Integrated Transcriptomic and Metabolomic Analysis of Color Changes in Maize Root Systems Treated with Methyl Jasmonate
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. The Cultivation and Treatment of Plant Materials
2.2. Library Construction and Sequencing for Transcriptome Analysis
2.3. Metabolomic Library Construction and Analysis
2.4. Metabolomic Profiling Analysis
2.5. Correlation Analysis
2.6. Identification of Real-Time qRT-PCR
3. Results
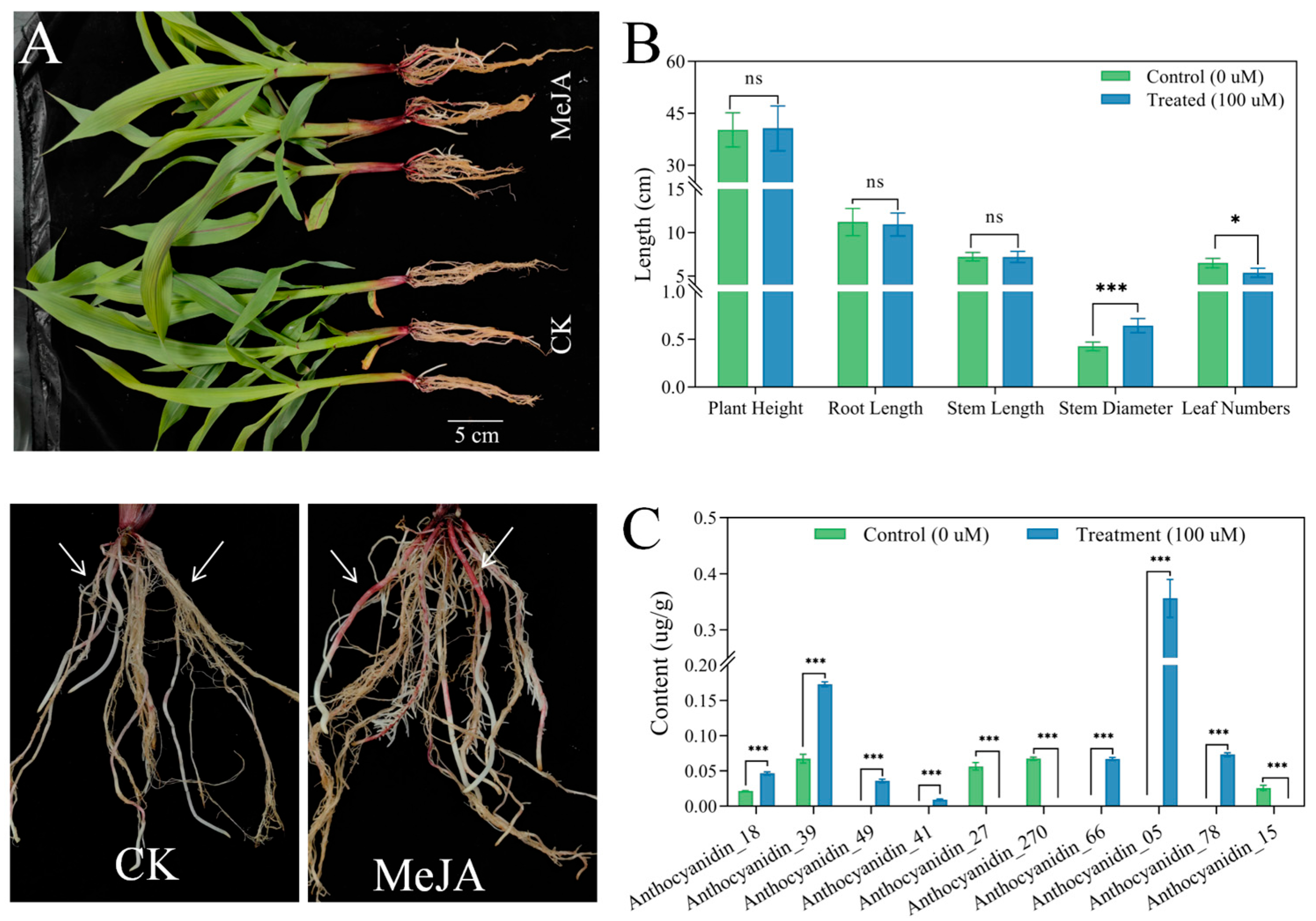
3.1. The Phenotypes and Anthocyanin Content Under the MeJA Treatment
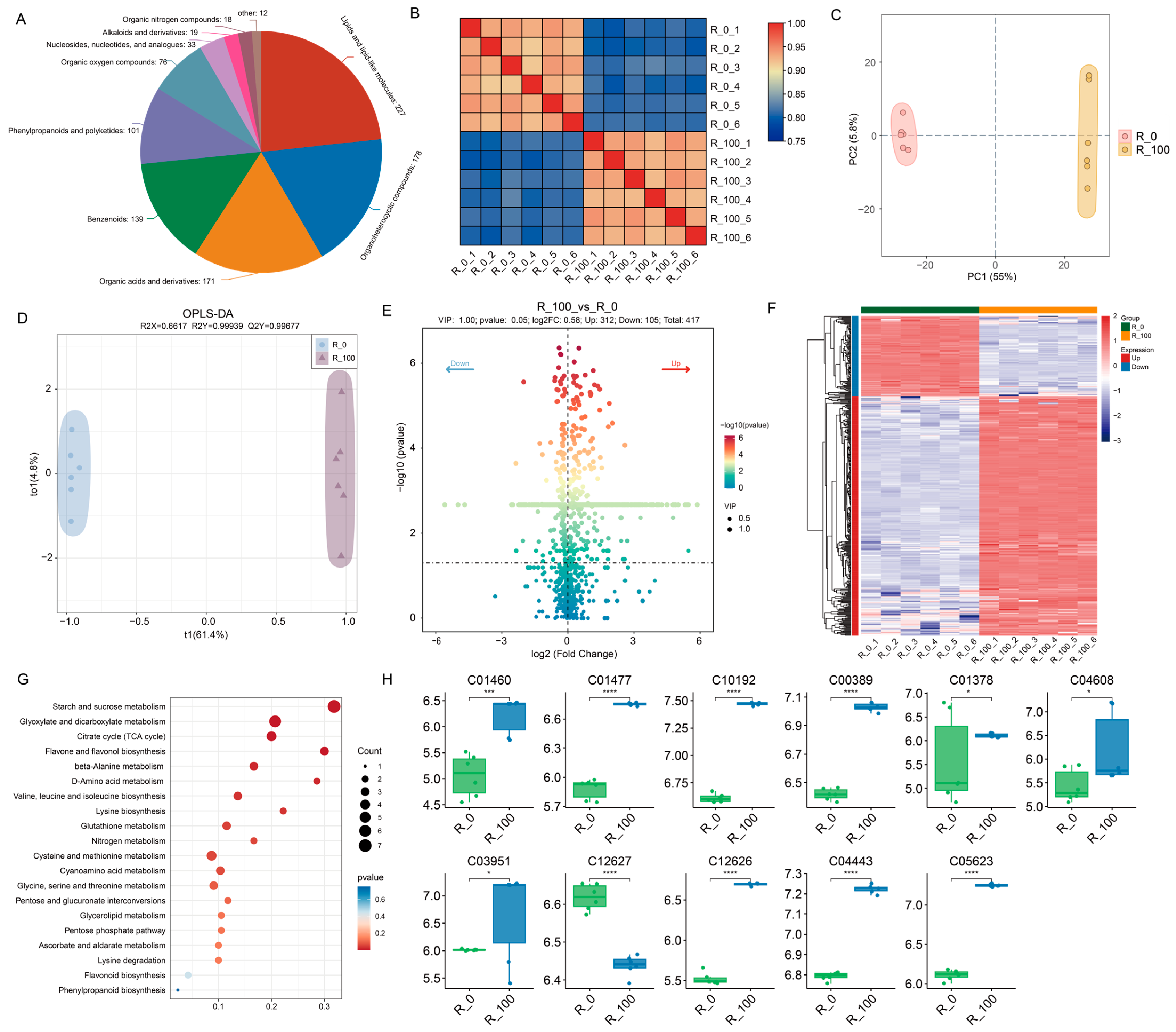
3.2. Transcriptome Analysis of Maize Seedlings Under MeJA Treatments
3.3. Analysis and Enrichment of Metabolic Processes Under MeJA Treatments
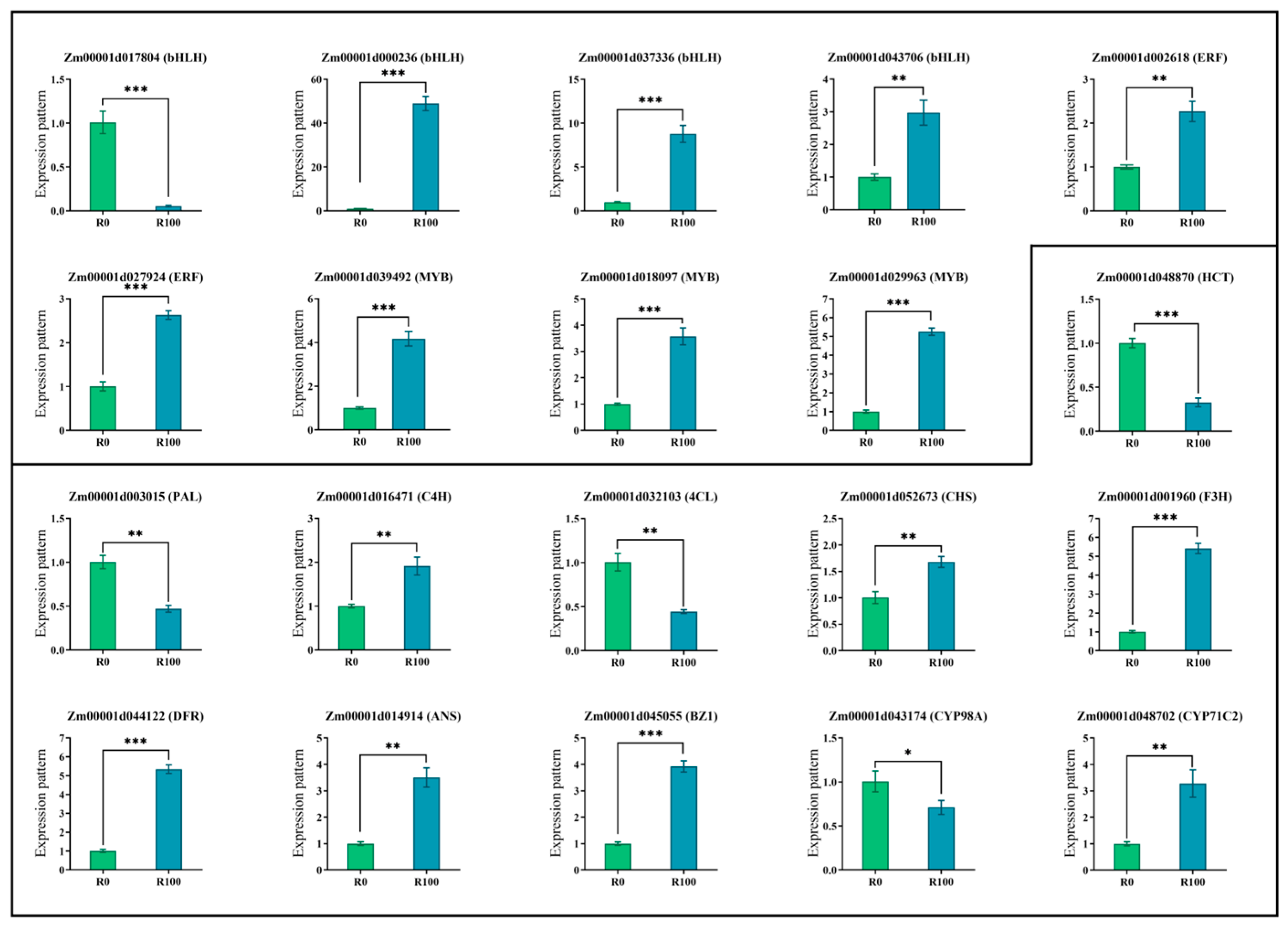
3.4. Combined Transcriptomics and Metabolomics Analysis of Maize Response to MeJA-Induced Gene
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, Y.; Gao, Z.; Zhang, C.; Li, N.; Liu, C. Research progress on the molecular regulation mechanism of anthocyanin biosynthesis pathway. Chin. J. Ecol. 2015, 34, 2937–2942. [Google Scholar] [CrossRef]
- Shoeva, O.Y.; Gordeeva, E.I.; Arbuzova, V.S.; Khlestkina, E.K. Anthocyanins Participate in Protection of Wheat Seedlings from Osmotic Stress. Cereal Res. Commun. 2017, 45, 47–56. [Google Scholar] [CrossRef]
- Chen, X.Y.; He, Y.Q.; Hong, G.J.; Li, J.M.; Li, L.Y.; Sun, Z.T.; Zhang, X.Y. SPX4 interacts with both PHR1 and PAP1 to regulate critical steps in phosphorus-status-dependent anthocyanin biosynthesis. New Phytol. 2021, 230, 205–217, Erratum in: New Phytol. 2021, 231, 1658. [Google Scholar] [CrossRef]
- Dong, Q.Q.; Zhao, X.H.; Zhou, D.Y.; Liu, Z.H.; Shi, X.L.; Yuan, Y.; Jia, P.Y.; Liu, Y.Y.; Song, P.H.; Wang, X.G.; et al. Maize and peanut intercropping improves the nitrogen accumulation and yield per plant of maize by promoting the secretion of flavonoids and abundance of Bradyrhizobium in rhizosphere. Front. Plant Sci. 2022, 13, 16. [Google Scholar] [CrossRef]
- Winkel-Shirley, B. Flavonoid biosynthesis. A colorful model for genetics, biochemistry, cell biology, and biotechnology. Plant Physiol. 2001, 126, 485–493. [Google Scholar] [CrossRef]
- Ma, C.Q.; Liang, B.W.; Chang, B.; Yan, J.Y.; Liu, L.; Wang, Y.; Yang, Y.Z.; Zhao, Z.Y. Transcriptome profiling of anthocyanin biosynthesis in the peel of “Granny Smith” apples (Malus domestica) after bag removal. BMC Genom. 2019, 20, 18. [Google Scholar] [CrossRef]
- Luo, L.; Molthoff, J.; Li, Q.; Liu, Y.; Luo, S.X.; Li, N.; Xuan, S.X.; Wang, Y.H.; Shen, S.X.; Bovy, A.G.; et al. Identification of candidate genes associated with less-photosensitive anthocyanin phenotype using an EMS mutant (pind) in eggplant (Solanum melongena L.). Front. Plant Sci. 2023, 14, 14. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.L.; Liu, C.L.; Song, L.L.; Dong, Y.X.; Chen, L.; Li, M. A Sweet Cherry Glutathione S-Transferase Gene, PavGST1, Plays a Central Role in Fruit Skin Coloration. Cells 2022, 11, 1170. [Google Scholar] [CrossRef]
- Ma, J.R.; Li, Z.; Liu, Y.L. Integrating Multi-Omics Analysis Reveals the Regulatory Mechanisms of White-Violet Mutant Flowers in Grape Hyacinth (Muscari latifolium). Int. J. Mol. Sci. 2023, 24, 5044. [Google Scholar] [CrossRef]
- Ke, Y.J.; Zheng, Q.D.; Yao, Y.H.; Ou, Y.; Chen, J.Y.; Wang, M.J.; Lai, H.P.; Yan, L.; Liu, Z.J.; Ai, Y. Genome-Wide Identification of the MYB Gene Family in Cymbidium ensifolium and Its Expression Analysis in Different Flower Colors. Int. J. Mol. Sci. 2021, 22, 13245. [Google Scholar] [CrossRef]
- Huang, G.Q.; Zeng, Y.C.; Wei, L.; Yao, Y.Q.; Dai, J.; Liu, G.; Gui, Z.Z. Comparative transcriptome analysis of mulberry reveals anthocyanin biosynthesis mechanisms in black (Morus atropurpurea Roxb.) and white (Morus alba L.) fruit genotypes. BMC Plant Biol. 2020, 20, 12. [Google Scholar] [CrossRef]
- Guo, L.P.; da Silva, J.A.T.; Pan, Q.H.; Liao, T.; Yu, X.N. Transcriptome Analysis Reveals Candidate Genes Involved in Anthocyanin Biosynthesis in Flowers of the Pagoda Tree (Sophora japonica L.). J. Plant Growth Regul. 2022, 41, 1–14. [Google Scholar] [CrossRef]
- Li, L.; Kong, Z.Y.; Huan, X.J.; Liu, Y.J.; Liu, Y.J.; Wang, Q.C.; Liu, J.N.; Zhang, P.; Guo, Y.R.; Qin, P. Transcriptomics Integrated With Widely Targeted Metabolomics Reveals the Mechanism Underlying Grain Color Formation in Wheat at the Grain-Filling Stage. Front. Plant Sci. 2021, 12, 14. [Google Scholar] [CrossRef] [PubMed]
- Zuñiga, P.E.; Castañeda, Y.; Arrey-Salas, O.; Fuentes, L.; Aburto, F.; Figueroa, C.R. Methyl Jasmonate Applications From Flowering to Ripe Fruit Stages of Strawberry (Fragaria × ananassa ‘Camarosa’) Reinforce the Fruit Antioxidant Response at Post-harvest. Front. Plant Sci. 2020, 11, 17. [Google Scholar] [CrossRef]
- Wang, N.; Xu, H.F.; Jiang, S.H.; Zhang, Z.Y.; Lu, N.L.; Qiu, H.R.; Qu, C.Z.; Wang, Y.C.; Wu, S.J.; Chen, X.S. MYB12 and MYB22 play essential roles in proanthocyanidin and flavonol synthesis in red-fleshed apple (Malus sieversii f. niedzwetzkyana). Plant J. 2017, 90, 276–292. [Google Scholar] [CrossRef]
- Li, P.; Tian, Z.N.; Zhang, Q.; Zhang, Y.; Wang, M.; Fang, X.A.; Shi, W.J.; Cai, X. MicroRNAome Profile of Euphorbia kansui in Response to Methyl Jasmonate. Int. J. Mol. Sci. 2019, 20, 1267. [Google Scholar] [CrossRef]
- He, S.M.; Dong, X.; Zhang, G.H.; Fan, W.; Duan, S.C.; Shi, H.; Li, D.W.; Li, R.; Chen, G.; Long, G.Q.; et al. High quality genome of Erigeron breviscapus provides a reference for herbal plants in Asteraceae. Mol. Ecol. Resour. 2021, 21, 153–169. [Google Scholar] [CrossRef]
- Yan, H.F.; Mao, P.S. Comparative Time-Course Physiological Responses and Proteomic Analysis of Melatonin Priming on Promoting Germination in Aged Oat (Avena sativa L.) Seeds. Int. J. Mol. Sci. 2021, 22, 811. [Google Scholar] [CrossRef]
- Wang, S.Y.; Bowman, L.; Ding, M. Methyl jasmonate enhances antioxidant activity and flavonoid content in blackberries (Rubus sp.) and promotes antiproliferation of human cancer cells. Food Chem. 2008, 107, 1261–1269. [Google Scholar] [CrossRef]
- Gil-Muñoz, R.; Fernández-Fernández, J.I.; Portu, J.; Garde-Cerdán, T. Methyl jasmonate: Effect on proanthocyanidin content in Monastrell and Tempranillo grapes and wines. Eur. Food Res. Technol. 2018, 244, 611–621. [Google Scholar] [CrossRef]
- Ribera-Fonseca, A.; Jiménez, D.; Leal, P.; Riquelme, I.; Roa, J.C.; Alberdi, M.; Peek, R.M.; Reyes-Díaz, M. The Anti-Proliferative and Anti-Invasive Effect of Leaf Extracts of Blueberry Plants Treated with Methyl Jasmonate on Human Gastric Cancer In Vitro Is Related to Their Antioxidant Properties. Antioxidants 2020, 9, 45. [Google Scholar] [CrossRef]
- Park, W.T.; Kim, Y.B.; Seo, J.M.; Kim, S.J.; Chung, E.; Lee, J.H.; Park, S.U. Accumulation of Anthocyanin and Associated Gene Expression in Radish Sprouts Exposed to Light and Methyl Jasmonate. J. Agric. Food Chem. 2013, 61, 4127–4132. [Google Scholar] [CrossRef]
- Qi, T.C.; Song, S.S.; Ren, Q.C.; Wu, D.W.; Huang, H.; Chen, Y.; Fan, M.; Peng, W.; Ren, C.M.; Xie, D.X. The Jasmonate-ZIM-Domain Proteins Interact with the WD-Repeat/bHLH/MYB Complexes to Regulate Jasmonate-Mediated Anthocyanin Accumulation and Trichome Initiation in Arabidopsis thaliana. Plant Cell 2011, 23, 1795–1814. [Google Scholar] [CrossRef]
- Wang, Y.C.; Liu, W.J.; Jiang, H.Y.; Mao, Z.L.; Wang, N.; Jiang, S.H.; Xu, H.F.; Yang, G.X.; Zhang, Z.Y.; Chen, X.S. The R2R3-MYB transcription factor MdMYB24-like is involved in methyl jasmonate-induced anthocyanin biosynthesis in apple. Plant Physiol. Biochem. 2019, 139, 273–282. [Google Scholar] [CrossRef] [PubMed]
- An, J.P.; Xu, R.R.; Liu, X.; Zhang, J.C.; Wang, X.F.; You, C.X.; Hao, Y.J. Jasmonate induces biosynthesis of anthocyanin and proanthocyanidin in apple by mediating the JAZ1-TRB1-MYB9 complex. Plant J. 2021, 106, 1414–1430. [Google Scholar] [CrossRef]
- Feng, S.Q.; Sun, J.J.; Sun, S.S.; Wang, Y.L.; Tian, C.P.; Sun, Q.T.; Chen, X.S. Transcriptional Profiles Underlying the Effects of Methyl Jasmonate on Apple Ripening. J. Plant Growth Regul. 2017, 36, 271–280. [Google Scholar] [CrossRef]
- Khramov, D.E.; Nedelyaeva, O.I.; Konoshenkova, A.O.; Volkov, V.S.; Balnokin, Y.V. Identification and selection of reference genes for analysis of gene expression by quantitative real-time PCR in the euhalophyte Suaeda altissima (L.) Pall. Commun. Integr. Biol. 2024, 17, 2372313. [Google Scholar] [CrossRef]
- Jiang, T.; Su, Q.; An, L. Screening and Validation of Reference Genes of qPCR in Maize under Multiple Stresses. Plant Physiol. J. 2015, 51, 1457–1464. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, L.L.; Wang, D.D.; Ma, H.L.; Liu, B.L.; Shi, Z.; Ma, X.H.; Chen, Y.; Chen, Q. Evolutionary History of the Glycoside Hydrolase 3 (GH3) Family Based on the Sequenced Genomes of 48 Plants and Identification of Jasmonic Acid-Related GH3 Proteins in Solanum tuberosum. Int. J. Mol. Sci. 2018, 19, 1850. [Google Scholar] [CrossRef]
- Nakajima, N.; Inoue, H.; Koshita, Y. Effects of exogenous methyl jasmonate and light condition on grape berry coloration and endogenous abscisic acid content. J. Pestic. Sci. 2021, 46, 322–332. [Google Scholar] [CrossRef]
- Jirakiattikul, Y.; Rithichai, P.; Kwanthong, P.; Itharat, A. Effect of jasmonic acid elicitation period on enhancement of bioactive compounds and antioxidant activity in callus cultures of Linn. Hortic. Environ. Biotechnol. 2021, 62, 629–636. [Google Scholar] [CrossRef]
- Wang, F.; Feng, Z.J.; Yang, X.Y.; Zhou, G.K.; Peng, Y.L. Physiological Parameters and Transcriptomic Levels Reveal the Response Mechanism of Maize to Deep Sowing and the Mechanism of Exogenous MeJA to Alleviate Deep Sowing Stress. Int. J. Mol. Sci. 2024, 25, 10718. [Google Scholar] [CrossRef]
- Leon-Cisneros, S.; Quirola-Garcés, A.; Alvarez-Santana, J.; Barriga, N.; Ramirez-Villacís, D.; Caviedes, M.; Ramirez-Cárdenas, L.; Leon-Reyes, A. Evaluation of Anthocyanin Production in White and Purple Maize (Zea mays L.) Using Methyl Jasmonate, Phosphorus Deficiency and High Concentration of Sucrose. Cereal Res. Commun. 2019, 47, 604–614. [Google Scholar] [CrossRef]
- Shan, X.Y.; Zhang, Y.S.; Peng, W.; Wang, Z.L.; Xie, D.X. Molecular mechanism for jasmonate-induction of anthocyanin accumulation in Arabidopsis. J. Exp. Bot. 2009, 60, 3849–3860. [Google Scholar] [CrossRef]
- Wang, X.; Wang, J.P.; Cui, H.T.; Yang, W.L.; Yu, B.; Zhang, C.; Wen, J.Q.; Kang, J.M.; Wang, Z.; Yang, Q.C. The UDP-glycosyltransferase MtUGT84A1 regulates anthocyanin accumulation and plant growth JA signaling in Medicago truncatula. Environ. Exp. Bot. 2022, 201, 104972. [Google Scholar] [CrossRef]
- Li, J.X.; Luo, M.M.; Tong, C.L.; Zhang, D.J.; Zha, Q. Advances in fruit coloring research in grapevine: An overview. Plant Growth Regul. 2024, 103, 51–63. [Google Scholar] [CrossRef]
- Zhou, W.; Shi, M.; Deng, C.; Lu, S.; Huang, F.; Wang, Y.; Kai, G. The methyl jasmonate-responsive transcription factor SmMYB1 promotes phenolic acid biosynthesis in Salvia miltiorrhiza. Hortic. Res. 2021, 8, 10. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Dong, Y.; Li, D.; Shi, S.; Zhao, N.; Liao, J.; Liu, Y.; Chen, H. Eggplant transcription factor SmMYB5 integrates jasmonate and light signaling during anthocyanin biosynthesis. Plant Physiol. 2024, 194, 1139–1165. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Chen, D.Y.; Yu, X.M.; Sun, Y.M.; Yu, H.Y.; Li, J.M.; Gao, H.Y.; Xu, H. Bioinformatics Analysis of LSD Transcription Factor in Maize and its Role in Resistance to Stem Base Rot. Int. J. Agric. Biol. 2019, 22, 1476–1482. [Google Scholar]
- Qin, W.Q.; Yu, Y.; Jin, Y.Y.; Wang, X.D.; Liu, J.; Xi, J.P.; Li, Z.; Li, H.Q.; Zhao, G.; Hu, W.; et al. Genome-Wide Analysis Elucidates the Role of CONSTANS-like Genes in Stress Responses of Cotton. Int. J. Mol. Sci. 2018, 19, 2658. [Google Scholar] [CrossRef]
- Wu, X.L.; Wang, Z.; Du, A.Q.; Gao, H.F.; Liang, J.H.; Yu, W.Y.; Yu, H.X.; Fan, S.H.; Chen, Q.J.; Guo, J.; et al. Transcription factor LBD16 targets cell wall modification/ion transport genes in peach lateral root formation. Plant Physiol. 2024, 194, 2472–2490. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.Y.; Zhang, L.L.; Liu, J.X. NFXL1 functions as a transcriptional activator required for thermotolerance at reproductive stage in Arabidopsis. J. Integr. Plant Biol. 2024, 66, 54–65. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, B.; Zhang, S.L.; Yao, J.; Rahman, M.U.; Hanif, M.; Zhu, Y.X.; Wang, X.P. Genomic Organization of the B3-Domain Transcription Factor Family in Grapevine (Vitis vinifera L.) and Expression during Seed Development in Seedless and Seeded Cultivars. Int. J. Mol. Sci. 2019, 20, 4553. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.C.; Wu, X.J.; Dong, Q.J.; Xiao, J.P. Screening and functional analysis of transcription factors in pigmented potato under low-temperature treatment. BMC Genom. 2024, 25, 283. [Google Scholar] [CrossRef]
- Liu, S.C.; Wang, Y.; Shi, M.; Maoz, I.; Gao, X.K.; Sun, M.H.; Yuan, T.P.; Li, K.L.; Zhou, W.; Guo, X.H.; et al. SmbHLH60 and SmMYC2 antagonistically regulate phenolic acids and anthocyanins biosynthesis in Salvia miltiorrhiza. J. Adv. Res. 2022, 42, 205–219. [Google Scholar] [CrossRef]
- An, J.P.; Xu, R.R.; Wang, X.N.; Zhang, X.W.; You, C.X.; Han, Y.P. MdbHLH162 connects the gibberellin and jasmonic acid signals to regulate anthocyanin biosynthesis in apple. J. Integr. Plant Biol. 2024, 66, 265–284. [Google Scholar] [CrossRef]
- Zhang, D.Y.; Sun, L.X.; Xi, D.D.; Li, X.F.; Gao, L.; Miao, L.M.; Luo, Y.; Tian, M.M.; Zhu, H.F. Methyl jasmonate-induced mediates tissue-specific accumulation of anthocyanins via regulating flavonoid metabolism-related pathways in Caitai. Physiol Plant. 2024, 176, e14434. [Google Scholar] [CrossRef]
- Yu, D.; Wei, W.; Fan, Z.Q.; Chen, J.Y.; You, Y.L.; Huang, W.D.; Zhan, J.C. VabHLH137 promotes proanthocyanidin and anthocyanin biosynthesis and enhances resistance to Colletotrichum gloeosporioides in grapevine. Hortic. Res. 2023, 10, uhac261. [Google Scholar] [CrossRef]
- Keller-Przybylkowicz, S.; Oskiera, M.; Liu, X.Q.; Song, L.Q.; Zhao, L.L.; Du, X.Y.; Kruczynska, D.; Walencik, A.; Kowara, N.; Bartoszewski, G. Transcriptome Analysis of White- and Red-Fleshed Apple Fruits Uncovered Novel Genes Related to the Regulation of Anthocyanin Biosynthesis. Int. J. Mol. Sci. 2024, 25, 1778. [Google Scholar] [CrossRef]
- Premathilake, A.T.; Ni, J.B.; Shen, J.Q.; Bai, S.L.; Teng, Y.W. Transcriptome analysis provides new insights into the transcriptional regulation of methyl jasmonate-induced flavonoid biosynthesis in pear calli. BMC Plant Biol. 2020, 20, 388. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Z.; Ahmad, N.; Sheng, X.; Iqbal, B.; Naeem, M.; Wang, N.; Li, F.; Yao, N.; Liu, X. Unraveling the functional characterization of a jasmonate-induced flavonoid biosynthetic CYP45082G24 gene in Carthamus tinctorius. Funct. Integr. Genom. 2023, 23, 172. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, C.; Zhang, L.; Guo, H. Integrated Transcriptomic and Metabolomic Analysis of Color Changes in Maize Root Systems Treated with Methyl Jasmonate. Biology 2025, 14, 1124. https://doi.org/10.3390/biology14091124
Zhang C, Zhang L, Guo H. Integrated Transcriptomic and Metabolomic Analysis of Color Changes in Maize Root Systems Treated with Methyl Jasmonate. Biology. 2025; 14(9):1124. https://doi.org/10.3390/biology14091124
Chicago/Turabian StyleZhang, Chao, Lili Zhang, and Huan Guo. 2025. "Integrated Transcriptomic and Metabolomic Analysis of Color Changes in Maize Root Systems Treated with Methyl Jasmonate" Biology 14, no. 9: 1124. https://doi.org/10.3390/biology14091124
APA StyleZhang, C., Zhang, L., & Guo, H. (2025). Integrated Transcriptomic and Metabolomic Analysis of Color Changes in Maize Root Systems Treated with Methyl Jasmonate. Biology, 14(9), 1124. https://doi.org/10.3390/biology14091124





