Dysregulation of Protein Kinase CaMKI Leads to Autism-Related Phenotypes in Synaptic Connectivity, Sleep, Sociality, and Aging-Dependent Degeneration in Drosophila
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Fly Stocks
2.2. CaMK Homologue Visualization
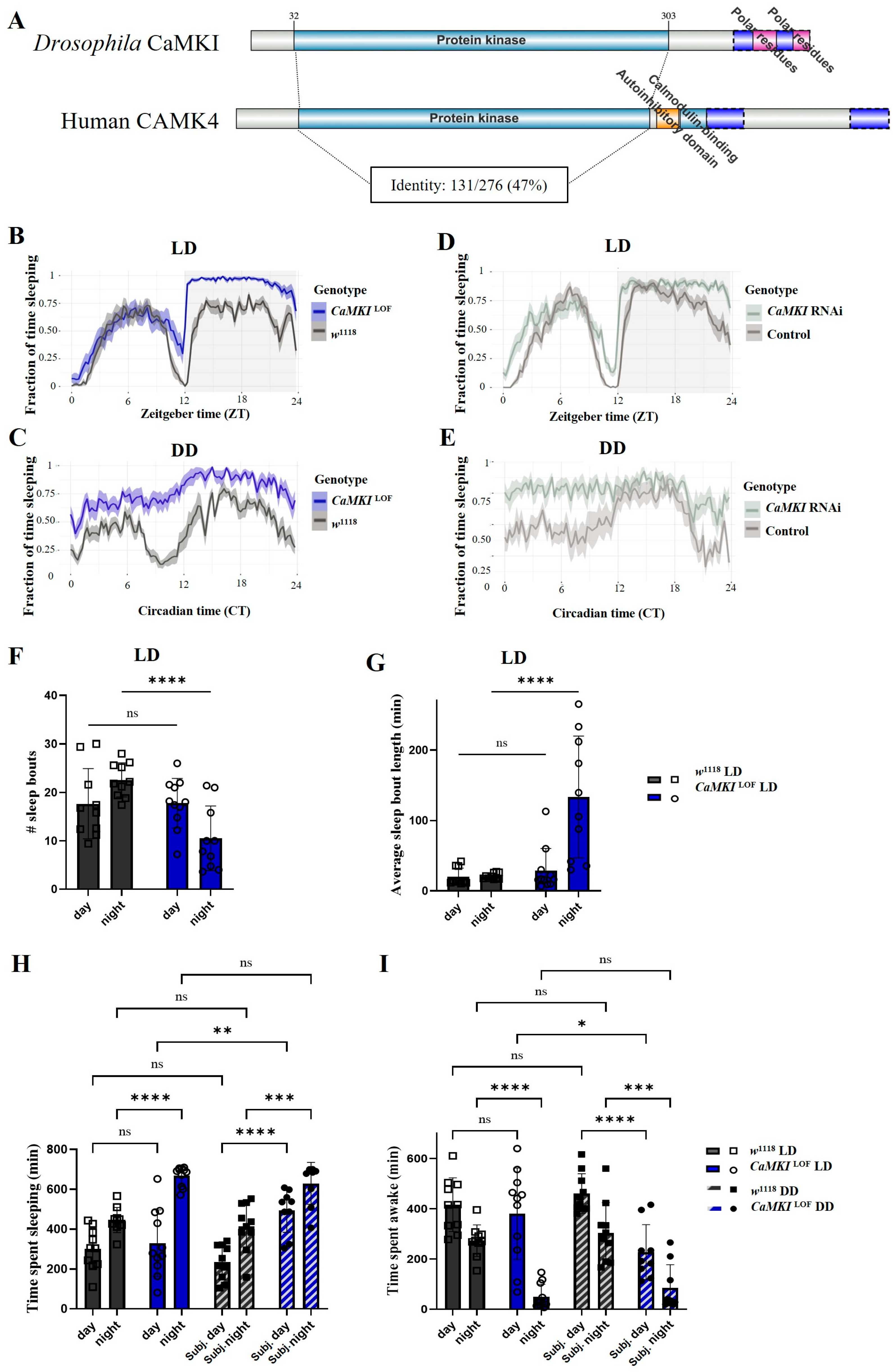
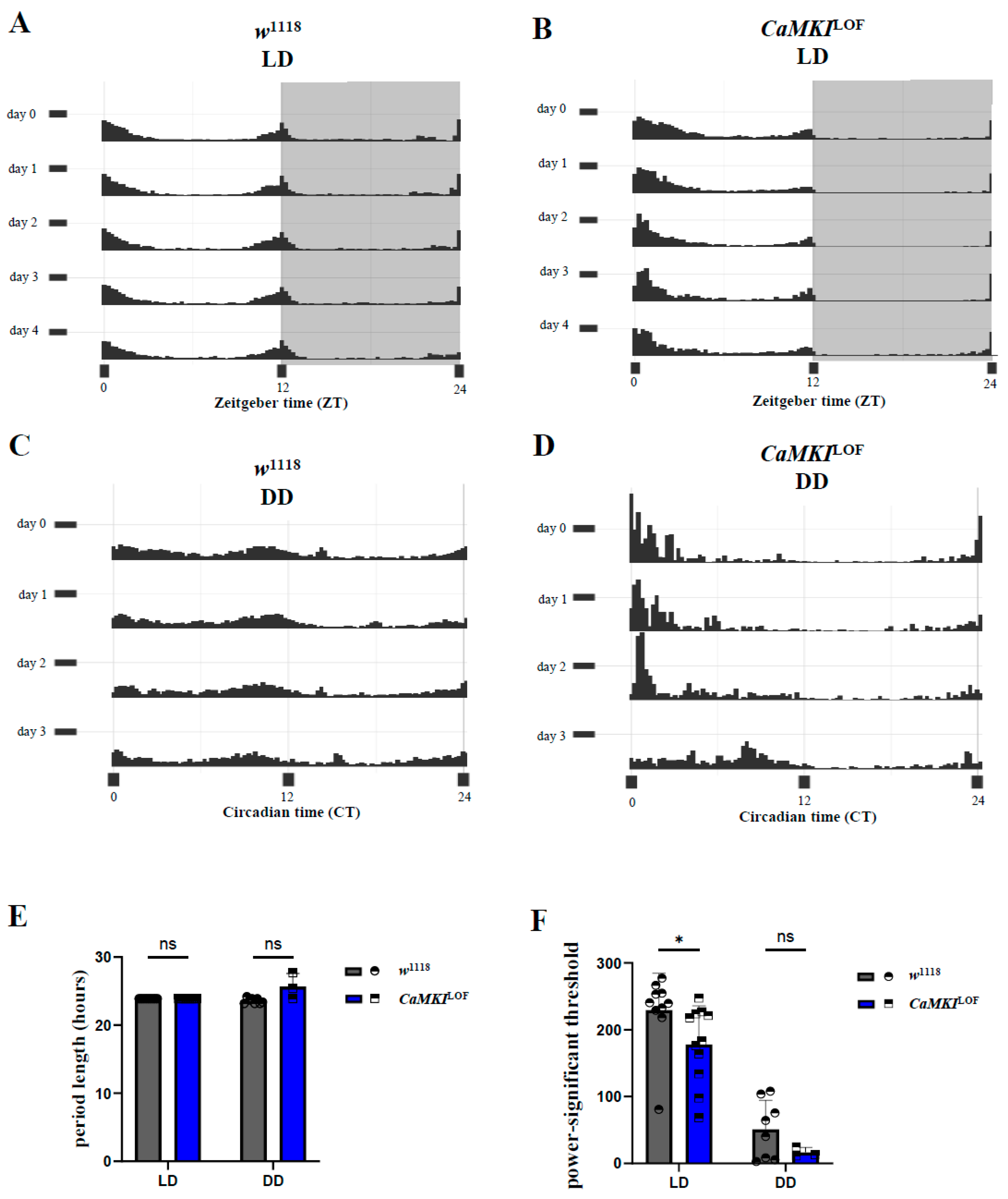
2.3. Sleep and Circadian Rhythm Experiments
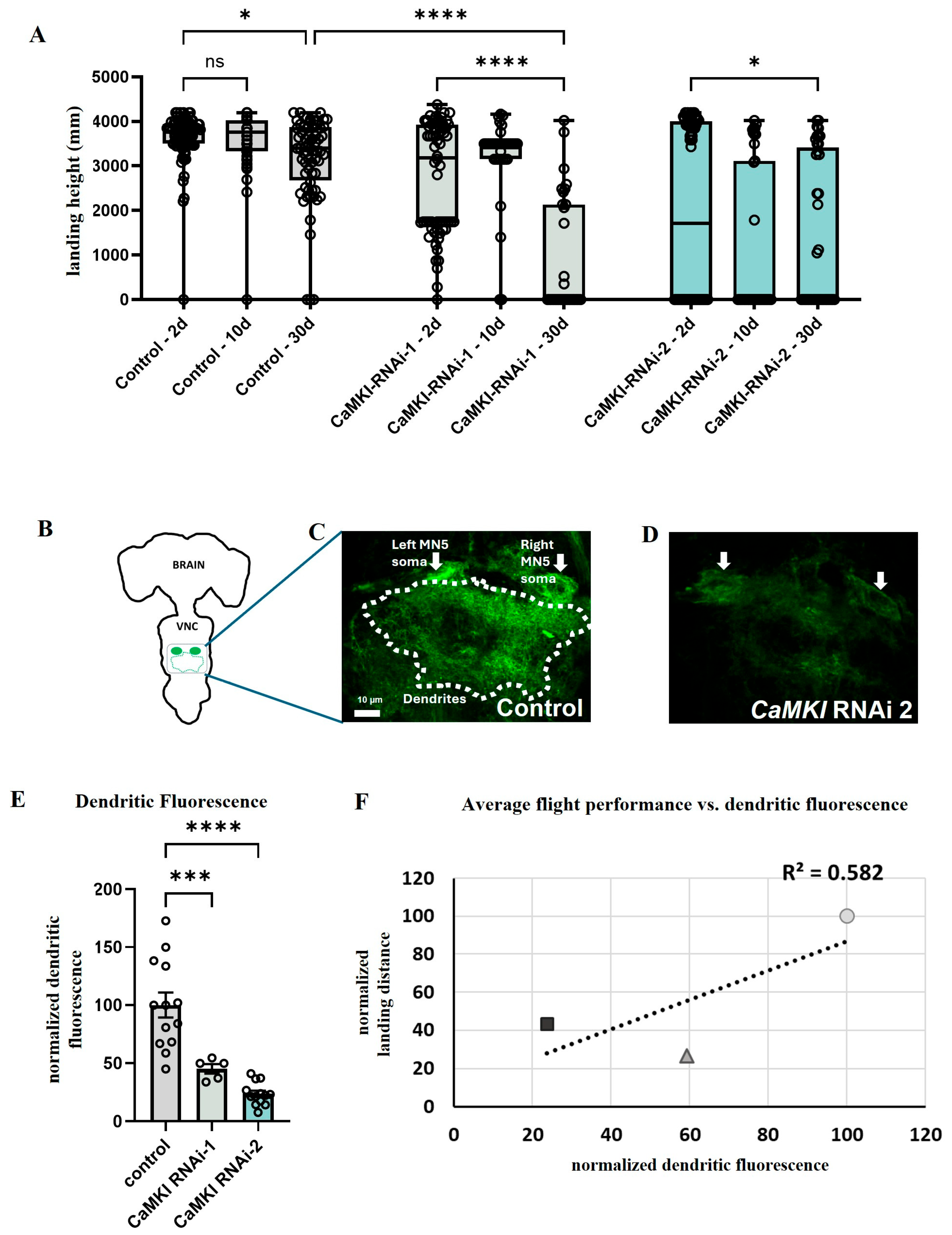
2.4. Anatomical Approaches and Flight Performance
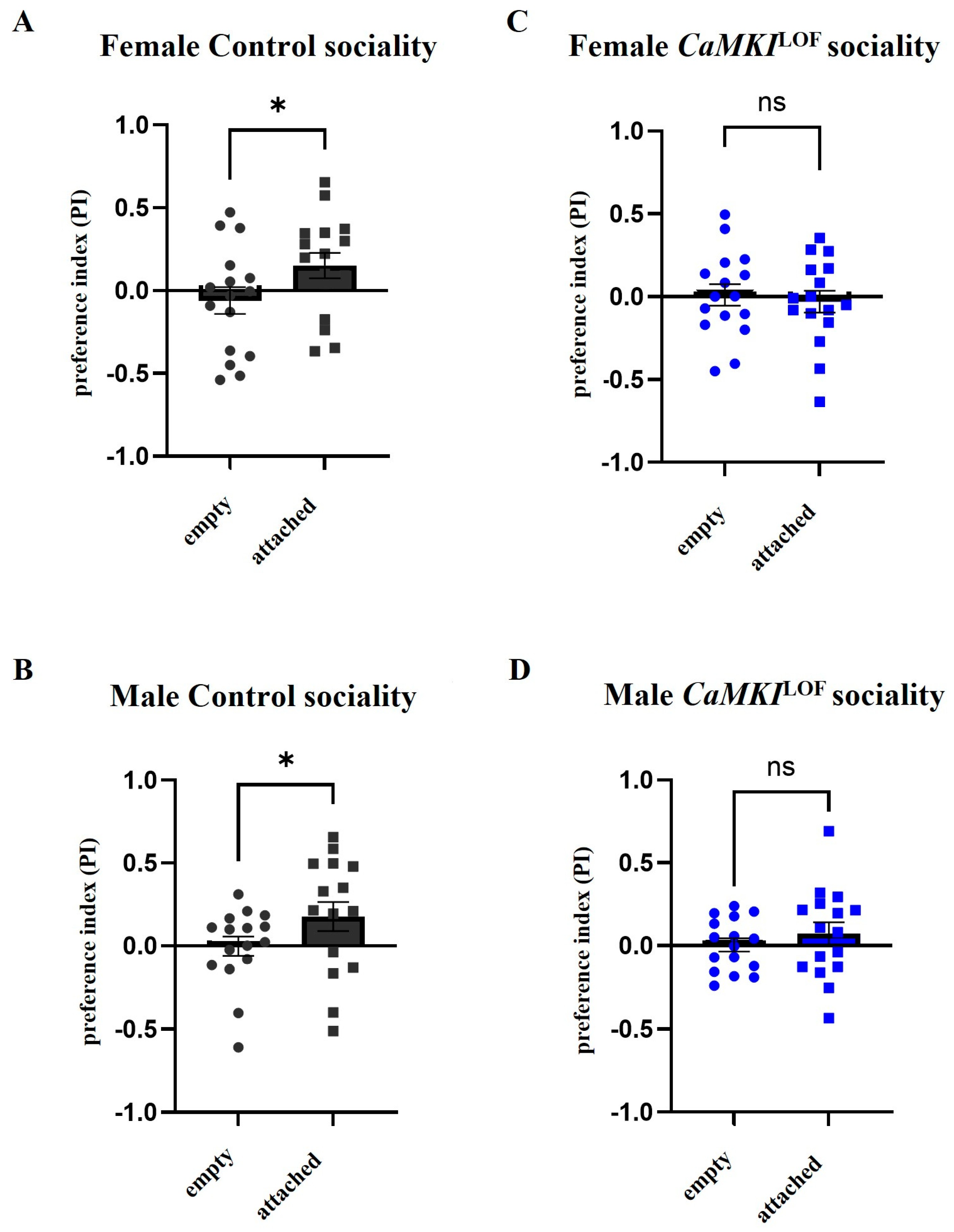
2.5. Social Assay
2.6. Proteomics Sample Preparation
2.7. Proteomics Mass Spectrometry Acquisition and Data Analysis
2.8. Statistical Analysis
3. Results
3.1. Loss of CaMKI Increases Sleep Duration and Alters Sleep Architecture in Drosophila
3.2. CaMKI Dysregulation Disrupts Circadian Locomotor Rhythms
3.3. Social Behavior Is Affected in Adult CaMKILOF Mutant Flies
3.4. CaMKI Dysregulation Enhances Behavioral Decline and Neurodegeneration
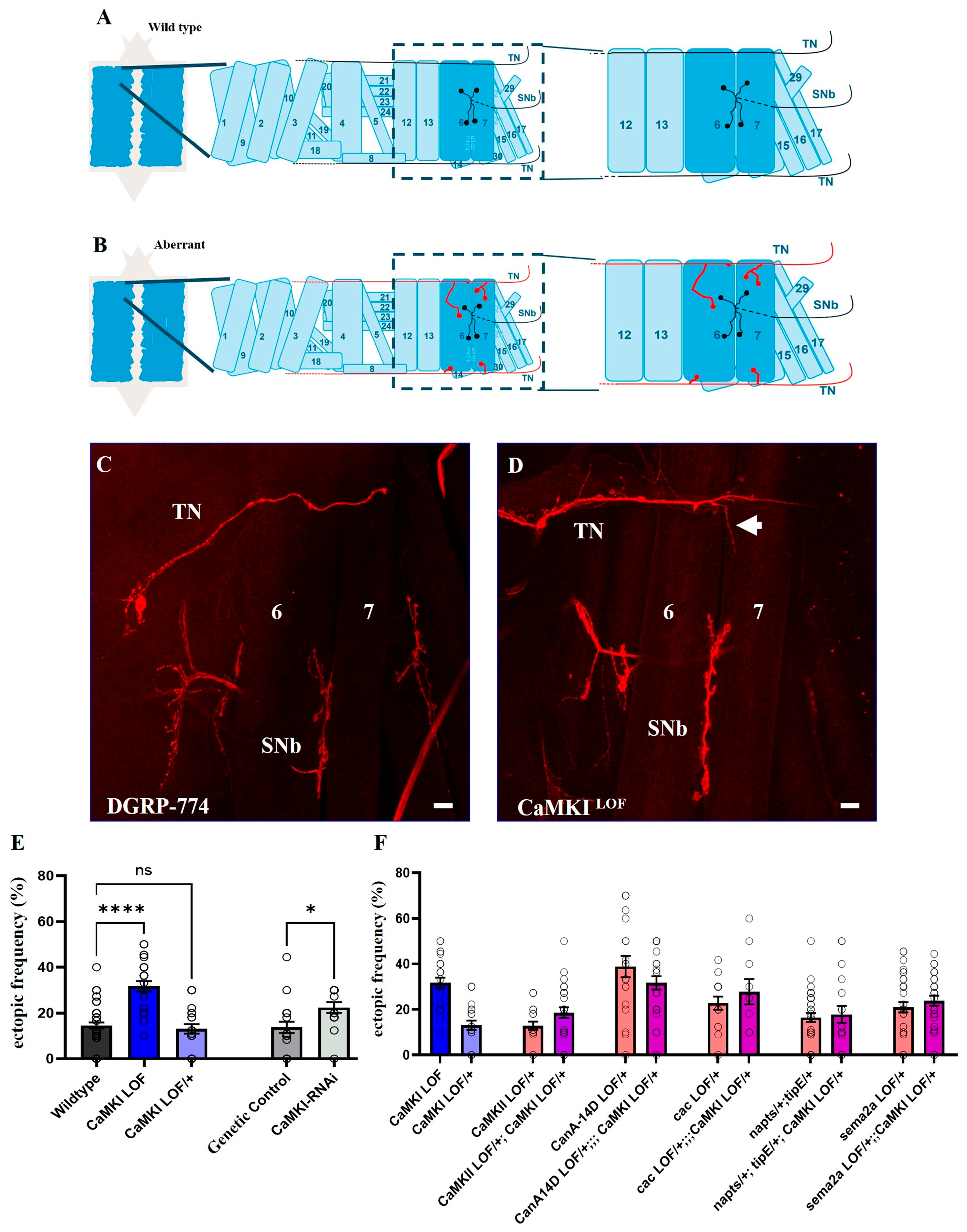
3.5. Loss of CaMKI Affects Synaptic Development Increasing Ectopic Synapses
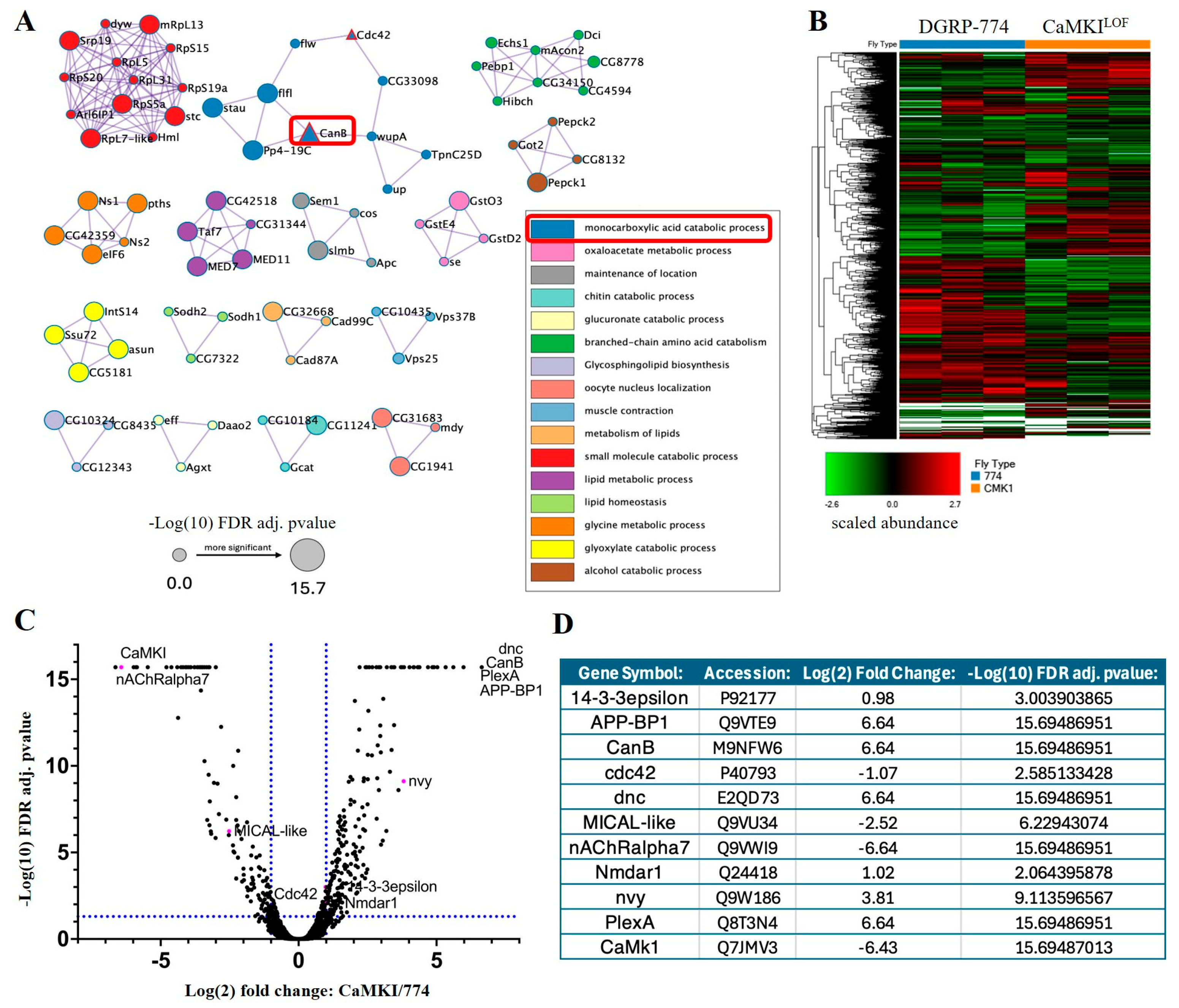
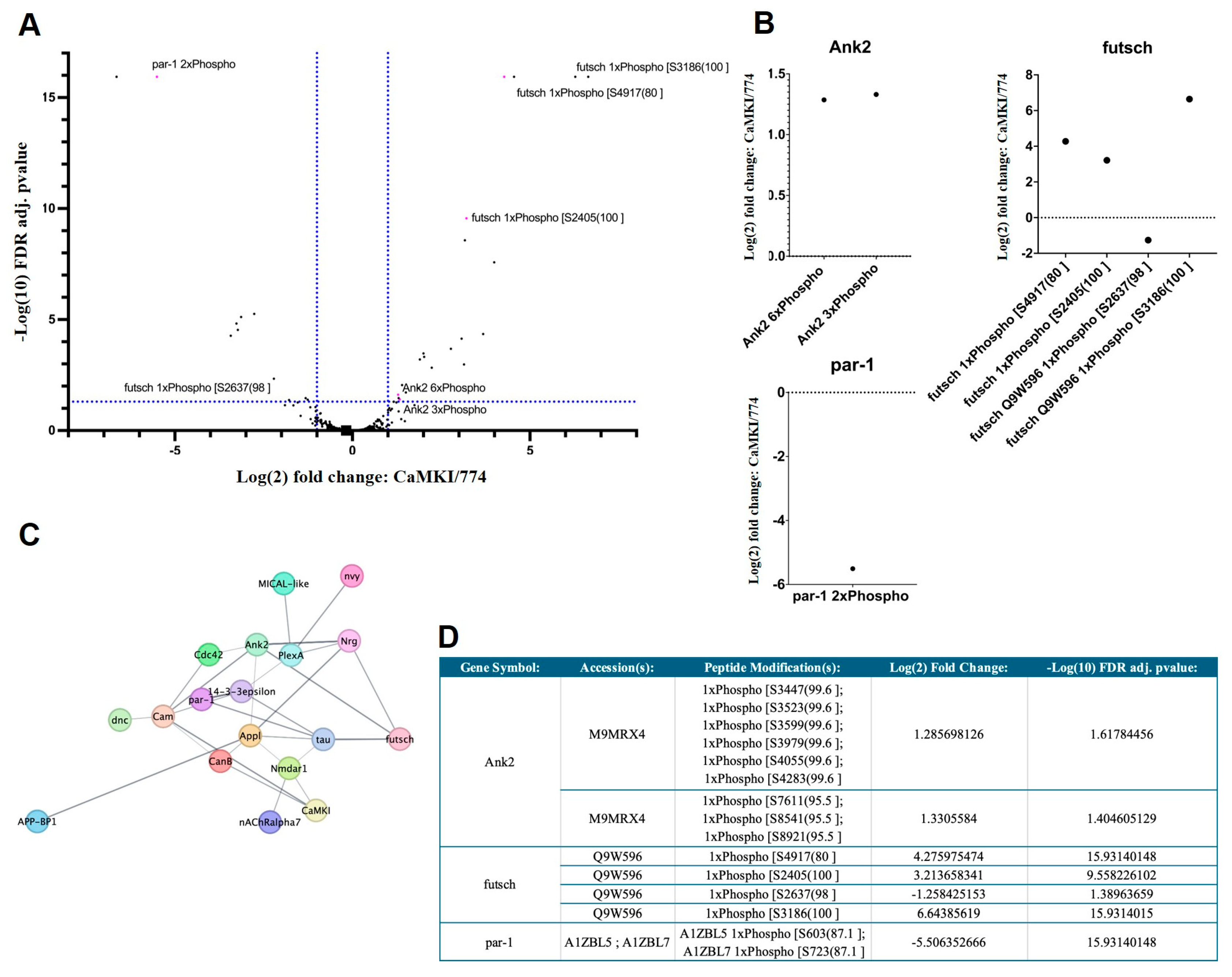
3.6. Proteomic Analyses Reveal Proteins Dysregulated by CaMKI Loss
4. Discussion
4.1. Drosophila as a Model to Study Autism-Associated Phenotypes
4.2. Link Between Developmental Deficits and Aging-Dependent Degeneration
4.3. Molecular Pathways Involving CaMKI During Neurodevelopment and Degeneration
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hutsler, J.J.; Zhang, H. Increased dendritic spine densities on cortical projection neurons in autism spectrum disorders. Brain Res. 2010, 1309, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Tang, G.; Gudsnuk, K.; Kuo, S.H.; Cotrina, M.L.; Rosoklija, G.; Sosunov, A.; Sonders, M.S.; Kanter, E.; Castagna, C.; Yamamoto, A.; et al. Loss of mTOR-dependent macroautophagy causes autistic-like synaptic pruning deficits. Neuron 2014, 83, 1131–1143. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Weir, R.K.; Bauman, M.D.; Jacobs, B.; Schumann, C.M. Protracted dendritic growth in the typically developing human amygdala and increased spine density in young ASD brains. J. Comp. Neurol. 2018, 526, 262–274. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zoghbi, H.Y.; Bear, M.F. Synaptic dysfunction in neurodevelopmental disorders associated with autism and intellectual disabilities. Cold Spring Harb. Perspect. Biol. 2012, 4, a009886. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bourgeron, T. A synaptic trek to autism. Curr. Opin. Neurobiol. 2009, 19, 231–234. [Google Scholar] [CrossRef] [PubMed]
- Faust, T.E.; Gunner, G.; Schafer, D.P. Mechanisms governing activity-dependent synaptic pruning in the developing mammalian CNS. Nat. Rev. Neurosci. 2021, 22, 657–673. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Penzes, P.; Cahill, M.E.; Jones, K.A.; VanLeeuwen, J.E.; Woolfrey, K.M. Dendritic spine pathology in neuropsychiatric disorders. Nat. Neurosci. 2011, 14, 285–293. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- James, E.J.; Gu, J.; Ramirez-Vizcarrondo, C.M.; Hasan, M.; Truszkowski, T.L.; Tan, Y.; Oupravanh, P.M.; Khakhalin, A.S.; Aizenman, C.D. Valproate-induced neurodevelopmental deficits in Xenopus laevis tadpoles. J. Neurosci. 2015, 35, 3218–3229. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Purves, D.; Lichtman, J.W. Elimination of synapses in the developing nervous system. Science 1980, 210, 153–157. [Google Scholar] [CrossRef] [PubMed]
- Rakic, P.; Bourgeois, J.P.; Eckenhoff, M.F.; Zecevic, N.; Goldman-Rakic, P.S. Concurrent overproduction of synapses in diverse regions of the primate cerebral cortex. Science 1986, 232, 232–235. [Google Scholar] [CrossRef] [PubMed]
- Cang, J.; Feldheim, D.A. Developmental mechanisms of topographic map formation and alignment. Annu. Rev. Neurosci. 2013, 36, 51–77. [Google Scholar] [CrossRef] [PubMed]
- Kano, M.; Hashimoto, K. Synapse elimination in the central nervous system. Curr. Opin. Neurobiol. 2009, 19, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Vonhoff, F.; Keshishian, H. Activity-Dependent Synaptic Refinement: New Insights from Drosophila. Front. Syst. Neurosci. 2017, 11, 23. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wiesel, T.N.; Hubel, D.H. Effects of Visual Deprivation on Morphology and Physiology of Cells in the Cats Lateral Geniculate Body. J. Neurophysiol. 1963, 26, 978–993. [Google Scholar] [CrossRef] [PubMed]
- Espinosa, J.S.; Stryker, M.P. Development and plasticity of the primary visual cortex. Neuron 2012, 75, 230–249. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Arroyo, D.A.; Feller, M.B. Spatiotemporal Features of Retinal Waves Instruct the Wiring of the Visual Circuitry. Front. Neural Circuits 2016, 10, 54. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ackman, J.B.; Burbridge, T.J.; Crair, M.C. Retinal waves coordinate patterned activity throughout the developing visual system. Nature 2012, 490, 219–225. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Carrillo, R.A.; Olsen, D.P.; Yoon, K.S.; Keshishian, H. Presynaptic activity and CaMKII modulate retrograde semaphorin signaling and synaptic refinement. Neuron 2010, 68, 32–44. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vonhoff, F.; Keshishian, H. In Vivo Calcium Signaling during Synaptic Refinement at the Drosophila Neuromuscular Junction. J. Neurosci. 2017, 37, 5511–5526. [Google Scholar] [CrossRef]
- Vonhoff, F.; Keshishian, H. Cyclic nucleotide signaling is required during synaptic refinement at the Drosophila neuromuscular junction. Dev. Neurobiol. 2017, 77, 39–60. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nicol, X.; Voyatzis, S.; Muzerelle, A.; Narboux-Nême, N.; Südhof, T.C.; Miles, R.; Gaspar, P. cAMP oscillations and retinal activity are permissive for ephrin signaling during the establishment of the retinotopic map. Nat. Neurosci. 2007, 10, 340–347. [Google Scholar] [CrossRef] [PubMed]
- Nicol, X.; Muzerelle, A.; Rio, J.P.; Métin, C.; Gaspar, P. Requirement of adenylate cyclase 1 for the ephrin-A5-dependent retraction of exuberant retinal axons. J. Neurosci. 2006, 26, 862–872. [Google Scholar] [CrossRef] [PubMed] [PubMed Central][Green Version]
- Stent, G.S. A physiological mechanism for Hebb’s postulate of learning. Proc. Natl. Acad. Sci. USA 1973, 70, 997–1001. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cheadle, L.; Tzeng, C.P.; Kalish, B.T.; Harmin, D.A.; Rivera, S.; Ling, E.; Nagy, M.A.; Hrvatin, S.; Hu, L.; Stroud, H.; et al. Visual Experience-Dependent Expression of Fn14 Is Required for Retinogeniculate Refinement. Neuron 2018, 99, 525–539.e10. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yap, E.L.; Greenberg, M.E. Activity-Regulated Transcription: Bridging the Gap between Neural Activity and Behavior. Neuron 2018, 100, 330–348. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Paulin, M.G.; Cahill-Lane, J. Events in Early Nervous System Evolution. Top. Cogn. Sci. 2021, 13, 25–44. [Google Scholar] [CrossRef] [PubMed]
- Sousa, A.M.M.; Meyer, K.A.; Santpere, G.; Gulden, F.O.; Sestan, N. Evolution of the Human Nervous System Function, Structure, and Development. Cell 2017, 170, 226–247. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Niven, J.E.; Chittka, L. Evolving understanding of nervous system evolution. Curr. Biol. 2016, 26, R937–R941. [Google Scholar] [CrossRef] [PubMed]
- Assaf, Y.; Bouznach, A.; Zomet, O.; Marom, A.; Yovel, Y. Conservation of brain connectivity and wiring across the mammalian class. Nat. Neurosci. 2020, 23, 805–808. [Google Scholar] [CrossRef] [PubMed]
- Kozol, R.A.; Conith, A.J.; Yuiska, A.; Cree-Newman, A.; Tolentino, B.; Benesh, K.; Paz, A.; Lloyd, E.; Kowalko, J.E.; Keene, A.C.; et al. A brain-wide analysis maps structural evolution to distinct anatomical module. eLife 2023, 12, e80777. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Burkhardt, P.; Sprecher, S.G. Evolutionary origin of synapses and neurons—Bridging the gap. Bioessays 2017, 39, 1700024. [Google Scholar] [CrossRef] [PubMed]
- Bellosta, P.; Soldano, A. Dissecting the genetics of autism spectrum disorders: A Drosophila perspective. Front. Physiol. 2019, 10, 987. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mariano, V.; Achsel, T.; Bagni, C.; Kanellopoulos, A.K. Modelling Learning and Memory in Drosophila to Understand Intellectual Disabilities. Neuroscience 2020, 445, 12–30. [Google Scholar] [CrossRef] [PubMed]
- Ueoka, I.; Pham, H.T.N.; Matsumoto, K.; Yamaguchi, M. Autism Spectrum Disorder-Related Syndromes: Modeling with Drosophila and Rodents. Int. J. Mol. Sci. 2019, 20, 4071. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Coll-Tané, M.; Krebbers, A.; Castells-Nobau, A.; Zweier, C.; Schenck, A. Intellectual disability and autism spectrum disorders ‘on the fly’: Insights from. Dis. Model. Mech. 2019, 12, dmm039180. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kaiser, J.; Risteska, A.; Muller, A.G.; Sun, H.; Lei, B.; Nay, K.; Means, A.R.; Cousin, M.A.; Drewry, D.H.; Oakhill, J.S.; et al. Convergence on CaMK4: A Key Modulator of Autism-Associated Signaling Pathways in Neurons. Biol. Psychiatry 2024, 97, 439–449. [Google Scholar] [CrossRef] [PubMed]
- Alawneh, I.; Amburgey, K.; Gonorazky, H.; Gorodetsky, C. CAMK4-related Case of Hyperkinetic Movement Disorder. Mov. Disord. Clin. Pract. 2023, 10, 707–709. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zech, M.; Lam, D.D.; Weber, S.; Berutti, R.; Poláková, K.; Havránková, P.; Fečíková, A.; Strom, T.M.; Růžička, E.; Jech, R.; et al. A unique de novo gain-of-function variant in CAMK4 associated with intellectual disability and hyperkinetic movement disorder. Cold Spring Harb. Mol. Case Stud. 2018, 4, a003293. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zech, M.; Bardakjian, T.M.; Stoklosa, M.; Ploski, R.; Jech, R.; Gonzalez-Alegre, P.; Winkelmann, J. A Neurodevelopmental Disorder with Dystonia and Chorea Resulting from Clustering CAMK4 Variants. Mov. Disord. 2021, 36, 520–521. [Google Scholar] [CrossRef] [PubMed]
- Iossifov, I.; O’Roak, B.J.; Sanders, S.J.; Ronemus, M.; Krumm, N.; Levy, D.; Stessman, H.A.; Witherspoon, K.T.; Vives, L.; Patterson, K.E.; et al. The contribution of de novo coding mutations to autism spectrum disorder. Nature 2014, 515, 216–221. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhao, S.; Deanhardt, B.; Barlow, G.T.; Schleske, P.G.; Rossi, A.M.; Volkan, P.C. Chromatin-based reprogramming of a courtship regulator by concurrent pheromone perception and hormone signaling. Sci. Adv. 2020, 6, eaba6913. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sethi, S.; Lin, H.H.; Shepherd, A.K.; Volkan, P.C.; Su, C.Y.; Wang, J.W. Social Context Enhances Hormonal Modulation of Pheromone Detection in Drosophila. Curr. Biol. 2019, 29, 3887–3898.e4. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ergaz, Z.; Weinstein-Fudim, L.; Ornoy, A. Genetic and non-genetic animal models for autism spectrum disorders (ASD). Reprod. Toxicol. 2016, 64, 116–140. [Google Scholar] [CrossRef] [PubMed]
- Alexander, R.D. The Evolution of Social Behavior. Annu. Rev. Ecol. Evol. Syst. 1974, 5, 325–383. [Google Scholar] [CrossRef]
- Hamilton, W.D. The Evolution of Altruistic Behavior. Am. Nat. 1963, 97, 354–356. [Google Scholar] [CrossRef]
- Wheeler, W.M. The Social Insects: Their Origin and Evolution; K. Paul, Trench, Trubner: London, UK, 1928. [Google Scholar]
- Keene, A.C.; Duboue, E.R. The origins and evolution of sleep. J. Exp. Biol. 2018, 221, jeb159533. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ballester, P.; Richdale, A.L.; Baker, E.K.; Peiró, A.M. Sleep in autism: A biomolecular approach to aetiology and treatment. Sleep Med. Rev. 2020, 54, 101357. [Google Scholar] [CrossRef] [PubMed]
- Carmassi, C.; Palagini, L.; Caruso, D.; Masci, I.; Nobili, L.; Vita, A.; Dell’Osso, L. Systematic Review of Sleep Disturbances and Circadian Sleep Desynchronization in Autism Spectrum Disorder: Toward an Integrative Model of a Self-Reinforcing Loop. Front. Psychiatry 2019, 10, 366. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Deliens, G.; Leproult, R.; Schmitz, R.; Destrebecqz, A.; Peigneux, P. Sleep Disturbances in Autism Spectrum Disorders. Rev. J. Autism Dev. Disord. 2015, 2, 343–356. [Google Scholar] [CrossRef]
- Krakowiak, P.; Goodlin-Jones, B.; Hertz-Picciotto, I.; Croen, L.A.; Hansen, R.L. Sleep problems in children with autism spectrum disorders, developmental delays, and typical development: A population-based study. J. Sleep Res. 2008, 17, 197–206. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Martinez-Cayuelas, E.; Gavela-Pérez, T.; Rodrigo-Moreno, M.; Losada-Del Pozo, R.; Moreno-Vinues, B.; Garces, C.; Soriano-Guillén, L. Sleep Problems, Circadian Rhythms, and Their Relation to Behavioral Difficulties in Children and Adolescents with Autism Spectrum Disorder. J. Autism Dev. Disord. 2024, 54, 1712–1726. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pagni, B.A.; Walsh, M.J.M.; Ofori, E.; Chen, K.; Sullivan, G.; Alvar, J.; Monahan, L.; Guerithault, N.; Delaney, S.; Braden, B.B. Effects of age on the hippocampus and verbal memory in adults with autism spectrum disorder: Longitudinal versus cross-sectional findings. Autism Res. 2022, 15, 1810–1823. [Google Scholar] [CrossRef] [PubMed]
- Vivanti, G.; Tao, S.; Lyall, K.; Robins, D.L.; Shea, L.L. The prevalence and incidence of early-onset dementia among adults with autism spectrum disorder. Autism Res. 2021, 14, 2189–2199. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vivanti, G.; Lee, W.L.; Ventimiglia, J.; Tao, S.; Lyall, K.; Shea, L.L. Prevalence of Dementia Among US Adults with Autism Spectrum Disorder. JAMA Netw. Open 2025, 8, e2453691. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rhodus, E.K.; Barber, J.; Abner, E.L.; Duff, D.M.C.; Bardach, S.H.; Caban-Holt, A.; Lightner, D.; Rowles, G.D.; Schmitt, F.A.; Jicha, G.A. Behaviors Characteristic of Autism Spectrum Disorder in a Geriatric Cohort with Mild Cognitive Impairment or Early Dementia. Alzheimer Dis. Assoc. Disord. 2020, 34, 66–71. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Powell, P.S.; Klinger, L.G.; Klinger, M.R. Patterns of Age-Related Cognitive Differences in Adults with Autism Spectrum Disorder. J. Autism Dev. Disord. 2017, 47, 3204–3219. [Google Scholar] [CrossRef] [PubMed]
- Shukla, A.K.; Johnson, K.; Giniger, E. Common features of aging fail to occur in Drosophila raised without a bacterial microbiome. iScience 2021, 24, 102703. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Piper, M.D.W.; Partridge, L. Drosophila as a model for ageing. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 2707–2717. [Google Scholar] [CrossRef] [PubMed]
- Maski, K.P.; Jeste, S.S.; Spence, S.J. Common neurological co-morbidities in autism spectrum disorders. Curr. Opin. Pediatr. 2011, 23, 609–615. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Van Waelvelde, H.; Oostra, A.; Dewitte, G.; Van Den Broeck, C.; Jongmans, M.J. Stability of motor problems in young children with or at risk of autism spectrum disorders, ADHD, and or developmental coordination disorder. Dev. Med. Child. Neurol. 2010, 52, e174–e178. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Kim, S.H.; Shim, S.O.; Park, E.; Kim, C.; Kim, K.; Tanouye, M.A.; Yim, J. Drosophila homolog of APP-BP1 (dAPP-BP1) interacts antagonistically with APPL during Drosophila development. Cell Death Differ. 2007, 14, 103–115. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, I.; Yang, Y.; Lu, B. PAR-1 Kinase Plays an Initiator Role in a Temporally Ordered Phosphorylation Process that Confers Tau Toxicity in Drosophila. Cell 2004, 116, 671–682. [Google Scholar] [CrossRef] [PubMed]
- DePew, A.T.; Mosca, T.J. Conservation and Innovation: Versatile Roles for LRP4 in Nervous System Development. J. Dev. Biol. 2021, 9, 9. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Menon, K.P.; Carrillo, R.A.; Zinn, K. Development and plasticity of the Drosophila larval neuromuscular junction. Wiley Interdiscip. Rev. Dev. Biol. 2013, 2, 647–670. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chou, V.T.; Johnson, S.A.; Van Vactor, D. Synapse development and maturation at the drosophila neuromuscular junction. Neural Dev. 2020, 15, 11. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vicidomini, R.; Serpe, M. Local BMP signaling: A sensor for synaptic activity that balances synapse growth and function. Curr. Top. Dev. Biol. 2022, 150, 211–254. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Harris, K.P.; Littleton, J.T. Transmission, Development, and Plasticity of Synapses. Genetics 2015, 201, 345–375. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Orr, B.O.; Hauswirth, A.G.; Celona, B.; Fetter, R.D.; Zunino, G.; Kvon, E.Z.; Zhu, Y.; Pennacchio, L.A.; Black, B.L.; Davis, G.W. Presynaptic Homeostasis Opposes Disease Progression in Mouse Models of ALS-Like Degeneration: Evidence for Homeostatic Neuroprotection. Neuron 2020, 107, 95–111.e6. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- DePew, A.T.; Bruckner, J.J.; O’Connor-Giles, K.M.; Mosca, T.J. Neuronal LRP4 directs the development, maturation and cytoskeletal organization of Drosophila peripheral synapses. Development 2024, 151, dev202517. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yu, X.M.; Gutman, I.; Mosca, T.J.; Iram, T.; Ozkan, E.; Garcia, K.C.; Luo, L.; Schuldiner, O. Plum, an immunoglobulin superfamily protein, regulates axon pruning by facilitating TGF-β signaling. Neuron 2013, 78, 456–468. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Restrepo, L.J.; DePew, A.T.; Moese, E.R.; Tymanskyj, S.R.; Parisi, M.J.; Aimino, M.A.; Duhart, J.C.; Fei, H.; Mosca, T.J. γ-secretase promotes Drosophila postsynaptic development through the cleavage of a Wnt receptor. Dev. Cell 2022, 57, 1643–1660.e7. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, Y.; Lobb-Rabe, M.; Ashley, J.; Anand, V.; Carrillo, R.A. Structural and Functional Synaptic Plasticity Induced by Convergent Synapse Loss in the. J. Neurosci. 2021, 41, 1401–1417. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Meng, J.L.; Wang, Y.; Carrillo, R.A.; Heckscher, E.S. Temporal transcription factors determine circuit membership by permanently altering motor neuron-to-muscle synaptic partnerships. eLife 2020, 9, e56898. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Harris, K.P.; Tepass, U. Cdc42 and Par proteins stabilize dynamic adherens junctions in the Drosophila neuroectoderm through regulation of apical endocytosis. J. Cell Biol. 2008, 183, 1129–1143. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Roos, J.; Hummel, T.; Ng, N.; Klämbt, C.; Davis, G.W. Drosophila Futsch Regulates Synaptic Microtubule Organization and Is Necessary for Synaptic Growth. Neuron 2000, 26, 371–382. [Google Scholar] [CrossRef]
- Chak, K.; Kolodkin, A.L. Function of the Drosophila receptor guanylyl cyclase Gyc76C in PlexA-mediated motor axon guidance. Development 2014, 141, 136–147. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Winberg, M.L.; Noordermeer, J.N.; Tamagnone, L.; Comoglio, P.M.; Spriggs, M.K.; Tessier-Lavigne, M.; Goodman, C.S. Plexin A Is a Neuronal Semaphorin Receptor that Controls Axon Guidance. Cell 1998, 95, 903–916. [Google Scholar] [CrossRef]
- Wu, H.; Yesilyurt, H.G.; Yoon, J.; Terman, J.R. The MICALs are a Family of F-actin Dismantling Oxidoreductases Conserved from Drosophila to Humans. Sci. Rep. 2018, 8, 937. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wildonger, J.; Mann, R.S. Evidence that nervy, the Drosophila homolog of ETO/MTG8, promotes mechanosensory organ development by enhancing Notch signaling. Dev. Biol. 2005, 286, 507–520. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yang, T.; Terman, J.R. 14-3-3ε couples protein kinase A to semaphorin signaling and silences plexin RasGAP-mediated axonal repulsion. Neuron 2012, 74, 108–121. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Oyeyinka, A.; Kansal, M.; O’Sullivan, S.M.; Gualtieri, C.; Smith, Z.M.; Vonhoff, F.J. Corazonin Neurons Contribute to Dimorphic Ethanol Sedation Sensitivity in Drosophila melanogaster. Front. Neural Circuits 2022, 16, 702901. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Remy, N.Q.; Guevarra, J.A.; Vonhoff, F.J. Food supplementation with wheat gluten leads to climbing performance decline in Drosophila melanogaster. MicroPubl. Biol. 2022, 2022, 10-17912. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mackay, T.F.; Richards, S.; Stone, E.A.; Barbadilla, A.; Ayroles, J.F.; Zhu, D.; Casillas, S.; Han, Y.; Magwire, M.M.; Cridland, J.M.; et al. The Drosophila melanogaster Genetic Reference Panel. Nature 2012, 482, 173–178. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Malpe, M.S.; McSwain, L.F.; Kudyba, K.; Ng, C.L.; Nicholson, J.; Brady, M.; Qian, Y.; Choksi, V.; Hudson, A.G.; Parrott, B.B.; et al. G-protein signaling is required for increasing germline stem cell division frequency in response to mating in Drosophila males. Sci. Rep. 2020, 10, 3888. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, Z.H.; Zhao, W.; Combs, C.A.; Zhang, F.; Knutson, J.R.; Lilly, M.A.; Xu, H. Mechanical stimulation from the surrounding tissue activates mitochondrial energy metabolism in Drosophila differentiating germ cells. Dev. Cell 2023, 58, 2249–2260.e9. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cho, J.H.; Jo, M.G.; Kim, E.S.; Lee, N.Y.; Kim, S.H.; Chung, C.G.; Park, J.H.; Lee, S.B. CBP-Mediated Acetylation of Importin α Mediates Calcium-Dependent Nucleocytoplasmic Transport of Selective Proteins in Drosophila Neurons. Mol. Cells 2022, 45, 855–867. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jarecki, J.; Keshishian, H. Role of neural activity during synaptogenesis in Drosophila. J. Neurosci. 1995, 15, 8177–8190. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vonhoff, F.; Kuehn, C.; Blumenstock, S.; Sanyal, S.; Duch, C. Temporal coherency between receptor expression, neural activity and AP-1-dependent transcription regulates Drosophila motoneuron dendrite development. Development 2013, 140, 606–616. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bellen, H.J.; Levis, R.W.; Liao, G.; He, Y.; Carlson, J.W.; Tsang, G.; Evans-Holm, M.; Hiesinger, P.R.; Schulze, K.L.; Rubin, G.M.; et al. The BDGP gene disruption project: Single transposon insertions associated with 40% of Drosophila genes. Genetics 2004, 167, 761–781. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Xie, Y.; Li, H.; Luo, X.; Gao, Q.; Zhang, L.; Teng, Y.; Zhao, Q.; Zuo, Z.; Ren, J. IBS 2.0: An upgraded illustrator for the visualization of biological sequences. Nucleic Acids Res. 2022, 50, W420–W426. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hu, Y.; Comjean, A.; Rodiger, J.; Chen, W.; Kim, A.R.; Qadiri, M.; Gao, C.; Zirin, J.; Mohr, S.E.; Perrimon, N. FlyRNAi.org 2025 update-expanded resources for new technologies and species. Nucleic Acids Res. 2025, 53, D958–D965. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vitaterna, M.H.; Takahashi, J.S.; Turek, F.W. Overview of circadian rhythms. Alcohol. Res. Health 2001, 25, 85–93. [Google Scholar] [PubMed] [PubMed Central]
- Ghosh, A.; Sheeba, V. VANESSA-Shiny Apps for Accelerated Time-series Analysis and Visualization of. J. Biol. Rhythm. 2022, 37, 222–231. [Google Scholar] [CrossRef] [PubMed]
- Hendricks, J.C.; Finn, S.M.; Panckeri, K.A.; Chavkin, J.; Williams, J.A.; Sehgal, A.; Pack, A.I. Rest in Drosophila Is a Sleep-like State. Neuron 2000, 25, 129–138. [Google Scholar] [CrossRef]
- Shaw, P.J.; Cirelli, C.; Greenspan, R.J.; Tononi, G. Correlates of sleep and waking in Drosophila melanogaster. Science 2000, 287, 1834–1837. [Google Scholar] [CrossRef] [PubMed]
- Harbison, S.T.; McCoy, L.J.; Mackay, T.F. Genome-wide association study of sleep in Drosophila melanogaster. BMC Genom. 2013, 14, 281. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mishra-Gorur, K.; Çağlayan, A.O.; Schaffer, A.E.; Chabu, C.; Henegariu, O.; Vonhoff, F.; Akgümüş, G.T.; Nishimura, S.; Han, W.; Tu, S.; et al. Mutations in KATNB1 cause complex cerebral malformations by disrupting asymmetrically dividing neural progenitors. Neuron 2014, 84, 1226–1239. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gabrawy, M.M.; Westbrook, R.; King, A.; Khosravian, N.; Ochaney, N.; DeCarvalho, T.; Wang, Q.; Yu, Y.; Huang, Q.; Said, A.; et al. Dual treatment with kynurenine pathway inhibitors and NAD precursors synergistically extends life span in Drosophila. Aging Cell 2024, 23, e14102. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ryglewski, S.; Vonhoff, F.; Scheckel, K.; Duch, C. Intra-neuronal Competition for Synaptic Partners Conserves the Amount of Dendritic Building Material. Neuron 2017, 93, 632–645.e6. [Google Scholar] [CrossRef] [PubMed] [PubMed Central][Green Version]
- Williams, A.A.; Mehler, V.J.; Mueller, C.; Vonhoff, F.; White, R.; Duch, C. Apoptotic Activity of MeCP2 Is Enhanced by C-Terminal Truncating Mutations. PLoS ONE 2016, 11, e0159632. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Drummond, D.R.; Hennessey, E.S.; Sparrow, J.C. Characterisation of missense mutations in the Act88F gene of Drosophila melanogaster. Mol. Gen. Genet. 1991, 226, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Nelson, H.B.; Heiman, R.G.; Bolduc, C.; Kovalick, G.E.; Whitley, P.; Stern, M.; Beckingham, K. Calmodulin point mutations affect Drosophila development and behavior. Genetics 1997, 147, 1783–1798. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Berke, B.; Wittnam, J.; McNeill, E.; Van Vactor, D.L.; Keshishian, H. Retrograde BMP signaling at the synapse: A permissive signal for synapse maturation and activity-dependent plasticity. J. Neurosci. 2013, 33, 17937–17950. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Berke, B.; Le, L.; Keshishian, H. Target-dependent retrograde signaling mediates synaptic plasticity at the Drosophila neuromuscular junction. Dev. Neurobiol. 2019, 79, 895–912. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sukhanova, T.; Renkemeyer, M.K.; Pritchett, N.; Berke, B.; Keshishian, H. The Roles of the Numb Protein in Synaptic Development and Plasticity. Dev. Neurobiol. 2025, 85, e22988. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sun, Y.; Qiu, R.; Li, X.; Cheng, Y.; Gao, S.; Kong, F.; Liu, L.; Zhu, Y. Author Correction: Social attraction in Drosophila is regulated by the mushroom body and serotonergic system. Nat. Commun. 2020, 11, 5738. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Orsburn, B.C. Proteome Discoverer-A Community Enhanced Data Processing Suite for Protein Informatics. Proteomes 2021, 9, 15. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Eng, J.K.; McCormack, A.L.; Yates, J.R. An approach to correlate tandem mass spectral data of peptides with amino acid sequences in a protein database. J. Am. Soc. Mass Spectrom. 1994, 5, 976–989. [Google Scholar] [CrossRef]
- Consortium, U. UniProt: The Universal Protein Knowledgebase in 2025. Nucleic Acids Res. 2025, 53, D609–D617. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- The, M.; MacCoss, M.J.; Noble, W.S.; Käll, L. Fast and Accurate Protein False Discovery Rates on Large-Scale Proteomics Data Sets with Percolator 3.0. J. Am. Soc. Mass Spectrom. 2016, 27, 1719–1727. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Käll, L.; Storey, J.D.; MacCoss, M.J.; Noble, W.S. Assigning Significance to Peptides Identified by Tandem Mass Spectrometry Using Decoy Databases. J. Proteome Res. 2008, 7, 29–34. [Google Scholar] [CrossRef]
- Taus, T.; Köcher, T.; Pichler, P.; Paschke, C.; Schmidt, A.; Henrich, C.; Mechtler, K. Universal and confident phosphorylation site localization using phosphoRS. J. Proteome Res. 2011, 10, 5354–5362. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhou, B.; Pache, L.; Chang, M.; Khodabakhshi, A.H.; Tanaseichuk, O.; Benner, C.; Chanda, S.K. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat. Commun. 2019, 10, 1523. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hu, Y.; Flockhart, I.; Vinayagam, A.; Bergwitz, C.; Berger, B.; Perrimon, N.; Mohr, S.E. An integrative approach to ortholog prediction for disease-focused and other functional studies. BMC Bioinform. 2011, 12, 357. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bayer, K.U.; Schulman, H. CaM Kinase: Still Inspiring at 40. Neuron 2019, 103, 380–394. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Manning, G.; Whyte, D.B.; Martinez, R.; Hunter, T.; Sudarsanam, S. The protein kinase complement of the human genome. Science 2002, 298, 1912–1934. [Google Scholar] [CrossRef] [PubMed]
- Manning, G.; Plowman, G.D.; Hunter, T.; Sudarsanam, S. Evolution of protein kinase signaling from yeast to man. Trends Biochem. Sci. 2002, 27, 514–520. [Google Scholar] [CrossRef]
- Stinchfield, M.J.; Weasner, B.P.; Weasner, B.M.; Zhitomersky, D.; Kumar, J.P.; O’Connor, M.B.; Newfeld, S.J. Fourth Chromosome Resource Project: A comprehensive resource for genetic analysis in Drosophila that includes humanized stocks. Genetics 2024, 226, iyad201. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sousa-Neves, R.; Lukacsovich, T.; Mizutani, C.M.; Locke, J.; Podemski, L.; Marsh, J.L. High-resolution mapping of the Drosophila fourth chromosome using site-directed terminal deficiencies. Genetics 2005, 170, 127–138. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Riddle, N.C.; Leung, W.; Haynes, K.A.; Granok, H.; Wuller, J.; Elgin, S.C. An investigation of heterochromatin domains on the fourth chromosome of Drosophila melanogaster. Genetics 2008, 178, 1177–1191. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shafer, O.T.; Keene, A.C. The Regulation of Drosophila Sleep. Curr. Biol. 2021, 31, R38–R49. [Google Scholar] [CrossRef] [PubMed]
- Al Lihabi, A. A literature review of sleep problems and neurodevelopment disorders. Front. Psychiatry 2023, 14, 1122344. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Winsky-Sommerer, R.; de Oliveira, P.; Loomis, S.; Wafford, K.; Dijk, D.J.; Gilmour, G. Disturbances of sleep quality, timing and structure and their relationship with other neuropsychiatric symptoms in Alzheimer’s disease and schizophrenia: Insights from studies in patient populations and animal models. Neurosci. Biobehav. Rev. 2019, 97, 112–137. [Google Scholar] [CrossRef] [PubMed]
- Singer, E.V.; Niarchou, M.; Maxwell-Horn, A.; Hucks, D.; Johnston, R.; Sutcliffe, J.S.; Davis, L.K.; Malow, B.A. Characterizing sleep disorders in an autism-specific collection of electronic health records. Sleep Med. 2022, 92, 88–95. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ryglewski, S.; Kadas, D.; Hutchinson, K.; Schuetzler, N.; Vonhoff, F.; Duch, C. Dendrites are dispensable for basic motoneuron function but essential for fine tuning of behavior. Proc. Natl. Acad. Sci. USA 2014, 111, 18049–18054. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vonhoff, F.; Williams, A.; Ryglewski, S.; Duch, C. Drosophila as a model for MECP2 gain of function in neurons. PLoS ONE. 2012, 7, e31835. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Scott, C.A.; Rossiter, J.P.; Andrew, R.D.; Jackson, A.C. Structural abnormalities in neurons are sufficient to explain the clinical disease and fatal outcome of experimental rabies in yellow fluorescent protein-expressing transgenic mice. J. Virol. 2008, 82, 513–521. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Halpern, M.E.; Chiba, A.; Johansen, J.; Keshishian, H. Growth cone behavior underlying the development of stereotypic synaptic connections in Drosophila embryos. J. Neurosci. 1991, 11, 3227–3238. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sink, H.; Whitington, P.M. Pathfinding in the central nervous system and periphery by identified embryonic Drosophila motor axons. Development 1991, 112, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Chiba, A.; Hing, H.; Cash, S.; Keshishian, H. Growth cone choices of Drosophila motoneurons in response to muscle fiber mismatch. J. Neurosci. 1993, 13, 714–732. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gögel, S.; Wakefield, S.; Tear, G.; Klämbt, C.; Gordon-Weeks, P.R. The Drosophila microtubule associated protein Futsch is phosphorylated by Shaggy/Zeste-white 3 at an homologous GSK3beta phosphorylation site in MAP1B. Mol. Cell. Neurosci. 2006, 33, 188–199. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, S.; Wilson, S.J.; Hale, T.K.; Fitzsimons, H.L. Ankyrin2 is essential for neuronal morphogenesis and long-term courtship memory in Drosophila. Mol. Brain 2023, 16, 42. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Doerflinger, H.; Benton, R.; Shulman, J.M.; St Johnston, D. The role of PAR-1 in regulating the polarised microtubule cytoskeleton in the Drosophila follicular epithelium. Development 2003, 130, 3965–3975. [Google Scholar] [CrossRef] [PubMed]
- Tener, S.J.; Lin, Z.; Park, S.J.; Oraedu, K.; Ulgherait, M.; Van Beek, E.; Martínez-Muñiz, A.; Pantalia, M.; Gatto, J.A.; Volpi, J.; et al. Neuronal knockdown of Cullin3 as a Drosophila model of autism spectrum disorder. Sci. Rep. 2024, 14, 1541. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- O’Roak, B.J.; Vives, L.; Girirajan, S.; Karakoc, E.; Krumm, N.; Coe, B.P.; Levy, R.; Ko, A.; Lee, C.; Smith, J.D.; et al. Sporadic autism exomes reveal a highly interconnected protein network of de novo mutations. Nature 2012, 485, 246–250. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chow, N.; Korenberg, J.R.; Chen, X.N.; Neve, R.L. APP-BP1, a novel protein that binds to the carboxyl-terminal region of the amyloid precursor protein. J. Biol. Chem. 1996, 271, 11339–11346. [Google Scholar] [CrossRef] [PubMed]
- Jalali, D.; Guevarra, J.A.; Martinez, L.; Hung, L.; Vonhoff, F.J. Nutraceutical and Probiotic Approaches to Examine Molecular Interactions of the Amyloid Precursor Protein APP in. Int. J. Mol. Sci. 2021, 22, 7022. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Association, A.P. Diagnostic and Statistical Manual of Mental Disorders; American Psychiatric Association: Washington, DC, USA, 2013. [Google Scholar]
- McDonald, T.A.M. The broader autism phenotype constellations-disability matrix paradigm: Theoretical model for autism and the broader autism phenotype. Med. Hypotheses 2021, 146, 110456. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kazdoba, T.M.; Leach, P.T.; Yang, M.; Silverman, J.L.; Solomon, M.; Crawley, J.N. Translational Mouse Models of Autism: Advancing Toward Pharmacological Therapeutics. Curr. Top. Behav. Neurosci. 2016, 28, 1–52. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Berg, E.L.; Silverman, J.L. Chapter 8—Animal models of autism. In The Neuroscience of Autism; Kana, R.K., Ed.; Academic Press: Cambridge, MA, USA, 2022; pp. 157–196. [Google Scholar]
- Silverman, J.L.; Thurm, A.; Ethridge, S.B.; Soller, M.M.; Petkova, S.P.; Abel, T.; Bauman, M.D.; Brodkin, E.S.; Harony-Nicolas, H.; Wöhr, M.; et al. Reconsidering animal models used to study autism spectrum disorder: Current state and optimizing future. Genes Brain Behav. 2022, 21, e12803. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ugur, B.; Chen, K.; Bellen, H.J. Drosophila tools and assays for the study of human diseases. Dis. Models Mech. 2016, 9, 235–244. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mirzoyan, Z.; Sollazzo, M.; Allocca, M.; Valenza, A.M.; Grifoni, D.; Bellosta, P. Drosophila melanogaster: A Model Organism to Study Cancer. Front. Genet. 2019, 10, 51. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Xin, M.; Davis, C.A.; Molkentin, J.D.; Lien, C.L.; Duncan, S.A.; Richardson, J.A.; Olson, E.N. A threshold of GATA4 and GATA6 expression is required for cardiovascular development. Proc. Natl. Acad. Sci. USA 2006, 103, 11189–11194. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Patkar, O.L.; Caruso, M.; Teakle, N.; Keshvari, S.; Bush, S.J.; Pridans, C.; Belmer, A.; Summers, K.M.; Irvine, K.M.; Hume, D.A. Analysis of homozygous and heterozygous Csf1r knockout in the rat as a model for understanding microglial function in brain development and the impacts of human CSF1R mutations. Neurobiol. Dis. 2021, 151, 105268. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lanctôt, C.; Moreau, A.; Chamberland, M.; Tremblay, M.L.; Drouin, J. Hindlimb patterning and mandible development require the Ptx1 gene. Development 1999, 126, 1805–1810. [Google Scholar] [CrossRef] [PubMed]
- Billeter, J.C.; Bailly, T.P.M.; Kohlmeier, P. The social life of Drosophila melanogaster. Insectes Sociaux 2024, 72, 127–140. [Google Scholar] [CrossRef]
- Cirelli, C.; Bushey, D. Sleep and Wakefulness inDrosophila melanogaster. Ann. N. Y. Acad. Sci. 2008, 1129, 323–329. [Google Scholar] [CrossRef]
- Couton, L.; Mauss, A.S.; Yunusov, T.; Diegelmann, S.; Evers, J.F.; Landgraf, M. Development of connectivity in a motoneuronal network in Drosophila larvae. Curr. Biol. 2015, 25, 568–576. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cheong, H.S.J.; Eichler, K.; Stürner, T.; Asinof, S.K.; Champion, A.S.; Marin, E.C.; Oram, T.B.; Sumathipala, M.; Venkatasubramanian, L.; Namiki, S.; et al. Transforming Descending Input into Behavior: The Organization of Premotor Circuits in the Drosophila Male Adult Nerve Cord Connectome; eLife Sciences Publications, Ltd.: Cambridge, UK, 2024. [Google Scholar]
- Hürkey, S.; Niemeyer, N.; Schleimer, J.H.; Ryglewski, S.; Schreiber, S.; Duch, C. Gap junctions desynchronize a neural circuit to stabilize insect flight. Nature 2023, 618, 118–125. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bell, C.; Kilo, L.; Gottschalk, D.; Arian, J.; Deneke, L.; Kern, H.; Rickert, C.; Kobler, O.; Strauß, J.; Heine, M.; et al. Specific presynaptic functions require distinct Drosophila Cav2 splice isoforms. eLife 2025, 13, RP100394. [Google Scholar] [CrossRef] [PubMed]
- Cukier, H.N.; Perez, A.M.; Collins, A.L.; Zhou, Z.; Zoghbi, H.Y.; Botas, J. Genetic modifiers of MeCP2 function in Drosophila. PLoS Genet. 2008, 4, e1000179. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ballester, P.; Martínez, M.J.; Javaloyes, A.; Inda, M.D.; Fernández, N.; Gázquez, P.; Aguilar, V.; Pérez, A.; Hernández, L.; Richdale, A.L.; et al. Sleep problems in adults with autism spectrum disorder and intellectual disability. Autism Res. 2019, 12, 66–79. [Google Scholar] [CrossRef] [PubMed]
- Parisky, K.M.; Agosto, J.; Pulver, S.R.; Shang, Y.; Kuklin, E.; Hodge, J.J.; Kang, K.; Liu, X.; Garrity, P.A.; Rosbash, M.; et al. PDF cells are a GABA-responsive wake-promoting component of the Drosophila sleep circuit. Neuron 2008, 60, 672–682. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Brown, M.P.; Verma, S.; Palmer, I.; Guerrero Zuniga, A.; Mehta, A.; Rosensweig, C.; Keles, M.F.; Wu, M.N. A subclass of evening cells promotes the switch from arousal to sleep at dusk. Curr. Biol. 2024, 34, 2186–2199.e3. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Furusawa, K.; Emoto, K. Scrap and Build for Functional Neural Circuits: Spatiotemporal Regulation of Dendrite Degeneration and Regeneration in Neural Development and Disease. Front. Cell Neurosci. 2020, 14, 613320. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wolff, J.J.; Jacob, S.; Elison, J.T. The journey to autism: Insights from neuroimaging studies of infants and toddlers. Dev. Psychopathol. 2018, 30, 479–495. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hazlett, H.C.; Gu, H.; Munsell, B.C.; Kim, S.H.; Styner, M.; Wolff, J.J.; Elison, J.T.; Swanson, M.R.; Zhu, H.; Botteron, K.N.; et al. Early brain development in infants at high risk for autism spectrum disorder. Nature 2017, 542, 348–351. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shen, M.D.; Li, D.D.; Keown, C.L.; Lee, A.; Johnson, R.T.; Angkustsiri, K.; Rogers, S.J.; Müller, R.A.; Amaral, D.G.; Nordahl, C.W. Functional Connectivity of the Amygdala Is Disrupted in Preschool-Aged Children with Autism Spectrum Disorder. J. Am. Acad. Child. Adolesc. Psychiatry 2016, 55, 817–824. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lombardo, M.V.; Pierce, K.; Eyler, L.T.; Carter Barnes, C.; Ahrens-Barbeau, C.; Solso, S.; Campbell, K.; Courchesne, E. Different functional neural substrates for good and poor language outcome in autism. Neuron 2015, 86, 567–577. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Schor, N.F.; Bianchi, D.W. Neurodevelopmental Clues to Neurodegeneration. Pediatr. Neurol. 2021, 123, 67–76. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, Y.; McPhie, D.L.; Hirschberg, J.; Neve, R.L. The amyloid precursor protein-binding protein APP-BP1 drives the cell cycle through the S-M checkpoint and causes apoptosis in neurons. J. Biol. Chem. 2000, 275, 8929–8935. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Zhang, J.; Su, Y.; Liu, M.; Ospina, J.K.; Yang, S.; Zhu, A.J. In vivo RNAi screen reveals neddylation genes as novel regulators of Hedgehog signaling. PLoS ONE 2011, 6, e24168. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cassar, M.; Kretzschmar, D. Analysis of Amyloid Precursor Protein Function in Drosophila melanogaster. Front. Mol. Neurosci. 2016, 9, 61. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Copenhaver, P.F.; Kögel, D. Role of APP Interactions with Heterotrimeric G Proteins: Physiological Functions and Pathological Consequences. Front. Mol. Neurosci. 2017, 10, 3. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Preat, T.; Goguel, V. Role of Drosophila Amyloid Precursor Protein in Memory Formation. Front. Mol. Neurosci. 2016, 9, 142. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Barber, K.R.; Hruska, M.; Bush, K.M.; Martinez, J.A.; Fei, H.; Levitan, I.B.; Dalva, M.B.; Wairkar, Y.P. Levels of Par-1 kinase determine the localization of Bruchpilot at the Drosophila neuromuscular junction synapses. Sci. Rep. 2018, 8, 16099. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, Y.; Guo, H.; Kwan, H.; Wang, J.W.; Kosek, J.; Lu, B. PAR-1 kinase phosphorylates Dlg and regulates its postsynaptic targeting at the Drosophila neuromuscular junction. Neuron 2007, 53, 201–215. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Maussion, G.; Carayol, J.; Lepagnol-Bestel, A.M.; Tores, F.; Loe-Mie, Y.; Milbreta, U.; Rousseau, F.; Fontaine, K.; Renaud, J.; Moalic, J.M.; et al. Convergent evidence identifying MAP/microtubule affinity-regulating kinase 1 (MARK1) as a susceptibility gene for autism. Hum. Mol. Genet. 2008, 17, 2541–2551. [Google Scholar] [CrossRef] [PubMed]
- Lund, H.; Gustafsson, E.; Svensson, A.; Nilsson, M.; Berg, M.; Sunnemark, D.; von Euler, G. MARK4 and MARK3 associate with early tau phosphorylation in Alzheimer’s disease granulovacuolar degeneration bodies. Acta Neuropathol. Commun. 2014, 2, 22. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Seshadri, S.; Fitzpatrick, A.L.; Ikram, M.A.; DeStefano, A.L.; Gudnason, V.; Boada, M.; Bis, J.C.; Smith, A.V.; Carassquillo, M.M.; Lambert, J.C.; et al. Genome-wide analysis of genetic loci associated with Alzheimer disease. JAMA 2010, 303, 1832–1840. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chin, J.Y.; Knowles, R.B.; Schneider, A.; Drewes, G.; Mandelkow, E.M.; Hyman, B.T. Microtubule-affinity regulating kinase (MARK) is tightly associated with neurofibrillary tangles in Alzheimer brain: A fluorescence resonance energy transfer study. J. Neuropathol. Exp. Neurol. 2000, 59, 966–971. [Google Scholar] [CrossRef] [PubMed]
- Terman, J.R.; Kolodkin, A.L. Nervy links protein kinase a to plexin-mediated semaphorin repulsion. Science 2004, 303, 1204–1207. [Google Scholar] [CrossRef] [PubMed]
- Marini-Davis, A.; Oliver, R.; Godwin, B.; Chase, S.; Khan, Z.; Vonhoff, F.J. Juvenile hormone regulates the maturation of sexually dimorphic naive ethanol olfactory preference in Drosophila melanogaster. R. Soc. Open Sci. 2025, 12, 242217. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gualtieri, C.; Smith, Z.M.; Cruz, A.; Khan, Z.; Jenkins, C.; Mishra-Gorur, K.; Vonhoff, F.J. Dysregulation of Protein Kinase CaMKI Leads to Autism-Related Phenotypes in Synaptic Connectivity, Sleep, Sociality, and Aging-Dependent Degeneration in Drosophila. Biology 2025, 14, 1228. https://doi.org/10.3390/biology14091228
Gualtieri C, Smith ZM, Cruz A, Khan Z, Jenkins C, Mishra-Gorur K, Vonhoff FJ. Dysregulation of Protein Kinase CaMKI Leads to Autism-Related Phenotypes in Synaptic Connectivity, Sleep, Sociality, and Aging-Dependent Degeneration in Drosophila. Biology. 2025; 14(9):1228. https://doi.org/10.3390/biology14091228
Chicago/Turabian StyleGualtieri, Claudia, Zachary M. Smith, Abby Cruz, Ziam Khan, Conor Jenkins, Ketu Mishra-Gorur, and Fernando J. Vonhoff. 2025. "Dysregulation of Protein Kinase CaMKI Leads to Autism-Related Phenotypes in Synaptic Connectivity, Sleep, Sociality, and Aging-Dependent Degeneration in Drosophila" Biology 14, no. 9: 1228. https://doi.org/10.3390/biology14091228
APA StyleGualtieri, C., Smith, Z. M., Cruz, A., Khan, Z., Jenkins, C., Mishra-Gorur, K., & Vonhoff, F. J. (2025). Dysregulation of Protein Kinase CaMKI Leads to Autism-Related Phenotypes in Synaptic Connectivity, Sleep, Sociality, and Aging-Dependent Degeneration in Drosophila. Biology, 14(9), 1228. https://doi.org/10.3390/biology14091228






