Responses to Voluntary Isocapnic Hyperpnea in Normoxia and Hypoxia: Insights from Blood Gas Analysis
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
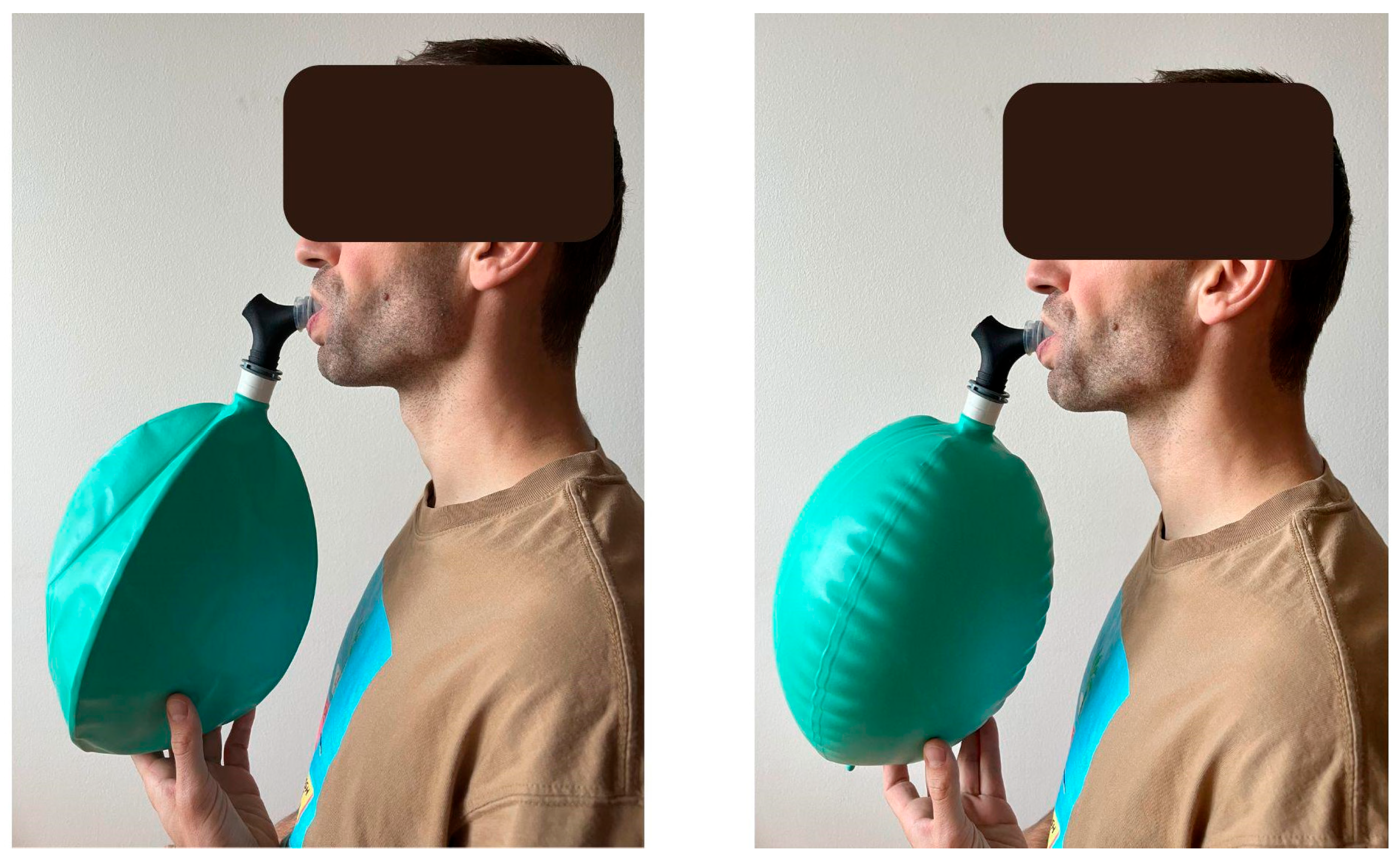
2.1. Applied Devices and Procedures
2.2. Experiment 1—VIH in Normoxia in Well-Trained Athletes
2.3. Experiment 2—VIH in Severe Hypoxia
2.4. Statistical Analyses
3. Results
3.1. Experiment 1
3.2. Experiment 2
4. Discussion
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Crisafulli, E.; Costi, S.; Fabbri, L.M.; Clini, E.M. Respiratory Muscles Training in COPD Patients. Int. J. Chron. Obstruct. Pulmon. Dis. 2007, 2, 19–25. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bozkurt, B.; Fonarow, G.C.; Goldberg, L.R.; Guglin, M.; Josephson, R.A.; Forman, D.E.; Lin, G.; Lindenfeld, J.; O’Connor, C.; Panjrath, G.; et al. Cardiac Rehabilitation for Patients with Heart Failure: JACC Expert Panel. J. Am. Coll. Cardiol. 2021, 77, 1454–1469. [Google Scholar] [CrossRef]
- Seixas, M.B.; Almeida, L.B.; Trevizan, P.F.; Martinez, D.G.; Laterza, M.C.; Vanderlei, L.C.M.; Silva, L.P. Effects of Inspiratory Muscle Training in Older Adults. Respir. Care 2020, 65, 535–544. [Google Scholar] [CrossRef]
- McConnell, A. Breathe Strong, Perform Better; Human Kinetics: Champaign, IL, USA, 2011; ISBN 9780736091695. [Google Scholar]
- Tosun, M.I.; Demirkan, E.; Kaplan, A.; Ari Yilmaz, Y.; Eker Arici, I.; Favre, M.; Aslan, V.; Kutlu, M. Respiratory Muscle Training Improves Aerobic Capacity and Respiratory Muscle Strength in Youth Wrestlers. Front. Physiol. 2025, 16, 1492446. [Google Scholar] [CrossRef]
- Sheel, A.W. Respiratory Muscle Training in Healthy Individuals: Physiological Rationale and Implications for Exercise Performance. Sports Med. 2002, 32, 567–581. [Google Scholar] [CrossRef] [PubMed]
- Sheel, A.W.; Boushel, R.; Dempsey, J.A. Competition for Blood Flow Distribution between Respiratory and Locomotor Muscles: Implications for Muscle Fatigue. J. Appl. Physiol. 2018, 125, 820–831. [Google Scholar] [CrossRef] [PubMed]
- Espinosa-Ramírez, M.; Riquelme, S.; Araya, F.; Rodríguez, G.; Figueroa-Martínez, F.; Gabrielli, L.; Viscor, G.; Reid, W.D.; Contreras-Briceño, F. Effectiveness of Respiratory Muscles Training by Voluntary Isocapnic Hyperpnea versus Inspiratory Threshold Loading on Intercostales and Vastus Lateralis Muscles Deoxygenation Induced by Exercise in Physically Active Adults. Biology 2023, 12, 219. [Google Scholar] [CrossRef]
- Kowalski, T.; Klusiewicz, A.; Rębiś, K.; Wilk, A.; Starczewski, M. Comparative Study of Different Respiratory Muscle Training Methods: Effects on Cardiopulmonary Indices and Athletic Performance in Elite Short-Track Speedskaters. Life 2024, 14, 1159. [Google Scholar] [CrossRef]
- Kowalski, T.; Kasiak, P.S.; Rebis, K.; Klusiewicz, A.; Granda, D.; Wiecha, S. Respiratory Muscle Training Induces Additional Stress and Training Load in Well-Trained Triathletes-Randomized Controlled Trial. Front. Physiol. 2023, 14, 1264265. [Google Scholar] [CrossRef]
- Kryvenko, V.; Vadász, I. Mechanisms of Hypercapnia-Induced Endoplasmic Reticulum Dysfunction. Front. Physiol. 2021, 12, 735580. [Google Scholar] [CrossRef]
- Kapuy, O. Mechanism of Decision Making between Autophagy and Apoptosis Induction upon Endoplasmic Reticulum Stress. Int. J. Mol. Sci. 2024, 25, 4368. [Google Scholar] [CrossRef]
- Duarte, C.M.; Jaremko, Ł.; Jaremko, M. Hypothesis: Potentially Systemic Impacts of Elevated CO2 on the Human Proteome and Health. Front. Public Health 2020, 8, 543322. [Google Scholar] [CrossRef]
- Parks, S.K. pH and Cell Signaling: How Intracellular and Extracellular Acidification Modulate MAPK Cascades and Cell Function. Cell Signal 2013. [Google Scholar]
- Kandrashina, S.; Sherstyukova, E.; Shvedov, M.; Inozemtsev, V.; Timoshenko, R.; Erofeev, A.; Dokukin, M.; Sergunova, V. The Effect of the Acid-Base Imbalance on the Shape and Structure of Red Blood Cells. Cells 2024, 13, 1813. [Google Scholar] [CrossRef]
- Lardner, A. The Effects of Extracellular pH on Immune Function. J. Leukoc. Biol. 2001, 69, 522–530. [Google Scholar] [CrossRef] [PubMed]
- Sommer, L.Z.; Iscoe, S.; Robicsek, A.; Kruger, J.; Silverman, J.; Rucker, J.; Dickstein, J.; Volgyesi, G.A.; Fisher, J.A. A Simple Breathing Circuit Minimizing Changes in Alveolar Ventilation during Hyperpnoea. Eur. Respir. J. 1998, 12, 698–701. [Google Scholar] [CrossRef] [PubMed]
- Feldman, J.L.; Mitchell, G.S.; Nattie, E.E. Breathing: Rhythmicity, Plasticity, Chemosensitivity. Annu. Rev. Neurosci. 2003, 26, 239–266. [Google Scholar] [CrossRef]
- Hallén, K.; Stenqvist, O.; Ricksten, S.-E.; Lindgren, S. A Simple Method for Isocapnic Hyperventilation Evaluated in a Lung Model. Acta Anaesthesiol. Scand. 2016, 60, 597–606. [Google Scholar] [CrossRef]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. STROBE Initiative The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for Reporting Observational Studies. Ann. Intern. Med. 2007, 147, 573–577. [Google Scholar] [CrossRef]
- McKay, A.K.A.; Stellingwerff, T.; Smith, E.S.; Martin, D.T.; Mujika, I.; Goosey-Tolfrey, V.L.; Sheppard, J.; Burke, L.M. Defining Training and Performance Caliber: A Participant Classification Framework. Int. J. Sports Physiol. Perform. 2022, 17, 317–331. [Google Scholar] [CrossRef]
- Bull, F.C.; Al-Ansari, S.S.; Biddle, S.; Borodulin, K.; Buman, M.P.; Cardon, G.; Carty, C.; Chaput, J.-P.; Chastin, S.; Chou, R.; et al. World Health Organization 2020 Guidelines on Physical Activity and Sedentary Behaviour. Br. J. Sports Med. 2020, 54, 1451–1462. [Google Scholar] [CrossRef]
- Dean, J.B.; Mulkey, D.K.; Henderson, R.A., 3rd; Potter, S.J.; Putnam, R.W. Hyperoxia, Reactive Oxygen Species, and Hyperventilation: Oxygen Sensitivity of Brain Stem Neurons. J. Appl. Physiol. 2004, 96, 784–791. [Google Scholar] [CrossRef] [PubMed]
- Arab, A.; Wang, J.; Bausch, K.; von Schmädel, K.; Bode, C.; Hehrlein, C. Transient Hyperoxic Reoxygenation Reduces Cytochrome C Oxidase Activity by Increasing Superoxide Dismutase and Nitric Oxide. J. Biol. Chem. 2010, 285, 11172–11177. [Google Scholar] [CrossRef] [PubMed]
- Checa, J.; Aran, J.M. Reactive Oxygen Species: Drivers of Physiological and Pathological Processes. J. Inflamm. Res. 2020, 13, 1057–1073. [Google Scholar] [CrossRef]
- Tretter, V.; Zach, M.-L.; Böhme, S.; Ullrich, R.; Markstaller, K.; Klein, K.U. Investigating Disturbances of Oxygen Homeostasis: From Cellular Mechanisms to the Clinical Practice. Front. Physiol. 2020, 11, 947. [Google Scholar] [CrossRef]
- Nummela, A.; Hämäläinen, I.; Rusko, H. Effect of Hyperoxia on Metabolic Responses and Recovery in Intermittent Exercise. Scand. J. Med. Sci. Sports 2002, 12, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Zoneff, E.; Wang, Y.; Jackson, C.; Smith, O.; Duchi, S.; Onofrillo, C.; Farrugia, B.; Moulton, S.E.; Williams, R.; Parish, C.; et al. Controlled Oxygen Delivery to Power Tissue Regeneration. Nat. Commun. 2024, 15, 4361. [Google Scholar] [CrossRef]
- Morawetz, D.; Dünnwald, T.; Faulhaber, M.; Gatterer, H.; Höllrigl, L.; Raschner, C.; Schobersberger, W. Can Hyperoxic Preconditioning in Normobaric Hypoxia (3500 M) Improve All-out Exercise Performance in Highly Skilled Skiers? A Randomized Crossover Study. Int. J. Sports Physiol. Perform. 2020, 15, 346–353. [Google Scholar] [CrossRef]
- Cyr-Kirk, S.; Billaut, F. Hyperoxia Improves Repeated-Sprint Ability and the Associated Training Load in Athletes. Front. Sports Act. Living 2022, 4, 817280. [Google Scholar] [CrossRef]
- Ortiz-Prado, E.; Dunn, J.F.; Vasconez, J.; Castillo, D.; Viscor, G. Partial Pressure of Oxygen in the Human Body: A General Review. Am. J. Blood Res. 2019, 9, 1–14. [Google Scholar]
- Iqbal, M.; Bliss, E.; Whiteside, E.J.; Hoffman, B.; Mills, D.E. The Effects of Volitional Hyperpnea on Biomarkers of Respiratory Muscle Damage in Healthy Young Men. Physiol. Rep. 2025, 13, e70277. [Google Scholar] [CrossRef]
- Dempsey, J.A.; Forster, H.V.; Birnbaum, M.L.; Reddan, W.G.; Thoden, J.; Grover, R.F.; Rankin, J. Control of Exercise Hyperpnea under Varying Durations of Exposure to Moderate Hypoxia. Respir. Physiol. 1972, 16, 213–231. [Google Scholar] [CrossRef]
- Verges, S.; Bachasson, D.; Wuyam, B. Effect of Acute Hypoxia on Respiratory Muscle Fatigue in Healthy Humans. Respir. Res. 2010, 11, 109. [Google Scholar] [CrossRef] [PubMed]
- Sato, S.; Ishida, K.; Tanaka, N.I.; Katayama, K. Impact of High-Intensity Interval Hyperpnea on Aerobic Energy Release and Inspiratory Muscle Fatigue. Respir. Physiol. Neurobiol. 2024, 319, 104170. [Google Scholar] [CrossRef] [PubMed]
- Teppema, L.J.; Dahan, A. The Ventilatory Response to Hypoxia in Mammals: Mechanisms, Measurement, and Analysis. Physiol. Rev. 2010, 90, 675–754. [Google Scholar] [CrossRef]
- Pamenter, M.E.; Powell, F.L. Time Domains of the Hypoxic Ventilatory Response and Their Molecular Basis. Compr. Physiol. 2016, 6, 1345–1385. [Google Scholar] [CrossRef] [PubMed]
- Kowalski, T.; Granda, D.; Klusiewicz, A. Practical Application of Respiratory Muscle Training in Endurance Sports. Strength Cond. J. 2024, 46, 686–695. [Google Scholar] [CrossRef]
- Tremblay, J.C.; Ainslie, P.N. Global and Country-Level Estimates of Human Population at High Altitude. Proc. Natl. Acad. Sci. USA 2021, 118, e2102463118. [Google Scholar] [CrossRef]
- Castro, D.; Patil, S.M.; Zubair, M.; Keenaghan, M. Arterial Blood Gas. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
| Session Number | Session Length | Breathing Frequency | Session Number | Session Length | Breathing Frequency |
|---|---|---|---|---|---|
| 1 | 3 | 20 | 12 | 13 | 22 |
| 2 | 4 | 20 | 13 | 14 | 24 |
| 3 | 5 | 20 | 14 | 15 | 24 |
| 4 | 5 | 20 | 15 | 16 | 24 |
| 5 | 6 | 22 | 16 | 17 | 24 |
| 6 | 7 | 22 | 17 | 18 | 44 |
| 7 | 8 | 22 | 18 | 18 | 26 |
| 8 | 9 | 22 | 19 | 19 | 26 |
| 9 | 10 | 22 | 20 | 20 | 26 |
| 10 | 11 | 22 | 21 | 20 | 26 |
| 11 | 12 | 22 |
| Variable | Value Before | Value After | Bayes Factor (BF10) | Bayesian Error % | Interpretation |
|---|---|---|---|---|---|
| Week 1 | |||||
| pH | 7.426 ± 0.01 | 7.423 ± 0.00 | 0.336 | 0.004 | Anecdotal evidence for H0 |
| HCO3− (mmol/L) | 27.089 ± 1.2 | 26.956 ± 1.4 | 0.435 | 0.008 | Anecdotal evidence for H0 |
| pO2 (mmHg) | 71.744 ± 6.2 | 81.667 ± 8.4 | 7.986 | <0.001 | Moderate evidence for H1 |
| pCO2 (mmHg) | 41.244 ± 1.5 | 41.478 ± 3.1 | 0.331 | 0.004 | Anecdotal evidence for H0 |
| Week 4 | |||||
| pH | 7.411 ± 0.00 | 7.409 ± 0.04 | 0.327 | 0.004 | Moderate evidence for H0 |
| HCO3− (mmol/L) | 26.386 ± 1.2 | 26.543 ± 1.5 | 0.397 | 0.004 | Anecdotal evidence for H0 |
| pO2 (mmHg) | 67.029 ± 10.4 | 75.814 ± 6.6 | 1.596 | <0.001 | Anecdotal evidence for H1 |
| pCO2 (mmHg) | 43.178 ± 4.2 | 41.456 ± 4.3 | 0.490 | 0.010 | Anecdotal evidence for H0 |
| Week 6 | |||||
| pH | 7.417 ± 0.02 | 7.417 ± 0.03 | 0.322 | 0.004 | Moderate evidence for H0 |
| HCO3− (mmol/L) | 26.386 ± 1.2 | 26.543 ± 1.5 | 0.323 | 0.004 | Moderate evidence for H0 |
| pO2 (mmHg) | 75.344 ± 5.5 | 85.578 ± 8.8 | 7.531 | <0.001 | Moderate evidence for H1 |
| pCO2 (mmHg) | 41.067 ± 2.8 | 41.189 ± 4.2 | 0.324 | 0.004 | Moderate evidence for H0 |
| Variable | Value Before | Value After | Bayes Factor (BF10) | Bayesian Error % | Interpretation |
|---|---|---|---|---|---|
| pH | 7.414 ± 0.02 | 7.443 ± 0.03 | 6.304 | <0.001 | Moderate evidence for H1 |
| HCO3− (mmol/L) | 24.156 ± 1.4 | 24.733 ± 1.3 | 0.836 | 0.023 | Anecdotal evidence for H0 |
| pO2 (mmHg) | 46.906 ± 3.7 | 51.761 ± 7.6 | 2.984 | <0.001 | Anecdotal evidence for H1 |
| pCO2 (mmHg) | 38.294 ± 2.7 | 35.783 ± 4.5 | 1.349 | 0.024 | Anecdotal evidence for H1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kowalski, T. Responses to Voluntary Isocapnic Hyperpnea in Normoxia and Hypoxia: Insights from Blood Gas Analysis. Biology 2025, 14, 1207. https://doi.org/10.3390/biology14091207
Kowalski T. Responses to Voluntary Isocapnic Hyperpnea in Normoxia and Hypoxia: Insights from Blood Gas Analysis. Biology. 2025; 14(9):1207. https://doi.org/10.3390/biology14091207
Chicago/Turabian StyleKowalski, Tomasz. 2025. "Responses to Voluntary Isocapnic Hyperpnea in Normoxia and Hypoxia: Insights from Blood Gas Analysis" Biology 14, no. 9: 1207. https://doi.org/10.3390/biology14091207
APA StyleKowalski, T. (2025). Responses to Voluntary Isocapnic Hyperpnea in Normoxia and Hypoxia: Insights from Blood Gas Analysis. Biology, 14(9), 1207. https://doi.org/10.3390/biology14091207






