Camel (Camelus dromedarius L. and Camelus bactrianus L.) Milk Composition and Effects on Human Type 1 and Type 2 Diabetes Mellitus: A Review
Simple Summary
Abstract
1. Introduction
- Inhibition of angiotensin-converting enzyme (ACE), indicating a potential role in blood pressure regulation.
- Antiproliferative activity against immortalized cancer cell lines, supporting its potential anticancer effects.
- Amelioration of metabolic symptoms associated with diabetes, including the following:
- Reduction in fasting blood glucose levels;
- Decreased insulin resistance, as measured by the Homeostasis Model Assessment of Insulin Resistance (HOMA-IR);
- Improvement of blood lipid profiles, notably through the reduction of triglycerides and cholesterol;
- Decreased plasma levels of LDL-C (Low-Density Lipoprotein Cholesterol) and VLDL-C (Very-Low-Density Lipoprotein Cholesterol), along with an increase in HDL-C (High-Density Lipoprotein Cholesterol) concentrations.
- Anticoagulant and antithrombotic properties, evidenced by the following:
- Reduction in coagulopathy manifestations;
- Improved platelet function.
- Antioxidant capacity, as indicated by the reduction in oxidative stress markers;
- Regenerative effects on pancreatic β-cells, potentially contributing to glycemic control in diabetic patients;
- Hepatoprotective and nephroprotective activities, including the attenuation of liver injuries and steatohepatitis, as well as protection against renal damage.
2. Materials and Methods
Bibliographic Search Description
3. Camel Milk Production
| Region/Country | Milk Yield (Liters per Lactation) | Lactation Duration | No. of Animals | Ref. |
|---|---|---|---|---|
| Mongolia | 477 | Up to 16 months | - | [10] |
| Africa | 1000–2700 | - | - | [11] |
| Egypt (Maghreb) | 1612 ± 710 | 353 ± 152 d | 43 (748 records) | [17] |
| Tunisia (Maghreb) | 2642 ± 523 | 390 d | 26 lactations | [18] |
| Ethiopia | 1123 | - | 5 | [19] |
| Pakistan | 2440–10,675 | 12–35 months | - | [21] |
4. Camel Milk’s Gross Composition
4.1. Protein
4.2. Fat
4.3. Lactose
4.4. Vitamins
4.5. Minerals
4.6. CM Composition Variation During Lactation
Camel Milk: A Putative Functional Food in Supporting Diabetes Mellitus?
5. Diabetes Mellitus in Humans: Classification and Prevalence
- Type 1 Diabetes Mellitus (T1DM): caused by autoimmune destruction of pancreatic β-cells, usually leading to absolute insulin deficiency. This category includes latent autoimmune diabetes in adults (LADA);
- Type 2 Diabetes Mellitus (T2DM): the most prevalent form of diabetes, characterized by insulin resistance combined with a relative and progressively worsening β-cell dysfunction. It is strongly associated with obesity, sedentary lifestyle, and genetic predisposition and often occurs in the context of metabolic syndrome;
- Specific types of diabetes due to other causes: these include monogenic forms of diabetes (e.g., neonatal diabetes, maturity-onset diabetes of the young [MODY]), diseases affecting the exocrine pancreas (e.g., cystic fibrosis, chronic pancreatitis), and diabetes secondary to medications or chemicals (e.g., prolonged glucocorticoid therapy, antiretroviral treatment, or immunosuppressive agents used in organ transplantation);
- Gestational diabetes mellitus (GDM): a form of hyperglycemia manifested during the second or third trimester of pregnancy, in individuals without previously diagnosed diabetes. It affects an estimated 21.1 million pregnancies worldwide, increasing the risk of complications for both mother and fetus, and elevating the mother’s future risk of developing T2DM.
6. Effects of Camel Milk on Diabetes Mellitus
6.1. Effects on Hyperglycemia
6.2. Effects on Hyperlipidemia
6.3. Effects on Diabetes Complications
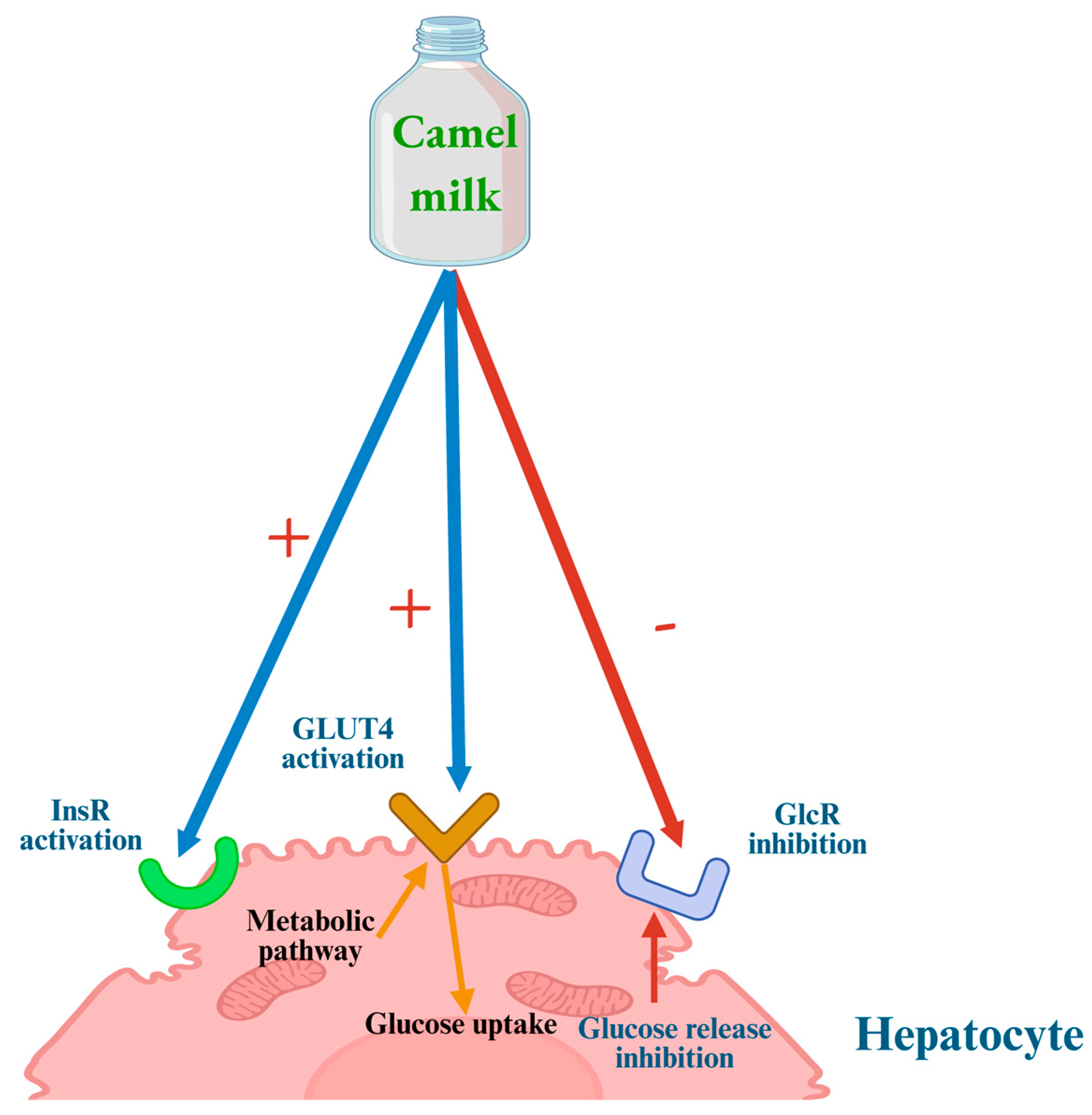
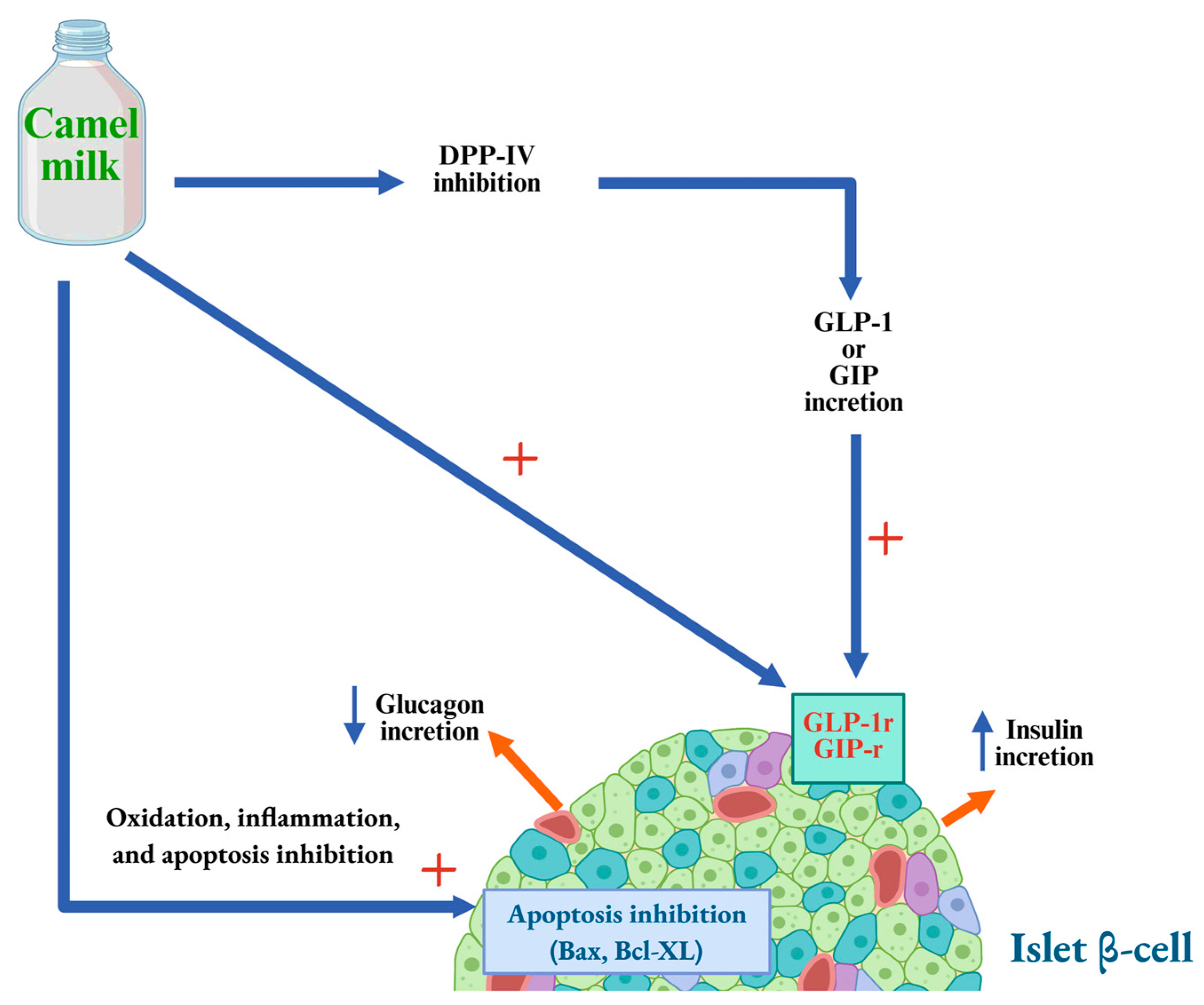
7. CM Anti-Diabetic Activity: Proposed Mechanisms of Action
7.1. Anti-Hyperglycemic Mechanisms
- (1)
- Direct modulation of insulin receptors and glucose transport across cell membranes;
- (2)
- Stimulation of insulin secretion by pancreatic β-cells through both direct and indirect mechanisms;
- (3)
- Support of pancreatic β-cell survival and function, thereby enhancing overall pancreatic activity.
7.2. Anti-Lipidemic Mechanisms
7.3. Antioxidant Mechanisms of CM
7.4. CM Wound Healing Mechanisms
7.5. Hepatoprotective Mechanisms of CM
7.6. CM Kidney Protective Mechanisms
7.7. The Role of Lactoferrin from CM
7.8. The Role of Protein-Derived Peptides
7.9. Camel Milk Exosomes: A Novel Nanocomponent in Glycemic Control and Diabetes Management
7.10. Non-Parenteral Administration of Insulin: Looking Forward
7.11. Additional Considerations: Potential Limitations, Safety Risks, and Regulatory Status
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CM | Camel milk |
| DM | Diabetes Mellitus |
| T1DM | Type 1 Diabetes Mellitus |
| T2DM | Type 2 Diabetes Mellitus |
References
- International Diabetes Federation. IDF Diabetes Atlas 2025; International Diabetes Federation: Brussels, Belgium, 2025. Available online: https://www.diabetesatlas.org (accessed on 12 July 2025).
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K.; et al. Global and Regional Diabetes Prevalence Estimates for 2019 and Projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th Edition. Diabetes Res. Clin. Pract. 2019, 157, 107843. [Google Scholar] [CrossRef]
- American Diabetes Association Professional Practice Committee. Diagnosis and Classification of Diabetes: Standards of Care in Diabetes-2025. Diabetes Care 2025, 48, S27–S49. [Google Scholar] [CrossRef]
- Mirzaei, M.; Alahyaribeik, S.; Mirdamadi, N.; Fang, Y.; Hazaveh, M.N. Chapter 20—The Role of Functional Foods in Diabetes Management. In Unleashing the Power of Functional Foods and Novel Bioactives; Sarkar, T., Smaoui, S., Petkoska, A.T., Eds.; Academic Press: Cambridge, MA, USA, 2025; pp. 397–422. ISBN 978-0-443-28862-3. [Google Scholar]
- Muthukumaran, M.S.; Mudgil, P.; Baba, W.N.; Ayoub, M.A.; Maqsood, S. A Comprehensive Review on Health Benefits, Nutritional Composition and Processed Products of Camel Milk. Food Rev. Int. 2023, 39, 3080–3116. [Google Scholar] [CrossRef]
- Seifu, E. Camel Milk Products: Innovations, Limitations and Opportunities. Food Prod. Process Nutr. 2023, 5, 15. [Google Scholar] [CrossRef]
- Korish, A.A.; Abdel Gader, A.G.M.; Alhaider, A.A. Comparison of the Hypoglycemic and Antithrombotic (Anticoagulant) Actions of Whole Bovine and Camel Milk in Streptozotocin-Induced Diabetes Mellitus in Rats. J. Dairy Sci. 2020, 103, 30–41. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Liu, L.-X.; Yang, J.; Liu, Y.-G. Exploration and Analysis of the Composition and Mechanism of Efficacy of Camel Milk. Food Biosci. 2023, 53, 102564. [Google Scholar] [CrossRef]
- Sharma, B.; Verma, P.; Singh, A.; Singh, T.P.; Sharma, S.; Sharma, P. The Multifaceted Therapeutic Properties of Camel Milk: From Neuroprotection to Anti-Cancer Effects. J. Food Sci. Technol. 2025, 62, 824–840. [Google Scholar] [CrossRef]
- El-Agamy, E.-S.I.; Milk, C. Handbook of Milk of Non-Bovine Mammals; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2017; pp. 409–480. ISBN 978-1-119-11031-6. [Google Scholar]
- Faye, B. Dairy Productivity Potential of Camels. Available online: https://agritrop.cirad.fr/559216/ (accessed on 8 July 2025).
- Faye, B.; Konuspayeva, G.; Magnan, C. L’élevage des Grands Camélidés; Ed. Quae: Versailles, France, 2022; ISBN 978-2-7592-3500-1. [Google Scholar]
- Faye, B. The Camel Today: Assets and Potentials. Anthropozoologica 2014, 49, 167–176. [Google Scholar] [CrossRef]
- Benmeziane-Derradji, F. Evaluation of Camel Milk: Gross Composition—A Scientific Overview. Trop. Anim. Health Prod. 2021, 53, 308. [Google Scholar] [CrossRef] [PubMed]
- Faye, B. Productivity Potential of Camels. Proc. of Intern. Workshop, «Desertification Combat and Food Safety: The Added Value of Camel Producers”. Ashkhabad (Turkmenistan), 19–22 April 2004. NATO Sci. Ser. Life Behav. Sci. 2005, 362, 127–134. [Google Scholar]
- Nagy, P.; Thomas, S.; Markó, O.; Juhász, J. Milk Production, Raw Milk Quality and Fertility of Dromedary Camels (Camelus dromedarius) under Intensive Management. Acta Vet. Hung. 2013, 61, 71–84. [Google Scholar] [CrossRef]
- Abdalla, E.B.; Anis Ashmawy, A.E.-H.; Farouk, M.H.; Abd El-Rahman Salama, O.; Khalil, F.A.; Seioudy, A.F. Milk Production Potential in Maghrebi She-Camels. Small Rumin. Res. 2015, 123, 129–135. [Google Scholar] [CrossRef]
- Jemmali, B.; Ferchichi, M.A.; Faye, B.; Kamoun, M. Milk Yield and Modeling of Lactation Curves of Tunisian She-Camel. Emir. J. Food Agric. 2016, 28, 1. [Google Scholar] [CrossRef]
- Richard, D.; Gérard, D. La Production Laitière Des Dromadaires Dankali (Ethiopie). Rev. D’élevage Médecine Vétérinaire Des Pays Trop. 1989, 42, 97–103. [Google Scholar] [CrossRef]
- Zhang, H.; Yao, J.; Zhao, D.; Liu, H.; Li, J.; Guo, M. Changes in Chemical Composition of Alxa Bactrian Camel Milk during Lactation. J. Dairy Sci. 2005, 88, 3402–3410. [Google Scholar] [CrossRef]
- Knoess, K.H.; Makhudum, A.J.; Rafiq, M.; Hafeez, M. Milk Production Potential of the Dromedary, with Special Reference to the Province of Punjab, Pakistan. World Anim. Rev. 1986, 57, 11–21. [Google Scholar]
- Roy, D.; Ye, A.; Moughan, P.J.; Singh, H. Composition, Structure, and Digestive Dynamics of Milk From Different Species—A Review. Front. Nutr. 2020, 7, 577759. [Google Scholar] [CrossRef] [PubMed]
- Farah, Z. Composition and Characteristics of Camel Milk. J. Dairy Res. 1993, 60, 603–626. [Google Scholar] [CrossRef]
- Ramet, J.P. La Technologie des Fromages au lait de Dromadaire (Camelus dromedarius); Food & Agriculture Org.: Rome, Italy, 1993; ISBN 978-92-5-203154-3. [Google Scholar]
- Shabo, Y.; Barzel, R.; Margoulis, M.; Yagil, R. Camel Milk for Food Allergies in Children. Isr. Med. Assoc. J. 2005, 7, 796–798. [Google Scholar] [PubMed]
- Magjeed, N.A.A. Corrective Effect of Milk Camel on Some Cancer Biomarkers in Blood of Rats Intoxicated with Aflatoxin B1. J. Saudi Chem. Soc. 2005, 9, 253–263. [Google Scholar]
- Agrawal, P.; Swami, S.; Kochar, D.; Sahani, M.; Tuteja, F.; Ghouri, S. Effect of Camel Milk on Glycemic Control, Risk Factors and Diabetes Quality of Life in Type-1 Diabetes: A Randomised Prospective Controlled Study. J. Camel Pract. Res. 2003, 10, 45–50. [Google Scholar]
- Konuspayeva, G.; Lemarie, É.; Faye, B.; Loiseau, G.; Montet, D. Fatty Acid and Cholesterol Composition of Camel’s (Camelus bactrianus, Camelus dromedarius and Hybrids) Milk in Kazakhstan. Dairy Sci. Technol. 2008, 88, 327–340. [Google Scholar] [CrossRef]
- Pastuszka, R.; Barłowska, J.; Litwińczuk, Z. Allergenicity of Milk of Different Animal Species in Relation to Human Milk. Postep. Hig. Med. Dosw. (Online) 2016, 70, 1451–1459. [Google Scholar] [CrossRef] [PubMed]
- Konuspayeva, G.; Faye, B.; Loiseau, G.; Levieux, D. Lactoferrin and Immunoglobulin Contents in Camel’s Milk (Camelus bactrianus, Camelus dromedarius, and Hybrids) from Kazakhstan. J. Dairy Sci. 2007, 90, 38–46. [Google Scholar] [CrossRef]
- Elagamy, E.I.; Ruppanner, R.; Ismail, A.; Champagne, C.P.; Assaf, R. Purification and Characterization of Lactoferrin, Lactoperoxidase, Lysozyme and Immunoglobulins from Camel’s Milk. Int. Dairy J. 1996, 6, 129–145. [Google Scholar] [CrossRef]
- Gnan, S.O.; Sheriha, A.M. Composition of Libyan Camel’s Milk. Aust. J. Dairy Technol. 1986, 41, 33–35. [Google Scholar]
- Konuspayeva, G.; Faye, B.; Loiseau, G. The Composition of Camel Milk: A Meta-Analysis of the Literature Data. J. Food Compos. Anal. 2009, 22, 95–101. [Google Scholar] [CrossRef]
- Al-Juboori, A.T.; Mohammed, M.; Rashid, J.; Kurian, J.; El Refaey, S. Nutritional and Medicinal Value of Camel (Camelus dromedarius) Milk. In Proceedings of the WIT Transactions on Ecology and The Environment; WIT Press: Budapest, Hungary, 2013. [Google Scholar]
- Ahmed, A.; Sayed, R. Nutritional Value and Sanitary Evaluation of Raw Camels Milk. Emir. J. Food Agric. 2014, 26, 317. [Google Scholar] [CrossRef]
- Fguiri, I.; Ziadi, M.; Sboui, A.; Ayeb, N.; Atigui, M.; Arroum, S.; Khorchani, T. Effect of the Production System and Stage of Lactation on the Microbiological and Biochemical Characteristics of Camel Milk. J. Camelid Sci. 2018, 11, 57–63. [Google Scholar]
- Bouhaddaoui, S.; Chabir, R.; Errachidi, F.; El Ghadraoui, L.; El Khalfi, B.; Benjelloun, M.; Soukri, A. Study of the Biochemical Biodiversity of Camel Milk. Sci. World J. 2019, 2019, 2517293. [Google Scholar] [CrossRef]
- Singh, R.; Mal, G.; Kumar, D.; Patil, N.; Pathak, K. Camel Milk: An Important Natural Adjuvant. Agric. Res. 2017, 6, 327–340. [Google Scholar] [CrossRef]
- Zennia, S.; Almi-Sebbane, D.; Chahra, S.; Boudjenah, S.; Mati, A. Separation and Characterization of Major Milk Proteins from Algerian Dromedary (Camelus dromedarius). Emir. J. Food Agric. 2012, 25, 257–264. [Google Scholar] [CrossRef]
- Mohamed, H.; Johansson, M.; Lundh, Å.; Nagy, P.; Kamal-Eldin, A. Short Communication: Caseins and α-Lactalbumin Content of Camel Milk (Camelus dromedarius) Determined by Capillary Electrophoresis. J. Dairy Sci. 2020, 103, 11094–11099. [Google Scholar] [CrossRef] [PubMed]
- Hinz, K.; O’Connor, P.M.; Huppertz, T.; Ross, R.P.; Kelly, A.L. Comparison of the Principal Proteins in Bovine, Caprine, Buffalo, Equine and Camel Milk. J. Dairy Res. 2012, 79, 185–191. [Google Scholar] [CrossRef]
- Ramet, J.-P.; Animal Production and Health Division. The Technology of Making Cheese from Camel Milk (Camelus dromedarius); Food & Agriculture Org.: Rome, Italy, 2001; ISBN 978-92-5-103154-4. [Google Scholar]
- El-Hatmi, H.; Girardet, J.-M.; Gaillard, J.-L.; Yahyaoui, M.H.; Attia, H. Characterisation of Whey Proteins of Camel (Camelus dromedarius) Milk and Colostrum. Small Rumin. Res. 2007, 70, 267–271. [Google Scholar] [CrossRef]
- Jilo, K. Medicinal Values of Camel Milk. Int. J. Vet. Sci. Res. 2016, 2, 018–025. [Google Scholar] [CrossRef]
- Yamina, M.; Chahrour, W.; Zarour, K.; Zergui, A.; Noureddine, S.; Eddine, H.; Mebrouk, K. Physico-Chemical and Microbiological Analysis of Algerian Raw Camel’s Milk and Identification of Predominating Thermophilic Lactic Acid Bacteria. J. Food Sci. Eng. 2013, 3, 55–63. [Google Scholar]
- Gorban, A.M.; Izzeldin, O.M. Fatty Acids and Lipids of Camel Milk and Colostrum. Int. J. Food Sci. Nutr. 2001, 52, 283–287. [Google Scholar] [CrossRef] [PubMed]
- Musaad Mustafa, A.; Faye, B.; Al-Mutairi, S. Seasonal and Physiological Variation of Gross Composition of Camel Milk in Saudi Arabia. Emir. J. Food Agric. 2013, 25, 618–624. [Google Scholar] [CrossRef]
- Mehaia, M.A.; Hablas, M.A.; Abdel-Rahman, K.M.; El-Mougy, S.A. Milk Composition of Majaheim, Wadah and Hamra Camels in Saudi Arabia. Food Chem. 1995, 52, 115–122. [Google Scholar] [CrossRef]
- Meribai, A.; Meklati, F.R.; Kouidri, A.; Nouani, A. Fatty Acid Profile Comparison and Hygienic Quality of Cow and Camel (Camelus dromedarius) Milk in Algeria. Emir. J. Food Agric. 2018, 30, 413–420. [Google Scholar] [CrossRef]
- Konuspayeva, G.; Faye, B.; Mussaad, A. Some Lipid Components of the Camel Milk and Blood in Intensive Farm in Saudi Arabia. Emir. J. Food Agric. 2013, 26, 367–371. [Google Scholar] [CrossRef]
- Ayadi, M.; Hammadi, M.; Casals, R.; Atigui, M.; Khorchani, T.; Samara, E.M.; Abdoun, K.A.; Al-Haidary, A.A.; Caja, G. Influence of Management Type and Stage of Lactation on the Performance and Milk Fatty Acid Profile of Dairy Camels (Camelus dromedaries). J. Agric. Sci. 2018, 156, 1111–1122. [Google Scholar] [CrossRef]
- Chamekh, L.; Calvo, M.; Khorchani, T.; Castro-Gómez, P.; Hammadi, M.; Fontecha, J.; Yahyaoui, M.H. Impact of Management System and Lactation Stage on Fatty Acid Composition of Camel Milk. J. Food Compos. Anal. 2020, 87, 103418. [Google Scholar] [CrossRef]
- Debouz, A.; Guerguer, L.; Hamid Oudjana, A.; Hadj Seyd, A. Etude comparative de la qualité physico-chimique et microbiologique du lait de vache et du lait camelin dans la wilaya de Ghardaïa. El-Wahat J. Res. Stud 2014, 7, 57–64. [Google Scholar]
- Shamsia, S.M. Nutritional and Therapeutic Properties of Camel and Human Milks. Int. J. Genet. Mol. Biol. 2009, 1, 052–058. [Google Scholar] [CrossRef]
- Shuiep, E.; El-Zubeir, I.; Musa, H.; El Owni, O. Influence of Season and Management on Composition of Raw Camel (Camelus dromedarius) Milk in Khartoum State, Sudan. Trop. Subtrop. Agroecosyst. 2008, 8, 101–106. [Google Scholar]
- Yoganandi, J.; Mehta, B.M.; Wadhwani, K.N.; Darji, V.B.; Aparnathi, K.D. Evaluation and Comparison of Camel Milk with Cow Milk and Buffalo Milk for Gross Composition. J. Camel Pract. Res. 2014, 21, 259. [Google Scholar] [CrossRef]
- Alaoui Ismaili, M.; Saidi, B.; Zahar, M.; Hamama, A.; Ezzaier, R. Composition and Microbial Quality of Raw Camel Milk Produced in Morocco. J. Saudi Soc. Agric. Sci. 2019, 18, 17–21. [Google Scholar] [CrossRef]
- Wang, S.Y.; Liang, J.P.; Shao, W.J.; Wen, H. Mineral, Vitamin and Fatty Acid Contents in the Camel Milk of Dromedaries in the Anxi Gansu China. J. Camel Pract. Res. 2011, 18, 273–276. [Google Scholar]
- Alkoofee, W.M. Retrospective Study on the Therapeutic Effects and Nutritional Values of Camels Milk. Adv. Anim. Vet. Sci. 2018, 6, 317–320. [Google Scholar] [CrossRef]
- Farah, Z.; Rettenmaier, R.; Atkins, D. Vitamin Content of Camel Milk. Int. J. Vitam. Nutr. Res. 1992, 62, 30–33. [Google Scholar] [PubMed]
- Konuspayeva, G.; Faye, B.; Loiseau, G.; Narmuratova, M.; Ivashchenko, A.; Meldebekova, A.; Davletov, S. Physiological Change in Camel Milk Composition (Camelus dromedarius) 1. Effect of Lactation Stage. Trop. Anim. Health Prod. 2009, 42, 495–499. [Google Scholar] [CrossRef] [PubMed]
- Mostafidi, M.; Moslehishad, M.; Piravivanak, Z.; Pouretedal, Z. Evaluation of Mineral Content and Heavy Metals of Dromedary Camel Milk in Iran. Food Sci. Technol. 2016, 36, 717–723. [Google Scholar] [CrossRef]
- Al-Wabel, N.A. Mineral Contents of Milk of Cattle, Camels, Goats and Sheep in the Central Region of Saudi Arabia. Asian J. Biochem. 2008, 3, 373–375. [Google Scholar] [CrossRef][Green Version]
- Gorban, A.M.; Izzeldin, O.M. Mineral Content of Camel Milk and Colostrum. J. Dairy Res. 1997, 64, 471–474. [Google Scholar] [CrossRef]
- Alhaj, O.A.; Altooq, N.J.; Alenezi, A.F.; Janahi, A.I.; Janahi, M.I.; Humood, A.M.; AlRasheed, M.M.; Bragazzi, N.L.; Jahrami, H.A.; Faye, B. Camel Milk Composition by Breed, Season, Publication Year, and Country: A Global Systematic Review, Meta-Analysis, and Meta-Regression. Compr. Rev. Food Sci. Food Saf. 2022, 21, 2520–2559. [Google Scholar] [CrossRef]
- Konuspayeva, G.; Faye, B.; Loiseau, G.; Narmuratova, M.; Ivashchenko, A.; Meldebekova, A.; Davletov, S. Physiological Change in Camel Milk Composition (Camelus dromedarius) 2: Physico-Chemical Composition of Colostrum. Trop. Anim. Health Prod. 2010, 42, 501–505. [Google Scholar] [CrossRef]
- Yagil, R.; van Creveld, C. Medicinal Use of Camel Milk: Fact or Fancy? In Proceedings of the 2nd International Camelid Conference: Agro-Economics of Camelid Farming, Almaty, Kazakhstan, 8 September 2000; p. 80. [Google Scholar]
- Merin, U.; Bernstein, S.; Bloch-Damti, A.; Yagil, R.; Creveld, C.; Lindner, P.; Gollop, N. A Comparative Study of Milk Serum Proteins in Camel (Camelus dromedarius) and Bovine Colostrum. Livest. Prod. Sci. 2001, 67, 297–301. [Google Scholar] [CrossRef]
- Al-Alawi, A.A.; Laleye, L.C. Characterization of Camel Milk Protein Isolates as Nutraceutical and Functional Ingredients. Collaborative Research Project SQU; UAEU CL/SQU-UAEU/01/08 SQU/UAEU 01-06-60/08; Sultan Qaboos University: Seeb, Oman, 2011. [Google Scholar]
- World Health Organization. Mean Fasting Blood Glucose. Available online: https://www.who.int/data/gho/indicator-metadata-registry/imr-details/2380 (accessed on 9 July 2025).
- Lindström, J.; Ilanne-Parikka, P.; Peltonen, M.; Aunola, S.; Eriksson, J.G.; Hemiö, K.; Hämäläinen, H.; Härkönen, P.; Keinänen-Kiukaanniemi, S.; Laakso, M.; et al. Sustained Reduction in the Incidence of Type 2 Diabetes by Lifestyle Intervention: Follow-up of the Finnish Diabetes Prevention Study. Lancet 2006, 368, 1673–1679. [Google Scholar] [CrossRef]
- Magliano, D.J.; Boyko, E.J.; IDF Diabetes Atlas 10th edition scientific committee. IDF Diabetes Atlas, 10th ed.; International Diabetes Federation: Brussels, Belgium, 2021; ISBN 978-2-930229-98-0. [Google Scholar]
- Khaliq, A.; Mishra, A.K.; Niroula, A.; Baba, W.N.; Shaukat, M.N.; Rabbani, A. An Updated Comprehensive Review of Camel Milk: Composition, Therapeutic Properties, and Industrial Applications. Food Biosci. 2024, 62, 105531. [Google Scholar] [CrossRef]
- Agrawal, R.P.; Budania, S.; Sharma, P.; Gupta, R.; Kochar, D.K.; Panwar, R.B.; Sahani, M.S. Zero Prevalence of Diabetes in Camel Milk Consuming Raica Community of North-West Rajasthan, India. Diabetes Res. Clin. Pract. 2007, 76, 290–296. [Google Scholar] [CrossRef] [PubMed]
- Mullaicharam, A.R. A Review on Medicinal Properties of Camel Milk. World J. Pharm. Sci. 2014, 2, 237–242. [Google Scholar]
- Agrawal, R.; Kochar, D.; Sahani, M.; Tuteja, F.; Ghorui, S. Hypoglycemic Activity of Camel Milk in Streptozotocin Induced Diabetic Rats. Int. J. Diabetes Dev. Ctries. 2004, 24, 47–49. [Google Scholar]
- Agrawal, R.P.; Saran, S.; Sharma, P.; Gupta, R.P.; Kochar, D.K.; Sahani, M.S. Effect of Camel Milk on Residual Beta-Cell Function in Recent Onset Type 1 Diabetes. Diabetes Res. Clin. Pract. 2007, 77, 494–495. [Google Scholar] [CrossRef] [PubMed]
- Hamers-Casterman, C.; Atarhouch, T.; Muyldermans, S.; Robinson, G.; Hamers, C.; Songa, E.B.; Bendahman, N.; Hamers, R. Naturally Occurring Antibodies Devoid of Light Chains. Nature 1993, 363, 446–448. [Google Scholar] [CrossRef]
- Malik, A.; Al-Senaidy, A.; Skrzypczak-Jankun, E.; Jankun, J. A Study of the Anti-Diabetic Agents of Camel Milk. Int. J. Mol. Med. 2012, 30, 585–592. [Google Scholar] [CrossRef]
- Al-Numair, K.S.; Chandramohan, G.; Alsaif, M.A. Effect of Camel Milk on Collagen Abnormalities in Streptozotocin-Diabetic Rats. Afr. J. Pharm. Pharmacol. 2011, 5, 238–243. [Google Scholar] [CrossRef][Green Version]
- Mohieldein, A.; Khan, A.; Alzohairy, M. Antidiabetic Effects of Camel Milk in Streptozotocin-Induced Diabetic Rats. Am. J. Biochem. Mol. Biol. 2013, 3, 151–158. [Google Scholar] [CrossRef]
- Badr, G. Camel Whey Protein Enhances Diabetic Wound Healing in a Streptozotocin-Induced Diabetic Mouse Model: The Critical Role of β-Defensin-1, -2 and -3. Lipids Health Dis. 2013, 12, 46. [Google Scholar] [CrossRef]
- Sboui, A.; Djegham, M.; Khorchani, T.; Hammadi, M.; Barhoumi, K.; Belhadj, O. Effect of Camel Milk on Blood Glucose, Cholesterol and Total Proteins Variationsin Alloxan-Induced Diabetic Dogs. Int. J. Diabetes Metab. 2010, 18, 5–11. [Google Scholar] [CrossRef]
- Arain, M.A.; Khaskheli, G.B.; Barham, G.S.; Shah, Q.A.; Nabi, F.; Almutairi, M.H.; Almutairi, B.O.; Marghazani, I.B. Exploring the Anti-Diabetic Properties of Camel Milk: Effects on Blood Glucose, Antioxidant Defense, and Organ Histo-Morphological Features in Rabbits. J. Mol. Histol. 2025, 56, 92. [Google Scholar] [CrossRef]
- Mohamad, R.H.; Zekry, Z.K.; Al-Mehdar, H.A.; Salama, O.; El-Shaieb, S.E.; El-Basmy, A.A.; Al-said, M.G.A.M.; Sharawy, S.M. Camel Milk as an Adjuvant Therapy for the Treatment of Type 1 Diabetes: Verification of a Traditional Ethnomedical Practice. J. Med. Food 2009, 12, 461–465. [Google Scholar] [CrossRef]
- Fallah, Z.; Ejtahed, H.-S.; Mirmiran, P.; Niasari Naslaji, A.; Moosavi Movahedi, A.; Azizi, F. Effect of Camel Milk on Glycaemic Control and Lipid Profile of Patients with Type 2 Diabetes: Randomised Controlled Clinical Trial. Int. Dairy J. 2020, 101, 104568. [Google Scholar] [CrossRef]
- Sboui, A.; Atig, C.; Khabir, A.; Hammadi, M.; Khorchani, T. Camel Milk Used as an Adjuvant Therapy to Treat Type 2 Diabetic Patients: Effects on Blood Glucose, HbA1c, Cholesterol, and TG Levels. J. Chem. 2022, 2022, 5860162. [Google Scholar] [CrossRef]
- AlKurd, R.; Hanash, N.; Khalid, N.; Abdelrahim, D.N.; Khan, M.A.B.; Mahrous, L.; Radwan, H.; Naja, F.; Madkour, M.; Obaideen, K.; et al. Effect of Camel Milk on Glucose Homeostasis in Patients with Diabetes: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients 2022, 14, 1245. [Google Scholar] [CrossRef]
- Agrawal, R.P.; Dogra, R.; Mohta, N.; Tiwari, R.; Singhal, S.; Sultania, S. Beneficial Effect of Camel Milk in Diabetic Nephropathy. Acta Biomed. 2009, 80, 131–134. [Google Scholar]
- Kotb-El-Sayed, M.-I.; Al-Shoeibi, Z.-Y.; El-Ghany, A.-A.A.; Atef, Z.-A. Effects of Camel’s Milk as a Vehicle for Insulin on Glycaemic Control and Lipid Profile in Type 1 Diabetics. Am. J. Biochem. Biotechnol. 2012, 7, 179–189. [Google Scholar] [CrossRef]
- Khalid, N.; Abdelrahim, D.N.; Hanach, N.; AlKurd, R.; Khan, M.; Mahrous, L.; Radwan, H.; Naja, F.; Madkour, M.; Obaideen, K.; et al. Effect of camel milk on lipid profile among patients with diabetes: A systematic review, meta-analysis, and meta-regression of randomized controlled trials. BMC Complement. Med. Ther. 2023, 23, 438. [Google Scholar] [CrossRef] [PubMed]
- Hamad, E.M.; Abdel-Rahi, E.A.; Romeih, E.A. Beneficial Effect of Camel Milk on Liver and Kidneys Function in Diabetic Sprague-Dawley Rats. Int. J. Dairy Sci. 2011, 6, 190–197. [Google Scholar] [CrossRef]
- Varvarovská, J.; Racek, J.; Stetina, R.; Sýkora, J.; Pomahacová, R.; Rusavý, Z.; Lacigová, S.; Trefil, L.; Siala, K.; Stozický, F. Aspects of Oxidative Stress in Children with Type 1 Diabetes Mellitus. Biomed. Pharmacother. 2004, 58, 539–545. [Google Scholar] [CrossRef]
- Abdel-Salam, A.; El-Mergawi, R.; A.I, A.-H.; Mousa, H. Chemical Composition and Antioxidant Activity of Dates and Dates-Camel-Milk Mixtures as a Protective Meal against Lipid Peroxidation in Rats. Am. J. Food Technol. 2010, 5, 22–30. [Google Scholar] [CrossRef]
- El-Sherbini El-Said, E.-S.; El-Sayed, G.R.; Tantawy, E. Effect of Camel Milk on Oxidative Stresses in Experimentally Induced Diabetic Rabbits. Vet. Res. Forum 2010, 1, 30–43. [Google Scholar]
- Saito, S.; Takayama, Y.; Mizumachi, K.; Suzuki, C. Lactoferrin Promotes Hyaluronan Synthesis in Human Dermal Fibroblasts. Biotechnol. Lett. 2011, 33, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Takayama, Y.; Aoki, R. Roles of Lactoferrin on Skin Wound Healing. Biochem. Cell Biol. 2012, 90, 497–503. [Google Scholar] [CrossRef] [PubMed]
- Jahani, S.; Shakiba, A.; Jahani, L. The Antimicrobial Effect of Lactoferrin on Gram-Negative and Gram-Positive Bacteria. Int. J. Infect. 2015, 2, e27954. [Google Scholar] [CrossRef]
- Mouritzen, M.V.; Petkovic, M.; Qvist, K.; Poulsen, S.S.; Alarico, S.; Leal, E.C.; Dalgaard, L.T.; Empadinhas, N.; Carvalho, E.; Jenssen, H. Improved Diabetic Wound Healing by LFcinB Is Associated with Relevant Changes in the Skin Immune Response and Microbiota. Mol. Ther. Methods Clin. Dev. 2021, 20, 726–739. [Google Scholar] [CrossRef]
- Ayoub, M.A.; Palakkott, A.R.; Ashraf, A.; Iratni, R. The Molecular Basis of the Anti-Diabetic Properties of Camel Milk. Diabetes Res. Clin. Pract. 2018, 146, 305–312. [Google Scholar] [CrossRef]
- Dou, Z.; Liu, C.; Feng, X.; Xie, Y.; Yue, H.; Dong, J.; Zhao, Z.; Chen, G.; Yang, J. Camel Whey Protein (CWP) Ameliorates Liver Injury in Type 2 Diabetes Mellitus Rats and Insulin Resistance (IR) in HepG2 Cells via Activation of the PI3K/Akt Signaling Pathway. Food Funct. 2022, 13, 255–269. [Google Scholar] [CrossRef]
- Agrawal, R.P.; Jain, S.; Shah, S.; Chopra, A.; Agarwal, V. Effect of Camel Milk on Glycemic Control and Insulin Requirement in Patients with Type 1 Diabetes: 2-Years Randomized Controlled Trial. Eur. J. Clin. Nutr. 2011, 65, 1048–1052. [Google Scholar] [CrossRef]
- Mihic, T.; Rainkie, D.; Wilby, K.J.; Pawluk, S.A. The Therapeutic Effects of Camel Milk: A Systematic Review of Animal and Human Trials. J. Evid. Based Complement. Altern. Med. 2016, 21, NP110–NP126. [Google Scholar] [CrossRef] [PubMed]
- Shori, A.B. Camel Milk as a Potential Therapy for Controlling Diabetes and Its Complications: A Review of in Vivo Studies. J. Food Drug Anal. 2015, 23, 609–618. [Google Scholar] [CrossRef] [PubMed]
- Seino, Y.; Fukushima, M.; Yabe, D. GIP and GLP-1, the Two Incretin Hormones: Similarities and Differences. J. Diabetes Investig. 2010, 1, 8–23. [Google Scholar] [CrossRef]
- Seino, Y.; Yabe, D. Glucose-Dependent Insulinotropic Polypeptide and Glucagon-like Peptide-1: Incretin Actions beyond the Pancreas. J. Diabetes Investig. 2013, 4, 108–130. [Google Scholar] [CrossRef]
- Abdulrahman, A.O.; Ismael, M.A.; Al-Hosaini, K.; Rame, C.; Al-Senaidy, A.M.; Dupont, J.; Ayoub, M.A. Differential Effects of Camel Milk on Insulin Receptor Signaling—Toward Understanding the Insulin-Like Properties of Camel Milk. Front. Endocrinol. 2016, 7, 4. [Google Scholar] [CrossRef]
- Badr, G.; Sayed, L.H.; Omar, H.E.-D.M.; Abd El-Rahim, A.M.; Ahmed, E.A.; Mahmoud, M.H. Camel Whey Protein Protects B and T Cells from Apoptosis by Suppressing Activating Transcription Factor-3 (ATF-3)-Mediated Oxidative Stress and Enhancing Phosphorylation of AKT and IκB-α in Type I Diabetic Mice. Cell Physiol. Biochem. 2017, 41, 41–54. [Google Scholar] [CrossRef]
- Mahmoud, M.H.; Badr, G.; El Shinnawy, N.A. Camel Whey Protein Improves Lymphocyte Function and Protects against Diabetes in the Offspring of Diabetic Mouse Dams. Int. J. Immunopathol. Pharmacol. 2016, 29, 632–646. [Google Scholar] [CrossRef] [PubMed]
- Sayed, L.H.; Badr, G.; Omar, H.M.; Abd El-Rahim, A.M.; Mahmoud, M.H. Camel Whey Protein Improves Oxidative Stress and Histopathological Alterations in Lymphoid Organs through Bcl-XL/Bax Expression in a Streptozotocin-Induced Type 1 Diabetic Mouse Model. Biomed. Pharmacother. 2017, 88, 542–552. [Google Scholar] [CrossRef]
- Moneim, A.A.; Helmy, H.; Abdel-Reheim, E.S.; Addaleel, W.W. Camel Milk Ameliorates Hyperglycemia-Mediated Hyperlipidemia and Oxidative Stress on Streptozotocin-Induced Diabetic Rats. Int. J. Diabetes Res. 2016, 5, 63–69. [Google Scholar]
- Zheng, Q.; Wang, Y.; Chen, G.; Mao, X. Camel Milk Endogenous Peptides Ameliorated Hyperglycemia in High-Fat Diet-Fed C57BL/6 J Mice in Association with Modulation of Gut Microbiota and the IRS/Akt and JNK/P38 Pathways. Food Res. Int. 2025, 212, 116471. [Google Scholar] [CrossRef]
- Tsalamandris, S.; Antonopoulos, A.S.; Oikonomou, E.; Papamikroulis, G.A.; Vogiatzi, G.; Papaioannou, S.; Deftereos, S.; Tousoulis, D. The Role of Inflammation in Diabetes: Current Concepts and Future Perspectives. Eur. Cardiol. 2019, 14, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Badr, G.; Badr, B.M.; Mahmoud, M.H.; Mohany, M.; Rabah, D.M.; Garraud, O. Treatment of Diabetic Mice with Undenatured Whey Protein Accelerates the Wound Healing Process by Enhancing the Expression of MIP-1α, MIP-2, KC, CX3CL1 and TGF-β in Wounded Tissue. BMC Immunol. 2012, 13, 32. [Google Scholar] [CrossRef] [PubMed]
- Ebaid, H.; Abdel-salam, B.; Hassan, I.; Al-Tamimi, J.; Metwalli, A.; Alhazza, I. Camel Milk Peptide Improves Wound Healing in Diabetic Rats by Orchestrating the Redox Status and Immune Response. Lipids Health Dis. 2015, 14, 132. [Google Scholar] [CrossRef]
- Ebaid, H.; Salem, A.; Sayed, A.; Metwalli, A. Whey Protein Enhances Normal Inflammatory Responses during Cutaneous Wound Healing in Diabetic Rats. Lipids Health Dis. 2011, 10, 235. [Google Scholar] [CrossRef] [PubMed]
- Santosh, H.; Alfred, C. Role of Ascorbic Acid in Diabetes Mellitus: A Comprehensive Review. J. Med. Radiol. Pathol. Surg. 2017, 4, 1–3. [Google Scholar] [CrossRef]
- Clement, S.; Still, J.G.; Kosutic, G.; McAllister, R.G. Oral Insulin Product Hexyl-Insulin Monoconjugate 2 (HIM2) in Type 1 Diabetes Mellitus: The Glucose Stabilization Effects of HIM2. Diabetes Technol. Ther. 2002, 4, 459–466. [Google Scholar] [CrossRef]
- Kappeler, S.R.; Heuberger, C.; Farah, Z.; Puhan, Z. Expression of the Peptidoglycan Recognition Protein, PGRP, in the Lactating Mammary Gland. J. Dairy Sci. 2004, 87, 2660–2668. [Google Scholar] [CrossRef]
- Darwish, H.A.; Abd Raboh, N.R.; Mahdy, A. Camel’s Milk Alleviates Alcohol-Induced Liver Injury in Rats. Food Chem. Toxicol. 2012, 50, 1377–1383. [Google Scholar] [CrossRef]
- Roy, A.; Srivastava, M.; Saqib, U.; Liu, D.; Faisal, S.M.; Sugathan, S.; Bishnoi, S.; Baig, M.S. Potential Therapeutic Targets for Inflammation in Toll-like Receptor 4 (TLR4)-Mediated Signaling Pathways. Int. Immunopharmacol. 2016, 40, 79–89. [Google Scholar] [CrossRef]
- Mohammed, W.; El Magdoub, H.M.; Schaalan, M. Renoprotective Effect of Camel Milk in Pediatric Diabetic Ketoacidosis: A Focus on TLR-4/MAPK Axis. Diabetes Res. Clin. Pract. 2019, 151, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Usman, M.; Qureshi, A.S.; Ali, M.Z.; Umer, Z.; Ateeq, M.K.; Sarfraz, A.; Hussain, M.; Anjum, F.R.; Mahmood, N.; Fakhar-I-Adil, M.; et al. The Effects of Long-Term Diabetes on the Haematological and Uterine Indicators and Their Association with Neonatal Nephrogenesis Counter-Protected by Camel Milk: A Time Dependent Study. Vet. Med. 2020, 65, 25–35. [Google Scholar] [CrossRef]
- Shaban, A.M.; Raslan, M.; Qahl, S.H.; Elsayed, K.; Abdelhameed, M.S.; Oyouni, A.A.A.; Al-Amer, O.M.; Hammouda, O.; El-Magd, M.A. Ameliorative Effects of Camel Milk and Its Exosomes on Diabetic Nephropathy in Rats. Membranes 2022, 12, 1060. [Google Scholar] [CrossRef]
- Khan, F.B.; Anwar, I.; Redwan, E.M.; Palakkott, A.; Ashraf, A.; Kizhakkayil, J.; Iratni, R.; Maqsood, S.; Akli Ayoub, M. Camel and Bovine Milk Lactoferrins Activate Insulin Receptor and Its Related AKT and ERK1/2 Pathways. J. Dairy Sci. 2022, 105, 1848–1861. [Google Scholar] [CrossRef]
- Mohamed, W.A.; Schaalan, M.F. Antidiabetic Efficacy of Lactoferrin in Type 2 Diabetic Pediatrics; Controlling Impact on PPAR-γ, SIRT-1, and TLR4 Downstream Signaling Pathway. Diabetol. Metab. Syndr. 2018, 10, 89. [Google Scholar] [CrossRef]
- Ashraf, A.; Mudgil, P.; Palakkott, A.; Iratni, R.; Gan, C.-Y.; Maqsood, S.; Ayoub, M.A. Molecular Basis of the Anti-Diabetic Properties of Camel Milk through Profiling of Its Bioactive Peptides on Dipeptidyl Peptidase IV (DPP-IV) and Insulin Receptor Activity. J. Dairy Sci. 2021, 104, 61–77. [Google Scholar] [CrossRef]
- Ayoub, M.A.; Yap, P.-G.; Mudgil, P.; Khan, F.B.; Anwar, I.; Muhammad, K.; Gan, C.-Y.; Maqsood, S. Invited Review: Camel Milk-Derived Bioactive Peptides and Diabetes-Molecular View and Perspectives. J. Dairy Sci. 2024, 107, 649–668. [Google Scholar] [CrossRef]
- Kamal, H.; Jafar, S.; Mudgil, P.; Murali, C.; Amin, A.; Maqsood, S. Inhibitory Properties of Camel Whey Protein Hydrolysates toward Liver Cancer Cells, Dipeptidyl Peptidase-IV, and Inflammation. J. Dairy Sci. 2018, 101, 8711–8720. [Google Scholar] [CrossRef] [PubMed]
- Mudgil, P.; Kamal, H.; Priya Kilari, B.; Mohd Salim, M.A.S.; Gan, C.-Y.; Maqsood, S. Simulated Gastrointestinal Digestion of Camel and Bovine Casein Hydrolysates: Identification and Characterization of Novel Anti-Diabetic Bioactive Peptides. Food Chem. 2021, 353, 129374. [Google Scholar] [CrossRef] [PubMed]
- Ayyash, M.; Al-Dhaheri, A.S.; Al Mahadin, S.; Kizhakkayil, J.; Abushelaibi, A. In Vitro Investigation of Anticancer, Antihypertensive, Antidiabetic, and Antioxidant Activities of Camel Milk Fermented with Camel Milk Probiotic: A Comparative Study with Fermented Bovine Milk. J. Dairy Sci. 2018, 101, 900–911. [Google Scholar] [CrossRef]
- Nongonierma, A.B.; Paolella, S.; Mudgil, P.; Maqsood, S.; FitzGerald, R.J. Identification of Novel Dipeptidyl Peptidase IV (DPP-IV) Inhibitory Peptides in Camel Milk Protein Hydrolysates. Food Chem. 2018, 244, 340–348. [Google Scholar] [CrossRef] [PubMed]
- Mudgil, P.; Maqsood, S. Bioactive Peptides Derived from Camel Milk Proteins. In Enzymes Beyond Traditional Applications in Dairy Science and Technology; Elsevier: Amsterdam, The Netherlands, 2023; pp. 233–288. [Google Scholar]
- Shukla, P.; Sakure, A.; Maurya, R.; Bishnoi, M.; Kondepudi, K.K.; Das, S.; Liu, Z.; Padhi, S.; Rai, A.K.; Hati, S. Antidiabetic, Angiotensin-Converting Enzyme Inhibitory and Anti-Inflammatory Activities of Fermented Camel Milk and Characterisation of Novel Bioactive Peptides from Lactic-Fermented Camel Milk with Molecular Interaction Study. Int. J. Dairy Technol. 2023, 76, 149–167. [Google Scholar] [CrossRef]
- Khakhariya, R.; Basaiawmoit, B.; Sakure, A.A.; Maurya, R.; Bishnoi, M.; Kondepudi, K.K.; Padhi, S.; Rai, A.K.; Liu, Z.; Hati, S. Production and Characterization of ACE Inhibitory and Anti-Diabetic Peptides from Buffalo and Camel Milk Fermented with Lactobacillus and Yeast: A Comparative Analysis with In Vitro, In Silico, and Molecular Interaction Study. Foods 2023, 12, 2006. [Google Scholar] [CrossRef]
- Mudgil, P.; Kamal, H.; Yuen, G.C.; Maqsood, S. Characterization and Identification of Novel Antidiabetic and Anti-Obesity Peptides from Camel Milk Protein Hydrolysates. Food Chem. 2018, 259, 46–54. [Google Scholar] [CrossRef]
- Tagliazucchi, D.; Martini, S.; Shamsia, S.; Helal, A.; Conte, A. Biological Activities and Peptidomic Profile of in Vitro-Digested Cow, Camel, Goat and Sheep Milk. Int. Dairy J. 2018, 81, 19–27. [Google Scholar] [CrossRef]
- Mozaffarian, D.; Cao, H.; King, I.B.; Lemaitre, R.N.; Song, X.; Siscovick, D.S.; Hotamisligil, G.S. Trans-Palmitoleic Acid, Metabolic Risk Factors, and New-Onset Diabetes in U.S. Adults: A Cohort Study. Ann. Intern. Med. 2010, 153, 790–799. [Google Scholar] [CrossRef]
- Gansevoort, R.T.; de Zeeuw, D.; de Jong, P.E. Is the Antiproteinuric Effect of ACE Inhibition Mediated by Interference in the Renin-Angiotensin System? Kidney Int. 1994, 45, 861–867. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, S.; Nakagomi, K.; Tomizuka, N.; Suzuki, H. Angiotensin I-Converting Enzyme Inhibitor Derived from an Enzymatic Hydrolysate of Casein. II. Isolation and Bradykinin-Potentiating Activity on the Uterus and the Ileum of Rats. Agric. Biol. Chem. 1985, 49, 1405–1409. [Google Scholar] [CrossRef]
- Anwar, I.; Khan, F.B.; Baby, B.; Antony, P.; Mudgil, P.; Gan, C.-Y.; Maqsood, S.; Vijayan, R.; Muhammad, K.; Ayoub, M.A. Functional Profiling of Synthetic Camel Milk-Derived Peptides with Implication in Glucose Transport and Diabetes. PLoS ONE 2025, 20, e0320812. [Google Scholar] [CrossRef] [PubMed]
- Cintio, M.; Polacchini, G.; Scarsella, E.; Montanari, T.; Stefanon, B.; Colitti, M. MicroRNA Milk Exosomes: From Cellular Regulator to Genomic Marker. Animals 2020, 10, 1126. [Google Scholar] [CrossRef]
- El-Kattawy, A.M.; Algezawy, O.; Alfaifi, M.Y.; Noseer, E.A.; Hawsawi, Y.M.; Alzahrani, O.R.; Algarni, A.; Kahilo, K.A.; El-Magd, M.A. Therapeutic Potential of Camel Milk Exosomes against HepaRG Cells with Potent Apoptotic, Anti-Inflammatory, and Anti-Angiogenesis Effects for Colostrum Exosomes. Biomed. Pharmacother. 2021, 143, 112220. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.Z.; Xiao, J.; Ma, Y.; Ma, J.; Liu, S.; Khan, A.; Khan, J.M.; Cao, Z. Research Development on Anti-Microbial and Antioxidant Properties of Camel Milk and Its Role as an Anti-Cancer and Anti-Hepatitis Agent. Antioxidants 2021, 10, 788. [Google Scholar] [CrossRef]
- Zheng, N.; Min, L.; Li, D.; Tan, S.; Gao, Y.; Wang, J. Occurrence of Aflatoxin M1 in Cow, Goat, Buffalo, Camel, and Yak Milk in China in 2016. Toxins 2022, 14, 870. [Google Scholar] [CrossRef]
- Yassin, A.M.; Abdel Hamid, M.I.; Farid, O.A.; Amer, H.; Warda, M. Dromedary Milk Exosomes as Mammary Transcriptome Nano-Vehicle: Their Isolation, Vesicular and Phospholipidomic Characterizations. J. Adv. Res. 2016, 7, 749–756. [Google Scholar] [CrossRef]
- Adriano, B.; Cotto, N.M.; Chauhan, N.; Jaggi, M.; Chauhan, S.C.; Yallapu, M.M. Milk Exosomes: Nature’s Abundant Nanoplatform for Theranostic Applications. Bioact. Mater. 2021, 6, 2479–2490. [Google Scholar] [CrossRef]
- Gu, Y.; Li, M.; Wang, T.; Liang, Y.; Zhong, Z.; Wang, X.; Zhou, Q.; Chen, L.; Lang, Q.; He, Z.; et al. Lactation-Related MicroRNA Expression Profiles of Porcine Breast Milk Exosomes. PLoS ONE 2012, 7, e43691. [Google Scholar] [CrossRef] [PubMed]
- Record, M.; Carayon, K.; Poirot, M.; Silvente-Poirot, S. Exosomes as New Vesicular Lipid Transporters Involved in Cell-Cell Communication and Various Pathophysiologies. Biochim. Biophys. Acta 2014, 1841, 108–120. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Bae, S.Y.; Oh, S.-J.; Lee, J.; Lee, J.H.; Lee, H.-C.; Lee, S.K.; Kil, W.H.; Kim, S.W.; Nam, S.J.; et al. Zerumbone Suppresses IL-1β-Induced Cell Migration and Invasion by Inhibiting IL-8 and MMP-3 Expression in Human Triple-Negative Breast Cancer Cells. Phytother. Res. 2014, 28, 1654–1660. [Google Scholar] [CrossRef]
- Elgazar, A.A.; Selim, N.M.; Abdel-Hamid, N.M.; El-Magd, M.A.; El Hefnawy, H.M. Isolates from Alpinia Officinarum Hance Attenuate LPS-Induced Inflammation in HepG2: Evidence from in Silico and in Vitro Studies. Phytother. Res. 2018, 32, 1273–1288. [Google Scholar] [CrossRef]
- Ibrahim, H.M.; Mohammed-Geba, K.; Tawfic, A.A.; El-Magd, M.A. Camel Milk Exosomes Modulate Cyclophosphamide-Induced Oxidative Stress and Immuno-Toxicity in Rats. Food Funct. 2019, 10, 7523–7532. [Google Scholar] [CrossRef]
- Arab, H.H.; Salama, S.A.; Maghrabi, I.A. Camel Milk Ameliorates 5-Fluorouracil-Induced Renal Injury in Rats: Targeting MAPKs, NF-κB and PI3K/Akt/eNOS Pathways. Cell Physiol. Biochem. 2018, 46, 1628–1642. [Google Scholar] [CrossRef]
- Ebaid, H.; Ahmed, O.M.; Mahmoud, A.M.; Ahmed, R.R. Limiting Prolonged Inflammation during Proliferation and Remodeling Phases of Wound Healing in Streptozotocin-Induced Diabetic Rats Supplemented with Camel Undenatured Whey Protein. BMC Immunol. 2013, 14, 31. [Google Scholar] [CrossRef]
- Grivennikov, S.I.; Greten, F.R.; Karin, M. Immunity, Inflammation, and Cancer. Cell 2010, 140, 883–899. [Google Scholar] [CrossRef] [PubMed]
- Badawy, A.A.; El-Magd, M.A.; AlSadrah, S.A. Therapeutic Effect of Camel Milk and Its Exosomes on MCF7 Cells In Vitro and In Vivo. Integr. Cancer Ther. 2018, 17, 1235–1246. [Google Scholar] [CrossRef] [PubMed]
- Laghi, L.; Bianchi, P.; Miranda, E.; Balladore, E.; Pacetti, V.; Grizzi, F.; Allavena, P.; Torri, V.; Repici, A.; Santoro, A.; et al. CD3+ Cells at the Invasive Margin of Deeply Invading (pT3-T4) Colorectal Cancer and Risk of Post-Surgical Metastasis: A Longitudinal Study. Lancet Oncol. 2009, 10, 877–884. [Google Scholar] [CrossRef]
- el Agamy, E.I.; Ruppanner, R.; Ismail, A.; Champagne, C.P.; Assaf, R. Antibacterial and Antiviral Activity of Camel Milk Protective Proteins. J. Dairy Res. 1992, 59, 169–175. [Google Scholar] [CrossRef]
- Wang, M.; Wang, C.; Ren, S.; Pan, J.; Wang, Y.; Shen, Y.; Zeng, Z.; Cui, H.; Zhao, X. Versatile Oral Insulin Delivery Nanosystems: From Materials to Nanostructures. Int. J. Mol. Sci. 2022, 23, 3362. [Google Scholar] [CrossRef]
- Karray, N.; Lopez, C.; Ollivon, M.; Attia, H. La matière grasse du lait de dromadaire: Composition, microstructure et polymorphisme. Une revue. Oléagineux Corps Gras Lipides 2005, 12, 439–446. [Google Scholar] [CrossRef]
- Prego, C.; García, M.; Torres, D.; Alonso, M.J. Transmucosal Macromolecular Drug Delivery. J. Control. Release 2005, 101, 151–162. [Google Scholar] [CrossRef]
- Vila, A.; Sánchez, A.; Tobío, M.; Calvo, P.; Alonso, M.J. Design of Biodegradable Particles for Protein Delivery. J. Control. Release 2002, 78, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Yadav, A.K.; Kumar, R.; Singh, L.P.J. Composition and Medicinal Properties of Camel Milk: A Review. Asian J. Dairy Food Res. 2015, 34, 83–91. [Google Scholar] [CrossRef]
- Ho, T.M.; Zou, Z.; Bansal, N. Camel Milk: A Review of Its Nutritional Value, Heat Stability, and Potential Food Products. Food Res. Int. 2022, 153, 110870. [Google Scholar] [CrossRef] [PubMed]
- Konuspayeva, G.S. Camel Milk Composition and Nutritional Value: In Practice, Progress, and Proficiency in Sustainability; Alhaj, O.A., Faye, B., Agrawal, R.P., Eds.; IGI Global: Hershey, PA, USA, 2020; pp. 15–40. ISBN 978-1-79981-604-1. [Google Scholar]
- Konuspayeva, G.; Al-Gedan, M.M.; Alzuraiq, F.; Faye, B. Some Variation Factors of Freezing Point in Camel Milk. Animals 2023, 13, 1657. [Google Scholar] [CrossRef] [PubMed]
- Al Haj, O.A.; Al Kanhal, H.A. Compositional, Technological and Nutritional Aspects of Dromedary Camel Milk. Int. Dairy J. 2010, 20, 811–821. [Google Scholar] [CrossRef]
| Country | Production (tons) |
|---|---|
| Kenya | 1,096,698 |
| Somalia | 987,842.9 |
| Pakistan | 944,000 |
| Mali | 294,248.6 |
| Ethiopia | 220,446 |
| Saudi Arabia | 135,540 |
| Niger | 106,597.4 |
| United Arab Emirates | 79,434.44 |
| Percentile | |||||||
|---|---|---|---|---|---|---|---|
| Variable | Mean | Median | Std Dev | Min | Max | 2.5th | 97.5th |
| Fat (g/dL) | 3.82 | 3.70 | 1.08 | 0.28 | 6.40 | 2.30 | 5.60 |
| Total protein (g/dL) | 3.36 | 3.30 | 0.63 | 2.15 | 4.90 | 2.28 | 4.61 |
| Lipids (g/dL) | 4.55 | 4.60 | 0.69 | 2.40 | 5.80 | 3.26 | 5.80 |
| Dry matter (g/dL) | 12.51 | 12.73 | 1.61 | 8.64 | 16.08 | 9.03 | 15.52 |
| Ash (g/dL) | 0.78 | 0.80 | 0.09 | 0.60 | 1.05 | 0.60 | 0.98 |
| Parameter | Camelus dromedarius | Camelus bactrianus | p-Value |
|---|---|---|---|
| Total Protein (%) | 3.10 [3.01; 3.20] | 3.92 [3.67; 4.17] | 0.0001 |
| Fat (%) | 3.34 [3.23; 3.44] | 5.49 [5.02; 5.96] | 0.0001 |
| Lactose (%) | 4.33 [4.21; 4.45] | 4.80 [4.13; 5.47] | n.s. |
| Ash (%) | 0.77 [0.75; 0.79] | 0.86 [0.82; 0.90] | 0.0003 |
| Total solids | 11.34 [10.93; 11.75] | 11.00 [9.62; 12.37] | n.s. |
| Ca (%) mg/100 g | 111.31 [104.97; 117.65] | 141.60 [117.87; 165.32] | 0.02 |
| Fe (%) mg/100 g | 0.46 [0.19; 0.72] | 0.21 [0.17; 0.25] | n.s. |
| K mg/100 g | 113.34 [94.31; 132.36] | 191.0 [189.70; 192.30] | 0.0001 |
| Mg mg/100 g | 9.65 [7.92; 11.39] | n.r. | n.r. |
| Na mg/100 g | 48.74 [39.66; 59.89] | n.r. | n.r. |
| Zn mg/100 g | 1.68 [1.45; 1.91] | n.r. | n.r. |
| Vit C (Ascorbic acid) mg/100 g | 5.22 [4.61; 5.83] | 10.26 [−4.18; 24.70] | n.s. |
| Vit A (Retinol) mg/100 g | 0.43 [0.05; 0.81] | 0.10 [0.09; 0.1] | n.s. |
| Vit B1 (Thiamine) mg/100 g | 0.06 [0.05; 0.08] | 0.01 [0.01; 0.013] | 0.0001 |
| Vit B2 (Riboflavin) mg/100 g | 0.13 [0.05; 0.21] | 0.12 [0.10; 0.15] | n.s. |
| Vit B3 (Niacin) mg/100 g | 0.51 [0.42; 0.59] | n.r. | n.r. |
| Vit B6 (Pyridoxine) mg/100 g | 0.14 [0.09; 0.18] | 0.05 [0.05; 0.06] | 0.0006 |
| Vit B12 (Cyanocobalamin) mg/100 g | 0.0039 [0.0015; 0.0064] | n.r. | n.r. |
| Parameters | Mean ± SD | Min | Max |
|---|---|---|---|
| Total protein, % | 6.03 ± 4.70 | 3.19 | 17.20 |
| Fat, % | 7.88 ± 8.23 | 1.56 | 25.94 |
| Lactose, % | 3.63 | – | – |
| Ca 1, g/L | 0.589 ± 0.700 | 0.104 | 1.877 |
| P 2, g/L | 0.404 ± 0.438 | 0.083 | 1.030 |
| Fe 3, mg/L | 2.50 ± 0.97 | 1.20 | 3.70 |
| Group/Subgroup | Studies Involved | Total Patients | Parameter | Estimated Mean Difference [95% CI] | p-Value |
|---|---|---|---|---|---|
| DM (total) | 15 | 641 | FBG (mg/dL) | −23.32 [−47.33, 0.70] | 0.06 |
| DM (total) | 12 | 585 | HbA1c (%) | −1.24 [−2.00, −0.48] | 0.001 |
| DM (total) | 7 | 214 | Insulin dose (%) | −16.72 [−22.09, −11.35] | <0.0001 |
| Type 1 DM | 7 | 217 | FBG (mg/dL) | −27.20 [−73.97, 19.57] | 0.25 |
| Type 1 DM | 7 | 217 | HbA1c (%) | −1.21 [−2.24, −0.19] | 0.02 |
| Type 2 DM | 8 | 400 | FBG (mg/dL) | −15.62 [−26.71, −4.54] | 0.006 |
| Type 2 DM | 5 | 368 | HbA1c (%) | −1.27 [−2.53, 0.00] | 0.05 |
| Author(s), Year | Specie | Diabetogen | Total n. of Subjects | Milk Dosage | Treatment time | Effects on FBG or Hb1Ac (if Indicated) | p-Value |
|---|---|---|---|---|---|---|---|
| Agrawal et al., 2003 | Human | T1DM | 24 | 500 mL/d (randomized) | 3 mo. | 118.16 ± 7.15 mg/dL (control) 100 ± 16.2 ±mg/dL (treated) | <0.001 |
| Agrawal et al., 2004 | Rat | STZ | 32 | 250 mL/d/head CM Vs. 250 mL/d/head cow milk | 3 wk | 191.33 ± 7.46 mg/dL (Cow milk) 86.25 ± 12.77 mg/dL (CM) | <0.05 |
| Agrawal et al., 2007 | Human | T1DM | 50 | 500 mL/d | 12 mo. | 104.00 ± 15.87 mg/dL (control) 100.20 ± 17.40 mg/dL (treated) | 0.002 |
| Mohamad et al., 2009 | Human | T1DM | 54 | 500 mL/d (randomized controlled) | 16 wk | FBG 227.2 ± 17.7 mg/dL (control) 98.9 ± 16.2 mg/dL (treated) HbA1c 9.59 ± 2.05 % (control) 7.16 ± 1.84 % (treated) | <0.05 <0.05 |
| Al Numair et al., 2011 | Rat | STZ | 30 | 250 mL/d/head | 45 d | 292.38 ± 19.20 mg/dL (before) 141.57 ± 12.82 (after) | <0.05 |
| Badr, 2013 | Mouse | STZ | 30 | 100 mg whey protein/kg b.w. | 13 d | 373.6 ± 32 mg/dL (control) 261 ± 25.5 mg/dL (treated) | <0.005 |
| Mohieldein et al., 2013 | Rat | STZ | 20 | 400 mL/d/cage | 30 d | 520.46 ± 8.90 mg/dL (control) 235.61 ± 7.10 mg/dL (treated) | <0.05 |
| Badr, 2013 | Mouse | STZ | 30 | 100 mg whey protein/kg b.w. | 13 d | 373.6 ± 32 mg/dL (control) 261 ± 25.5 mg/dL (treated) | <0.005 |
| Fallah et al., 2020 | Human | T2DM | 40 | 500 mL/d (randomized controlled) | 3 mo. | FBG 169.3 ± 78.9 mg/dL (before treat.) 148.4 ± 59.5 mg/dL (after treat.) HbA1c 12.7 ± 2.6% (before treat.) 9.4 ± 0.3% (after treat.) | 0.02 0.001 |
| Sboui et al., 2022 | Human | T2DM | 60 | 500 mL/d | 3 mo. | 8.37 ± 0.79 (control) 6.13 ± 0.55 mmol/dL (treated) | <0.05 |
| Arain et al., 2025 | Rabbit | STZ | 36 | 100 mg/kg b.w. | 42 d | 583.3 ± 3.58 mg/dL (control at d 42) 201 ± 3.31 mg/dL (treated at d 42) | <0.05 |
| Studies Involved | Total Patients | Parameter | Estimated Mean Difference [95% CI] | p-Value |
|---|---|---|---|---|
| 10 | 322 | Total cholesterol (%) | −21.69 [−41.05, −2.33] | 0.03 |
| 10 | 322 | Triglycerides (%) | −18.79 [−36.16, −3.42] | 0.02 |
| 7 | 218 | Low-density lipoprotein (%) | −11.92 [−20.57, −3.26] | 0.007 |
| 7 | 218 | High-density lipoprotein (%) | 10.37 [1.90, 18.84] | 0.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Faustini, M.; Vigo, D.; Brecchia, G.; Agradi, S.; Draghi, S.; Curone, G.; Atigui, M.; Sboui, A.; Quattrone, A.; Fehri, N.E. Camel (Camelus dromedarius L. and Camelus bactrianus L.) Milk Composition and Effects on Human Type 1 and Type 2 Diabetes Mellitus: A Review. Biology 2025, 14, 1162. https://doi.org/10.3390/biology14091162
Faustini M, Vigo D, Brecchia G, Agradi S, Draghi S, Curone G, Atigui M, Sboui A, Quattrone A, Fehri NE. Camel (Camelus dromedarius L. and Camelus bactrianus L.) Milk Composition and Effects on Human Type 1 and Type 2 Diabetes Mellitus: A Review. Biology. 2025; 14(9):1162. https://doi.org/10.3390/biology14091162
Chicago/Turabian StyleFaustini, Massimo, Daniele Vigo, Gabriele Brecchia, Stella Agradi, Susanna Draghi, Giulio Curone, Moufida Atigui, Amel Sboui, Alda Quattrone, and Nour Elhouda Fehri. 2025. "Camel (Camelus dromedarius L. and Camelus bactrianus L.) Milk Composition and Effects on Human Type 1 and Type 2 Diabetes Mellitus: A Review" Biology 14, no. 9: 1162. https://doi.org/10.3390/biology14091162
APA StyleFaustini, M., Vigo, D., Brecchia, G., Agradi, S., Draghi, S., Curone, G., Atigui, M., Sboui, A., Quattrone, A., & Fehri, N. E. (2025). Camel (Camelus dromedarius L. and Camelus bactrianus L.) Milk Composition and Effects on Human Type 1 and Type 2 Diabetes Mellitus: A Review. Biology, 14(9), 1162. https://doi.org/10.3390/biology14091162













