StemBell Therapy Does Not Significantly Affect Atherosclerotic Plaque Characteristics in a Streptozotocin-Induced Diabetes Mellitus Mouse Model
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Stem Cell Isolation
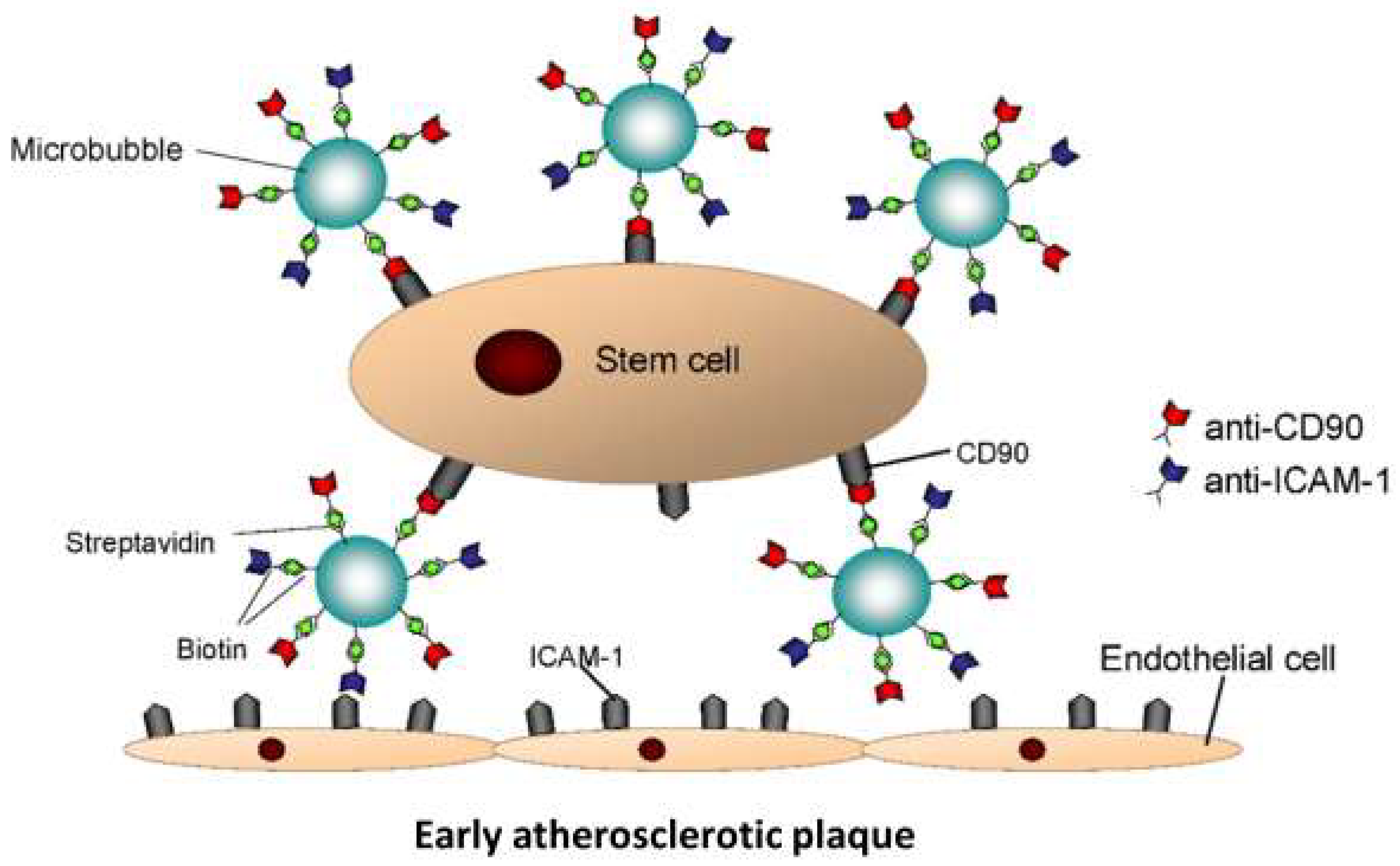
2.2. StemBell Preparation
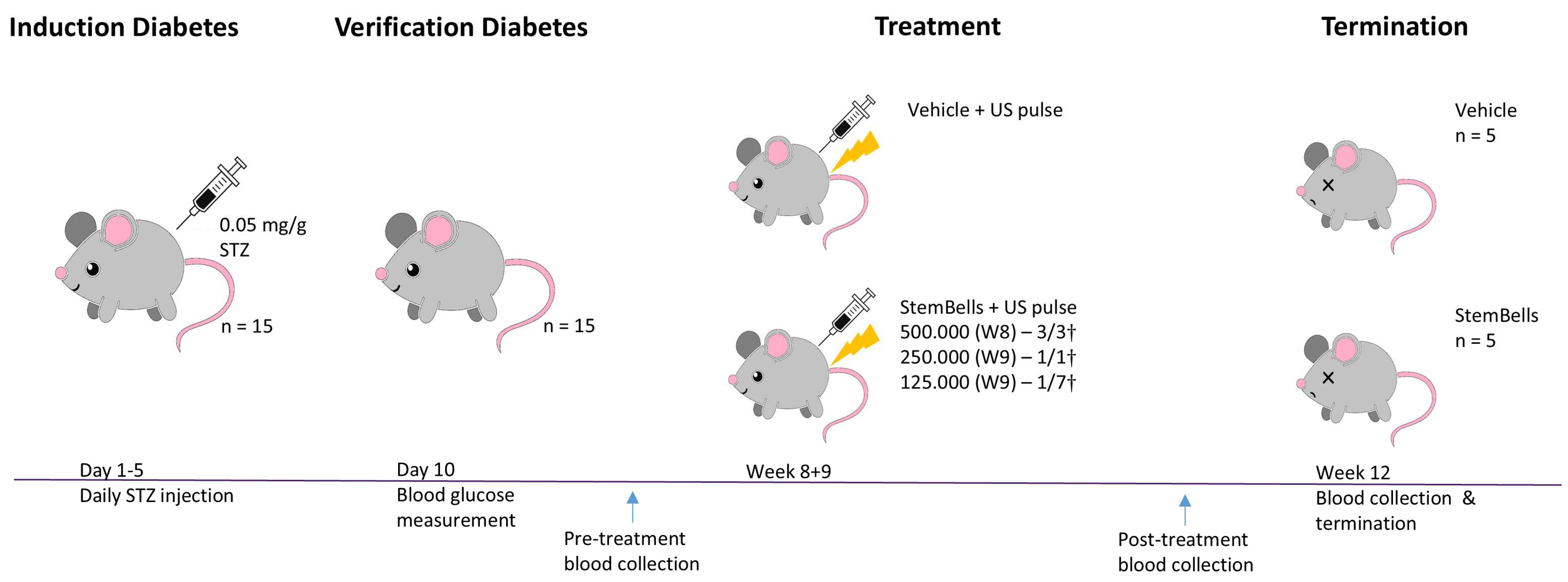
2.3. Animal Procedures
2.4. Tissue Processing
2.5. (Immuno)histochemistry
2.6. (Immuno)histochemical Scoring
2.7. PBMC Isolation
2.8. Flow Cytometry
2.9. Statistical Analysis
3. Results
3.1. Body Weight and Blood Glucose Measurements
3.2. StemBell Therapy Complications
3.3. Atherosclerotic Plaque Features
3.4. Circulating Monocyte Subsets
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Morrish, N.J.; Wang, S.L.; Stevens, L.K.; Fuller, J.H.; Keen, H. Mortality and causes of death in the WHO Multinational Study of Vascular Disease in Diabetes. Diabetologia 2001, 44 (Suppl. S2), S14–S21. [Google Scholar] [CrossRef]
- Matheus, A.S.; Tannus, L.R.; Cobas, R.A.; Palma, C.C.; Negrato, C.A.; Gomes, M.B. Impact of diabetes on cardiovascular disease: An update. Int. J. Hypertens. 2013, 2013, 653789. [Google Scholar] [CrossRef]
- Einarson, T.R.; Acs, A.; Ludwig, C.; Panton, U.H. Prevalence of cardiovascular disease in type 2 diabetes: A systematic literature review of scientific evidence from across the world in 2007–2017. Cardiovasc. Diabetol. 2018, 17, 83. [Google Scholar] [CrossRef] [PubMed]
- Virmani, R.; Burke, A.P.; Kolodgie, F.D.; Farb, A. Vulnerable plaque: The pathology of unstable coronary lesions. J. Interv. Cardiol. 2002, 15, 439–446. [Google Scholar] [CrossRef]
- Yahagi, K.; Kolodgie, F.D.; Lutter, C.; Mori, H.; Romero, M.E.; Finn, A.V.; Virmani, R. Pathology of Human Coronary and Carotid Artery Atherosclerosis and Vascular Calcification in Diabetes Mellitus. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 191–204. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, T.; Yamamoto, E.; Bryniarski, K.; Xing, L.; Fracassi, F.; Lee, H.; Jang, I.K. Coronary Plaque Characteristics in Patients With Diabetes Mellitus Who Presented With Acute Coronary Syndromes. J. Am. Heart Assoc. 2018, 7, e009245. [Google Scholar] [CrossRef] [PubMed]
- Fadini, G.P.; Simoni, F.; Cappellari, R.; Vitturi, N.; Galasso, S.; Vigili de Kreutzenberg, S.; Previato, L.; Avogaro, A. Pro-inflammatory monocyte-macrophage polarization imbalance in human hypercholesterolemia and atherosclerosis. Atherosclerosis 2014, 237, 805–808. [Google Scholar] [CrossRef]
- Wang, Z.X.; Wang, C.Q.; Li, X.Y.; Feng, G.K.; Zhu, H.L.; Ding, Y.; Jiang, X.J. Mesenchymal stem cells alleviate atherosclerosis by elevating number and function of CD4+CD25+FOXP3+ regulatory T-cells and inhibiting macrophage foam cell formation. Mol. Cell. Biochem. 2015, 400, 163–172. [Google Scholar] [CrossRef]
- Ma, Y.; Gu, T.H.; He, S.Q.; He, S.Y.; Jiang, Z.S. Development of stem cell therapy for atherosclerosis. Mol. Cell. Biochem. 2024, 479, 779–791. [Google Scholar] [CrossRef]
- Markina, Y.V.; Kirichenko, T.V.; Tolstik, T.V.; Bogatyreva, A.I.; Zotova, U.S.; Cherednichenko, V.R.; Postnov, A.Y.; Markin, A.M. Target and Cell Therapy for Atherosclerosis and CVD. Int. J. Mol. Sci. 2023, 24, 10308. [Google Scholar] [CrossRef]
- Mahdavi Gorabi, A.; Banach, M.; Reiner, Ž.; Pirro, M.; Hajighasemi, S.; Johnston, T.P.; Sahebkar, A. The role of mesenchymal stem cells in atherosclerosis: Prospects for therapy via the modulation of inflammatory milieu. J. Clin. Med. 2019, 8, 1413. [Google Scholar] [CrossRef]
- Aggarwal, S.; Pittenger, M.F. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood 2005, 105, 1815–1822. [Google Scholar] [CrossRef] [PubMed]
- Frodermann, V.; van Duijn, J.; van Pel, M.; van Santbrink, P.J.; Bot, I.; Kuiper, J.; de Jager, S.C. Mesenchymal Stem Cells Reduce Murine Atherosclerosis Development. Sci. Rep. 2015, 5, 15559. [Google Scholar] [CrossRef]
- Ezquer, F.E.; Ezquer, M.E.; Parrau, D.B.; Carpio, D.; Yanez, A.J.; Conget, P.A. Systemic administration of multipotent mesenchymal stromal cells reverts hyperglycemia and prevents nephropathy in type 1 diabetic mice. Biol. Blood Marrow Transplant. 2008, 14, 631–640. [Google Scholar] [CrossRef]
- Li, H.L.; Chen, C.; Wang, Y.S.; Yi, W.; Guo, P.P.; Yao, C.G.; Liu, J.B.; Wei, Y.H.; Hu, K.H.; Shang, X.K.; et al. A meta-analysis on application and prospect of cell therapy in the treatment of diabetes mellitus. Stem Cell Res. Ther. 2025, 16, 249. [Google Scholar] [CrossRef]
- Woudstra, L.; Krijnen, P.A.; Bogaards, S.J.; Meinster, E.; Emmens, R.W.; Kokhuis, T.J.; Bollen, I.A.; Baltzer, H.; Baart, S.M.; Parbhudayal, R.; et al. Development of a new therapeutic technique to direct stem cells to the infarcted heart using targeted microbubbles: StemBells. Stem Cell Res. 2016, 17, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Benson, V.; McMahon, A.C.; Lowe, H.C. ICAM-1 in acute myocardial infarction: A potential therapeutic target. Curr. Mol. Med. 2007, 7, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Woudstra, L.; Meinster, E.; van Haren, L.; Kay, A.M.; Koopman, M.; Belien, J.A.M.; Morrison, M.C.; AC, V.A.N.R.; Helder, M.N.; Juffermans, L.J.M.; et al. StemBell therapy stabilizes atherosclerotic plaques after myocardial infarction. Cytotherapy 2018, 20, 1143–1154. [Google Scholar] [CrossRef] [PubMed]
- van Dijk, A.; Naaijkens, B.A.; Jurgens, W.J.; Nalliah, K.; Sairras, S.; van der Pijl, R.J.; Vo, K.; Vonk, A.B.; van Rossum, A.C.; Paulus, W.J.; et al. Reduction of infarct size by intravenous injection of uncultured adipose derived stromal cells in a rat model is dependent on the time point of application. Stem Cell Res. 2011, 7, 219–229. [Google Scholar] [CrossRef]
- Korn, A.; Simsek, S.; Fiet, M.D.; Waas, I.S.E.; Niessen, H.W.M.; Krijnen, P.A.J. Application of adipose tissue-derived stem cell therapy with a clinically relevant dose does not significantly affect atherosclerotic plaque characteristics in a streptozotocin-induced hyperglycaemia mouse model. J. Mol. Cell. Cardiol. Plus 2024, 9, 100083. [Google Scholar] [CrossRef]
- Hanssen, N.M.; Brouwers, O.; Gijbels, M.J.; Wouters, K.; Wijnands, E.; Cleutjens, J.P.; De Mey, J.G.; Miyata, T.; Biessen, E.A.; Stehouwer, C.D. Glyoxalase 1 overexpression does not affect atherosclerotic lesion size and severity in ApoE−/− mice with or without diabetes. Cardiovasc. Res. 2014, 104, 160–170. [Google Scholar] [CrossRef]
- Ganguly, R.; Sahu, S.; Ohanyan, V.; Haney, R.; Chavez, R.J.; Shah, S.; Yalamanchili, S.; Raman, P. Oral chromium picolinate impedes hyperglycemia-induced atherosclerosis and inhibits proatherogenic protein TSP-1 expression in STZ-induced type 1 diabetic ApoE−/− mice. Sci. Rep. 2017, 7, 45279. [Google Scholar] [CrossRef]
- Kokhuis, T.J.; Skachkov, I.; Naaijkens, B.A.; Juffermans, L.J.; Kamp, O.; Kooiman, K.; van der Steen, A.F.; Versluis, M.; de Jong, N. Intravital microscopy of localized stem cell delivery using microbubbles and acoustic radiation force. Biotechnol. Bioeng. 2015, 112, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Petrovich, E.; Feigelson, S.W.; Stoler-Barak, L.; Hatzav, M.; Solomon, A.; Bar-Shai, A.; Ilan, N.; Li, J.P.; Engelhardt, B.; Vlodavsky, I.; et al. Lung ICAM-1 and ICAM-2 support spontaneous intravascular effector lymphocyte entrapment but are not required for neutrophil entrapment or emigration inside endotoxin-inflamed lungs. FASEB J. 2016, 30, 1767–1778. [Google Scholar] [CrossRef]
- Lessieur, E.M.; Liu, H.; Saadane, A.; Du, Y.; Kiser, J.; Kern, T.S. ICAM-1 on the luminal surface of endothelial cells is induced to a greater extent in mouse retina than in other tissues in diabetes. Diabetologia 2022, 65, 1734–1744. [Google Scholar] [CrossRef] [PubMed]
- Müller, B.; Lang, S.; Dominietto, M.; Rudin, M.; Schulz, G.; Deyhle, H.; Germann, M.; Pfeiffer, F.; David, C.; Weitkamp, T. High-resolution tomographic imaging of microvessels. In Proceedings of the Developments in X-Ray Tomography VI, San Diego, CA, USA, 10–14 August 2008; SPIE: Bellingham, WA, USA, 2008; pp. 89–98. [Google Scholar]
- Alvaro-Gracia, J.M.; Jover, J.A.; Garcia-Vicuna, R.; Carreno, L.; Alonso, A.; Marsal, S.; Blanco, F.; Martinez-Taboada, V.M.; Taylor, P.; Martin-Martin, C.; et al. Intravenous administration of expanded allogeneic adipose-derived mesenchymal stem cells in refractory rheumatoid arthritis (Cx611): Results of a multicentre, dose escalation, randomised, single-blind, placebo-controlled phase Ib/IIa clinical trial. Ann. Rheum. Dis. 2017, 76, 196–202. [Google Scholar] [CrossRef]
- Butler, J.; Epstein, S.E.; Greene, S.J.; Quyyumi, A.A.; Sikora, S.; Kim, R.J.; Anderson, A.S.; Wilcox, J.E.; Tankovich, N.I.; Lipinski, M.J.; et al. Intravenous Allogeneic Mesenchymal Stem Cells for Nonischemic Cardiomyopathy: Safety and Efficacy Results of a Phase II-A Randomized Trial. Circ. Res. 2017, 120, 332–340. [Google Scholar] [CrossRef] [PubMed]
- Fang, B.; Mai, L.; Li, N.; Song, Y. Favorable response of chronic refractory immune thrombocytopenic purpura to mesenchymal stem cells. Stem Cells Dev. 2012, 21, 497–502. [Google Scholar] [CrossRef]
- Fang, B.; Song, Y.P.; Liao, L.M.; Han, Q.; Zhao, R.C. Treatment of severe therapy-resistant acute graft-versus-host disease with human adipose tissue-derived mesenchymal stem cells. Bone Marrow Transplant. 2006, 38, 389–390. [Google Scholar] [CrossRef]
- Uccelli, A.; Laroni, A.; Brundin, L.; Clanet, M.; Fernandez, O.; Nabavi, S.M.; Muraro, P.A.; Oliveri, R.S.; Radue, E.W.; Sellner, J.; et al. MEsenchymal StEm cells for Multiple Sclerosis (MESEMS): A randomized, double blind, cross-over phase I/II clinical trial with autologous mesenchymal stem cells for the therapy of multiple sclerosis. Trials 2019, 20, 263. [Google Scholar] [CrossRef]
- Abu-Shahba, N.; Mahmoud, M.; El-Erian, A.M.; Husseiny, M.I.; Nour-Eldeen, G.; Helwa, I.; Amr, K.; ElHefnawi, M.; Othman, A.I.; Ibrahim, S.A.; et al. Impact of type 2 diabetes mellitus on the immunoregulatory characteristics of adipose tissue-derived mesenchymal stem cells. Int. J. Biochem. Cell Biol. 2021, 140, 106072. [Google Scholar] [CrossRef] [PubMed]
- Juntunen, M.; Heinonen, S.; Huhtala, H.; Rissanen, A.; Kaprio, J.; Kuismanen, K.; Pietilainen, K.H.; Miettinen, S.; Patrikoski, M. Evaluation of the effect of donor weight on adipose stromal/stem cell characteristics by using weight-discordant monozygotic twin pairs. Stem Cell Res. Ther. 2021, 12, 516. [Google Scholar] [CrossRef]
- Serena, C.; Keiran, N.; Ceperuelo-Mallafre, V.; Ejarque, M.; Fradera, R.; Roche, K.; Nunez-Roa, C.; Vendrell, J.; Fernandez-Veledo, S. Obesity and Type 2 Diabetes Alters the Immune Properties of Human Adipose Derived Stem Cells. Stem Cells 2016, 34, 2559–2573. [Google Scholar] [CrossRef]
- Ishizuka, T.; Hinata, T.; Watanabe, Y. Superoxide induced by a high-glucose concentration attenuates production of angiogenic growth factors in hypoxic mouse mesenchymal stem cells. J. Endocrinol. 2011, 208, 147–159. [Google Scholar] [CrossRef]
- Yin, M.; Zhang, Y.; Yu, H.B.; Li, X. Role of Hyperglycemia in the Senescence of Mesenchymal Stem Cells. Front. Cell Dev. Biol. 2021, 9, 665412. [Google Scholar] [CrossRef]
- Liu, M.H.; Li, Y.; Han, L.; Zhang, Y.Y.; Wang, D.; Wang, Z.H.; Zhou, H.M.; Song, M.; Li, Y.H.; Tang, M.X.; et al. Adipose-derived stem cells were impaired in restricting CD4+T cell proliferation and polarization in type 2 diabetic ApoE-/- mouse. Mol. Immunol. 2017, 87, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Cramer, C.; Freisinger, E.; Jones, R.K.; Slakey, D.P.; Dupin, C.L.; Newsome, E.R.; Alt, E.U.; Izadpanah, R. Persistent high glucose concentrations alter the regenerative potential of mesenchymal stem cells. Stem Cells Dev. 2010, 19, 1875–1884. [Google Scholar] [CrossRef] [PubMed]
- Keats, E.; Khan, Z.A. Unique responses of stem cell-derived vascular endothelial and mesenchymal cells to high levels of glucose. PLoS ONE 2012, 7, e38752. [Google Scholar] [CrossRef]
- Yu, S.Y.; Cheng, Y.; Zhang, L.X.; Yin, Y.Q.; Xue, J.; Li, B.; Gong, Z.Y.; Gao, J.Q.; Mu, Y.M. Treatment with adipose tissue-derived mesenchymal stem cells exerts anti-diabetic effects, improves long-term complications, and attenuates inflammation in type 2 diabetic rats. Stem Cell Res. Ther. 2019, 10, 333. [Google Scholar] [CrossRef]
- Saleh, F.A.; Jaber, H.; Eid, A. Effect of Adipose derived mesenchymal stem cells on multiple Organ Injuries in diet-induced obese mice. Tissue Barriers 2021, 9, 1952150. [Google Scholar] [CrossRef]
- Miklosz, A.; Chabowski, A. Efficacy of adipose-derived mesenchymal stem cell therapy in the treatment of chronic micro- and macrovascular complications of diabetes. Diabetes Obes. Metab. 2024, 26, 793–808. [Google Scholar] [CrossRef] [PubMed]


| Treatment | Body Weight (g) | Blood Glucose (mmol/L) | |||
|---|---|---|---|---|---|
| Start | End | Verification | Pre-treatment | Post-treatment | |
| Vehicle | 24.8 ± 1.6 | 22.3 ± 3.4 | 20.8 ± 4.3 | 27.8 ± 0.0 | 24.2 ± 8.1 * |
| StemBells | 24.0 ± 1.4 | 23.7 ± 3.5 | 18.3 ± 6.0 | 24.5 ± 6.8 | 27.8 ± 0.0 * |
| Target | Marker | Antigen Retrieval | Blocking Serum | Antibody Solvent | Primary Antibody Reference | Secondary Antibody Reference | Tertiary Antibody Reference | Visualisation (min) |
|---|---|---|---|---|---|---|---|---|
| Activated endothelium | ICAM-1 | Citrate | - | TBT | 1:500 Abcam, 119871 | 1:400 Dako, E0468 | 1:100 Vector Laboratories, PK6000 | 1–3 |
| Anti-inflammatory M2 macrophages | CD163 | Citrate | NMS | NAD | 1:200 Dako, M0794 Rockland, 810-4102 | 1:50 Immunologic, DPVR-120HRP | - | 7–10 |
| Leukocytes | CD45 | Citrate | NRS | NAD | 1:50 BD Biosciences, 553076 | 1:100 Jackson ImmunoResearch, 212-065-04 | 1:100 Dako, P0397 | 7–10 |
| Neutrophilic granulocytes | Ly6G | Pepsin | NRS | NAD | 1:200 BD Biosciences, 551459 | 1:100 Jackson ImmunoResearch, 212-065-04 | 1:100 Dako, P0397 | 7–10 |
| Pan-macrophages | Mac3 | Citrate | NRS | NAD | 1:30 o/n BD Biosciences, 553322 | 1:50 Dako, P0162 | - | 7–10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Korn, A.; Simsek, S.; Fiet, M.D.; Waas, I.S.E.; Kooiman, K.; Niessen, H.W.M.; Krijnen, P.A.J. StemBell Therapy Does Not Significantly Affect Atherosclerotic Plaque Characteristics in a Streptozotocin-Induced Diabetes Mellitus Mouse Model. Biology 2025, 14, 1130. https://doi.org/10.3390/biology14091130
Korn A, Simsek S, Fiet MD, Waas ISE, Kooiman K, Niessen HWM, Krijnen PAJ. StemBell Therapy Does Not Significantly Affect Atherosclerotic Plaque Characteristics in a Streptozotocin-Induced Diabetes Mellitus Mouse Model. Biology. 2025; 14(9):1130. https://doi.org/10.3390/biology14091130
Chicago/Turabian StyleKorn, Amber, Suat Simsek, Mitchell D. Fiet, Ingeborg S. E. Waas, Klazina Kooiman, Hans W. M. Niessen, and Paul A. J. Krijnen. 2025. "StemBell Therapy Does Not Significantly Affect Atherosclerotic Plaque Characteristics in a Streptozotocin-Induced Diabetes Mellitus Mouse Model" Biology 14, no. 9: 1130. https://doi.org/10.3390/biology14091130
APA StyleKorn, A., Simsek, S., Fiet, M. D., Waas, I. S. E., Kooiman, K., Niessen, H. W. M., & Krijnen, P. A. J. (2025). StemBell Therapy Does Not Significantly Affect Atherosclerotic Plaque Characteristics in a Streptozotocin-Induced Diabetes Mellitus Mouse Model. Biology, 14(9), 1130. https://doi.org/10.3390/biology14091130






