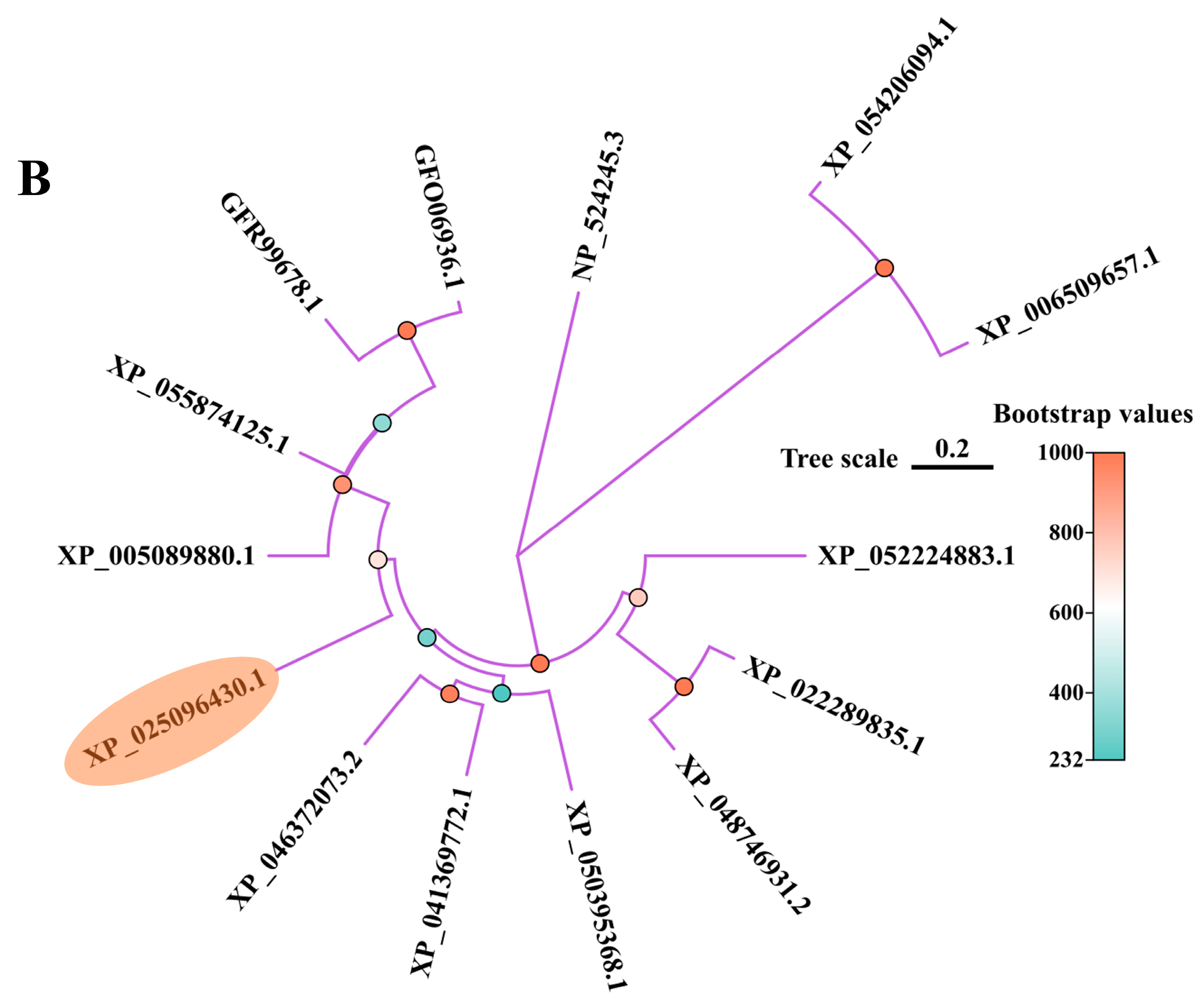
3.1. Sequence Characterization and Phylogeny Analysis for PcNPFR
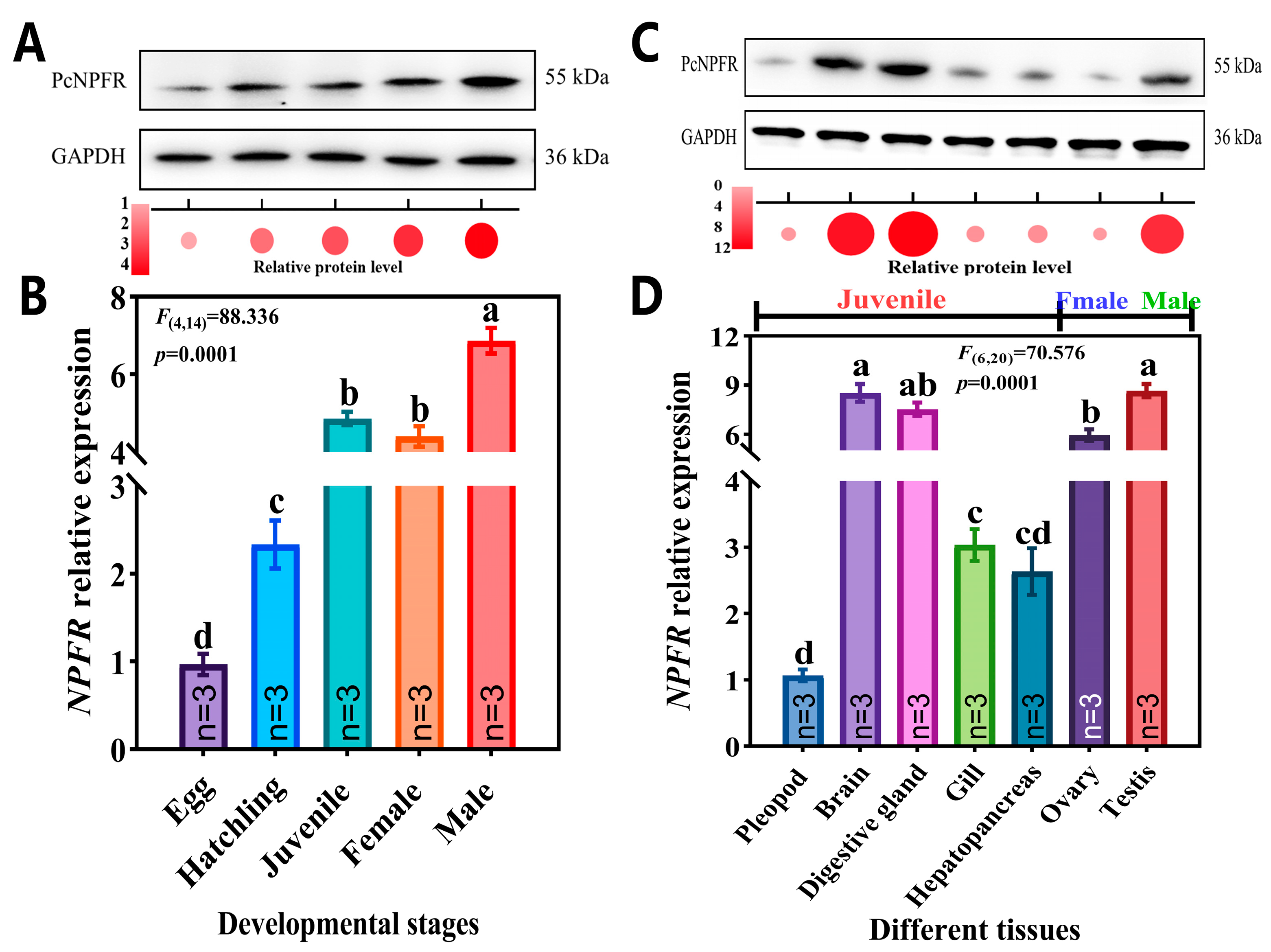
3.2. Expression Patterns of PcNPFR
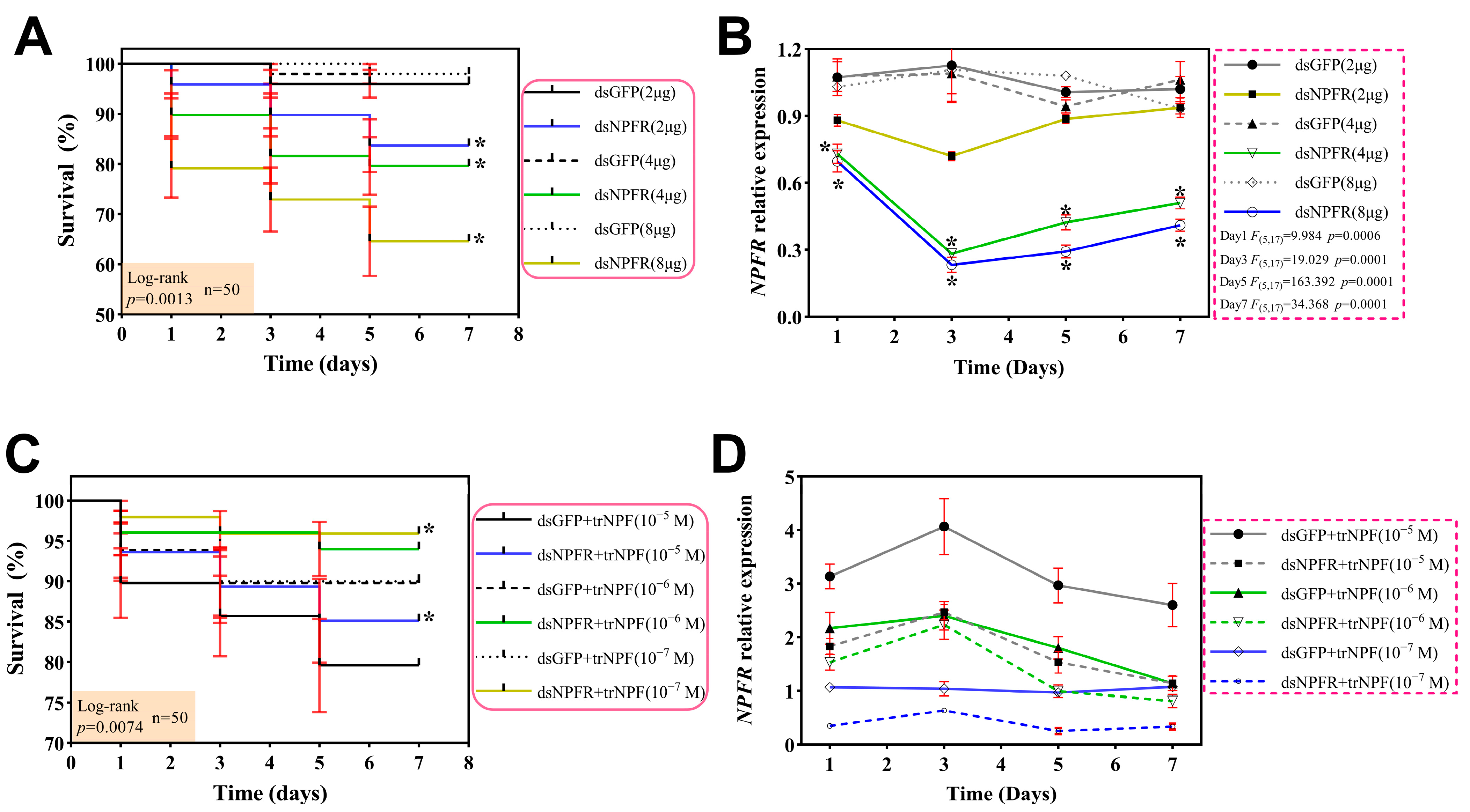
3.3. Determination of Application Concentration and Sampling Time
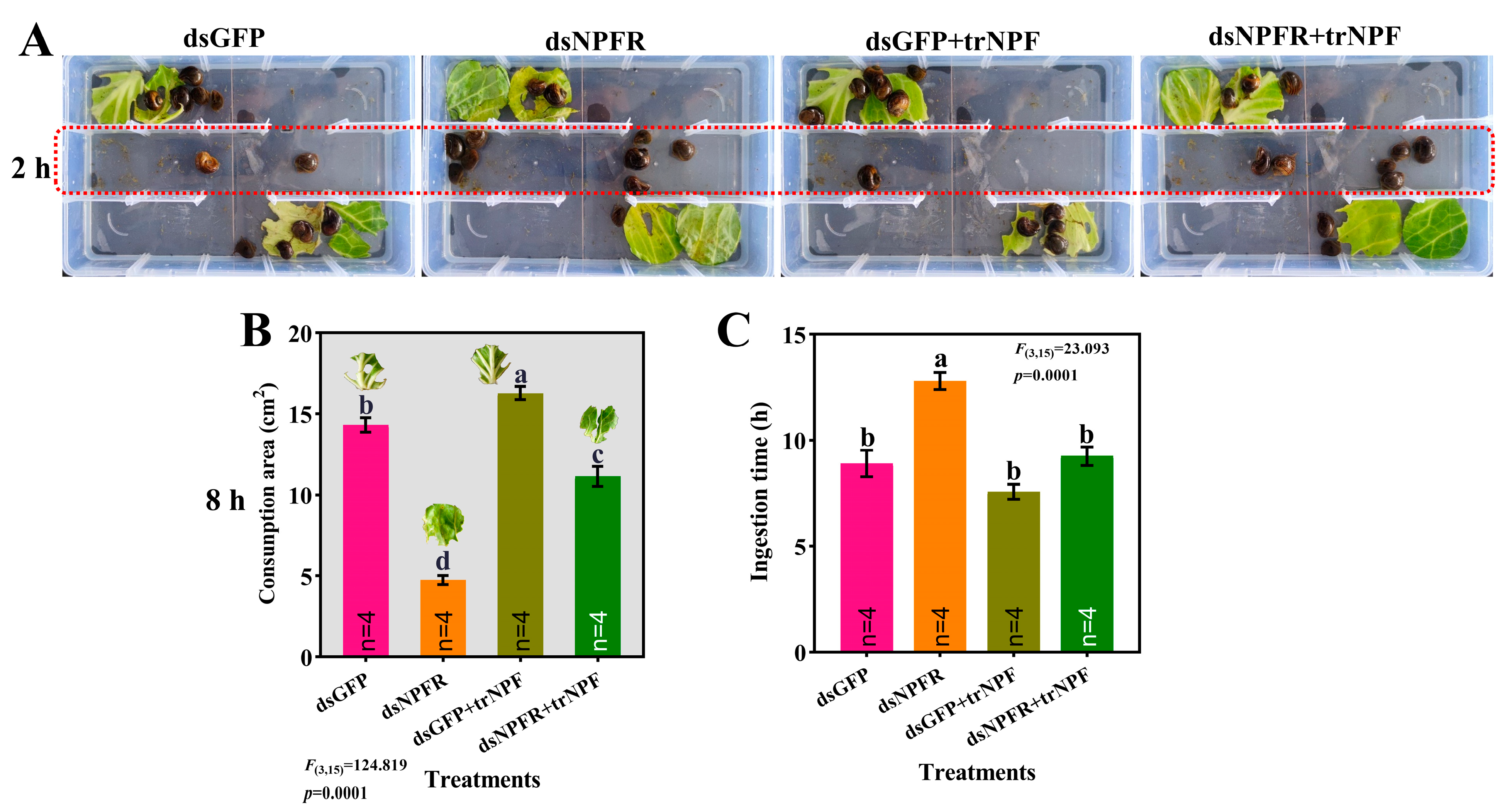
3.4. Effects of dsNPFR and trNPF Treatments on Feeding Activity
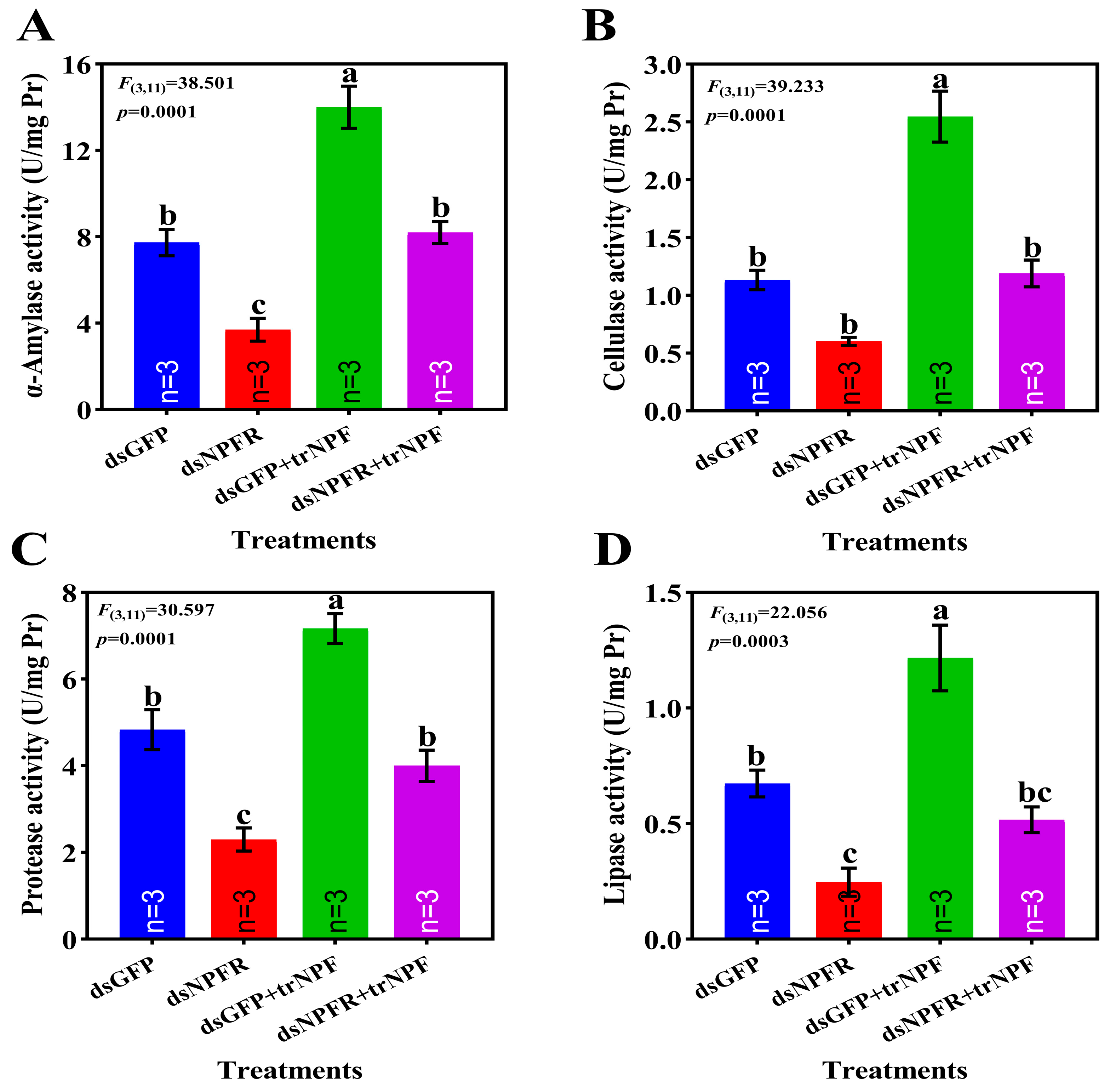
3.5. Alterations in Digestive System Post NPFR Silencing/trNPF Injection
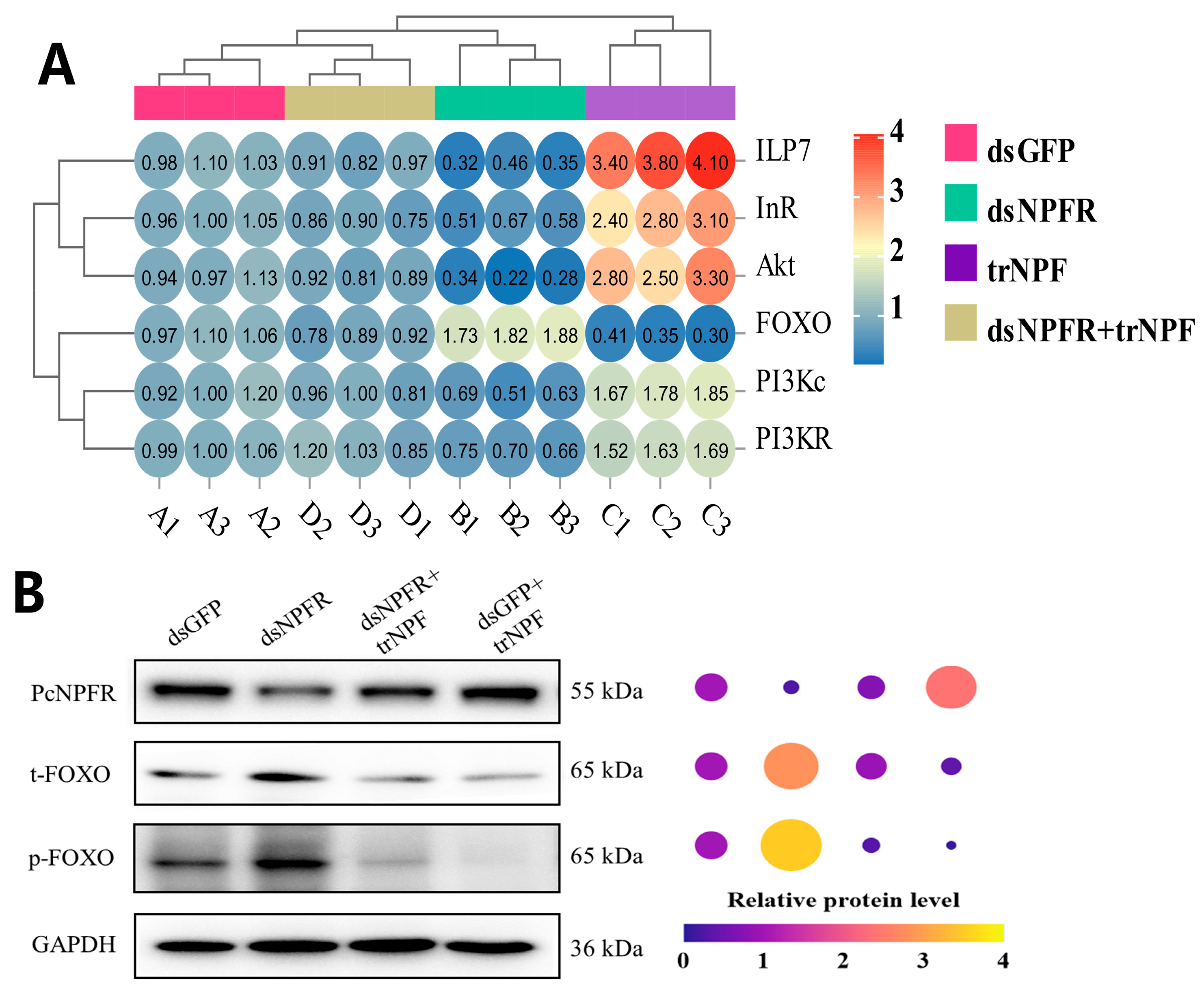
3.6. NPF–NPFR Functioning by Communication with the ISP Pathway
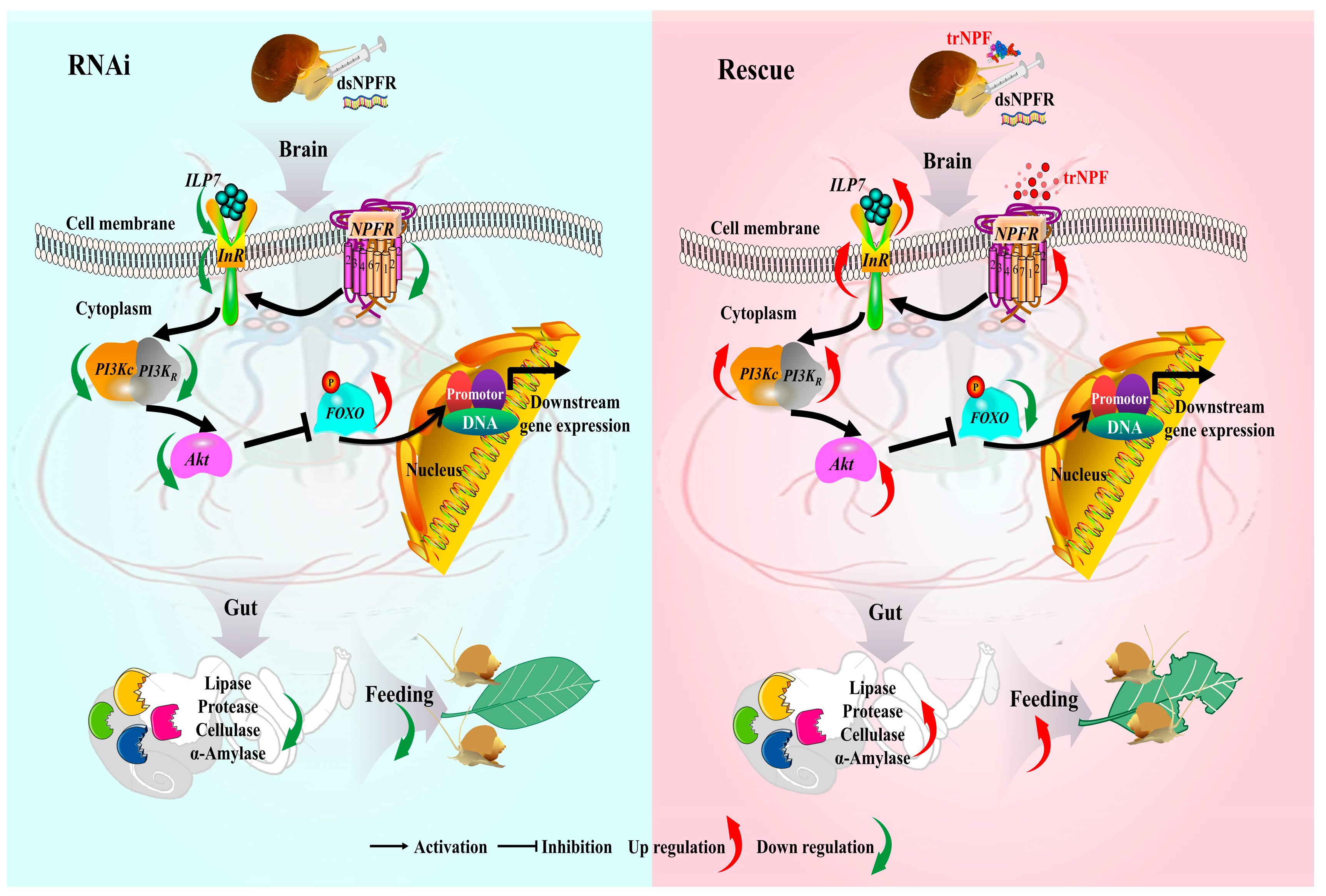
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marciniak, P.; Urbański, A.; Lubawy, J.; Szymczak, M.; Pacholska-Bogalska, J.; Chowański, S.; Kuczer, M.; Rosiński, G. Short neuropeptide F signaling regulates functioning of male reproductive system in Tenebrio molitor beetle. J. Comp. Physiol. B 2020, 190, 521–534. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Shi, J.; Cui, H.; Wang, C.Z.; Zhao, Z. Effects of NPF on larval taste responses and feeding behaviors in Ostrinia furnacalis. J. Insect Physiol. 2021, 133, 104276. [Google Scholar] [CrossRef]
- Liu, N.; Wang, Y.; Li, T.; Feng, X. G-protein coupled receptors (GPCRs): Signaling pathways, characterization, and functions in insect physiology and toxicology. Int. J. Mol. Sci. 2021, 22, 5260. [Google Scholar] [CrossRef] [PubMed]
- Nagata, S.; Neuropeptide, F. Handbook of Hormones; Academic Press: Cambridge, MA, USA, 2016; pp. 355–356. [Google Scholar]
- Fadda, M.; Hasakiogullari, I.; Temmerman, L.; Beets, I.; Zels, S.; Schoofs, L. Regulation of feeding and metabolism by neuropeptide F and short neuropeptide F in invertebrates. Front. Endocrinol. 2019, 10, 64. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Li, M.; Shen, P. A G-protein-coupled neuropeptide Y-like receptor suppresses behavioral and sensory response to multiple stressful stimuli in Drosophila. J. Neurosci. 2010, 30, 2504–2512. [Google Scholar] [CrossRef]
- He, C.; Cong, X.; Zhang, R.; Wu, D.; An, C.; Zhao, Z. Regulation of circadian locomotor rhythm by neuropeptide Y-like system in Drosophila melanogaster. Insect Mol. Biol. 2013, 22, 376–388. [Google Scholar] [CrossRef]
- Chung, B.Y.; Ro, J.; Hutter, S.A.; Miller, K.M.; Guduguntla, L.S.; Kondo, S.; Pletcher, S.D. Drosophila neuropeptide F signaling independently regulates feeding and sleep-wake behavior. Cell Rep. 2017, 19, 2441–2450. [Google Scholar] [CrossRef]
- Shen, C.; Wu, J.; Huang, Z.; He, M.; Chen, W.; Ilyas, N.; Zhang, X.; Chen, C.; Xu, C.; Xie, Y.; et al. Effects of neuropeptide F signaling on feeding, growth and development of Plutella xylostella (L.) larvae. Int. J. Biol. Macromol. 2025, 293, 139339. [Google Scholar] [CrossRef]
- Wu, Q.; Zhao, Z.; Shen, P. Regulation of aversion to noxious food by Drosophila neuropeptide Y—And insulin-like systems. Nat. Neurosci. 2005, 8, 1350–1355. [Google Scholar] [CrossRef]
- Van Wielendaele, P.; Dillen, S.; Zels, S.; Badisco, L.; Vanden Broeck, J. Regulation of feeding by Neuropeptide F in the desert locust, Schistocerca gregaria. Insect Biochem. Mol. Biol. 2013, 43, 102–114. [Google Scholar] [CrossRef]
- Yue, Z.; Zhao, Z. Feeding regulation by neuropeptide Y on Asian corn borer Ostrinia furnacalis. Arch. Insect Biochem. Physiol. 2017, 95, e21396. [Google Scholar] [CrossRef] [PubMed]
- Jing, J.; Vilim, F.S.; Horn, C.C.; Alexeeva, V.; Hatcher, N.G.; Sasaki, K.; Yashina, I.; Zhurov, Y.; Kupfermann, I.; Sweedler, J.V.; et al. From hunger to satiety: Reconfiguration of a feeding network by Aplysia neuropeptide Y. J. Neurosci. 2007, 27, 3490–3502. [Google Scholar] [CrossRef]
- Kim, K.S.; Kim, M.A.; Park, K.; Sohn, Y.C. NPF activates a specific NPF receptor and regulates food intake in Pacific abalone Haliotis discus hannai. Sci. Rep. 2021, 11, 1. [Google Scholar] [CrossRef]
- Wang, X.; Miao, J.; Liu, P.; Pan, L. Role of neuropeptide F in regulating filter feeding of Manila clam, Ruditapes philippinarum. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2017, 205, 30–38. [Google Scholar] [CrossRef]
- De Jong-Brink, M.; Ter Maat, A.; Tensen, C.P. NPY in invertebrates: Molecular answers to altered functions during evolution. Peptides 2001, 22, 309–315. [Google Scholar] [CrossRef]
- Nässel, D.R.; Kubrak, O.I.; Liu, Y.; Luo, J.; Lushchak, O.V. Factors that regulate insulin producing cells and their output in Drosophila. Front. Physiol. 2013, 4, 252. [Google Scholar] [CrossRef]
- Lin, X.; Yu, N.; Smagghe, G. Insulin receptor regulates food intake through sulfakinin signaling in the red flour beetle, Tribolium castaneum. Peptides 2016, 80, 89–95. [Google Scholar] [CrossRef]
- Zhao, J.; Song, Y.; Jiang, X.; He, L.; Wei, L.; Zhao, Z. Synergism of Feeding and Digestion Regulated by the Neuropeptide F System in Ostrinia furnacalis Larvae. Cells 2023, 12, 194. [Google Scholar] [CrossRef]
- Kannangara, J.R.; Henstridge, M.A.; Parsons, L.M.; Kondo, S.; Mirth, C.K.; Warr, C.G. A new role for neuropeptide F signaling in controlling developmental timing and body size in Drosophila melanogaster. Genetics 2020, 216, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Lingo, P.R.; Zhao, Z.; Shen, P. Co-regulation of cold resistant food acquisition by insulin- and neuropeptide Y-like systems in Drosophila melanogaster. Neuroscience 2007, 148, 371–374. [Google Scholar] [CrossRef] [PubMed]
- Global Invasive Species Database. Species Profile: Pomacea Canaliculata. Available online: http://www.iucngisd.org/gisd/species.php?sc=135 (accessed on 14 July 2025).
- Yang, C.; Ma, Y.; Wang, B.; Wang, Y.; Liu, J.; Jiang, C.; Zhang, M.; Qiu, X.; Luo, L.; Chen, H. Identification and functional verification of the target protein of pedunsaponin A in the gills of Pomacea canaliculata. Pest Manag. Sci. 2022, 78, 947–954. [Google Scholar] [CrossRef]
- Yang, C.; Zhang, Y.; Zhou, Y.; Chen, H.; Lv, T.; Luo, L.; Qiu, X.; Zhang, M.; Qin, G.; Gong, G. Screening and functional verification of the target protein of pedunsaponin A in the killing of Pomacea canaliculata. Ecotoxicol. Environ. Saf. 2021, 220, 112393. [Google Scholar] [CrossRef] [PubMed]
- Qu, G.; Yao, J.; Wang, J.; Zhang, X.; Dai, J.; Yu, H.; Dai, Y.; Xing, Y. Molluscicide screening and identification of novel targets against Pomacea canaliculata. Pest Manag. Sci. 2024, 80, 4264–4272. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Wang, Y.; Liang, J.; Du, J.; Zhao, Z.W. Effects of knocking down neuropeptide F gene (npf) on the feeding, growth and reproduction of Ostrinia furnacalis (Lepidoptera: Pyralididae). Acta Entomol. Sin. 2021, 64, 1080–1091. [Google Scholar]
- Basit, A.; Haq, I.U.; Hyder, M.; Humza, M.; Younas, M.; Akhtar, M.R.; Ghafar, M.A.; Liu, T.-X.; Hou, Y. Microbial Symbiosis in Lepidoptera: Analyzing the Gut Microbiota for Sustainable Pest Management. Biology 2025, 14, 937. [Google Scholar] [CrossRef]
- Mamta, B.; Rajam, M.V. RNAi technology: A new platform for crop pest control. Physiol. Mol. Biol. Plants 2017, 23, 487–501. [Google Scholar] [CrossRef]
- Liu, G. Physiological Responses, Screening and Functional Studies of Differentially Expressed Genes of Pomacea canaliculata Under Low Temperature Stress. Master’s Thesis, Anhui Agricultural University, Hefei, China, 2019. [Google Scholar]
- Matsukura, K.; Okuda, M.; Cazzaniga, N.J.; Wada, T. Genetic exchange between two freshwater apple snails, Pomacea canaliculata and Pomacea maculata invading East and Southeast Asia. Biol. Invasions 2013, 15, 2039–2048. [Google Scholar] [CrossRef]
- Aizaki, K.; Yusa, Y. Field observations of the alarm response to crushed conspecifics in the freshwater snail Pomacea canaliculata: Effects of habitat, vegetation, and body size. J. Ethol. 2008, 27, 175–180. [Google Scholar] [CrossRef]
- Zhang, C.; Guo, J.; Saveanu, L.; Martín, P.R.; Shi, Z.; Zhang, J. Invasiveness of Pomacea canaliculata: The differences in life history traits of snail populations from invaded and native areas. Agronomy 2023, 13, 1259. [Google Scholar] [CrossRef]
- Kijprayoon, S.; Tolieng, V.; Petsom, A.; Chaicharoenpong, C. Molluscicidal activity of Camellia oleifera seed meal. Sci. Asia 2014, 40, 393–399. [Google Scholar] [CrossRef]
- Giraud-Billoud, M.; Castro-Vazquez, A. Aging and retinoid X receptor agonists on masculinization of female Pomacea canaliculata, with a critical appraisal of imposex evaluation in the Ampullariidae. Ecotoxicol. Environ. Saf. 2019, 169, 573–582. [Google Scholar] [CrossRef]
- Marciniak, P.; Urbański, A.; Kudlewska, M.; Szymczak, M.; Rosiński, G. Peptide hormones regulate the physiological functions of reproductive organs in Tenebrio molitor males. Peptides 2017, 98, 35–42. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed]
- Getman-Pickering, Z.L.; Campbell, A.; Aflitto, N.; Grele, A.; Davis, J.K.; Ugine, T.A.; Ramula, S. LeafByte: A mobile application that measures leaf area and herbivory quickly and accurately. Methods Ecol. Evol. 2020, 11, 215–221. [Google Scholar] [CrossRef]
- Dai, L.; Qian, X.; Nan, X.; Zhang, Y. Effect of cardiac glycosides from Nerium indicum on feeding rate, digestive enzymes activity and ultrastructural alterations of hepatopancreas in Pomacea canaliculata. Environ. Toxicol. Pharmacol. 2014, 37, 220–227. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Cadierno, M.P.; Dreon, M.S.; Heras, H. Validation by qPCR of Reference Genes for Reproductive Studies in the Invasive Apple Snail Pomacea canaliculata. Malacologia 2018, 62, 163–170. [Google Scholar] [CrossRef]
- Zhao, J.; Yan, S.; Li, M.; Sun, L.; Dong, M.; Yin, M.; Shen, J.; Zhao, Z. NPFR regulates the synthesis and metabolism of lipids and glycogen via AMPK: Novel targets for efficient corn borer management. Int. J. Biol. Macromol. 2023, 247, 125816. [Google Scholar] [CrossRef]
- Tang, Q.Y.; Zhang, C.X. Data Processing System (DPS) software with experimental design, statistical analysis and data mining developed for use in entomological research. Insect Sci. 2013, 20, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chang, L.; Sun, Z.; Zhang, Y.; Yao, L. Analysis of earthworm Eisenia fetida proteomes during cadmium exposure: An ecotoxicoproteomics approach. Proteomics 2010, 7, 4476–4490. [Google Scholar] [CrossRef]
- Hill, C.A.; Sharan, S.; Watts, V.J. Genomics, GPCRs and new targets for the control of insect pests and vectors. Curr. Opin. Insect Sci. 2018, 30, 99–106. [Google Scholar] [CrossRef]
- Jiang, Y.; Loker, E.S.; Zhang, S.M. In vivo and in vitro knockdown of FREP2 gene expression in the snail Biomphalaria glabrata using RNA interference. Dev. Comp. Immunol. 2006, 30, 855–866. [Google Scholar] [CrossRef] [PubMed]
- Xiu, L.; Wang, X.; Cheng, S.; Liu, W.; Wang, L.; Li, J.; Zhang, J.; Xuan, Y.; Hu, W. Evaluating the pathogenic and immunological effects of ds-GFP as a control in in vivo RNA interference studies of Schistosoma japonicum. Parasite 2025, 32, 16. [Google Scholar] [CrossRef]
- Song, C.P. Molecular Cloning, Expression, Identification, and Preliminary Functional Study of Neuropeptide F and Its Receptor in Sepiella maindroni. Master’s Thesis, Zhejiang Ocean University, Zhoushan, China, 2021. [Google Scholar]
- Feng, G.; Reale, V.; Chatwin, H.; Kennedy, K.; Venard, R.; Ericsson, C.; Yu, K.; Evans, P.D.; Hall, L.M. Functional characterization of a neuropeptide F-like receptor from Drosophila melanogaster. Eur. J. Neurosci. 2003, 18, 227–238. [Google Scholar] [CrossRef]
- Deng, X.; Yang, H.; He, X.; Liao, Y.; Zheng, C.; Zhou, Q.; Zhu, C.; Zhang, G.; Gao, J.; Zhou, N. Activation of Bombyx neuropeptide G protein-coupled receptor A4 via a Gαi-dependent signaling pathway by direct interaction with neuropeptide F from silkworm, Bombyx mori. Insect Biochem. Mol. Biol. 2014, 45, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Du, J. Effects of NPY on Feeding and Related Gene Expression in Grass Carp. Master’s Thesis, Jinan University, Jinan, China, 2012. [Google Scholar]
- Yue, Z.; Liu, X.; Zhou, Z.; Hou, G.; Hua, J.; Zhao, Z. Development of a novel-type transgenic cotton plant for control of cotton bollworm. Plant Biotechnol. J. 2016, 14, 1747–1755. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Wang, W.; Deng, M.; Xiao, T.; Ma, W.; Huang, X.; Lu, K. Characterization of neuropeptides from Spodoptera litura and functional analysis of NPF in diet intake. J. Agric. Food Chem. 2024, 72, 10304–10313. [Google Scholar] [CrossRef]
- Luo, M.; Zhang, J.; Hu, J.; Zhao, B. Comparative Study on the Digestive Enzyme Activities between Golden Apple Snails (Pomacea canaliculata) and Local Field Snails (Cipangopaludina chinensis). Acta Ecol. Sin. 2015, 35, 3580–3587. [Google Scholar] [CrossRef][Green Version]
- Weidlich, S.; Hoffmann, K.H.; Woodring, J. Secretion of lipases in the digestive tract of the cricket Gryllus bimaculatus. Arch. Insect Biochem. Physiol. 2015, 90, 209–217. [Google Scholar] [CrossRef]
- Yu, Z.; Shi, J.; Jiang, X.; Song, Y.; Du, J.; Zhao, Z. Neuropeptide F regulates feeding via the juvenile hormone pathway in Ostrinia furnacalis larvae. Pest Manag. Sci. 2022, 79, 1193–1203. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Shi, J.; Yang, H.; Zhao, Z. The cholinergic pathway transmits signals of neuropeptide F to regulate feeding of Ostrinia furnacalis larvae. Pest Manag. Sci. 2023, 79, 3593–3601. [Google Scholar] [CrossRef] [PubMed]
- Yoshinari, Y.; Kosakamoto, H.; Kamiyama, T.; Hoshino, R.; Matsuoka, R.; Kondo, S.; Tanimoto, H.; Nakamura, A.; Obata, F.; Niwa, R. The sugar-responsive enteroendocrine neuropeptide F regulates lipid metabolism through glucagon-like and insulin-like hormones in Drosophila melanogaster. Nat. Commun. 2021, 12, 4818. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gu, H.; Teng, H.; Zhang, T.; Yuan, Y. Molecular and Functional Characterization of Neuropeptide F Receptor in Pomacea canaliculata: Roles in Feeding and Digestion and Communication with the Insulin Pathway. Biology 2025, 14, 1241. https://doi.org/10.3390/biology14091241
Gu H, Teng H, Zhang T, Yuan Y. Molecular and Functional Characterization of Neuropeptide F Receptor in Pomacea canaliculata: Roles in Feeding and Digestion and Communication with the Insulin Pathway. Biology. 2025; 14(9):1241. https://doi.org/10.3390/biology14091241
Chicago/Turabian StyleGu, Haotian, Haiyuan Teng, Tianshu Zhang, and Yongda Yuan. 2025. "Molecular and Functional Characterization of Neuropeptide F Receptor in Pomacea canaliculata: Roles in Feeding and Digestion and Communication with the Insulin Pathway" Biology 14, no. 9: 1241. https://doi.org/10.3390/biology14091241
APA StyleGu, H., Teng, H., Zhang, T., & Yuan, Y. (2025). Molecular and Functional Characterization of Neuropeptide F Receptor in Pomacea canaliculata: Roles in Feeding and Digestion and Communication with the Insulin Pathway. Biology, 14(9), 1241. https://doi.org/10.3390/biology14091241




