Integrated Taxonomy and Species Diversity of the Historical Chondrichthyan Collection of the Zoology Museum “Pietro Doderlein” at the University of Palermo (Italy)
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Comparative Assessment of the Historical Records
- Height (H): the maximum vertical distance from the lowest point of the specimen (or its supporting base) to its highest point.
- Length (L): the horizontal distance from the leftmost to the right extremity of the specimen.
- Width (l): the depth, or front-to-back dimension, of the specimen’s spatial footprint.
2.2. Morphological and Molecular Species Identification
2.3. Digitization of the Chondrichthyan Collection
- -
- Class (Holocephali/Elasmobranchii)
- -
- Superorder (Batoidea/Selachimorpha)
- -
- Order
- -
- Family and author
- -
- Genus and author
- -
- Species and author
- -
- Collection number
- -
- Year of acquisition
- -
- Historical number
- -
- Specimen (Digestive system/Respiratory system/Ocular system/Branchial and circulatory system/Tail/Skull/Taxidermized specimen/Jaw/Oviduct/Rostrum/Skeleton/Splanchnocranium)
- -
- Sex (♂ or ♀), maturity stage (juvenile, sub-adult, adult), length/total length (cm).
3. Results
3.1. Comparative Assessment of the Historical Records
3.2. Morphological and Molecular Species Identification
3.3. Catalogue Digitization
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pauly, D. Anecdotes and the shifting baseline syndrome of fisheries. Trends Ecol. Evol. 1995, 10, 430. [Google Scholar] [CrossRef] [PubMed]
- Soga, M.; Gaston, K.J. Global synthesis indicates widespread occurrence of shifting baseline syndrome. BioScience 2024, 74, 686–694. [Google Scholar] [CrossRef]
- McClenachan, L.; Ferretti, F.; Baum, J.K. From archives to conservation: Why historical data are needed to set baselines for marine animals and ecosystems. Conserv. Lett. 2012, 5, 349–359. [Google Scholar] [CrossRef]
- Gatti, G.; Bianchi, C.N.; Parravicini, V.; Rovere, A.; Peirano, A.; Montefalcone, M.; Massa, F.; Morri, C. Ecological change, sliding baselines and the importance of historical data: Lessons from combining observational and quantitative data on a temperate reef over 70 years. PLoS ONE 2015, 10, e0118581. [Google Scholar] [CrossRef]
- Johnson, K.G.; Brooks, S.J.; Fenberg, P.B.; Glover, A.G.; James, K.E.; Lister, A.M.; Michel, E.; Spencer, M.; Todd, J.A.; Valsami-Jones, E.; et al. Climate change and biosphere response: Unlocking the collections vault. Trends Ecol. Evol. 2011, 26, 84–91. [Google Scholar] [CrossRef]
- Rodrigues, A.S.L.; Monsarrat, S.; Charpentier, A.; Brooks, T.M.; Hoffmann, M.; Reeves, R.; Palomares, M.L.D.; Turvey, S.T. Unshifting the baseline: A framework for documenting historical population changes and assessing long-term anthropogenic impacts. Philos. Trans. R. Soc. B Biol. Sci. 2019, 374, 20170391. [Google Scholar] [CrossRef]
- Gaubert, P.; Papes, M.; Peterson, A.T. Natural history collections and the conservation of poorly known taxa: Ecological niche modeling in central African rainforest genets (Genetta spp.). Biol. Conserv. 2006, 130, 106–117. [Google Scholar] [CrossRef]
- Millien, V.; Lyons, S.K.; Olson, L.; Smith, F.A.; Wilson, A.B.; Yom-Tov, Y. Ecotypic variation in the context of global climate change: Revisiting the rules. Ecol. Lett. 2006, 9, 853–869. [Google Scholar] [CrossRef]
- Catullo, T.A. Memoria Geognostico-Paleozoica sulle Alpi Venete (parte prima con 4 tavole litografiche). Mem. Mat. Fis. Soc. Ital. Sci. 1848, 24, 187–342. [Google Scholar]
- Mari, M.; Ansaloni, I. Reperti dal Sud America dei medici Luigi Bompani (1814–1879) e Giuseppe Casari (1852–?) nei musei modenesi. Atti Soc. Nat. Mat. Modena 2016, 147, 351–370. [Google Scholar]
- De Brignoli Di Brünnhoff, G.; Doderlein, P.; Gaddi, P. Notizie Degli Aumenti Generosamente Procurati All’orto Botanico ed ai Musei di Storia Naturale e di Anatomia nella R. Università di Modena dal Sig. Dottore Luigi Bompani Modenese; Tip. Antonio & Angelo Cappelli: Modena, Italy, 1845; pp. 1–15. [Google Scholar]
- Doderlein, P. Donativi Fatti Nel 1852 ai Vari Gabinetti Presso la R. Università Degli Studi in Modena Dal Prof. Dott. Luigi Bompani Modenese; Tipografia Moneti & Pelloni: Modena, Italy, 1852; pp. 1–37. [Google Scholar]
- Arcoleo, G. Cronaca della Istruzione. Sul Gabinetto di zoologia nella R. Università. La Favilla, Giorn. Sc. Lett. Arti E Pedagogia 1863, 1, 248–249. [Google Scholar]
- Massa, B.; Cerasa, G.; Bellia, E.; Lo Brutto, S. In ricordo di Pietro Doderlein (2 febbraio 1809-28 marzo 1895). Nat. Sicil. S. IV XLII(2) 2018, 4, 195–236. [Google Scholar]
- Doderlein, P. Manuale Ittiologico del Mediterraneo Ossia Sinossi Metodica Delle Varie Specie di Pesci Riscontrate Sin Qui Nel Mediterraneo ed in Particolare Nelle Acque di Sicilia; Soc. Sc. nat. econom. & Min. Agr. Ind. Comm, Tipografia del Giornale di Sicilia. Italia: Palermo, Italy, 1879–1891. [Google Scholar] [CrossRef]
- Doderlein, P. Avifauna del Modenese e della Sicilia. Giorn. Sci. Nat. Econom. 1874, 10, 35–71+133–148. [Google Scholar]
- Doderlein, P. Sul passaggio autunnale di alcune specie nordiche di Uccelli per l’isola d’Ustica. Giorn. Uff. Sicil. 1872. [Google Scholar]
- Doderlein, P. Alcune generalità sulla fauna sicula dei Vertebrati. Ann. Soc. Natur. Modena 1872, 6, 1–60. [Google Scholar]
- Doderlein, P. Rivista della fauna sicula dei Vertebrati. Nuove Effem. Sicil. 1881, 11, 1–92. [Google Scholar]
- Doderlein, P. Descrizione di una notevole specie di Sgomberoide (Cybium veranyi Dod.), presa nelle acque marine della Sicilia. Giorn. Sci. Nat. Econom. 1871, 8, 125–136. [Google Scholar]
- Doderlein, P. Descrizione di alcune particolarità zoologiche-anatomiche di uno dei più rari pesci del Mediterraneo (Lophotes Cepedianus Giorna,1803). Atti Accad. Sc. Lett. Arti Palermo 1877, 5, 1–10. [Google Scholar]
- Doderlein, P. Rivista delle specie del Gen. Epinephelus Bloch (Cerna Bonap.) riscontrate sin’ora nei mari della Sicilia. Mem. Soc. Sci. Nat. Econ. Palermo 1882, 15, 1–90. [Google Scholar]
- Bellia, E.; Cerasa, G.; Cigna, V.; Brutto, S.L.; Massa, B. Epinephelus sicanus (Doderlein, 1882) (Perciformes: Serranidae: Epinephelinae), a valid species of grouper from the Mediterranean Sea. Zootaxa 2020, 4758, 191–195. [Google Scholar] [CrossRef]
- Doderlein, P. Descrizione zoologico-zootomica del Pteridium armatum. Giorn. Sicilia 1886, 5, 1–10. [Google Scholar]
- Doderlein, P.; Riggio, G. Rinvenimento di Callionymus phaeton, Günther nelle acque del Golfo di Palermo. Naturalista Sicil. 1890, 9, 133–139. [Google Scholar]
- Sarà, M. La collezione di apparati anatomici del Museo di Zoologia dell’Università di Palermo. Nat. Sicil. 1985, 9, 3–18. [Google Scholar]
- Sarà, R.; Sarà, M. La collezione ittiologica Doderlein del museo di Zoologia di Palermo. Museol. Scient. 1990, 6, 1–23. [Google Scholar]
- Spencer, M.; Vincent, S.; Smith, D.; Buschbom, J.; Collier, B.; Ellis, L.; Grinberg, I.; Hsu, T.-T.; Hunn, B.; Humphries, J.; et al. Pioneering Museum Data Transformation: Unifying Legacy Systems and Enhancing Data Integrity. In Proceedings of the Biodiversity Information Science and Standards (BISS) 2024, Meise, Belgium, 5–8 November 2024. [Google Scholar]
- Doderlein, P. Prospetto Metodico Delle Varie Specie di Pesci Riscontrate Sinora Nelle Acque Marine e Fluviali Della sicilia, e Catalogo Delle Relative Preparazioni Tassidermiche e Anatomiche Che Si Conservano Nel Museo Zoologico-Zootomico Della Regia Università Di Palermo; Tip. Giorn. Sicilia: Palermo, Italy, 1879. [Google Scholar]
- ICCD. Scheda BNZ—Beni Naturalistici—Zoologia. In Strutturazione dei Dati Delle Schede di Catalogo (Zoological Naturalistic Heritage Data Sheets: Data Structuring of Catalog Records); ICCD: Rome, Italy, 2007; pp. 1–110. [Google Scholar]
- Conservazione e Gestione Delle Risorse Marine: Le Specie Ittiche. Available online: https://elearning.uniroma1.it/pluginfile.php/254863/mod_folder/content/0/Specie%20Ittiche.pdf?forcedownload=1 (accessed on 18 March 2024).
- Marongiu, M.F. La Riproduzione nei Condroitti Come Elemento Chiave per la Loro Conservazione e Gestione nel Mediterraneo Centro-Occidentale. Ph.D. Thesis, University of Cagliari, Cagliari, Italy, 2014. [Google Scholar]
- Otero, M.; Serena, F.; Gerovasileiou, V.; Barone, M.; Bo, M.; Arcos, J.M.; Vulcano, A.; Xavier, J. Guida All’Identificazione Delle Specie Vulnerabili Oggetto di Catture Accidentali Della Pesca Nel Mediterraneo; IUCN: Malaga, Spain, 2021. [Google Scholar]
- Ebert, D.A.; Dando, M.; Fowler, S. Sharks of the World: A Complete Guide; Princeton University Press: Princeton, NJ, USA, 2021. [Google Scholar]
- Gordon, C. A Guide to Angel Shark Identification; The Shark Trust: Plymouth, UK, 2022. [Google Scholar]
- Tortonese, E. Fauna of Italy: Leptocardia, Cyclostomata, Selachii; Italian National Academy of Entomology: Florence, Italy; Italian Zoological Union: Rome, Italy; Publishing Calderini: Bologna, Italy, 1956. [Google Scholar]
- Last, P.R.; White, W.T.; de Carvalho, M.R.; Séret, B.; Stehmann, M.F.W.; Naylor, G.J.P. Rays of the World; CSIRO Publishing & Cornell University Press: Clayton, Australia, 2016. [Google Scholar]
- Ebert, D.A.; Dando, M. Blackspot Smothhound Mustelus punctulatus. In Field Guide to Sharks, Rays & Chimaeras of Europe and the Mediterranean; Wild Nature Press; Princeton University Press: Princeton, NJ, USA, 2021; Volume 20, pp. 1–2. [Google Scholar]
- Barone, M.; Mazzoldi, C.; Serena, F. Sharks, Rays and Chimaeras in Mediterranean and Black Seas—Key to Identification; Food and Agriculture Organization: Rome, Italy, 2022. [Google Scholar] [CrossRef]
- Naylor, G.J.P.; Marcus, L.F. Identifying isolated shark teeth of the genus Carcharhinus to species: Relevance for tracking phyletic change through the fossil record. Am. Mus. Novit. 1994, 3109, 53. [Google Scholar]
- Purdy, R.W. A Key to the Common Genera of Neogene Shark Teeth; National Museum of Natural History: Washington, DC, USA, 2003. [Google Scholar]
- Pollerspöck, J.; Straube, N. An Identification Key to Elasmobranch Genera Based on Dental Morphological Characters Part A: Squalomorph Sharks (Superorder Squalomorphii). Bull. Fish Biol. 2018, 18, 77–105. [Google Scholar]
- Türtscher, J.; Jambura, P.L.; López-Romero, F.A.; Kindlimann, R.; Sato, K.; Tomita, T.; Kriwet, J. Heterodonty and ontogenetic shift dynamics in the dentition of the tiger shark Galeocerdo cuvier (Chondrichthyes, Galeocerdidae). J. Anat. 2022, 241, 372–392. [Google Scholar] [CrossRef]
- Campos, P.F.; Gilbert, T.M.P. Ancient DNA: Methods and Protocols. Methods Mol. Biol. 2012, 42, 81–85. [Google Scholar]
- Gilbert, M.T.P.; Bandelt, H.J.; Hofreiter, M.; Barnes, I. Assessing ancient DNA studies. Trends Ecol. Evol. 2005, 20, 541–544. [Google Scholar] [CrossRef] [PubMed]
- Llamas, B.; Valverde, G.; Fehren-Schmitz, L.; Weyrich, L.S.; Cooper, A.; Haak, W. From the field to the laboratory: Controlling DNA contamination in human ancient DNA research in the high-throughput sequencing era. Sci. Technol. Archaeol. Res. 2017, 3, 1–14. [Google Scholar] [CrossRef]
- Fulton, T.L. Setting Up an Ancient DNA Laboratory. Methods Mol. Biol. 2012, 840, 1–11. [Google Scholar] [PubMed]
- Cilli, E.; Gabanini, G.; Ciucani, M.M.; De Fanti, S.; Serventi, P.; Bazaj, A.; Sarno, S.; Ferri, G.; Fregnani, A.; Cornaglia, G.; et al. A Multifaceted Approach Towards Investigating Childbirth Deaths in Double Burials: Anthropology, Paleopathology and Ancient DNA. J. Archaeol. Sci. 2020, 122, 105219. [Google Scholar] [CrossRef]
- Dabney, J.; Knapp, M.; Glocke, I.; Gansauge, M.T.; Weihmann, A.; Nickel, B.; Valdiosera, C.; García, N.; Pääbo, S.; Arsuaga, J.L.; et al. Complete Mitochondrial Genome Sequence of a Middle Pleistocene Cave Bear Re-Constructed from Ultrashort DNA Fragments. Proc. Natl. Acad. Sci. USA 2013, 110, 15758–15763. [Google Scholar] [CrossRef]
- Miya, M.; Sato, Y.; Fukunaga, T.; Sado, T.; Poulsen, J.Y.; Sato, K.; Minamoto, T.; Yamamoto, S.; Yamanaka, H.; Araki, H.; et al. MiFish, a Set of Universal PCR Primers for Metabarcoding Environmental DNA from Fishes: Detection of More Than 230 Subtropical Marine Species. R. Soc. Open Sci. 2015, 2, 150088. [Google Scholar] [CrossRef]
- Taberlet, P.; Bonin, A.; Zinger, L.; Coissac, E. Sampling. In Environmental DNA: For Biodiversity Research and Monitoring; Taberlet, P., Bonin, A., Zinger, L., Coissac, E., Eds.; Oxford University Press: Oxford, UK, 2018; pp. 28–34. [Google Scholar]
- Liu, Z.; Collins, R.A.; Baillie, C.; Rainbird, S.; Brittain, R.; Griffiths, A.M.; Sims, D.W.; Mariani, S.; Genner, M.J. Environmental DNA Captures Elasmobranch Diversity in a Temperate Marine Ecosystem. Environ. DNA 2022, 4, 1024–1038. [Google Scholar] [CrossRef]
- Albonetti, L.; Maiello, G.; Cariani, A.; Carpentieri, P.; Ferrari, A.; Sbrana, A.; Shum, P.; Talarico, L.; Russo, T.; Mariani, S. DNA Metabarcoding of Trawling Bycatch Reveals Diversity and Distribution Patterns of Sharks and Rays in the Central Tyrrhenian Sea. ICES J. Mar. Sci. 2023, 80, 664–674. [Google Scholar] [CrossRef]
- Maiello, G.; Bellodi, A.; Cariani, A.; Carpentieri, P.; Carugati, L.; Cicala, D.; Ferrari, A.; Follesa, C.; Ligas, A.; Sartor, P.; et al. Fishing in the Gene-Pool: Implementing Trawl-Associated eDNA Metaprobe for Large Scale Monitoring of Fish Assemblages. Rev. Fish Biol. Fish. 2024, 34, 1293–1307. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Ratnasingham, S.; Wei, C.; Chan, D.; Agda, J.; Agda, J.; Ballesteros-Mejia, L.; Ait Boutou, H.; El Bastami, Z.M.; Ma, E.; Manjunath, R.; et al. BOLD v4: A Centralized Bioinformatics Platform for DNA-Based Biodiversity Data. In DNA Barcoding. Methods in Molecular Biology; DeSalle, R., Ed.; Humana: New York, NY, USA, 2024; Volume 2744, pp. 403–441. [Google Scholar] [CrossRef]
- Thompson, J.D.; Higgins, D.G.; Gibson, T.J. CLUSTAL W: Improving the Sensitivity of Progressive Multiple Sequence Alignment Through Sequence Weighting, Position-Specific Gap Penalties and Weight Matrix Choice. Nucleic Acids Res. 1994, 22, 4673–4680. [Google Scholar] [CrossRef] [PubMed]
- Adobe. Adobe Photoshop 2022 & Adobe Lightroom Classic: User Guide; Adobe Inc.: San Jose, CA, USA, 2022. [Google Scholar]
- Crobe, V. Towards the Conservation of Vulnerable Marine Large Predators: Morphometric, Molecular and Microchemical Variation of Historical Sawfish Rostra from Mediterranean Collections. Ph.D. Thesis, University of Bologna, Bologna, Italy, 2022. [Google Scholar]
- Meriç, N.; Eryilmaz, L.; Özuluğ, M. A Catalogue of the Fishes Held in the Istanbul University, Science Faculty, Hydrobiology Museum. Zootaxa 2007, 1472, 29–54. [Google Scholar] [CrossRef]
- Agnelli, P.; Ducci, L.; Funaioli, U.; Cagnolaro, L. La Collezione dei Cetacei Attuali del Museo di Storia Naturale dell’Università di Firenze: Indagine Storica e Revisione Sistematica. Museol. Scient. Mem. 2014, 12, 194–212. [Google Scholar]
- Podestà, M.; Bardelli, G.; Cagnolaro, L. Catalogo dei cetacei attuali del Museo di Storia Naturale di Milano. Museol. Scient. Mem. 2014, 12, 24–51. [Google Scholar]
- Sacdanaku, E.; Bajrami, A.; Saliaj, O.; Mullaj, A. Museum of Natural Sciences “Sabiha Kasimati”: Past and Present. Museol. Scient. 2018, 12, 130–137. [Google Scholar]
- Cagnolaro, L.; Podestà, M.; Affronte, M.; Agnelli, P.; Cancelli, F.; Capanna, E.; Carlini, R.; Cataldini, G.; Cozzi, B.; Insacco, G.; et al. Collections of Extant Cetaceans in Italian Museums and Other Scientific Institutions: A Comparative Review. Atti Soc. Ital. Sci. Nat. Mus. Civ. Stor. Nat. Milano 2012, 153, 145–202. [Google Scholar] [CrossRef][Green Version]
- Faria, V.V.; McDavitt, M.T.; Charvet, P.; Wiley, T.R.; Simpfendorfer, C.A.; Naylor, G.J.P. Species Delineation and Global Population Structure of Critically Endangered Sawfishes (Pristidae). Zool. J. Linn. Soc. 2013, 167, 136–164. [Google Scholar] [CrossRef]
- Fearing, A.; Smith, K.; Wiley, T.; Whitty, J.; Feldheim, K.; Kyne, P.; Phillips, N. Looking Back for the Future: Utilizing Sawfish Saws from Natural History Collections to Conserve the Critically Endangered Largetooth Sawfish (Pristis pristis). Biodivers. Data J. 2018, e25806. [Google Scholar] [CrossRef]
- Bevacqua, L.; Reinero, F.R.; Becerril-García, E.E.; Elorriaga-Verplancken, F.R.; Juaristi-Videgaray, D.; Micarelli, P.; Galván-Magaña, F.; Curiel-Godoy, P.; Giglio, G.; Tripepi, S.; et al. Trace Elements and Isotopes Analyses on Historical Samples of White Sharks from the Mediterranean Sea. Eur. Zool. J. 2021, 88, 132–141. [Google Scholar] [CrossRef]
- Manuzzi, A.; Jiménez-Mena, B.; Henriques, R.; Holmes, B.J.; Pepperell, J.; Edson, J.; Bennett, M.B.; Huveneers, C.; Ovenden, J.R.; Nielsen, E.E. Retrospective Genomics Highlights Changes in Genetic Composition of Tiger Sharks (Galeocerdo cuvier) and Potential Loss of a South-Eastern Australia Population. Sci. Rep. 2022, 12, 6582. [Google Scholar] [CrossRef]
- Pasino, M.; Cariani, A.; Crobe, V.; Cilli, E.; Iacovelli, M.V.; Mazzini, A.; Perini, E.M.; Bundone, L.E.A.; Fioravanti, T.; Arneri, E.; et al. Le Collezioni Museali Naturalistiche Come Fondamentale Risorsa Nella Ricerca Scientifica: Distribuzione Spazio-Temporale Dei Reperti Di Grandi Vertebrati Marini Nei Musei Italiani. Quad. Mus. Civ. Stor. Nat. Ferrara 2023, 11, 33–48. [Google Scholar]
- Srinivasan, M.; Sedmak, D.; Jewell, S. Effect of fixatives and tissue processing on the content and integrity of nucleic acids. Am. J. Pathol. 2002, 161, 1961–1971. [Google Scholar] [CrossRef]
- Zimmermann, J.; Hajibabaei, M.; Blackburn, D.C.; Hanken, J.; Cantin, E.; Posfai, J.; Evans, T.C., Jr. DNA damage in preserved specimens and tissue samples: A molecular assessment. Front. Zool. 2008, 5, 18. [Google Scholar] [CrossRef]
- Lynghammar, A.; Christiansen, J.S.; Griffiths, A.M.; Fevolden, S.-E.; Hop, H.; Bakken, T. DNA barcoding of the Northern Northeast Atlantic skates (Chondrichthyes, Rajiformes), with remarks on the widely distributed starry ray. Zool. Scr. 2014, 43, 485–495. [Google Scholar] [CrossRef]
- Carugati, L.; Melis, R.; Cariani, A.; Cau, A.; Crobe, V.; Ferrari, A.; Follesa, M.C.; Geraci, M.L.; Iglésias, S.P.; Pesci, P.; et al. Combined COI barcode-based methods to avoid mislabelling of threatened species of deep-sea skates. Anim. Conserv. 2022, 25, 38–52. [Google Scholar] [CrossRef]
- Ferrari, A.; Crobe, V.; Cannas, R.; Leslie, R.W.; Serena, F.; Stagioni, M.; Costa, F.O.; Golani, D.; Hemida, F.; Zaera-Perez, D.; et al. To Be, or Not to Be: That Is the Hamletic Question of Cryptic Evolution in the Eastern Atlantic and Mediterranean Raja miraletus Species Complex. Animals 2023, 13, 2139. [Google Scholar] [CrossRef]
- Dulvy, N.K.; Allen, D.J.; Ralph, G.M.; Walls, R.H.L. The Conservation Status of Sharks, Rays and Chimaeras in the Mediterranean Sea; IUCN: Malaga, Spain, 2016; pp. 1–15. [Google Scholar]
- Bertrand, J.A.; Leonori, I.; Dremière, P.-Y.; Cosimi, G. Depth trajectory and performance of a trawl used for an international bottom trawl survey in the Mediterranean. Sci. Mar. 2002, 66 (Suppl. S2), 169–182. [Google Scholar] [CrossRef]
- Spedicato, M.T.; Massutí, E.; Mérigot, B.; Tserpes, G.; Jadaud, A.; Relini, G. The MEDITS trawl survey specifications in an ecosystem approach to fishery management. Sci. Mar. 2019, 83 (Suppl. S1), 9–20. [Google Scholar] [CrossRef]
- Cannas, R.; Follesa, M.C.; Cabiddu, S.; Porcu, C.; Salvadori, S.; Iglésias, S.P.; Deiana, A.M.; Cau, A. Molecular and morphological evidence of the occurrence of the Norwegian skate Dipturus nidarosiensis (Storm, 1881) in the Mediterranean Sea. Mar. Biol. Res. 2010, 6, 341–350. [Google Scholar] [CrossRef]
- Cariani, A.; Messinetti, S.; Ferrari, A.; Arculeo, M.; Bonello, J.J.; Bonnici, L.; Cannas, R.; Carbonara, P.; Cau, A.; Charilaou, C.; et al. Improving the Conservation of Mediterranean Chondrichthyans: The ELASMOMED DNA Barcode Reference Library. PLoS ONE 2017, 12, e0170244. [Google Scholar] [CrossRef] [PubMed]
- Carbonara, P.; Cannas, R.; Donnaloia, M.; Melis, R.; Porcu, C.; Spedicato, M.T.; Zupa, W.; Follesa, M.C. On the presence of Dipturus nidarosiensis (Storm, 1881) in the Central Mediterranean area. PeerJ 2019, 7, e7009. [Google Scholar] [CrossRef] [PubMed]
| Register | n |
|---|---|
| Historical Record 1863–1880 | 16 |
| Acquisitions 1880–1922 | 53 |
| Additions up to 1922 | 7 |
| CODE | 1880 | 1880–1890 | 1922 | 2024 |
|---|---|---|---|---|
| 118 | 2 | – | 1 | 1 |
| 119 | 18 | – | 18 | 16 |
| 120 | 37 | – | 32 | 29 |
| 121 | 53 | – | 46 | 41 |
| 129 | 23 | – | 23 | – |
| 139 | 36 | – | 36 | 33 |
| 143 | 27 | – | 27 | 25 |
| 147 | 45 | – | 45 | 42 |
| 148 | 3 | – | 3 | 5 |
| 151 | 169 | – | 185 | 37 |
| 152 | 96 | – | 96 | 1 |
| 153 | 30 | – | 30 | 1 |
| 154 | 9 | – | 9 | 1 |
| 158 | 6 | – | 3 | 1 |
| 119 | – | 1 | – | – |
| 435 | – | 1 | 1 | – |
| 487 | – | 1 | – | – |
| 564 | – | 3 | – | – |
| 589 | – | 1 | 1 | – |
| 594 | – | 1 | 1 | – |
| 595 | – | 1 | – | 1 |
| 638 | – | 1 | – | – |
| 693 | – | 1 | – | – |
| 695 | – | 1 | 1 | – |
| 710 | – | 2 | – | – |
| 744 | – | 1 | 1 | – |
| 765 | – | 1 | 1 | – |
| 805 | – | 1 | 1 | – |
| 807 | – | 1 | 1 | – |
| 852 | – | 1 | 1 | – |
| 1062 | – | 1 | – | – |
| 1115 | – | 1 | 1 | – |
| 1120 | – | 1 | – | 1 |
| 440-441 | – | 1 | 1 | – |
| 2648 bis | – | – | 4 | 6 |
| 2650 bis | – | – | 25 | 29 |
| A | Code | Specimen Label | Morphological Identification | Molecular Species Identification |
|---|---|---|---|---|
| AN | 7 | Leucoraja circularis | Rostroraja alba | |
| AN | 31 | Squatina squatina | Squatina squatina * | – |
| AN | 32 | Squatina squatina | Squatina squatina * | – |
| AN | 33 | Squatina oculata | Squatina oculata * | – |
| AN | 35 | Rostroraja alba | Raja brachyura | |
| AN | 39 | Dipturus batis | Dipturus batis * | Dipturus batis |
| AN | 40 | Mustelus mustelus | Mustelus asterias | |
| AN | 41 | Squalus acanthias | Squalus blainville | |
| AN | 42 | Centrophorus granulosus | Centrophorus uyato | |
| AN | 51 | Raja asterias | Raja brachyura | |
| AN | 59 | Mustelus asterias | Mustelus mustelus | |
| AN | 69 | Raja asterias | Raja brachyura | |
| AN | 75 | Scyliorhinus stellaris | Scyliorhinus canicula | |
| AN | 76 | Aetomylaeus bovinus | Dasyatis centroura | |
| AN | 83 | Squatina squatina | Squatina aculeata * | Squatina aculeata |
| AN | 84 | Squatina squatina | Squatina oculate * | – |
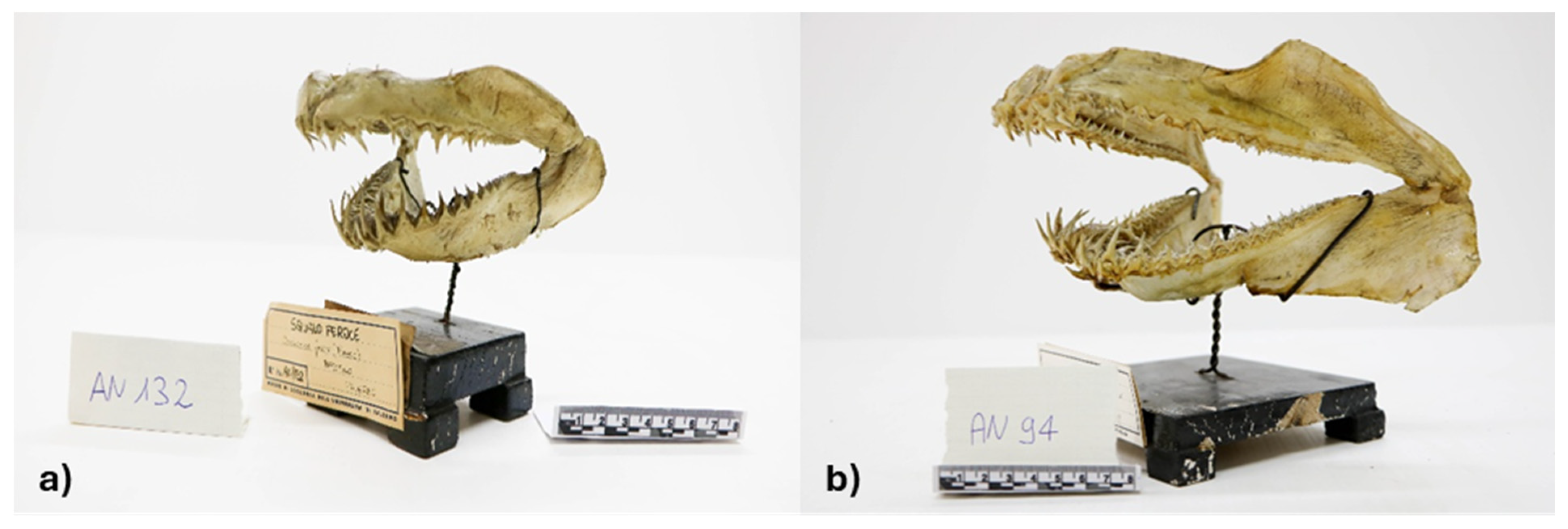
| AN | 94 | Carcharias taurus | Odontaspis ferox | |
| AN | 103 | Raja undulata | Raja clavata | |
| AN | 105 | Prionace glauca | Carcharhinus plumbeus | |
| AN | 109 | Prionace glauca | Carcharhinus plumbeus | |
| AN | 110 | Prionace glauca | Echinorhinus brucus | |
| AN | 111 | Prionace glauca | Carcharhinus plumbeus | |
| AN | 117 | Prionace glauca | Carcharhinus plumbeus | |
| AN | 119 | Centrophorus granulosus | Centrophorus uyato | |
| AN | 122 | Centrophorus granulosus | Centrophorus uyato | |
| AN | 123 | Prionace glauca | Carcharhinus plumbeus | |
| AN | 129 | Centrophorus granulosus | Centrophorus uyato | |
| AN | 131 | Prionace glauca | Carcharhinus plumbeus | |
| AN | 132 | Odontaspis ferox | Carcharias taurus | |
| AN | 135 | Scyliorhinus canicula | Scyliorhinus stellaris | |
| AN | 140 | Squatina squatina | Squatina aculeata * | Squatina aculeata |
| AN | 142 | Squatina oculata | Squatina aculeata * | – |
| AN | 144 | Prionace glauca | Carcharhinus plumbeus | |
| AN | 182 | Pristis pristis | Anoxypristis cuspidata | Anoxypristis cuspidata [59] |
| AN | 183 | Pristis pristis | Pristis zijsron | Pristis zijsron [59] |
| AN | 184 | Pristis pristis | Pristis pectinata | Pristis pectinata [59] |
| AN | 185 | Pristis pristis | Pristis pectinata | Pristis pectinata [59] |
| AN | 186 | Pristis pristis | Pristis zijsron | Pristis zijsron [59] |
| AN | 405 | Squatina oculata | Squatina aculeata * | Squatina aculeata |
| AN | 406 | Leucoraja circularis | Rostroraja alba * | Rostroraja alba |
| AN | 1235 | Isurus oxyrinchus | Prionace glauca | |
| AN | 1236 | Isurus oxyrinchus | Odontaspis ferox | |
| P | 518 | Odontaspis ferox | Carcharias taurus | |
| P | 520 | Mustelus asterias | Mustelus mustelus | |
| P | 532 | Centrophorus granulosus | Centrophorus uyato | |
| P | 538 | Centrophorus granulosus | Centrophorus uyato | |
| P | 542 | Scyliorhinus stellaris | Scyliorhinus canicula | |
| P | 543 | Scyliorhinus stellaris | Scyliorhinus canicula | |
| P | 544 | Scyliorhinus stellaris | Scyliorhinus canicula | |
| P | 562 | Rhinobatos rhinobatos | Rhinobatos cemiculus | |
| P | 563 | Squatina oculata | Squatina aculeata | |
| P | 602 | Raja brachyura | Raja polystigma | |
| P | 604 | Raja brachyura | Raja polystigma | |
| P | 606 | Raja montagui | – | Raja radula |
| P | 612 | Raja asterias | Raja polystigma | |
| P | 616 | Raja montagui | – | Raja radula |
| P | 618 | Raja asterias | Raja brachyura | |
| P | 622 | Raja asterias | Raja polystigma | |
| P | 632 | Centrophorus granulosus | Centrophorus uyato | |
| P | 645 | Raja polystigma | Raja brachyura | |
| P | 649 | Rhinobatos rhinobatos | Rhinobatos cemiculus | |
| P | 650 | Rhinobatos rhinobatos | Rhinobatos cemiculus | |
| P | 651 | Dipturus nidarosiensis | Dipturus nidarosiensis * | Dipturus nidarosiensis |
| P | 653 | Dipturus intermedius | Dipturus batis | |
| P | 660 | Dipturus oxyrinchus | Dipturus batis |
| FAMILY | n | s | TAX | SKE | J | SKU | R | DP | LP |
|---|---|---|---|---|---|---|---|---|---|
| Alopiidae | 7 | 1 | 2 | 4 | 1 | ||||
| Carcharhinidae | 19 | 3 | 4 | 1 | 13 | 1 | |||
| Centrophoridae | 10 | 2 | 4 | 1 | 4 | 1 | |||
| Cetorhinidae | 2 | 1 | 1 | 1 | |||||
| Chimaeridae | 7 | 1 | 3 | 2 | 1 | 1 | |||
| Dalatiidae | 10 | 1 | 4 | 1 | 2 | 3 | |||
| Dasyatidae | 23 | 3 | 6 | 3 | 8 | 5 | 1 | ||
| Echinorhinidae | 5 | 1 | 1 | 3 | 1 | ||||
| Etmopteridae | 4 | 1 | 2 | 1 | 1 | ||||
| Gymnuridae | 7 | 1 | 3 | 1 | 3 | ||||
| Hexanchidae | 11 | 2 | 4 | 2 | 4 | 1 | |||
| Lamnidae | 16 | 3 | 2 | 13 | 1 | ||||
| Mobulidae | 3 | 1 | 1 | 1 | 1 | ||||
| Myliobatidae | 17 | 2 | 4 | 3 | 3 | 1 | 5 | 1 | |
| Odontaspididae | 18 | 2 | 4 | 1 | 9 | 2 | 2 | ||
| Oxynotidae | 3 | 1 | 2 | 1 | |||||
| Pentanchidae | 1 | 1 | 1 | ||||||
| Pristidae | 6 | 3 | 6 | ||||||
| Rajidae | 96 | 13 | 45 | 18 | 22 | 10 | 1 | ||
| Rhinobatidae | 14 | 2 | 8 | 2 | 4 | ||||
| Scyliorhinidae | 7 | 2 | 4 | 1 | 2 | ||||
| Somniosidae | 1 | 1 | 1 | ||||||
| Sphyrnidae | 11 | 1 | 4 | 2 | 2 | 2 | 1 | ||
| Squalidae | 5 | 3 | 2 | 1 | 1 | 1 | |||
| Squatinidae | 14 | 3 | 4 | 2 | 6 | 1 | 1 | ||
| Torpedinidae | 13 | 3 | 7 | 2 | 2 | 2 | |||
| Triakidae | 12 | 3 | 5 | 2 | 1 | 3 | 1 | ||
| Total | 342 | 62 | 127 | 49 | 98 | 8 | 6 | 45 | 16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iacovelli, M.V.; Bellia, E.; Caruso, M.; Zaffuto, E.; Crobe, V.; Marrone, F.; Mazzotti, S.; Tinti, F. Integrated Taxonomy and Species Diversity of the Historical Chondrichthyan Collection of the Zoology Museum “Pietro Doderlein” at the University of Palermo (Italy). Biology 2025, 14, 1129. https://doi.org/10.3390/biology14091129
Iacovelli MV, Bellia E, Caruso M, Zaffuto E, Crobe V, Marrone F, Mazzotti S, Tinti F. Integrated Taxonomy and Species Diversity of the Historical Chondrichthyan Collection of the Zoology Museum “Pietro Doderlein” at the University of Palermo (Italy). Biology. 2025; 14(9):1129. https://doi.org/10.3390/biology14091129
Chicago/Turabian StyleIacovelli, Maria Vittoria, Enrico Bellia, Martina Caruso, Ettore Zaffuto, Valentina Crobe, Federico Marrone, Stefano Mazzotti, and Fausto Tinti. 2025. "Integrated Taxonomy and Species Diversity of the Historical Chondrichthyan Collection of the Zoology Museum “Pietro Doderlein” at the University of Palermo (Italy)" Biology 14, no. 9: 1129. https://doi.org/10.3390/biology14091129
APA StyleIacovelli, M. V., Bellia, E., Caruso, M., Zaffuto, E., Crobe, V., Marrone, F., Mazzotti, S., & Tinti, F. (2025). Integrated Taxonomy and Species Diversity of the Historical Chondrichthyan Collection of the Zoology Museum “Pietro Doderlein” at the University of Palermo (Italy). Biology, 14(9), 1129. https://doi.org/10.3390/biology14091129







