Introduced Western Honeybees Dramatically Reduce the Abundance of Wild Bees in Alpine Meadows, Eastern Tibet Plateau
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site and Natural History
2.2. Field Sampling
2.3. Identification of Pollen Carried by Bee Individuals
2.4. Perceived Apparent Competition
2.5. Data Analysis
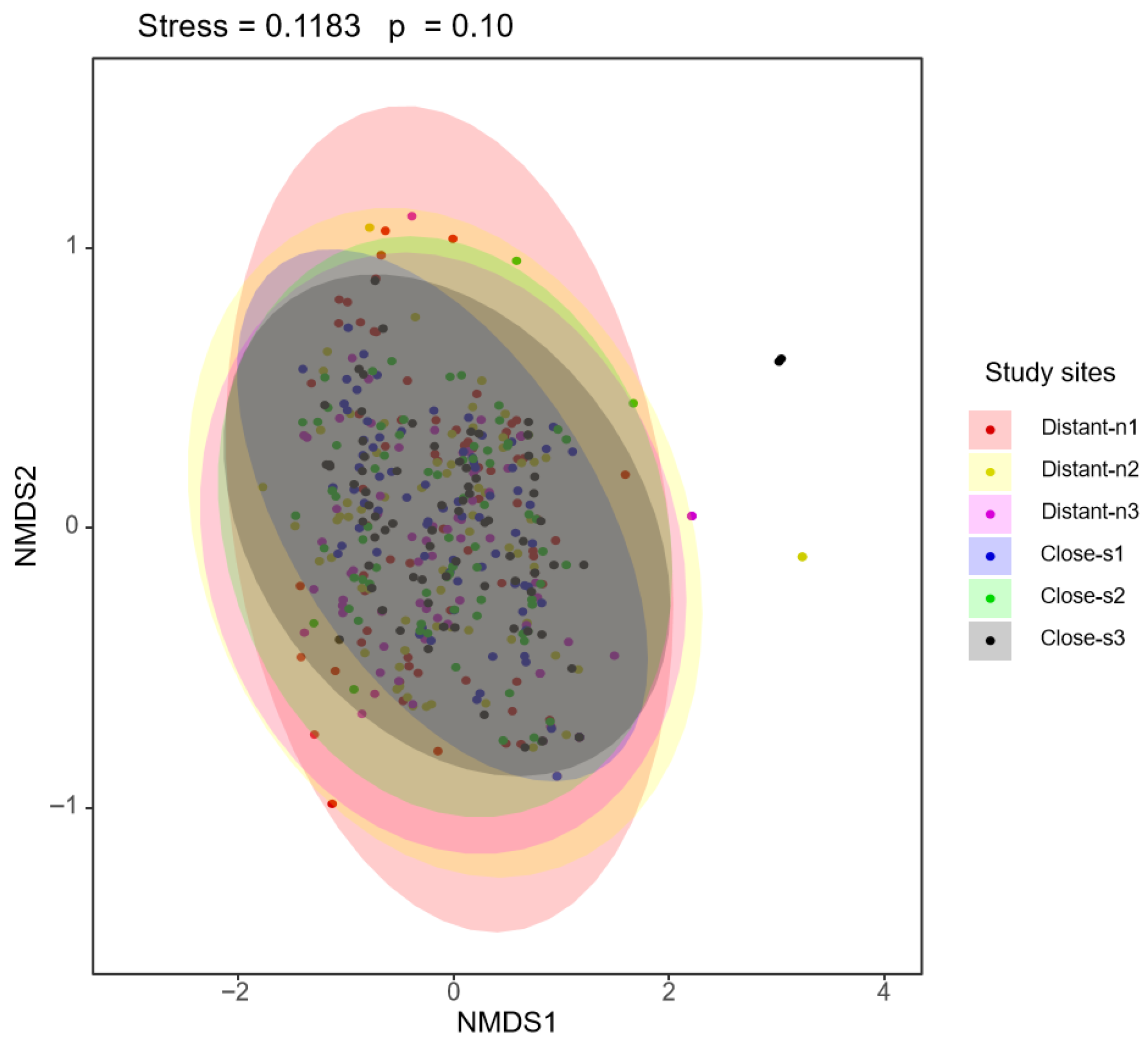
3. Results
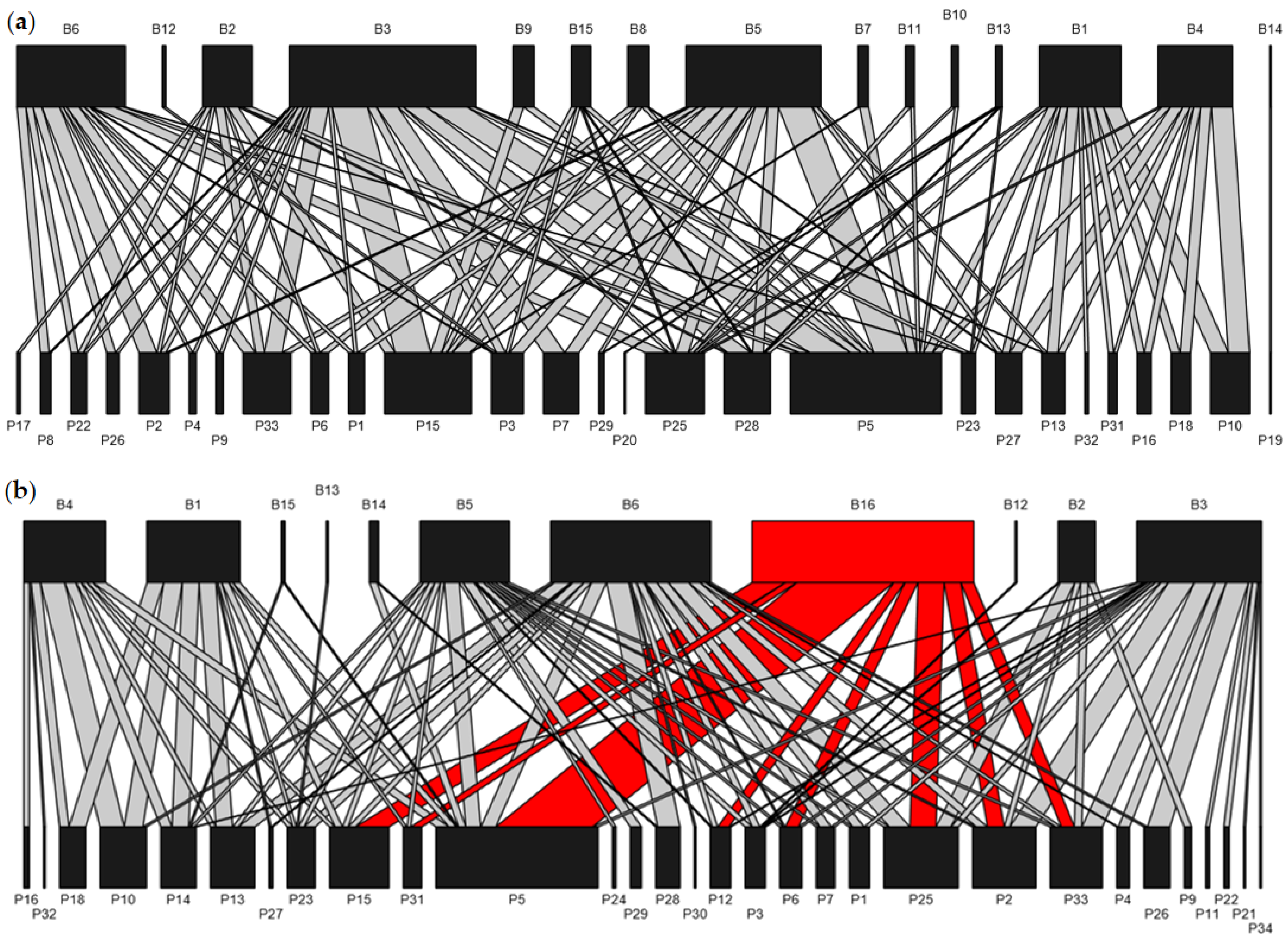
3.1. The Plant–Bee Interaction Network
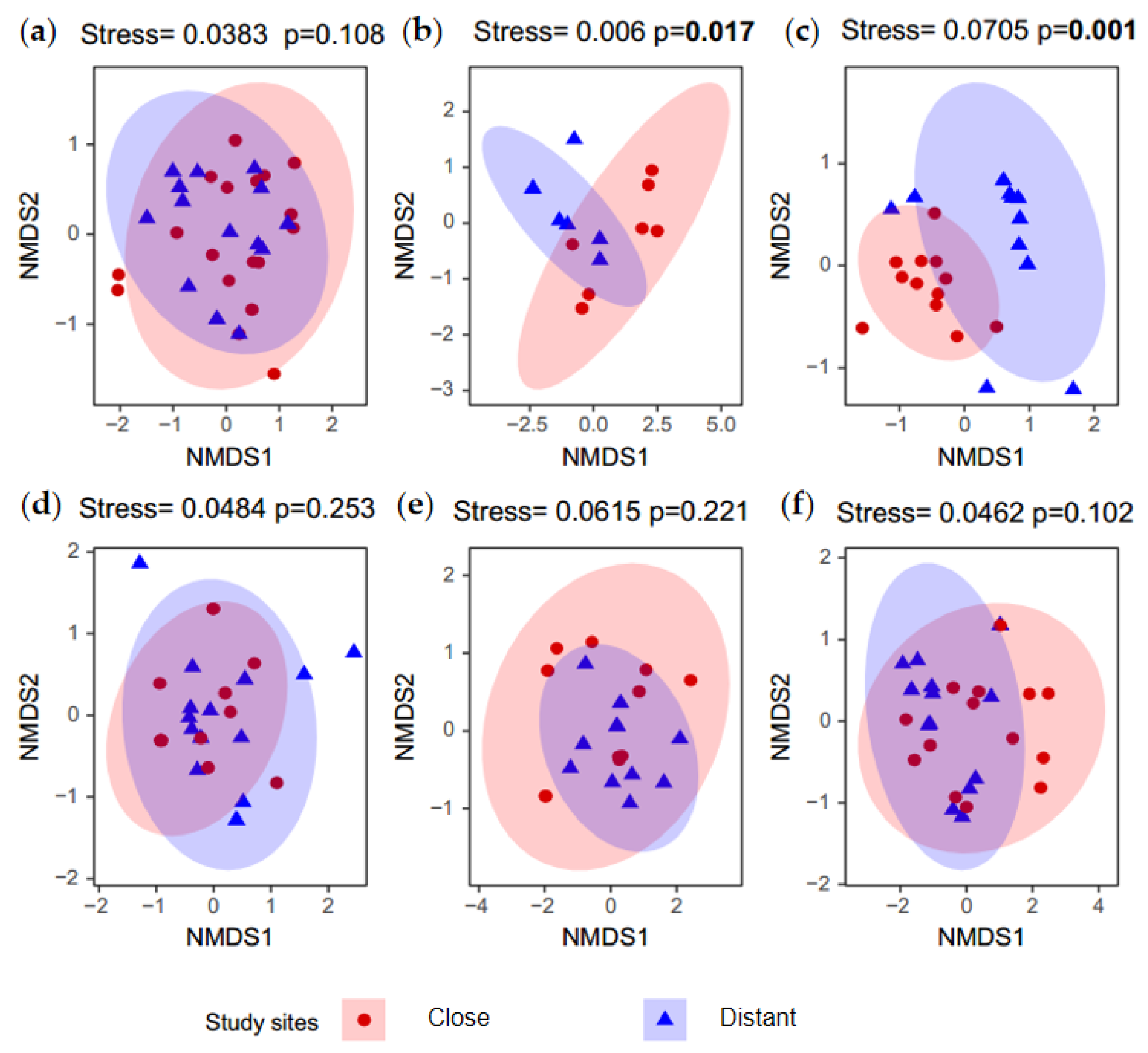
3.2. The Difference in Native Bee Species Abundance Between Nearby and Distant Plots
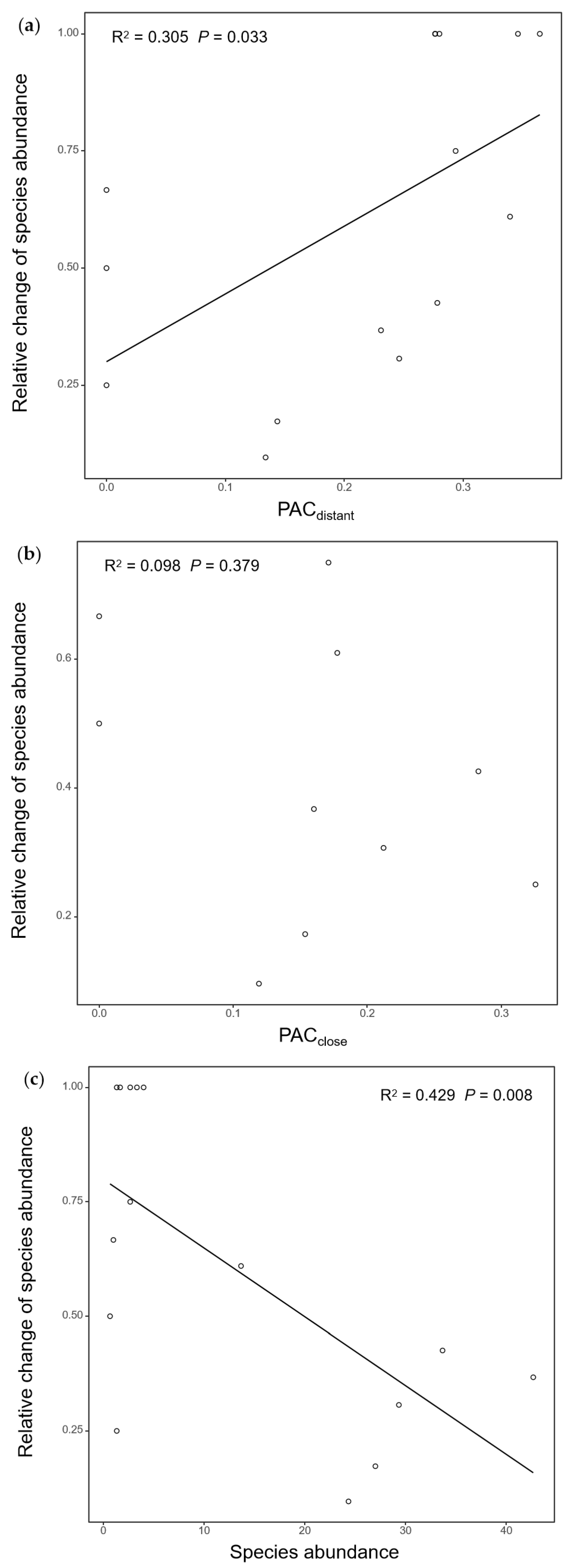
3.3. PAC and Species Abundance on Relative Change in Bee Abundance
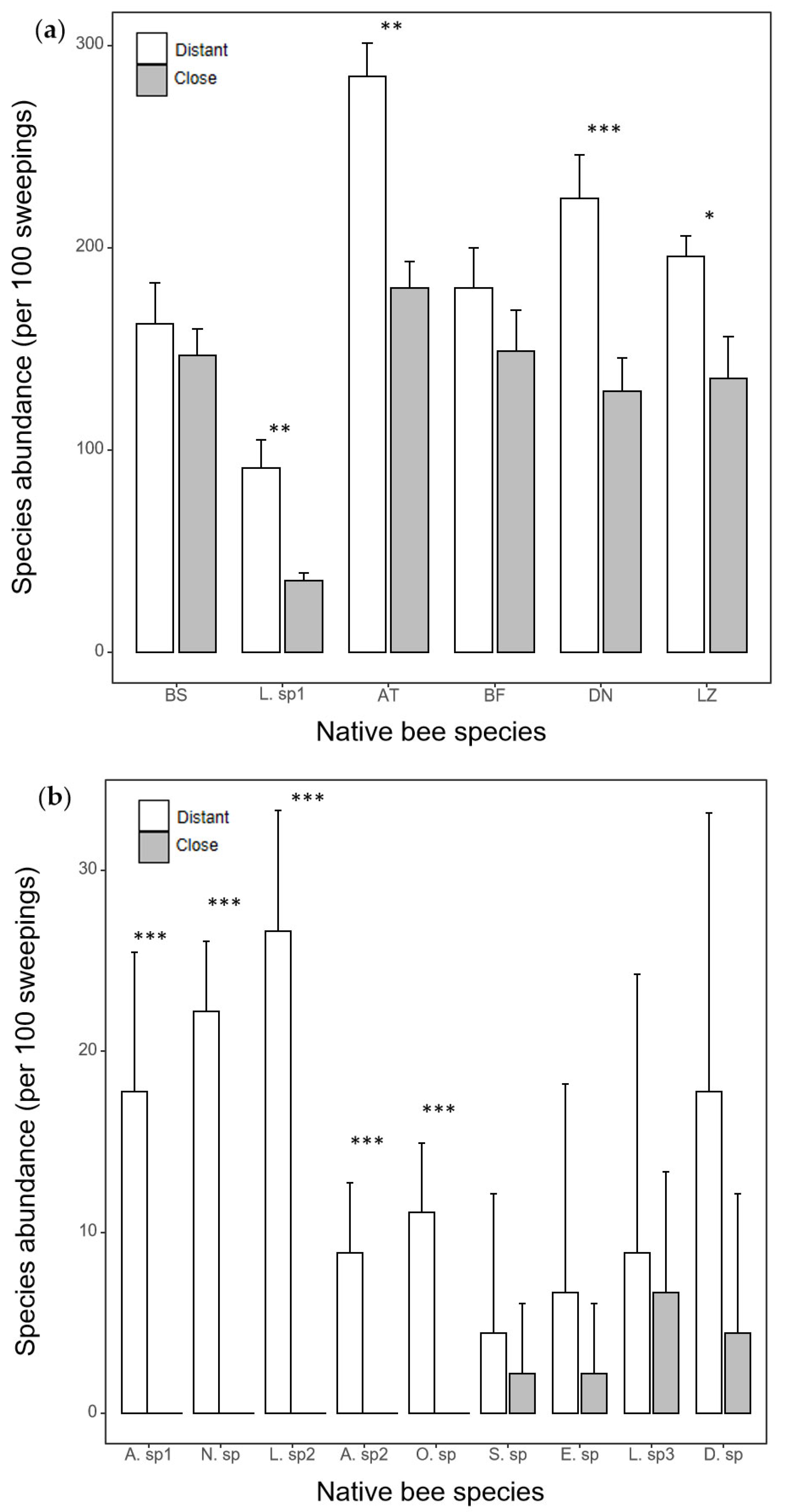
3.4. The Shift in the Feeding Niche of Native Bees
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| ID | Family | Species | Abbreviation |
|---|---|---|---|
| B1 | Apidae | Bombus supremus | BS |
| B2 | Halictidae | Lasioglossum sp1 | L. sp1 |
| B3 | Andrenidae | Andrena tarsata | AT |
| B4 | Apidae | Bombus filchnerae | BF |
| B5 | Halictidae | Dufourea novaeangliae | DN |
| B6 | Halictidae | Lasioglossum zonulum | LZ |
| B7 | Andrenidae | Andrena sp1 | A. sp1 |
| B8 | Apidae | Nomada sp. | N. sp. |
| B9 | Halictidae | Lasioglossum sp2 | L. sp2 |
| B10 | Andrenidae | Andrena sp2 | A. sp2 |
| B11 | Megachilidae | Osmia sp. | O. sp. |
| B12 | Halictidae | Sphecodes sp. | S. sp. |
| B13 | Apidae | Epeolus sp. | E. sp. |
| B14 | Halictidae | Lasioglossum sp3 | L. sp3 |
| B15 | Halictidae | Dufourea sp. | D. sp. |
| ID | Family | Species |
|---|---|---|
| P1 | Gentianaceae | Gentiana abaensis |
| P2 | Ranunculaceae | Trollius farreri |
| P3 | Apiaceae | Angelica dahurica |
| P4 | Ranunculaceae | Anemone rivularis |
| P5 | Asteraceae | Saussurea nigrescens |
| P6 | Apiaceae | Angelica apaensis |
| P7 | Apiaceae | Semenovia malcolmii |
| P8 | Rosaceae | Potentilla anserina |
| P9 | Rosaceae | Potentilla discolor |
| P10 | Orobanchaceae | Pedicularis kansuensis |
| P11 | Apiaceae | Carum carvi |
| P12 | Geraniaceae | Geranium pylzowianum |
| P13 | Amaryllidaceae | Allium sikkimense |
| P14 | Fabaceae | Oxytropis kansuensis |
| P15 | Amaryllidaceae | Allium chrysanthum |
| P16 | Gentianaceae | Halenia elliptica |
| P17 | Gentianaceae | Comastoma pulmonarium |
| P18 | Orobanchaceae | Pedicularis longiflora |
| P19 | Asteraceae | Leontopodium souliei |
| P20 | Rosaceae | Dasiphora fruticosa |
| P21 | Rosaceae | Geum japonicum |
| P22 | Ranunculaceae | Ranunculus tanguticus |
| P23 | Gentianaceae | Gentiana formosa |
| P24 | Geraniaceae | Geranium pratense |
| P25 | Asteraceae | Taraxacum sikkimense |
| P26 | Ranunculaceae | Anemone trullifolia |
| P27 | Fabaceae | Oxytropis glabra |
| P28 | Asteraceae | Ajania przewalskii |
| P29 | Asteraceae | Anaphalis flavescens |
| P30 | Asteraceae | Saussurea stella |
| P31 | Fabaceae | Hedysarum tanguticum |
| P32 | Orobanchaceae | Pedicularis sp. |
| P33 | Asteraceae | Aster alpinus |
| P34 | Polygonaceae | Polygonum viviparum |
Appendix B
References
- Crane, E. Recent research on the world history of beekeeping. Bee World 1999, 80, 174–186. [Google Scholar] [CrossRef]
- Requier, F.; Garnery, L.; Kohl, P.L.; Njovu, H.K.; Pirk, C.W.W.; Crewe, R.M.; Steffan-Dewenter, I. The Conservation of Native Honey Bees Is Crucial. Trends Ecol. Evol. 2019, 34, 789–798. [Google Scholar] [CrossRef] [PubMed]
- Yang, G. Harm of introducing the western honeybee Apis mellifera L. to the Chinese honeybee Apis cerana F. and its ecolocjcal impact. Acta Entomol. Sin. 2005, 42, 401–406. [Google Scholar] [CrossRef]
- Chen, C.; Liu, Z.; Pan, Q.; Chen, X.; Wang, H.; Guo, H.; Liu, S.; Lu, H.; Tian, S.; Li, R.; et al. Genomic Analyses Reveal Demographic History and Temperate Adaptation of the Newly Discovered Honey Bee Subspecies Apis mellifera sinisxinyuan n. ssp. Mol. Biol. Evol. 2016, 33, 1337–1348. [Google Scholar] [CrossRef] [PubMed]
- Goulson, D. Effects of introduced bees on native ecosystems. Annu. Rev. Ecol. Evol. Syst. 2003, 34, 1–26. [Google Scholar] [CrossRef]
- Paini, D.R. Impact of the introduced honey bee (Apis mellifera) (Hymenoptera: Apidae) on native bees:: A review. Austral Ecol. 2004, 29, 399–407. [Google Scholar] [CrossRef]
- Roubik, D.W.; Villanueva-Gutierrez, R. Invasive Africanized honey bee impact on native solitary bees: A pollen resource and trap nest analysis. Biol. J. Linn. Soc. 2009, 98, 152–160. [Google Scholar] [CrossRef]
- Thomson, D.M. Detecting the effects of introduced species: A case study of competition between Apis and Bombus. Oikos 2006, 114, 407–418. [Google Scholar] [CrossRef]
- Goulson, D. Bumblebees: Bahavior, Ecology, and Conservation; Online edn; Oxford Academic: Oxford, UK, 2023. [Google Scholar] [CrossRef]
- Suzuki, K.; Dohzono, I.; Hiei, K. Evolution of pollinator generalization in bumblebee-pollinated plants. Plant Species Biol. 2007, 22, 141–159. [Google Scholar] [CrossRef]
- Page, M.L.; Williams, N.M. Evidence of exploitative competition between honey bees and native bees in two California landscapes. J. Anim. Ecol. 2023, 92, 1802–1814. [Google Scholar] [CrossRef]
- Stenc, J.; Janosik, L.; Freudenfeld, M.; Matouskova, E.; Hadrava, J.; Mikat, M.; Dankova, K.; Hadravova, T.; Rysan, T.; Simonova, J.; et al. Pollen presentation mitigates competition for pollinators due to diurnal stratification of pollen transfer. Perspect. Plant Ecol. Evol. Syst. 2025, 67, 125868. [Google Scholar] [CrossRef]
- Thomas, C.D.; Mallorie, H.C. Rarity, species richness and conservation-butterflies of the Atlas Mountains in Morocco. Biol. Conserv. 1985, 33, 95–117. [Google Scholar] [CrossRef]
- Kunin, W.E.; Gaston, K.J. The biology of rarity-patterns, causes and consequences. Trends Ecol. Evol. 1993, 8, 298–301. [Google Scholar] [CrossRef]
- Valido, A.; Rodriguez-Rodriguez, M.C.; Jordano, P. Honeybees disrupt the structure and functionality of plant-pollinator networks. Sci. Rep. 2019, 9, 4711. [Google Scholar] [CrossRef]
- Magrach, A.; Gonzalez-Varo, J.P.; Boiffier, M.; Vila, M.; Bartomeus, I. Honeybee spillover reshuffles pollinator diets and affects plant reproductive success. Nat. Ecol. Evol. 2017, 1, 1299–1307. [Google Scholar] [CrossRef]
- Wang, L.-L.; Huang, Z.Y.; Dai, W.-F.; Yang, Y.-P.; Duan, Y.-W. Mixed effects of honey bees on pollination function in the Tibetan alpine grasslands. Nat. Commun. 2024, 15, 8164. [Google Scholar] [CrossRef] [PubMed]
- Montero-Castano, A.; Vila, M. Influence of the honeybee and trait similarity on the effect of a non-native plant on pollination and network rewiring. Funct. Ecol. 2017, 31, 142–152. [Google Scholar] [CrossRef]
- Thomson, D.M. Local bumble bee decline linked to recovery of honey bees, drought effects on floral resources. Ecol. Lett. 2016, 19, 1247–1255. [Google Scholar] [CrossRef] [PubMed]
- Macior, L.W.; Ya, T.; Zhang, J. Reproductive biology of Pedicularis (Scrophulariaceae) in the Sichuan Himalaya. Plant Species Biol. 2001, 16, 83–89. [Google Scholar] [CrossRef]
- Hu, X.; Zhou, W.; Li, X.; Niklas, K.J.; Sun, S. Changes in Community Composition Induced by Experimental Warming in an Alpine Meadow: Beyond Plant Functional Type. Front. Ecol. Evol. 2021, 9, 569422. [Google Scholar] [CrossRef]
- Wu, X.; Duffy, J.E.; Reich, P.B.; Sun, S. A brown-world cascade in the dung decomposer food web of an alpine meadow: Effects of predator interactions and warming. Ecol. Monogr. 2011, 81, 313–328. [Google Scholar] [CrossRef]
- Peng, Y.; Dong, Y.; Xu, H.; Xi, X.; Niklas, K.J.; Sun, S. Domesticated honeybees facilitate interspecific hybridization between two (Taraxacum) congeners. J. Ecol. 2018, 106, 1204–1216. [Google Scholar] [CrossRef]
- Mu, J.; Peng, Y.; Xi, X.; Wu, X.; Griffin, J.N.; Niklas, K.J.; Sun, S. Domesticated honey bees evolutionarily reduce flower nectar volume in a Tibetan lotus. Ecology 2014, 95, 3161–3172. [Google Scholar] [CrossRef]
- Zhao, Y.-H.; Lazaro, A.; Ren, Z.-X.; Zhou, W.; Li, H.-D.; Tao, Z.-B.; Xu, K.; Wu, Z.-K.; Wolfe, L.M.; Li, D.-Z.; et al. The topological differences between visitation and pollen transport networks: A comparison in species rich communities of the Himalaya-Hengduan Mountains. Oikos 2019, 128, 551–562. [Google Scholar] [CrossRef]
- Dormann, C.F.; Fründ, J.; Blüthgen, N.; Gruber, B. Indices, Graphs and Null Models: Analyzing Bipartite Ecological Networks. Open Ecol. J. 2009, 2, 7–24. [Google Scholar] [CrossRef]
- Dormann, C.F. How to be a specialist? Quantifying specialisation in pollination networks. Netw. Biol. 2011, 1, 1–20. [Google Scholar]
- Morris, R.J.; Lewis, O.T.; Godfray, H.C.J. Apparent competition and insect community structure: Towards a spatial perspective. Ann. Zool. Fenn. 2005, 42, 449–462. [Google Scholar]
- Müller, C.B.; Adriaanse, I.C.T.; Belshaw, R.; Godfray, H.C.J. The structure of an aphid–parasitoid community. J. Anim. Ecol. 1999, 68, 346–370. [Google Scholar] [CrossRef]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D. R Package, Version 2.5–4; Vegan: Community Ecology Package. Available online: https://CRAN.R-project.org/package=vegan (accessed on 14 August 2025).
- Carvell, C.; Bourke, A.F.G.; Dreier, S.; Freeman, S.N.; Hulmes, S.; Jordan, W.C.; Redhead, J.W.; Sumner, S.; Wang, J.; Heard, M.S. Bumblebee family lineage survival is enhanced in high-quality landscapes. Nature 2017, 543, 547–549. [Google Scholar] [CrossRef]
- Goodell, K. Food availability affects Osmia pumila (Hymenoptera: Megachilidae) foraging, reproduction, and brood parasitism. Oecologia 2003, 134, 518–527. [Google Scholar] [CrossRef]
- Williams, N.M.; Kremen, C. Resource distributions among habitats determine solitary bee offspring production in a mosaic landscape. Ecol. Appl. 2007, 17, 910–921. [Google Scholar] [CrossRef]
- Crone, E.E.; Williams, N.M. Bumble bee colony dynamics: Quantifying the importance of land use and floral resources for colony growth and queen production. Ecol. Lett. 2016, 19, 460–468. [Google Scholar] [CrossRef] [PubMed]
- Malfi, R.L.; Crone, E.; Williams, N. Demographic benefits of early season resources for bumble bee (B. vosnesenskii) colonies. Oecologia 2019, 191, 377–388. [Google Scholar] [CrossRef] [PubMed]
- Thomson, D.M.; Page, M.L. The importance of competition between insect pollinators in the Anthropocene. Curr. Opin. Insect Sci. 2020, 38, 55–62. [Google Scholar] [CrossRef]
- Forrest, J.R.K.; Chisholm, S.P.M. Direct benefits and indirect costs of warm temperatures for high-elevation populations of a solitary bee. Ecology 2017, 98, 359–369. [Google Scholar] [CrossRef] [PubMed]
- Steffan-Dewenter, I.; Schiele, S. Do resources or natural enemies drive bee population dynamics in fragmented habitats? Ecology 2008, 89, 1375–1387. [Google Scholar] [CrossRef]
- Thomson, D. Competitive interactions between the invasive European honey bee and native bumble bees. Ecology 2004, 85, 458–470. [Google Scholar] [CrossRef]
- Hudewenz, A.; Klein, A.-M. Red mason bees cannot compete with honey bees for floral resources in a cage experiment. Ecol. Evol. 2015, 5, 5049–5056. [Google Scholar] [CrossRef]
- Di Pasquale, G.; Salignon, M.; Le Conte, Y.; Belzunces, L.P.; Decourtye, A.; Kretzschmar, A.; Suchail, S.; Brunet, J.-L.; Alaux, C. Influence of Pollen Nutrition on Honey Bee Health: Do Pollen Quality and Diversity Matter? PLoS ONE 2013, 8, e72016. [Google Scholar] [CrossRef]
- Fruend, J.; Dormann, C.F.; Holzschuh, A.; Tscharntke, T. Bee diversity effects on pollination depend on functional complementarity and niche shifts. Ecology 2013, 94, 2042–2054. [Google Scholar] [CrossRef]
- Roulston, T.H.; Cane, J.H. Pollen nutritional content and digestibility for animals. Plant Syst. Evol. 2000, 222, 187–209. [Google Scholar] [CrossRef]
- Spiesman, B.J.; Gratton, C. Flexible foraging shapes the topology of plant-pollinator interaction networks. Ecology 2016, 97, 1431–1441. [Google Scholar] [CrossRef]
- Zurbuchen, A.; Cheesman, S.; Klaiber, J.; Mueller, A.; Hein, S.; Dorn, S. Long foraging distances impose high costs on offspring production in solitary bees. J. Anim. Ecol. 2010, 79, 674–681. [Google Scholar] [CrossRef]
- Goodell, K. The Impact of Introduced Honey Bees on Native Solitary Bees: Competition and Indirect Effects. Ph.D. Thesis, State University of New York at Stony Brook, Stony Brook, NY, USA, 2000. [Google Scholar]
- Walther-Hellwig, K.; Fokul, G.; Frankl, R.; Buechler, R.; Ekschmitt, K.; Wolters, V. Increased density of honeybee colonies affects foraging bumblebees. Apidologie 2006, 37, 517–532. [Google Scholar] [CrossRef]
- Lande, R. Risks of population extinction from demographic and environmental stochasticity and random catastrophes. Am. Nat. 1993, 142, 911–927. [Google Scholar] [CrossRef]
- Magrach, A.; Holzschuh, A.; Bartomeus, I.; Riedinger, V.; Roberts, S.P.M.; Rundlof, M.; Vujic, A.; Wickens, J.B.; Wickens, V.J.; Bommarco, R.; et al. Plant-pollinator networks in semi-natural grasslands are resistant to the loss of pollinators during blooming of mass-flowering crops. Ecography 2018, 41, 62–74. [Google Scholar] [CrossRef]
- Gong, Z.T. Chinese Soil Taxonomy: Theory, Method and Application; Science Press: Beijing, China; New York, NY, USA, 1999; pp. 1–903. [Google Scholar]
| Response | Predictor | Estimate | df | p |
|---|---|---|---|---|
| Relative change of species abundance | Species abundance | −0.024 | 1 | 0.036 * |
| PACD | 1.476 | 1 | 0.002 * | |
| Interaction | 0.036 | 1 | 0.432 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
An, R.; Sun, S. Introduced Western Honeybees Dramatically Reduce the Abundance of Wild Bees in Alpine Meadows, Eastern Tibet Plateau. Biology 2025, 14, 1186. https://doi.org/10.3390/biology14091186
An R, Sun S. Introduced Western Honeybees Dramatically Reduce the Abundance of Wild Bees in Alpine Meadows, Eastern Tibet Plateau. Biology. 2025; 14(9):1186. https://doi.org/10.3390/biology14091186
Chicago/Turabian StyleAn, Ruimin, and Shucun Sun. 2025. "Introduced Western Honeybees Dramatically Reduce the Abundance of Wild Bees in Alpine Meadows, Eastern Tibet Plateau" Biology 14, no. 9: 1186. https://doi.org/10.3390/biology14091186
APA StyleAn, R., & Sun, S. (2025). Introduced Western Honeybees Dramatically Reduce the Abundance of Wild Bees in Alpine Meadows, Eastern Tibet Plateau. Biology, 14(9), 1186. https://doi.org/10.3390/biology14091186







