Pain Chronicity and Relief: From Molecular Basis to Exercise-Based Rehabilitation
Simple Summary
Abstract
1. Introduction
2. Search Strategy
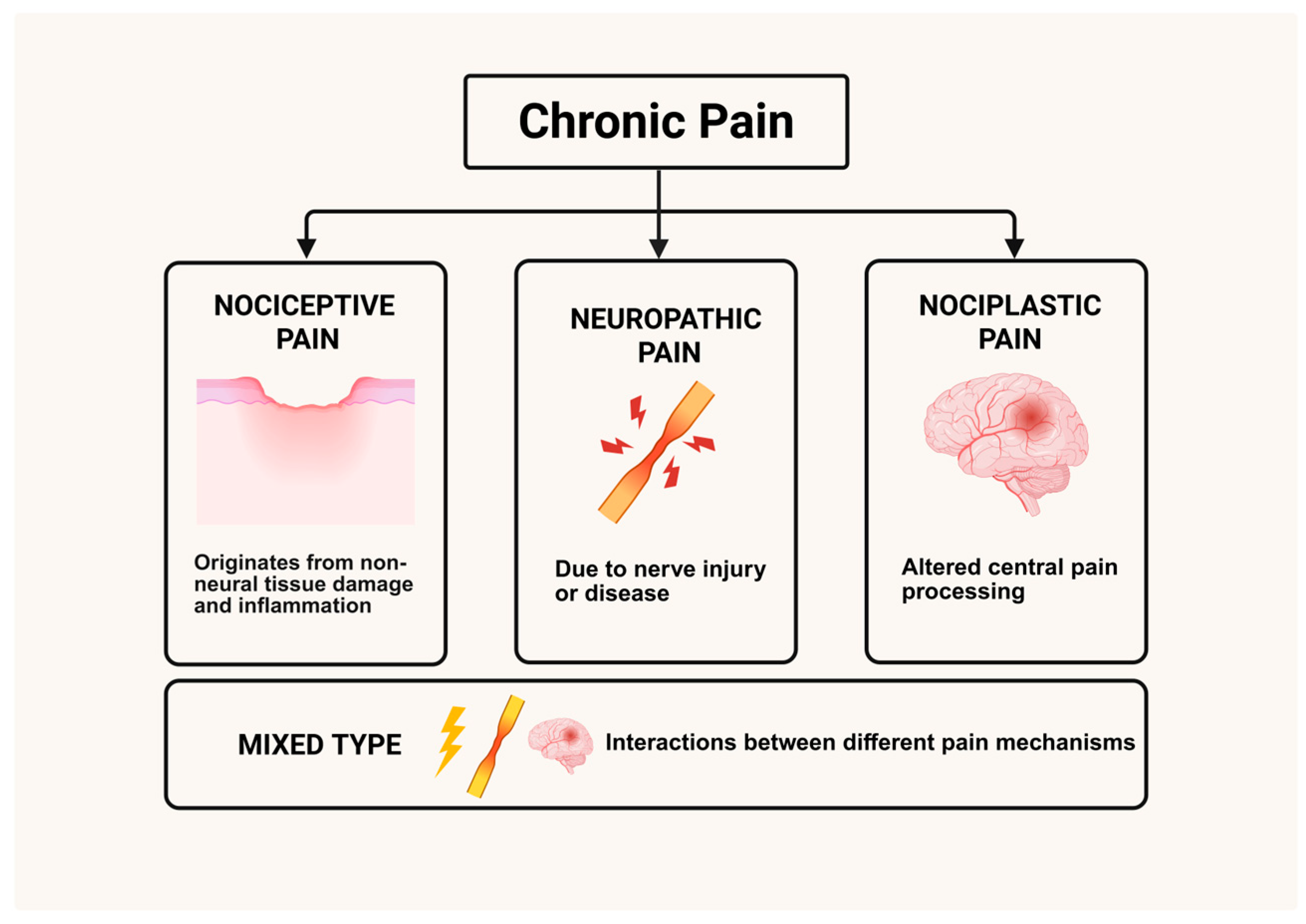
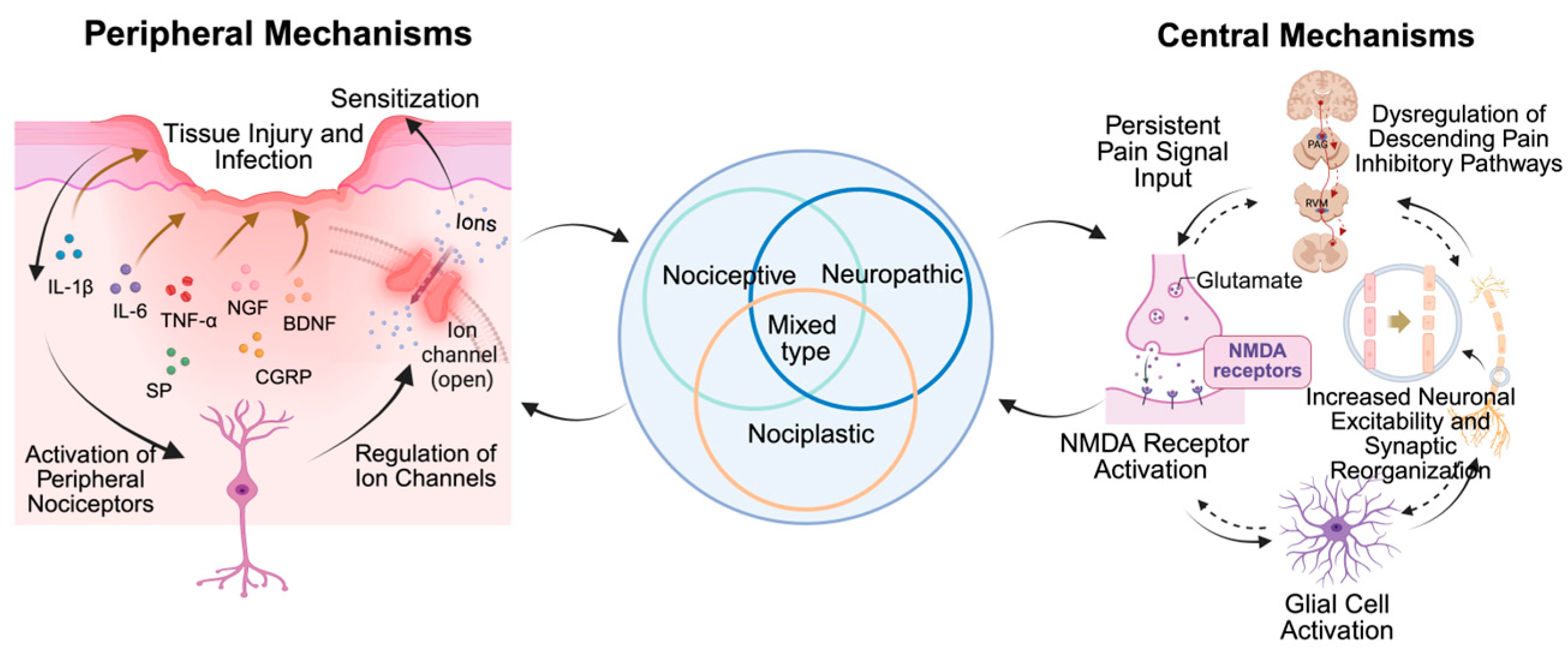
3. Classification and Mechanisms of Chronic Pain
3.1. Classification of Chronic Pain
3.2. Peripheral Mechanisms of Chronic Pain
3.3. Central Mechanisms of Chronic Pain
3.4. Sex Differences in Chronic Pain
4. Efficacy and Potential Mechanisms of Exercise in Chronic Pain Relief
4.1. Different Exercise Types in Chronic Pain Management
| Exercise Type | Primary Goal | Potential Mechanisms of Action | Prescription Parameters | Considerations and Limitations | References |
|---|---|---|---|---|---|
| Aerobic Exercise | Improve cardiovascular endurance; reduce widespread pain; improve mood and sleep | Systemic anti-inflammatory effects (↓CRP, ↓TNF-α); release of endogenous opioids; improvement in mood (↑5-HT, ↑BDNF) | Intensity: moderate (able to talk but not sing) Frequency/Duration: 150 min per week can be divided into 30 min/day or 5 days/week | For severe arthritis or low physical capacity, start with low-intensity; “start low, go slow” principle is important | [88,94,95,96] |
| Resistance Training | Increase muscle strength and function; improve localized pain; improve metabolic level | Release of anti-inflammatory myokines; improvement in local biomechanics; reduction in joint load and increase in pain threshold | Intensity: Weight that can be lifted with 8–15 repetitions; Frequency: 2–3 times/week, primarily for targeted muscle groups | More technical requirements; incorrect posture may cause injury; professional guidance recommended; not suitable for acute inflammation | [95,97] |
| Mind–Body Exercise | Improve central sensitization; reduce anxiety, fear, and avoidance; enhance body awareness and balance | Regulation of ANS (↑parasympathetic activity); enhancement in descending inhibitory pathways (↑GABA, ↑5-HT); reduction in maladaptive pain-related cognition | Intensity: Low with focus on breathing and movement coordination; Frequency/Duration: 2–3 times/week, with each lasting session 45–60 min | Effects influenced by physical confidence and psychological factors; limited mechanistic studies; more high-quality evidence needed | [98,99] |
4.2. Mechanistic Insights from Preclinical Animal Research
4.2.1. Peripheral Mechanisms of Exercise-Induced Chronic Pain Relief
- Systemic and Local Anti-Inflammation
- 2.
- Pain-Associated Ion Channels
4.2.2. Central Mechanisms of Exercise-Induced Chronic Pain Relief
- The Endogenous Opioids System
- 2.
- The serotonergic system
- 3.
- The NMDA receptor
- 4.
- Glial cells
5. Conclusions
6. Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| IASP | International Association for the Study of Pain |
| TLR4 | Toll-like receptor 4 |
| AR | Axonal reflex |
| DRR | Dorsal root reflex |
| CGRP | Calcitonin gene-related peptide |
| CNS | Central nervous system |
| NMDA | N-methyl-D-aspartate |
| LTP | Long-term potentiation |
| IL-1β | Interleukin-1β |
| IL-1 | Interleukin-1 |
| IL-4 | Interleukin-4 |
| IL-6 | Interleukin-6 |
| IL-10 | Interleukin-10 |
| IL-1ra | Interleukin-1 receptor antagonist |
| TNF-α | Tumor necrosis factor-α |
| NGF | Nerve growth factor |
| BDNF | Brain-derived neurotrophic factor |
| MAPK | Mitogen-activated protein kinase |
| TRP | Transient receptor potential |
| DRG | Dorsal root ganglia |
| PAG | Periaqueductal gray |
| RVM | Rostral ventromedial medulla |
| VLM | Ventrolateral medulla |
| 5-HT | Serotonin |
| 5-HIAA | 5-Hydroxyindoleacetic acid |
| ACC | Anterior cingulate cortex |
| C3 | Component 3 |
| GABA | Gamma-aminobutyric acid |
| ANS | Autonomic nervous system |
| HRV | Heart rate variability |
| CLBP | Chronic low back pain |
References
- Treede, R.-D.; Rief, W.; Barke, A.; Aziz, Q.; Bennett, M.I.; Benoliel, R.; Cohen, M.; Evers, S.; Finnerup, N.B.; First, M.B.; et al. Chronic Pain as a Symptom or a Disease: The IASP Classification of Chronic Pain for the International Classification of Diseases (ICD-11). Pain 2019, 160, 19–27. [Google Scholar] [CrossRef]
- Rikard, S.M.; Strahan, A.E.; Schmit, K.M.; Guy, G.P. Chronic Pain among Adults—United States, 2019–2021. Mmwr. Morb. Mortal. Wkly. Rep. 2023, 72, 379–385. [Google Scholar] [CrossRef]
- Galvez-Sánchez, C.M.; Montoro, C.I. Chronic Pain: Clinical Updates and Perspectives. J. Clin. Med. 2022, 11, 3474. [Google Scholar] [CrossRef]
- De La Rosa, J.S.; Brady, B.R.; Ibrahim, M.M.; Herder, K.E.; Wallace, J.S.; Padilla, A.R.; Vanderah, T.W. Co-Occurrence of Chronic Pain and Anxiety/Depression Symptoms in U.S. Adults: Prevalence, Functional Impacts, and Opportunities. Pain 2023, 165, 666–673. [Google Scholar] [CrossRef]
- Barrett, J.E.; Kohut, A.R. A Historical Perspective and Recent Advances on the Evolution of the Relationship between Acute and Chronic Pain and Cardiovascular Disease. Biochem. Pharmacol. 2024, 228, 116357. [Google Scholar] [CrossRef]
- Cohen, S.P.; Vase, L.; Hooten, W.M. Chronic Pain: An Update on Burden, Best Practices, and New Advances. Lancet 2021, 397, 2082–2097. [Google Scholar] [CrossRef]
- Schumacher, M.A. Peripheral Neuroinflammation and Pain: How Acute Pain Becomes Chronic. Curr. Neuropharmacol. 2024, 22, 6–14. [Google Scholar] [CrossRef] [PubMed]
- Willemen, H.L.D.M.; Santos Ribeiro, P.S.; Broeks, M.; Meijer, N.; Versteeg, S.; Tiggeler, A.; de Boer, T.P.; Małecki, J.M.; Falnes, P.Ø.; Jans, J.; et al. Inflammation-Induced Mitochondrial and Metabolic Disturbances in Sensory Neurons Control the Switch from Acute to Chronic Pain. Cell Rep. Med. 2023, 4, 101265. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.-J.; Gao, Y.-J. Astrocytes in Chronic Pain: Cellular and Molecular Mechanisms. Neurosci. Bull. 2022, 39, 425–439. [Google Scholar] [CrossRef] [PubMed]
- Benson, C.A.; King, J.F.; Reimer, M.L.; Kauer, S.D.; Waxman, S.G.; Tan, A.M. Dendritic Spines and Pain Memory. Neuroscientist 2024, 30, 294–314. [Google Scholar] [CrossRef] [PubMed]
- Parisien, M.; Lima, L.V.; Dagostino, C.; El-Hachem, N.; Drury, G.L.; Grant, A.V.; Huising, J.; Verma, V.; Meloto, C.B.; Silva, J.R.; et al. Acute Inflammatory Response via Neutrophil Activation Protects against the Development of Chronic Pain. Sci. Transl. Med. 2022, 14, eabj9954. [Google Scholar] [CrossRef]
- Ghazisaeidi, S.; Muley, M.M.; Salter, M.W. Neuropathic Pain: Mechanisms, Sex Differences, and Potential Therapies for a Global Problem. Annu. Rev. Pharmacol. Toxicol. 2023, 63, 565–583. [Google Scholar] [CrossRef]
- Viellard, J.; Bouali-Benazzouz, R.; Benazzouz, A.; Fossat, P. Modulating Neural Circuits of Pain in Preclinical Models: Recent Insights for Future Therapeutics. Cells 2024, 13, 997. [Google Scholar] [CrossRef]
- Balanaser, M.; Carley, M.; Baron, R.; Finnerup, N.B.; Moore, R.A.; Rowbotham, M.C.; Chaparro, L.E.; Gilron, I. Combination Pharmacotherapy for the Treatment of Neuropathic Pain in Adults: Systematic Review and Meta-Analysis. Pain 2023, 164, 230–251. [Google Scholar] [CrossRef]
- Gebhart, G.; Bielefeldt, K. Physiology of Visceral Pain. Compr. Physiol. 2016, 6, 1609–1633. [Google Scholar] [CrossRef]
- Dubin, A.E.; Patapoutian, A. Nociceptors: The Sensors of the Pain Pathway. J. Clin. Investig. 2010, 120, 3760–3772. [Google Scholar] [CrossRef]
- Finnerup, N.B.; Kuner, R.; Jensen, T.S. Neuropathic Pain: From Mechanisms to Treatment. Physiol. Rev. 2021, 101, 259–301. [Google Scholar] [CrossRef] [PubMed]
- Fitzcharles, M.-A.; Cohen, S.P.; Clauw, D.J.; Littlejohn, G.; Usui, C.; Häuser, W. Nociplastic Pain: Towards an Understanding of Prevalent Pain Conditions. Lancet 2021, 397, 2098–2110. [Google Scholar] [CrossRef] [PubMed]
- Kosek, E.; Clauw, D.; Nijs, J.; Baron, R.; Gilron, I.; Harris, R.E.; Mico, J.-A.; Rice, A.S.C.; Sterling, M. Chronic Nociplastic Pain Affecting the Musculoskeletal System: Clinical Criteria and Grading System. Pain 2021, 162, 2629–2634. [Google Scholar] [CrossRef] [PubMed]
- Yarns, B.C.; Cassidy, J.T.; Jimenez, A.M. At the Intersection of Anger, Chronic Pain, and the Brain: A Mini-Review. Neurosci. Biobehav. Rev. 2022, 135, 104558. [Google Scholar] [CrossRef]
- Soares, F.F.C.; Ferreira, D.M.A.O.; Raimundini, A.A.; Dionísio, T.J.; Dos Santos, C.F.; Conti, P.C.R.; Costa, Y.M.; Bonjardim, L.R. Influence of Genetic Polymorphisms on Mechanical Pain Sensitivity and Endogenous Pain Modulation of Trigeminal and Spinal Areas. J. Oral Rehabil. 2023, 50, 39–53. [Google Scholar] [CrossRef]
- Freynhagen, R.; Parada, H.A.; Calderon-Ospina, C.A.; Chen, J.; Rakhmawati Emril, D.; Fernández-Villacorta, F.J.; Franco, H.; Ho, K.-Y.; Lara-Solares, A.; Li, C.C.-F.; et al. Current Understanding of the Mixed Pain Concept: A Brief Narrative Review. Curr. Med. Res. Opin. 2019, 35, 1011–1018. [Google Scholar] [CrossRef]
- Wang, Y.; Aaron, R.; Attal, N.; Colloca, L. An Update on Non-Pharmacological Interventions for Pain Relief. Cell Rep. Med. 2025, 6, 101940. [Google Scholar] [CrossRef]
- Park, J.; Roh, J.; Pan, J.; Kim, Y.H.; Park, C.-K.; Jo, Y.Y. Role of Resolvins in Inflammatory and Neuropathic Pain. Pharmaceuticals 2023, 16, 1366. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, S.; Wu, L.; Liu, Y.; Du, J.; Luo, Z.; Xu, J.; Guo, L.; Liu, Y. Epigenetic Regulation of Chemokine (CC-Motif) Ligand 2 in Inflammatory Diseases. Cell Proliferation 2023, 56, e13428. [Google Scholar] [CrossRef]
- Zheng, H.; Lim, J.Y.; Kim, Y.; Jung, S.T.; Hwang, S.W. The Role of Oxytocin, Vasopressin, and Their Receptors at Nociceptors in Peripheral Pain Modulation. Front. Neuroendoc. 2021, 63, 100942. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.J.; Isensee, J.; Neel, D.; Quadros, A.U.; Zhang, H.-X.B.; Lauzadis, J.; Liu, S.M.; Shiers, S.; Belu, A.; Palan, S.; et al. Anthrax Toxins Regulate Pain Signaling and Can Deliver Molecular Cargoes into Antxr2+ DRG Sensory Neurons. Nat. Neurosci. 2022, 25, 168–179. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.; Yan, F.; Yang, A.; Liu, J.; Ma, J. Activation of G Protein-Coupled Receptor 39 Alleviates Neuropathic Pain and Chronic Inflammation. J. Biochem. Mol. Toxicol. 2024, 38, e23545. [Google Scholar] [CrossRef]
- Maximiano, T.K.E.; Carneiro, J.A.; Fattori, V.; Verri, W.A. TRPV1: Receptor Structure, Activation, Modulation and Role in Neuro-Immune Interactions and Pain. Cell Calcium 2024, 119, 102870. [Google Scholar] [CrossRef]
- Osteen, J.D.; Immani, S.; Tapley, T.L.; Indersmitten, T.; Hurst, N.W.; Healey, T.; Aertgeerts, K.; Negulescu, P.A.; Lechner, S.M. Pharmacology and Mechanism of Action of Suzetrigine, a Potent and Selective NaV1.8 Pain Signal Inhibitor for the Treatment of Moderate to Severe Pain. Pain Ther. 2025, 14, 655–674. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Luo, H.; Peng, X.; Chen, J. Spider and Scorpion Knottins Targeting Voltage-Gated Sodium Ion Channels in Pain Signaling. Biochem. Pharmacol. 2024, 227, 116465. [Google Scholar] [CrossRef] [PubMed]
- García-Domínguez, M. NGF in Neuropathic Pain: Understanding Its Role and Therapeutic Opportunities. Curr. Issues Mol. Biol. 2025, 47, 93. [Google Scholar] [CrossRef] [PubMed]
- O-Sullivan, I.; Kc, R.; Singh, G.; Das, V.; Ma, K.; Li, X.; Mwale, F.; Votta-Velis, G.; Bruce, B.; Natarajan Anbazhagan, A.; et al. Sensory Neuron-Specific Deletion of Tropomyosin Receptor Kinase a (TrkA) in Mice Abolishes Osteoarthritis (OA) Pain via NGF/TrkA Intervention of Peripheral Sensitization. Int. J. Mol. Sci. 2022, 23, 12076. [Google Scholar] [CrossRef] [PubMed]
- Raveh, A.; Pen, Y.; Silberman, A.; Peretz, A.; Attali, B.; Maile, L.; Davidson, S.; Brown, A.D.; Kennedy, J.D.; Belinson, H. Dual Kv7. 2/3-TRPV1 Modulators Inhibit Nociceptor Hyperexcitability and Alleviate Pain without Target-Related Side Effects. Pain 2022, 166, 793–811. [Google Scholar] [CrossRef]
- Yang, J.; Ding, H.; Shuai, B.; Zhang, Y.; Zhang, Y. Mechanism and Effects of STING–IFN-I Pathway on Nociception: A Narrative Review. Front. Mol. Neurosci. 2023, 15, 1081288. [Google Scholar] [CrossRef]
- Bayliss, W.M. On the Origin from the Spinal Cord of the Vaso-Dilator Fibres of the Hind-Limb, and on the Nature of These Fibres. J. Physiol. 1901, 26, 173–209. [Google Scholar] [CrossRef]
- Hoegh, M. Pain Science in Practice (Part 3): Peripheral Sensitization. J. Orthop. Sports Phys. Ther. 2022, 52, 303–306. [Google Scholar] [CrossRef]
- Hanani, M. How Do Peripheral Neurons and Glial Cells Participate in Pain Alleviation by Physical Activity? Cells 2025, 14, 462. [Google Scholar] [CrossRef]
- Matsuda, M.; Huh, Y.; Ji, R.-R. Roles of Inflammation, Neurogenic Inflammation, and Neuroinflammation in Pain. J. Anesth. 2019, 33, 131–139. [Google Scholar] [CrossRef]
- Zheng, Q.; Dong, X.; Green, D.P.; Dong, X. Peripheral Mechanisms of Chronic Pain. Med. Rev. 2022, 2, 251–270. [Google Scholar] [CrossRef]
- Kim, J.W.; Yong, A.J.; Aisenberg, E.E.; Lobel, J.H.; Wang, W.; Dawson, T.M.; Dawson, V.L.; Gao, R.; Jan, Y.N.; Bateup, H.S.; et al. Molecular Recording of Calcium Signals via Calcium-Dependent Proximity Labeling. Nat. Chem. Biol. 2024, 20, 894–905. [Google Scholar] [CrossRef]
- Yin, C.; Lyu, Q.; Dong, Z.; Liu, B.; Zhang, K.; Liu, Z.; Yu, Q.; Li, P.; Wei, Z.; Tai, Y.; et al. Well-Defined Alginate Oligosaccharides Ameliorate Joint Pain and Inflammation in a Mouse Model of Gouty Arthritis. Theranostics 2024, 14, 3082–3103. [Google Scholar] [CrossRef]
- Pokhilko, A.; Nash, A.; Cader, M.Z. Common Transcriptional Signatures of Neuropathic Pain. Pain 2020, 161, 1542–1554. [Google Scholar] [CrossRef]
- Singh, M.; Kim, A.; Young, A.; Nguyen, D.; Monroe, C.L.; Ding, T.; Gray, D.; Venketaraman, V. The Mechanism and Inflammatory Markers Involved in the Potential Use of N-Acetylcysteine in Chronic Pain Management. Life 2024, 14, 1361. [Google Scholar] [CrossRef]
- Li, X.-H.; Miao, H.-H.; Zhuo, M. NMDA Receptor Dependent Long-Term Potentiation in Chronic Pain. Neurochem. Res. 2019, 44, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-J.; Li, Y.-L.; Fang, Z.-H.; Liao, H.-L.; Zhang, Y.-Y.; Lin, J.; Liu, F.; Shen, J.-F. NMDARs Mediate Peripheral and Central Sensitization Contributing to Chronic Orofacial Pain. Front. Cell. Neurosci. 2022, 16, 999509. [Google Scholar] [CrossRef]
- Willis, W.D., Jr. Dorsal Root Potentials and Dorsal Root Reflexes: A Double-Edged Sword. Exp. Brain Res. 1999, 124, 395–421. [Google Scholar] [CrossRef] [PubMed]
- Sorkin, L.S.; Eddinger, K.A.; Woller, S.A.; Yaksh, T.L. Origins of Antidromic Activity in Sensory Afferent Fibers and Neurogenic Inflammation. Semin. Immunopathol. 2018, 40, 237–247. [Google Scholar] [CrossRef]
- Hagains, C.E.; Trevino, L.A.; He, J.-W.; Liu, H.; Peng, Y.B. Contributions of Dorsal Root Reflex and Axonal Reflex to Formalin-Induced Inflammation. Brain Res. 2010, 1359, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Ji, R.-R.; Nackley, A.; Huh, Y.; Terrando, N.; Maixner, W. Neuroinflammation and Central Sensitization in Chronic and Widespread Pain. Anesthesiology 2018, 129, 343–366. [Google Scholar] [CrossRef]
- Donnelly, C.R.; Andriessen, A.S.; Chen, G.; Wang, K.; Jiang, C.; Maixner, W.; Ji, R.-R. Central Nervous System Targets: Glial Cell Mechanisms in Chronic Pain. Neurotherapeutics 2020, 17, 846–860. [Google Scholar] [CrossRef]
- Pajarillo, E.; Rizor, A.; Lee, J.; Aschner, M.; Leea, E. The Role of Astrocytic Glutamate Transporters GLT-1 and GLAST in Neurological Disorders: Potential Targets for Neurotherapeutics. Neuropharmacology 2019, 161, 107559. [Google Scholar] [CrossRef]
- Kitayama, T. The Role of Astrocytes in the Modulation ofK+-Cl−-Cotransporter-2 Function. Int. J. Mol. Sci. 2020, 21, 9539. [Google Scholar] [CrossRef]
- Coull, J.A.M.; Beggs, S.; Boudreau, D.; Boivin, D.; Tsuda, M.; Inoue, K.; Gravel, C.; Salter, M.W.; De Koninck, Y. BDNF from Microglia Causes the Shift in Neuronal Anion Gradient Underlying Neuropathic Pain. Nature 2005, 438, 1017–1021. [Google Scholar] [CrossRef] [PubMed]
- Richner, M.; Pallesen, L.T.; Ulrichsen, M.; Poulsen, E.T.; Holm, T.H.; Login, H.; Castonguay, A.; Lorenzo, L.-E.; Gonçalves, N.P.; Andersen, O.M.; et al. Sortilin Gates Neurotensin and BDNF Signaling to Control Peripheral Neuropathic Pain. Sci. Adv. 2019, 5, eaav9946. [Google Scholar] [CrossRef] [PubMed]
- Marcos, J.L.; Galleguillos, D.; Pelissier, T.; Hernández, A.; Velásquez, L.; Villanueva, L.; Constandil, L. Role of the Spinal TrkB-NMDA Receptor Link in the BDNF-Induced Long-Lasting Mechanical Hyperalgesia in the Rat: A Behavioural Study. Eur. J. Pain 2017, 21, 1688–1696. [Google Scholar] [CrossRef]
- Ossipov, M.H.; Morimura, K.; Porreca, F. Descending Pain Modulation and Chronification of Pain. Curr. Opin. Support. Palliat. Care 2014, 8, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Tao, Z.-Y.; Wang, P.-X.; Wei, S.-Q.; Traub, R.J.; Li, J.-F.; Cao, D.-Y. The Role of Descending Pain Modulation in Chronic Primary Pain: Potential Application of Drugs Targeting Serotonergic System. Neural Plast. 2019, 2019, 1389296. [Google Scholar] [CrossRef]
- Ossipov, M.H.; Dussor, G.O.; Porreca, F. Central Modulation of Pain. J. Clin. Investig. 2010, 120, 3779–3787. [Google Scholar] [CrossRef]
- Patel, R.; Dickenson, A.H. Modality Selective Roles of Pro-Nociceptive Spinal 5-HT2A and 5-HT3 Receptors in Normal and Neuropathic States. Neuropharmacology 2018, 143, 29–37. [Google Scholar] [CrossRef]
- Heijmans, L.; Mons, M.R.; Joosten, E.A. A Systematic Review on Descending Serotonergic Projections and Modulation of Spinal Nociception in Chronic Neuropathic Pain and after Spinal Cord Stimulation. Mol. Pain 2021, 17, 17448069211043965. [Google Scholar] [CrossRef]
- Chia, J.S.M.; Omar Farouk, A.A.; Mohamad, A.S.; Sulaiman, M.R.; Perimal, E.K. Zerumbone Alleviates Chronic Constriction Injury-Induced Allodynia and Hyperalgesia through Serotonin 5-HT Receptors. Biomed. Pharmacother. 2016, 83, 1303–1310. [Google Scholar] [CrossRef]
- Bravo, L.; Llorca-Torralba, M.; Berrocoso, E.; Micó, J.A. Monoamines as Drug Targets in Chronic Pain: Focusing on Neuropathic Pain. Front. Neurosci. 2019, 13, 1268. [Google Scholar] [CrossRef]
- Kim, J.-R.; Kim, H.A. Molecular Mechanisms of Sex-Related Differences in Arthritis and Associated Pain. Int. J. Mol. Sci. 2020, 21, 7938. [Google Scholar] [CrossRef]
- Smith, Y.R.; Stohler, C.S.; Nichols, T.E.; Bueller, J.A.; Koeppe, R.A.; Zubieta, J.-K. Pronociceptive and Antinociceptive Effects of Estradiol through Endogenous Opioid Neurotransmission in Women. J. Neurosci. 2006, 26, 5777–5785. [Google Scholar] [CrossRef] [PubMed]
- Paige, C.; Barba-Escobedo, P.A.; Mecklenburg, J.; Patil, M.; Goffin, V.; Grattan, D.R.; Dussor, G.; Akopian, A.N.; Price, T.J. Neuroendocrine Mechanisms Governing Sex Differences in Hyperalgesic Priming Involve Prolactin Receptor Sensory Neuron Signaling. J. Neurosci. 2020, 40, 7080–7090. [Google Scholar] [CrossRef]
- Liu, J.; Li, Z.; Ju, J.; Chu, T.; Gao, F. Sex Differences in the Regulation of Interleukins in Chronic Pain: A Widely Recognized but Difficult-to-Tackle Factor. Int. J. Mol. Sci. 2025, 26, 3835. [Google Scholar] [CrossRef]
- Lee, J.; Chung, S.; Hwang, M.; Kwon, Y.; Han, S.H.; Lee, S.J. Estrogen Mediates the Sexual Dimorphism of GT1b-Induced Central Pain Sensitization. Cells 2023, 12, 808. [Google Scholar] [CrossRef]
- Patil, M.J.; Ruparel, S.B.; Henry, M.A.; Akopian, A.N. Prolactin Regulates TRPV1, TRPA1, and TRPM8 in Sensory Neurons in a Sex-Dependent Manner: Contribution of Prolactin Receptor to Inflammatory Pain. Am. J. Physiol. Endocrinol. Metab. 2013, 305, E1154–E1164. [Google Scholar] [CrossRef] [PubMed]
- Patil, M.; Belugin, S.; Mecklenburg, J.; Wangzhou, A.; Paige, C.; Barba-Escobedo, P.A.; Boyd, J.T.; Goffin, V.; Grattan, D.; Boehm, U.; et al. Prolactin Regulates Pain Responses via a Female-Selective Nociceptor-Specific Mechanism. Iscience 2019, 20, 449–465. [Google Scholar] [CrossRef] [PubMed]
- Sorge, R.E.; Mapplebeck, J.C.S.; Rosen, S.; Beggs, S.; Taves, S.; Alexander, J.K.; Martin, L.J.; Austin, J.-S.; Sotocinal, S.G.; Chen, D.; et al. Different Immune Cells Mediate Mechanical Pain Hypersensitivity in Male and Female Mice. Nat. Neurosci. 2015, 18, 1081–1083. [Google Scholar] [CrossRef]
- Lacagnina, M.J.; Willcox, K.F.; Boukelmoune, N.; Bavencoffe, A.; Sankaranarayanan, I.; Barratt, D.T.; Zuberi, Y.A.; Dayani, D.; Chavez, M.V.; Lu, J.T.; et al. B Cells Drive Neuropathic Pain–Related Behaviors in Mice through IgG–Fc Gamma Receptor Signaling. Sci. Transl. Med. 2024, 16, eadj1277. [Google Scholar] [CrossRef]
- Fiore, N.T.; Yin, Z.; Guneykaya, D.; Gauthier, C.D.; Hayes, J.; D’hary, A.; Butovsky, O.; Moalem-Taylor, G. Sex-Specific Transcriptome of Spinal Microglia in Neuropathic Pain Due to Peripheral Nerve Injury. Glia 2022, 70, 675–696. [Google Scholar] [CrossRef]
- Su, S.; Li, M.; Wu, D.; Cao, J.; Ren, X.; Tao, Y.-X.; Zang, W. Gene Transcript Alterations in the Spinal Cord, Anterior Cingulate Cortex, and Amygdala in Mice Following Peripheral Nerve Injury. Front. Cell Dev. Biol. 2021, 9, 634810. [Google Scholar] [CrossRef]
- Deal, B.; Phillips, K.; Crelli, C.; Janjic, J.M.; Pollock, J.A. RNA-Seq Reveals Sex Differences in Gene Expression during Peripheral Neuropathic Inflammation and in Pain Relief from a COX-2 Inhibiting Theranostic Nanoemulsion. Int. J. Mol. Sci. 2023, 24, 9163. [Google Scholar] [CrossRef]
- Ray, P.R.; Shiers, S.; Caruso, J.P.; Tavares-Ferreira, D.; Sankaranarayanan, I.; Uhelski, M.L.; Li, Y.; North, R.Y.; Tatsui, C.; Dussor, G.; et al. RNA Profiling of Human Dorsal Root Ganglia Reveals Sex Differences in Mechanisms Promoting Neuropathic Pain. Brain 2022, 146, 749–766. [Google Scholar] [CrossRef] [PubMed]
- Geneen, L.J.; Moore, R.A.; Clarke, C.; Martin, D.; Colvin, L.A.; Smith, B.H. Physical Activity and Exercise for Chronic Pain in Adults: An Overview of Cochrane Reviews. Cochrane Database Syst. Rev. 2017, 42, i1–i57. [Google Scholar] [CrossRef]
- Tilbrook, H.E.; Cox, H.; Hewitt, C.E.; Kang’ombe, A.R.; Chuang, L.-H.; Jayakody, S.; Aplin, J.D.; Semlyen, A.; Trewhela, A.; Watt, I.; et al. Yoga for Chronic Low Back Pain. Ann. Intern. Med. 2011, 155, 569–578. [Google Scholar] [CrossRef]
- Bruehl, S.; Burns, J.W.; Koltyn, K.; Gupta, R.; Buvanendran, A.; Edwards, D.; Chont, M.; Wu, Y.H.; Stone, A. Does Aerobic Exercise Training Alter Responses to Opioid Analgesics in Individuals with Chronic Low Back Pain? A Randomized Controlled Trial. Pain 2021, 162, 2204–2213. [Google Scholar] [CrossRef] [PubMed]
- Saleem, Y.; Noushad, S.; Ahmed, S.; Ansari, B. Psychophysiological Biomarkers for the Effects of Yoga as an Alternative Therapy among Healthcare Professionals with Chronic Low Back Pain: A Randomized Controlled Trial. J. Nat. Sci. Med. 2025, 8, 74–80. [Google Scholar] [CrossRef]
- Hooten, M.W.; Qu, W.; Townsend, C.O.; Judd, J.W. Effects of Strength vs Aerobic Exercise on Pain Severity in Adults with Fibromyalgia: A Randomized Equivalence Trial. Pain 2012, 153, 915–923. [Google Scholar] [CrossRef]
- Bjersing, J.L.; Dehlin, M.; Erlandsson, M.; Bokarewa, M.I.; Mannerkorpi, K. Changes in Pain and Insulin-like Growth Factor 1 in Fibromyalgia during Exercise: The Involvement of Cerebrospinal Inflammatory Factors and Neuropeptides. Arthritis Res. Ther. 2012, 14, R162. [Google Scholar] [CrossRef]
- Andrade, C.P.; Zamunér, A.R.; Forti, M.; Tamburús, N.Y.; Silva, E. Effects of Aquatic Training and Detraining on Women with Fibromyalgia: Controlled Randomized Clinical Trial. Eur. J. Phys. Rehabil. Med. 2019, 55, 79–88. [Google Scholar] [CrossRef]
- Wang, C.; Schmid, C.H.; Iversen, M.D.; Harvey, W.F.; Fielding, R.A.; Driban, J.B.; Price, L.L.; Wong, J.B.; Reid, K.F.; Rones, R.; et al. Comparative Effectiveness of Tai Chi Versus Physical Therapy for Knee Osteoarthritis. Ann. Intern. Med. 2016, 165, 77–86. [Google Scholar] [CrossRef]
- Aguiar, G.C.; Rocha, S.G.; Rezende, G.A.D.S.; Nascimento, M.R.D.; Scalzo, P.L. Effects of Resistance Training in Individuals with Knee Osteoarthritis. Fisioter. Em Mov. 2016, 29, 589–596. [Google Scholar] [CrossRef]
- De Araujo Cazotti, L.; Jones, A.; Roger-Silva, D.; Ribeiro, L.H.C.; Natour, J. Effectiveness of the Pilates Method in the Treatment of Chronic Mechanical Neck Pain: A Randomized Controlled Trial. Arch. Phys. Med. Rehabil. 2018, 99, 1740–1746. [Google Scholar] [CrossRef] [PubMed]
- Stegner, A.J.; Almassi, N.E.; Dougherty, R.J.; Ellingson, L.D.; Gretzon, N.P.; Lindheimer, J.B.; Ninneman, J.V.; Van Riper, S.M.; O’Connor, P.J.; Cook, D.B. Safety and Efficacy of Short-Term Structured Resistance Exercise in Gulf War Veterans with Chronic Unexplained Muscle Pain: A Randomized Control Trial. Life Sci. 2021, 282, 119810. [Google Scholar] [CrossRef]
- Bruehl, S.; Burns, J.W.; Koltyn, K.; Gupta, R.; Buvanendran, A.; Edwards, D.; Chont, M.; Wu, Y.H.; Qu’d, D.; Stone, A. Are Endogenous Opioid Mechanisms Involved in the Effects of Aerobic Exercise Training on Chronic Low Back Pain: A Randomized Controlled Trial. Pain 2020, 161, 2887–2897. [Google Scholar] [CrossRef]
- Hughes, L.; Patterson, S.D. The Effect of Blood Flow Restriction Exercise on Exercise-Induced Hypoalgesia and Endogenous Opioid and Endocannabinoid Mechanisms of Pain Modulation. J. Appl. Physiol. 2020, 128, 914–924. [Google Scholar] [CrossRef] [PubMed]
- Hughes, L.; Grant, I.; Patterson, S.D. Aerobic Exercise with Blood Flow Restriction Causes Local and Systemic Hypoalgesia and Increases Circulating Opioid and Endocannabinoid Levels. J. Appl. Physiol. 2021, 131, 1460–1468. [Google Scholar] [CrossRef] [PubMed]
- Murtezani, A.; Hundozi, H.; Orovcanec, N.; Sllamniku, S.; Osmani, T. A Comparison of High Intensity Aerobic Exercise and Passive Modalities for the Treatment of Workers with Chronic Low Back Pain: A Randomized, Controlled Trial. Eur. J. Phys. Rehabil. Med. 2011, 47, 359–366. [Google Scholar]
- Pedersen, B.K.; Febbraio, M.A. Muscles, Exercise and Obesity: Skeletal Muscle as a Secretory Organ. Nat. Rev. Endocrinol. 2012, 8, 457–465. [Google Scholar] [CrossRef]
- Docherty, S.; Harley, R.; McAuley, J.J.; Crowe, L.A.N.; Pedret, C.; Kirwan, P.D.; Siebert, S.; Millar, N.L. The Effect of Exercise on Cytokines: Implications for Musculoskeletal Health: A Narrative Review. BMC Sports Sci. Med. Rehabil. 2022, 14, 5. [Google Scholar] [CrossRef] [PubMed]
- Ortega, E.; Bote, M.E.; Giraldo, E.; García, J.J. Aquatic Exercise Improves the Monocyte Pro- and Anti-inflammatory Cytokine Production Balance in Fibromyalgia Patients. Scand. J. Med. Sci. Sports 2012, 22, 104–112. [Google Scholar] [CrossRef]
- American College of Sports Medicine. ACSM’s Guidelines for Exercise Testing and Prescription; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2013. [Google Scholar]
- Zimmer, P.; Stritt, C.; Bloch, W.; Schmidt, F.-P.; Hübner, S.T.; Binnebößel, S.; Schenk, A.; Oberste, M. The Effects of Different Aerobic Exercise Intensities on Serum Serotonin Concentrations and Their Association with Stroop Task Performance: A Randomized Controlled Trial. Eur. J. Appl. Physiol. 2016, 116, 2025–2034. [Google Scholar] [CrossRef] [PubMed]
- Zunner, B.E.M.; Wachsmuth, N.B.; Eckstein, M.L.; Scherl, L.; Schierbauer, J.R.; Haupt, S.; Stumpf, C.; Reusch, L.; Moser, O. Myokines and Resistance Training: A Narrative Review. Int. J. Mol. Sci. 2022, 23, 3501. [Google Scholar] [CrossRef]
- Streeter, C.C.; Whitfield, T.H.; Owen, L.; Rein, T.; Karri, S.K.; Yakhkind, A.; Perlmutter, R.; Prescot, A.; Renshaw, P.F.; Ciraulo, D.A.; et al. Effects of Yoga versus Walking on Mood, Anxiety, and Brain GABA Levels: A Randomized Controlled MRS Study. J. Altern. Complement. Med. 2010, 16, 1145–1152. [Google Scholar] [CrossRef]
- Villemure, C.; ÄŒeko, M.; Cotton, V.A.; Bushnell, M.C. Neuroprotective Effects of Yoga Practice: Age-, Experience-, and Frequency-Dependent Plasticity. Front. Hum. Neurosci. 2015, 9, 281. [Google Scholar] [CrossRef] [PubMed]
- Wood, L.; Bejarano, G.; Csiernik, B.; Miyamoto, G.C.; Mansell, G.; Hayden, J.A.; Lewis, M.; Cashin, A.G. Pain Catastrophising and Kinesiophobia Mediate Pain and Physical Function Improvements with Pilates Exercise in Chronic Low Back Pain: A Mediation Analysis of a Randomised Controlled Trial. J. Physiother. 2023, 69, 168–174. [Google Scholar] [CrossRef]
- Crevelário De Melo, R.; Victoria Ribeiro, A.Â.; Luquine, C.D., Jr.; De Bortoli, M.C.; Toma, T.S.; Barreto, J.O.M. Effectiveness and Safety of Yoga to Treat Chronic and Acute Pain: A Rapid Review of Systematic Reviews. BMJ Open 2021, 11, e048536. [Google Scholar] [CrossRef]
- Nakamura, Y.; Shibata, M.; Morisaki, Y.; Hirabayashi, N.; Higashioka, M.; Hata, J.; Hosoi, M.; Sudo, N.; Yamaura, K.; Ninomiya, T. Autonomic Nervous System Function Assessed by Heart Rate Variability and the Presence of Symptoms Affecting Activities of Daily Living in Community-dwelling Residents with Chronic Pain: The Hisayama Study. Eur. J. Pain 2023, 28, 831–844. [Google Scholar] [CrossRef]
- Koenig, J.; Loerbroks, A.; Jarczok, M.N.; Fischer, J.E.; Thayer, J.F. Chronic Pain and Heart Rate Variability in a Cross-Sectional Occupational Sample: Evidence for Impaired Vagal Control. Clin. J. Pain 2016, 32, 218–225. [Google Scholar] [CrossRef]
- Krishna, B.H.; Pal, P. Effect of Yoga Therapy on Heart Rate, Blood Pressure and Cardiac Autonomic Function in Heart Failure. J. Clin. Diagn. Res. JCDR 2014, 8, 14–16. [Google Scholar] [CrossRef]
- Liu, J.; Xie, H.; Liu, M.; Wang, Z.; Zou, L.; Yeung, A.S.; Hui, S.S.; Yang, Q. The Effects of Tai Chi on Heart Rate Variability in Older Chinese Individuals with Depression. Int. J. Environ. Res. Public Health 2018, 15, 2771. [Google Scholar] [CrossRef] [PubMed]
- Geraci, I.; Bargeri, S.; Basso, G.; Castellini, G.; Chiarotto, A.; Gianola, S.; Ostelo, R.; Testa, M.; Innocenti, T. Therapeutic Quality of Exercise Interventions for Chronic Low Back Pain: A Meta-Research Study Using i-CONTENT Tool. BMJ Evid.-Based Med. 2025, 30, 194–201. [Google Scholar] [CrossRef]
- Mosser, D.M.; Edwards, J.P. Exploring the Full Spectrum of Macrophage Activation. Nat. Rev. Immunol. 2008, 8, 958–969. [Google Scholar] [CrossRef]
- Grace, P.M.; Fabisiak, T.J.; Green-Fulgham, S.M.; Anderson, N.D.; Strand, K.A.; Kwilasz, A.J.; Galer, E.L.; Walker, F.R.; Greenwood, B.N.; Maier, S.F.; et al. Prior Voluntary Wheel Running Attenuates Neuropathic Pain. Pain 2016, 157, 2012–2023. [Google Scholar] [CrossRef] [PubMed]
- Bobinski, F.; Teixeira, J.M.; Sluka, K.A.; Santos, A.R.S. IL-4 Mediates the Analgesia Produced by Low-Intensity Exercise in Mice with Neuropathic Pain. Pain 2018, 159, 437–450. [Google Scholar] [CrossRef]
- Leung, A.; Gregory, N.S.; Allen, L.-A.H.; Sluka, K.A. Regular Physical Activity Prevents Chronic Pain by Altering Resident Muscle Macrophage Phenotype and Increasing IL-10 in Mice. Pain 2016, 157, 70–79. [Google Scholar] [CrossRef] [PubMed]
- De Azambuja, G.; Jorge, C.O.; Gomes, B.B.; Lourenço, H.R.; Simabuco, F.M.; Oliveira-Fusaro, M.C.G. Regular Swimming Exercise Prevented the Acute and Persistent Mechanical Muscle Hyperalgesia by Modulation of Macrophages Phenotypes and Inflammatory Cytokines via PPARγ Receptors. Brain. Behav. Immun. 2021, 95, 462–476. [Google Scholar] [CrossRef]
- Lu, Z.; Wang, Z.; Zhang, X.-A.; Ning, K. Myokines May Be the Answer to the Beneficial Immunomodulation of Tailored Exercise—A Narrative Review. Biomolecules 2024, 14, 1205. [Google Scholar] [CrossRef]
- Slate-Romano, J.J.; Yano, N.; Zhao, T.C. Irisin Reduces Inflammatory Signaling Pathways in Inflammation-Mediated Metabolic Syndrome. Mol. Cell. Endocrinol. 2022, 552, 111676. [Google Scholar] [CrossRef]
- Xie, X.; Yu, X.; Zhang, H.; Dai, H.; Huang, Y.; Wu, F. Irisin Alleviates Chronic Constriction Injury-Induced Hyperalgesia and Affective Disorders in Mice through NF-κB and Nrf2 Signaling Pathways. IBRO Neurosci. Rep. 2024, 17, 280–289. [Google Scholar] [CrossRef]
- Rahman, M.M.; Hwang, S.-M.; Go, E.J.; Kim, Y.H.; Park, C.-K. Irisin Alleviates CFA-Induced Inflammatory Pain by Modulating Macrophage Polarization and Spinal Glial Cell Activation. Biomed. Pharmacother. 2024, 178, 117157. [Google Scholar] [CrossRef]
- Cooper, M.A.; Ryals, J.M.; Wu, P.Y.; Wright, K.D.; Walter, K.R.; Wright, D.E. Modulation of Diet-Induced Mechanical Allodynia by Metabolic Parameters and Inflammation. J. Peripher. Nerv. Syst. Jpns 2017, 22, 39–46. [Google Scholar] [CrossRef]
- Yoon, H.; Thakur, V.; Isham, D.; Fayad, M.; Chattopadhyay, M. Moderate Exercise Training Attenuates Inflammatory Mediators in DRG of Type 1 Diabetic Rats. Exp. Neurol. 2015, 267, 107–114. [Google Scholar] [CrossRef]
- Sumizono, M.; Yoshizato, Y.; Imai, T.; Tani, A.; Nakanishi, K.; Nojima, N.; Kakimoto, S.; Sakakima, H. Effects of Pain Relief through Minimal Exercise Intervention in a Rat Model of Neuropathic Pain. Cureus 2024, 16, e62897. [Google Scholar] [CrossRef] [PubMed]
- Stagg, N.J.; Mata, H.P.; Ibrahim, M.M.; Henriksen, E.J.; Porreca, F.; Vanderah, T.W.; Malan, T.P. Regular Exercise Reverses Sensory Hypersensitivity in a Rat Neuropathic Pain Model: Role of Endogenous Opioids. Anesthesiology 2011, 114, 940–948. [Google Scholar] [CrossRef] [PubMed]
- Lima, L.V.; DeSantana, J.M.; Rasmussen, L.A.; Sluka, K.A. Short-Duration Physical Activity Prevents the Development of Activity-Induced Hyperalgesia through Opioid and Serotoninergic Mechanisms. Pain 2017, 158, 1697–1710. [Google Scholar] [CrossRef] [PubMed]
- Brito, R.G.; Rasmussen, L.A.; Sluka, K.A. Regular Physical Activity Prevents Development of Chronic Muscle Pain through Modulation of Supraspinal Opioid and Serotonergic Mechanisms. Pain Rep. 2017, 2, e618. [Google Scholar] [CrossRef] [PubMed]
- Korb, A.; Bonetti, L.V.; Da Silva, S.A.; Marcuzzo, S.; Ilha, J.; Bertagnolli, M.; Partata, W.A.; Faccioni-Heuser, M.C. Effect of Treadmill Exercise on Serotonin Immunoreactivity in Medullary Raphe Nuclei and Spinal Cord Following Sciatic Nerve Transection in Rats. Neurochem. Res. 2010, 35, 380–389. [Google Scholar] [CrossRef]
- Bobinski, F.; Ferreira, T.A.A.; Córdova, M.M.; Dombrowski, P.A.; Da Cunha, C.; Santo, C.C.D.E.; Poli, A.; Pires, R.G.W.; Martins-Silva, C.; Sluka, K.A.; et al. Role of Brainstem Serotonin in Analgesia Produced by Low-Intensity Exercise on Neuropathic Pain after Sciatic Nerve Injury in Mice. Pain 2015, 156, 2595–2606. [Google Scholar] [CrossRef]
- Lopez-Alvarez, V.M.; Puigdomenech, M.; Navarro, X.; Cobianchi, S. Monoaminergic Descending Pathways Contribute to Modulation of Neuropathic Pain by Increasing-Intensity Treadmill Exercise after Peripheral Nerve Injury. Exp. Neurol. 2018, 299, 42–55. [Google Scholar] [CrossRef]
- Zhou, Y.-S.; Meng, F.-C.; Cui, Y.; Xiong, Y.-L.; Li, X.-Y.; Meng, F.-B.; Niu, Z.-X.; Zheng, J.-X.; Quan, Y.-Q.; Wu, S.-X.; et al. Regular Aerobic Exercise Attenuates Pain and Anxiety in Mice by Restoring Serotonin-Modulated Synaptic Plasticity in the Anterior Cingulate Cortex. Med. Sci. Sports Exercise 2022, 54, 566–581. [Google Scholar] [CrossRef] [PubMed]
- Maguire, D.R. Interactions between Fentanyl and 2, 5-Dimethoxy-4-Methylamphetamine (DOM), a 5-HT2AReceptor Agonist, in Rhesus Monkeys Responding under a Food versus Drug Choice Procedure. FASEB J. 2022, 36, R788. [Google Scholar] [CrossRef]
- Nikbakhtzadeh, M.; Borzadaran, F.M.; Zamani, E.; Shabani, M. Protagonist Role of Opioidergic System on Post-Traumatic Stress Disorder and Associated Pain. Psychiatry Investig. 2020, 17, 506–516. [Google Scholar] [CrossRef]
- Loyd, D.R.; Morgan, M.M.; Murphy, A.Z. Morphine Preferentially Activates the Periaqueductal Gray –Rostral Ventromedial Medullary Pathway in the Male Rat: A Potential Mechanism for Sex Differences in Antinociception. Neuroscience 2007, 147, 456–468. [Google Scholar] [CrossRef]
- Hiroki, T.; Suto, T.; Ohta, J.; Saito, S.; Obata, H. Spinal γ-Aminobutyric Acid Interneuron Plasticity Is Involved in the Reduced Analgesic Effects of Morphine on Neuropathic Pain. J. Pain 2022, 23, 547–557. [Google Scholar] [CrossRef] [PubMed]
- Laumet, G.; Chen, S.-R.; Pan, H.-L. NMDA Receptors and Signaling in Chronic Neuropathic Pain. In The Nmda Receptors; Hashimoto, K., Ed.; Springer International Publishing: Cham, Switzerland, 2017; pp. 103–119. ISBN 978-3-319-49793-8. [Google Scholar]
- Sluka, K.A.; Danielson, J.; Rasmussen, L.; Dasilva, L.F. Exercise-Induced Pain Requires NMDA Receptor Activation in the Medullary Raphe Nuclei. Med. Sci. Sports Exercise 2012, 44, 420–427. [Google Scholar] [CrossRef]
- Wang, X.-Q.; Zhong, X.-L.; Li, Z.-B.; Wang, H.-T.; Zhang, J.; Li, F.; Zhang, J.-Y.; Dai, R.-P.; Xin-Fu, Z.; Li, C.-Q.; et al. Differential Roles of Hippocampal Glutamatergic Receptors in Neuropathic Anxiety-like Behavior after Partial Sciatic Nerve Ligation in Rats. BMC Neurosci. 2015, 16, 14. [Google Scholar] [CrossRef]
- Li, Q.; Yue, N.; Liu, S.-B.; Wang, Z.-F.; Mi, W.-L.; Jiang, J.-W.; Wu, G.-C.; Yu, J.; Wang, Y.-Q. Effects of Chronic Electroacupuncture on Depression- and Anxiety-like Behaviors in Rats with Chronic Neuropathic Pain. Evid. -based Complement. Altern. Med. 2014, 2014, 158987. [Google Scholar] [CrossRef]
- Benson, C.; Paylor, J.W.; Tenorio, G.; Winship, I.; Baker, G.; Kerr, B.J. Voluntary Wheel Running Delays Disease Onset and Reduces Pain Hypersensitivity in Early Experimental Autoimmune Encephalomyelitis (EAE). Exp. Neurol. 2015, 271, 279–290. [Google Scholar] [CrossRef]
- Sluka, K.A.; O’Donnell, J.M.; Danielson, J.; Rasmussen, L.A. Regular Physical Activity Prevents Development of Chronic Pain and Activation of Central Neurons. J. Appl. Physiol. 2013, 114, 725–733. [Google Scholar] [CrossRef]
- Chen, Y.-W.; Lin, M.-F.; Chen, Y.-C.; Hung, C.-H.; Tzeng, J.-I.; Wang, J.-J. Exercise Training Attenuates Postoperative Pain and Expression of Cytokines and N-Methyl-D-Aspartate Receptor Subunit 1 in Rats. Reg. Anesth. Pain Med. 2013, 38, 282–288. [Google Scholar] [CrossRef] [PubMed]
- Sumizono, M.; Sakakima, H.; Otsuka, S.; Terashi, T.; Nakanishi, K.; Ueda, K.; Takada, S.; Kikuchi, K. The Effect of Exercise Frequency on Neuropathic Pain and Pain-Related Cellular Reactions in the Spinal Cord and Midbrain in a Rat Sciatic Nerve Injury Model. J. Pain Res. 2018, 11, 281–291. [Google Scholar] [CrossRef]
- Almeida, C.; DeMaman, A.; Kusuda, R.; Cadetti, F.; Ravanelli, M.I.; Queiroz, A.L.; Sousa, T.A.; Zanon, S.; Silveira, L.R.; Lucas, G. Exercise Therapy Normalizes BDNF Upregulation and Glial Hyperactivity in a Mouse Model of Neuropathic Pain. Pain 2015, 156, 504–513. [Google Scholar] [CrossRef]
- Gong, X.; Fan, R.; Zhu, Q.; Ye, X.; Chen, Y.; Zhang, M. Exercise Reduces Morphine-Induced Hyperalgesia and Antinociceptive Tolerance. Biomed Res. Int. 2021, 2021, 6667474. [Google Scholar] [CrossRef] [PubMed]
- He, W.-C.; Hou, S.-L.; Wang, K.-B.; Xu, N.; Li, K.; Xiong, T.; Luo, J. Treadmill Running on Neuropathic Pain: Via Modulation of Neuroinflammation. Front. Mol. Neurosci. 2024, 17, 1345864. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.-J.; Jing, X.-Y.; Wang, Y.-Z.; Yang, B.-R.; Lu, Q.; Hu, H.; Kang, L. Exercise, Spinal Microglia and Neuropathic Pain: Potential Molecular Mechanisms. Neurochem. Res. 2024, 49, 29–37. [Google Scholar] [CrossRef]
- Wang, C.; He, H.; Gao, T.; Sun, X.; Du, L.; Yang, Y.; Zhu, J.; Yang, Y.; Wang, Y.; Mi, W. Analgesic Effect of Exercise on Neuropathic Pain via Regulating the Complement Component 3 of Reactive Astrocytes. Anesth. Analg. 2024, 139, 840–850. [Google Scholar] [CrossRef]
- Jia, X.; Li, Z.; Shen, X.; Zhang, Y.; Zhang, L.; Zhang, L. High-Intensity Swimming Alleviates Nociception and Neuroinflammation in a Mouse Model of Chronic Post-Ischemia Pain by Activating the Resolvin E1-Chemerin Receptor 23 Axis in the Spinal Cord. Neural Regener. Res. 2023, 18, 2535–2542. [Google Scholar] [CrossRef] [PubMed]
- Balchin, C.; Tan, A.L.; Golding, J.; Bissell, L.-A.; Wilson, O.J.; McKenna, J.; Stavropoulos-Kalinoglou, A. Acute Effects of Exercise on Pain Symptoms, Clinical Inflammatory Markers and Inflammatory Cytokines in People with Rheumatoid Arthritis: A Systematic Literature Review. Ther. Adv. Musculoskelet. Dis. 2022, 14, 1759720X221114104. [Google Scholar] [CrossRef] [PubMed]
- Burma, N.E.; Leduc-Pessah, H.; Fan, C.Y.; Trang, T. Animal Models of Chronic Pain: Advances and Challenges for Clinical Translation. J. Neurosci. Res. 2016, 95, 1242–1256. [Google Scholar] [CrossRef]
- Bushnell, M.C.; Čeko, M.; Low, L.A. Cognitive and Emotional Control of Pain and Its Disruption in Chronic Pain. Nat. Rev. Neurosci. 2013, 14, 502–511. [Google Scholar] [CrossRef]
- Gunn, J. An Analysis of Biomarkers in Patients withChronic Pain. Pain Physician 2020, 23, E41–E49. [Google Scholar] [CrossRef]
- Polaski, A.M.; Phelps, A.L.; Kostek, M.C.; Szucs, K.A.; Kolber, B.J. Exercise-Induced Hypoalgesia: A Meta-Analysis of Exercise Dosing for the Treatment of Chronic Pain. PLoS ONE 2019, 14, e0210418. [Google Scholar] [CrossRef] [PubMed]

| Reference | Pain Condition | Sample (N) | Exercise Type | Exercise Form | Intensity | Duration | Pain Outcome and Findings |
|---|---|---|---|---|---|---|---|
| Tilbrook et al. (2011) [78] | CLBP | 313 | Mind–body | Yoga | Gradually progressing | 1 × wk, 12 wks | No change in pain intensity |
| Bruehl et al. (2021) [79] | CLBP | 83 | Aerobic | Not specified | 70–85% HRR | 3 × wk, 6 wks | ↓Pain intensity; ↓HPI; ↑HPT; no change in PPI |
| Saleem et al. (2025) [80] | CLBP | 140 | Mind–body | Yoga | Moderate | 5 × wk, 12 wks | ↓Pain intensity |
| Hooten et al. (2012) [81] | FM | 72 | Aerobic vs. Resistance | Cycling vs. Not specified | Progressive, to maximal tolerance | 7 × wk, 3 wks | Both groups showed equivalent ↓Pain severity |
| Bjersing et al. (2012) [82] | FM | 49 | Aerobic | Walking | Moderate to high vs. low | 2 × wk, 15 wks | No change in pain intensity; ↑PPT |
| Andrade et al. (2019) [83] | FM | 54 | Aerobic | Aquatic training | 80–110% VAT HR | 2 × wk, 16 wks | ↓Pain intensity; ↑PPT |
| Wang et al. (2016) [84] | KOA | 204 | Mind–body | Tai Chi | — | 2 × wk, 12 wks | ↓Pain intensity |
| Aguiar et al. (2016) [85] | KOA | 22 | Resistance | — | 80% 10 RM | 12 weeks | ↓Pain intensity |
| De Araujo Cazotti et al. (2018) [86] | chronic neck pain | 64 | Mind–body | Pilates | 6–12 repetitions | 2 × wk, 12 wks | ↓Pain intensity |
| Stegner et al. (2021) [87] | CMP | 54 | Resistance | Machines | Low-start, gradual progression | 2 × wk, 16 wk | No change in pain intensity |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ni, W.; Kuang, X.; Zhu, Z. Pain Chronicity and Relief: From Molecular Basis to Exercise-Based Rehabilitation. Biology 2025, 14, 1116. https://doi.org/10.3390/biology14091116
Ni W, Kuang X, Zhu Z. Pain Chronicity and Relief: From Molecular Basis to Exercise-Based Rehabilitation. Biology. 2025; 14(9):1116. https://doi.org/10.3390/biology14091116
Chicago/Turabian StyleNi, Weidi, Xin Kuang, and Zheng Zhu. 2025. "Pain Chronicity and Relief: From Molecular Basis to Exercise-Based Rehabilitation" Biology 14, no. 9: 1116. https://doi.org/10.3390/biology14091116
APA StyleNi, W., Kuang, X., & Zhu, Z. (2025). Pain Chronicity and Relief: From Molecular Basis to Exercise-Based Rehabilitation. Biology, 14(9), 1116. https://doi.org/10.3390/biology14091116






