Exploring Metabolic and Immunological Biomarkers for Oral Squamous Cell Carcinoma: Potential Targets for Precision Therapy
Simple Summary
Abstract
1. Introduction
2. Metabolic Alterations and Therapeutic Targeting in OSCC
2.1. Dysfunctional Amino Acid Metabolism
2.2. Lipid-Choline Metabolism
2.3. Glycolysis and the Warburg Effect
2.4. Pentose Phosphate Pathway
2.5. Others Salivary Biomarkers: Insights and Applications
3. Immunological Biomarkers Associated with OSCC
3.1. Key Immune Biomarkers Identified in OSCC
3.1.1. Cytokines
3.1.2. Chemokines
3.1.3. Tumor Microenvironment (TME) Components
3.1.4. Tumor-Infiltrating Immune Cells
| Cell Type | Markers | Role | Clinical Significance | Reference |
|---|---|---|---|---|
| CD8+ T cells | CD8 | Anti-tumor | Good prognosis | Mittrücker et al., 2014 [106] |
| Tregs | FOXP3, CD25 | Immunosuppression | Poor prognosis | Chaudhary & Elkord, 2016 [105] |
| M2 TAMs | CD163, CD206 | Tumor promotion | Poor prognosis | Chaudhari et al., 2020 [121] |
| CAFs | αSMA, FAP | ECM remodeling, immunosuppression | Poor prognosis | Dourado et al., 2018 [111] |
| MDSCs | CD11b, CD33 | Immune suppression | Disease progression | Pang et al., 2020 [113] |
3.1.5. Hypoxia and Inflammation Mediators as Biomarkers
4. Altered Immunological Biomarkers as Therapeutic Target
4.1. Immune Check Points Inhibitors
4.2. Adoptive Cellular Immunotherapy (ACI)
5. The Role of Metabolism in Shaping Immune Responses
6. Comparative Insights with Other Head and Neck Cancers
7. Challenges and Limitation for Biomarkers Research
8. Future Directions: Emerging Technologies in OSCC Biomarker Research
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tandon, P.; Dadhich, A.; Saluja, H.; Bawane, S.; Sachdeva, S. The prevalence of squamous cell carcinoma in different sites of oral cavity at our Rural Health Care Centre in Loni, Maharashtra—A retrospective 10-year study. Contemp. Oncol./Współczesna Onkol. 2017, 21, 178–183. [Google Scholar] [CrossRef]
- Bugshan, A.; Farooq, I. Oral squamous cell carcinoma: Metastasis, potentially associated malignant disorders, etiology and recent advancements in diagnosis. FResearch 2020, 9, 229. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Pérez-Sayáns, M.; Somoza-Martín, J.M.; Barros-Angueira, F.; Reboiras-López, M.D.; Gándara Rey, J.M.; García-García, A. Genetic and molecular alterations associated with oral squamous cell cancer. Oncol. Rep. 2009, 22, 1277–1282. [Google Scholar] [CrossRef]
- Uzawa, N.; Akanuma, D.; Negishi, A.; Iwaki, H.; Uzawa, Y.; Amagasa, T.; Yoshida, M. Homozygous deletions on the short arm of chromosome 3 in human oral squamous cell carcinomas. Oral Oncol. 2001, 37, 351–356. [Google Scholar] [CrossRef]
- Somoza-Martín, J.M.; García-García, A.; Barros-Angueira, F.; Otero-Rey, E.; Torres-Español, M.; Gándara-Vila, P.; Reboiras-López, M.D.; Blanco-Carrión, A.; Gándara-Rey, J.M. Gene expression profile in oral squamous cell carcinoma: A pilot study. J. Oral Maxillofac. Surg. 2005, 63, 786–792. [Google Scholar] [CrossRef]
- Yanamoto, S.; Kawasaki, G.; Yoshitomi, I.; Iwamoto, T.; Hirata, K.; Mizuno, A. Clinicopathologic significance of EpCAM expression in squamous cell carcinoma of the tongue and its possibility as a potential target for tongue cancer gene therapy. Oral Oncol. 2007, 43, 869–877. [Google Scholar] [CrossRef] [PubMed]
- Ohta, S.; Uemura, H.; Matsui, Y.; Ishiguro, H.; Fujinami, K.; Kondo, K.; Miyamoto, H.; Yazawa, T.; Danenberg, K.; Danenberg, P.V.; et al. Alterations of p16 and p14ARF genes and their 9p21 locus in oral squamous cell carcinoma. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2009, 107, 81–91. [Google Scholar] [CrossRef]
- Kaur, J.; Srivastava, A.; Ralhan, R. Overexpression of p53 protein in betel-and tobacco-related human oral dysplasia and sqInternational journal of canceruamous-cell carcinoma in India. Int. J. Cancer 1994, 58, 340–345. [Google Scholar] [CrossRef]
- Azad, N.; Maurya, M.K.; Kar, M.; Goel, M.M.; Singh, A.K.; Sagar, M.; Mehrotra, D.; Kumar, V.J.J.o.o.b.; research, c. Expression of GLUT-1 in oral squamous cell carcinoma in tobacco and non-tobacco users. J. Oral Biol. Craniofacial Res. 2016, 6, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Costa, A.d.L.L.; de Araújo, N.S.; Santos Pinto, D.d., Jr.; de Araújo, V.C. PCNA/AgNOR and Ki-67/AgNOR double staining in oral squamous cell carcinoma. J. Oral Pathol. Med. 1999, 28, 438–441. [Google Scholar] [CrossRef] [PubMed]
- Stephens, H.; Vaughan, R.; Collins, R.; Kondeatis, E.; Theron, J.; Payne, A. Towards a molecular phototyping system for allelic variants of MICA, encoded by polymorphisms in exons 2, 3 and 4 of MHC class I chain-related genes. Tissue Antigens 1999, 53, 167–174. [Google Scholar] [CrossRef]
- Imai, T.; Toyota, M.; Suzuki, H.; Akino, K.; Ogi, K.; Sogabe, Y.; Kashima, L.; Maruyama, R.; Nojima, M.; Mita, H. Epigenetic inactivation of RASSF2 in oral squamous cell carcinoma. Cancer Sci. 2008, 99, 958–966. [Google Scholar] [CrossRef]
- Dumache, R.; Rogobete, A.F.; Andreescu, N.; Puiu, M. Genetic and Epigenetic Biomarkers of Molecular Alterations in Oral Carcinogenesis. Clin. Lab. 2015, 61, 1373–1381. [Google Scholar] [CrossRef]
- Gómez, I.; Seoane, J.; Varela-Centelles, P.; Diz, P.; Takkouche, B. Is diagnostic delay related to advanced-stage oral cancer? A meta-analysis. Eur. J. Oral Sci. 2009, 117, 541–546. [Google Scholar] [CrossRef]
- Scott, S.; Grunfeld, E.; McGurk, M. The idiosyncratic relationship between diagnostic delay and stage of oral squamous cell carcinoma. Oral Oncol. 2005, 41, 396–403. [Google Scholar] [CrossRef]
- Silberring, J.; Ciborowski, P. Biomarker discovery and clinical proteomics. TrAC Trends Anal. Chem. 2010, 29, 128–140. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yang, Z.; Liu, Y.; Pei, J.; Li, R.; Yang, Y. Targeting lipid metabolism: Novel insights and therapeutic advances in pancreatic cancer treatment. Lipids Health Dis. 2025, 24, 12. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Sun, H.; Wang, X.J.A.b. Saliva metabolomics opens door to biomarker discovery, disease diagnosis, and treatment. Appl. Biochem. Biotechnol. 2012, 168, 1718–1727. [Google Scholar] [CrossRef]
- Aharoni, A.; Ric de Vos, C.; Verhoeven, H.A.; Maliepaard, C.A.; Kruppa, G.; Bino, R.; Goodenowe, D.B. Nontargeted metabolome analysis by use of Fourier transform ion cyclotron mass spectrometry. Omics J. Integr. Biol. 2002, 6, 217–234. [Google Scholar] [CrossRef]
- Tang, Y.; Li, Z.; Lazar, L.; Fang, Z.; Tang, C.; Zhao, J. Metabolomics workflow for lung cancer: Discovery of biomarkers. Clin. Chim. Acta 2019, 495, 436–445. [Google Scholar] [CrossRef]
- Zhou, Q.; Zhang, L.-Y.; Xie, C.; Zhang, M.-L.; Wang, Y.-J.; Liu, G.-H. Metabolomics as a potential method for predicting thyroid malignancy in children and adolescents. Pediatr. Surg. Int. 2020, 36, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Roberts, S.; Bhamra, R.; Ilankovan, V.J.B.J.o.O.; Surgery, M. The malignant transformation rate of erosive oral lichen planus-a retrospective study. Br. J. Oral Maxillofac. Surg. 2024, 62, 788–793. [Google Scholar] [CrossRef] [PubMed]
- Glunde, K.; Bhujwalla, Z.M.; Ronen, S.M. Choline metabolism in malignant transformation. Nat. Rev. Cancer 2011, 11, 835–848. [Google Scholar] [CrossRef]
- Ling, Z.-N.; Jiang, Y.-F.; Ru, J.-N.; Lu, J.-H.; Ding, B.; Wu, J. Amino acid metabolism in health and disease. Signal Transduct. Target. Ther. 2023, 8, 345. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.S.; Nandimath, K.R.; Hiremath, S.; Burde, K. Alteration of plasma free amino acids’ level in oral squamous cell carcinoma patients using High Performance Liquid Chromatography. J. Oral Maxillofac. Surg. Med. Pathol. 2020, 32, 65–71. [Google Scholar] [CrossRef]
- Hu, Z.; Xu, Z.; Yue, Q.; Pan, X.; Shi, P.; Zhang, D.; Zhang, J.; Deng, R.; Lin, Z. The role of blood metabolites in oral cancer: Insights from a Mendelian randomization approach. Front. Oncol. 2024, 14, 1305684. [Google Scholar] [CrossRef]
- Li, X.; Liu, L.; Li, N.; Jia, Q.; Wang, X.; Zuo, L.; Long, J.; Xue, P.; Sun, Z.; Zhao, H. Metabolomics based plasma biomarkers for diagnosis of oral squamous cell carcinoma and oral erosive lichen planus. J. Cancer 2022, 13, 76. [Google Scholar] [CrossRef]
- Xie, G.X.; Chen, T.L.; Qiu, Y.P.; Shi, P.; Zheng, X.J.; Su, M.M.; Zhao, A.H.; Zhou, Z.T.; Jia, W. Urine metabolite profiling offers potential early diagnosis of oral cancer. Metabolomics 2012, 8, 220–231. [Google Scholar] [CrossRef]
- DeBerardinis, R.J.; Mancuso, A.; Daikhin, E.; Nissim, I.; Yudkoff, M.; Wehrli, S.; Thompson, C.B. Beyond aerobic glycolysis: Transformed cells can engage in glutamine metabolism that exceeds the requirement for protein and nucleotide synthesis. Proc. Natl. Acad. Sci. USA 2007, 104, 19345–19350. [Google Scholar] [CrossRef]
- Jiang, J.; Srivastava, S.; Zhang, J. Starve cancer cells of glutamine: Break the spell or make a hungry monster? Cancers 2019, 11, 804. [Google Scholar] [CrossRef] [PubMed]
- Cetindis, M.; Biegner, T.; Munz, A.; Teriete, P.; Reinert, S.; Grimm, M. Glutaminolysis and carcinogenesis of oral squamous cell carcinoma. Eur. Arch. Oto-Rhino-Laryngol. 2016, 273, 495–503. [Google Scholar] [CrossRef]
- Luo, Y.; Li, W.; Ling, Z.; Hu, Q.; Fan, Z.; Cheng, B.; Tao, X. ASCT2 overexpression is associated with poor survival of OSCC patients and ASCT2 knockdown inhibited growth of glutamine-addicted OSCC cells. Cancer Med. 2020, 9, 3489–3499. [Google Scholar] [CrossRef]
- Gan, M.; Liu, N.; Li, W.; Chen, M.; Bai, Z.; Liu, D.; Liu, S. Metabolic targeting of regulatory T cells in oral squamous cell carcinoma: New horizons in immunotherapy. Mol. Cancer 2024, 23, 273. [Google Scholar] [CrossRef]
- Bag, S.; Banerjee, D.R.; Basak, A.; Das, A.K.; Pal, M.; Banerjee, R.; Paul, R.R.; Chatterjee, J.J.B.; communications, b.r. NMR (1H and 13C) based signatures of abnormal choline metabolism in oral squamous cell carcinoma with no prominent Warburg effect. Biochem. Biophys. Res. Commun. 2015, 459, 574–578. [Google Scholar] [CrossRef]
- Ohshima, M.; Sugahara, K.; Kasahara, K.; Katakura, A. Metabolomic analysis of the saliva of Japanese patients with oral squamous cell carcinoma. Oncol. Rep. 2017, 37, 2727–2734. [Google Scholar] [CrossRef]
- Ishikawa, S.; Sugimoto, M.; Edamatsu, K.; Sugano, A.; Kitabatake, K.; Iino, M. Discrimination of oral squamous cell carcinoma from oral lichen planus by salivary metabolomics. Oral Dis. 2020, 26, 35–42. [Google Scholar] [CrossRef]
- Song, X.; Yang, X.; Narayanan, R.; Shankar, V.; Ethiraj, S.; Wang, X.; Duan, N.; Ni, Y.-H.; Hu, Q.; Zare, R.N. Oral squamous cell carcinoma diagnosed from saliva metabolic profiling. Proc. Natl. Acad. Sci. USA 2020, 117, 16167–16173. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Gao, P.; Wang, X.; Duan, Y. The early diagnosis and monitoring of squamous cell carcinoma via saliva metabolomics. Sci. Rep. 2014, 4, 6802. [Google Scholar] [CrossRef] [PubMed]
- Santos, C.R.; Schulze, A. Lipid metabolism in cancer. FEBS J. 2012, 279, 2610–2623. [Google Scholar] [CrossRef]
- Haidari, S.; Tröltzsch, M.; Knösel, T.; Liokatis, P.; Kasintsova, A.; Eberl, M.; Ortner, F.; Otto, S.; Fegg, F.; Boskov, M. Fatty acid receptor cd36 functions as a surrogate parameter for lymph node metastasis in oral squamous cell carcinoma. Cancers 2021, 13, 4125. [Google Scholar] [CrossRef]
- Sakurai, K.; Tomihara, K.; Yamazaki, M.; Heshiki, W.; Moniruzzaman, R.; Sekido, K.; Tachinami, H.; Ikeda, A.; Imaue, S.; Fujiwara, K. CD36 expression on oral squamous cell carcinoma cells correlates with enhanced proliferation and migratory activity. Oral Dis. 2020, 26, 745–755. [Google Scholar] [CrossRef]
- Fang, L.Y.; Wong, T.Y.; Chiang, W.F.; Chen, Y.L. Fatty-acid-binding protein 5 promotes cell proliferation and invasion in oral squamous cell carcinoma. J. Oral Pathol. Med. 2010, 39, 342–348. [Google Scholar] [CrossRef]
- Silva, S.D.; Cunha, I.W.; Rangel, A.L.C.; Jorge, J.; Zecchin, K.G.; Agostini, M.; Kowalski, L.P.; Coletta, R.D.; Graner, E. Differential expression of fatty acid synthase (FAS) and ErbB2 in nonmalignant and malignant oral keratinocytes. Virchows Arch. 2008, 453, 57–67. [Google Scholar] [CrossRef]
- Agostini, M.; Almeida, L.Y.; Bastos, D.C.; Ortega, R.M.; Moreira, F.S.; Seguin, F.; Zecchin, K.G.; Raposo, H.F.; Oliveira, H.C.; Amoêdo, N.D. The fatty acid synthase inhibitor orlistat reduces the growth and metastasis of orthotopic tongue oral squamous cell carcinomas. Mol. Cancer Ther. 2014, 13, 585–595. [Google Scholar] [CrossRef] [PubMed]
- Almeida, L.Y.d.; Moreira, F.D.S.; Santos, G.A.S.d.; Cuadra Zelaya, F.J.M.; Ortiz, C.A.; Agostini, M.; Mariano, F.S.; Bastos, D.C.; Daher, U.R.N.; Kowalski, L.P. FASN inhibition sensitizes metastatic OSCC cells to cisplatin and paclitaxel by downregulating cyclin B1. Oral Dis. 2023, 29, 649–660. [Google Scholar] [CrossRef] [PubMed]
- Patmanathan, S.N.; Johnson, S.P.; Lai, S.L.; Panja Bernam, S.; Lopes, V.; Wei, W.; Ibrahim, M.H.; Torta, F.; Narayanaswamy, P.; Wenk, M.R. Aberrant expression of the S1P regulating enzymes, SPHK1 and SGPL1, contributes to a migratory phenotype in OSCC mediated through S1PR2. Sci. Rep. 2016, 6, 25650. [Google Scholar] [CrossRef]
- Wongviriya, A.; Shelton, R.M.; Cooper, P.R.; Milward, M.R.; Landini, G. The relationship between sphingosine-1-phosphate receptor 2 and epidermal growth factor in migration and invasion of oral squamous cell carcinoma. Cancer Cell Int. 2023, 23, 65. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.; Zhang, H.; Qian, Y.; Zhou, X.; Li, J.; Zhang, L.; Sun, Z.; Wang, W. Prognostic Analysis of Lactic Acid Metabolism Genes in Oral Squamous Cell Carcinoma. Int. Dent. J. 2024, 74, 1053–1063. [Google Scholar] [CrossRef]
- Gatenby, R.A.; Gillies, R.J. Why do cancers have high aerobic glycolysis? Nat. Rev. Cancer 2004, 4, 891–899. [Google Scholar] [CrossRef]
- Warburg, O. On the origin of cancer cells. Science 1956, 123, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Guduguntla, P.; Guttikonda, V.R. Estimation of serum pyruvic acid levels in oral squamous cell carcinoma. J. Oral Maxillofac. Pathol. 2020, 24, 585. [Google Scholar] [CrossRef] [PubMed]
- Wolf, A.; Agnihotri, S.; Micallef, J.; Mukherjee, J.; Sabha, N.; Cairns, R.; Hawkins, C.; Guha, A. Hexokinase 2 is a key mediator of aerobic glycolysis and promotes tumor growth in human glioblastoma multiforme. J. Exp. Med. 2011, 208, 313–326. [Google Scholar] [CrossRef]
- Chen, W.; Gueron, M. The inhibition of bovine heart hexokinase by 2-deoxy-D-glucose-6-phosphate: Characterization by 31P NMR and metabolic implications. Biochimie 1992, 74, 867–873. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Outschoorn, U.E.; Peiris-Pagés, M.; Pestell, R.G.; Sotgia, F.; Lisanti, M.P. Cancer metabolism: A therapeutic perspective. Nat. Rev. Clin. Oncol. 2017, 14, 11–31. [Google Scholar] [CrossRef]
- Pajak, B.; Siwiak, E.; Sołtyka, M.; Priebe, A.; Zieliński, R.; Fokt, I.; Ziemniak, M.; Jaśkiewicz, A.; Borowski, R.; Domoradzki, T. 2-Deoxy-d-glucose and its analogs: From diagnostic to therapeutic agents. Int. J. Mol. Sci. 2019, 21, 234. [Google Scholar] [CrossRef]
- Ancey, P.B.; Contat, C.; Meylan, E. Glucose transporters in cancer–from tumor cells to the tumor microenvironment. FEBS J. 2018, 285, 2926–2943. [Google Scholar] [CrossRef]
- Ohba, S.; Fujii, H.; Ito, S.; Fujimaki, M.; Matsumoto, F.; Furukawa, M.; Yokoyama, J.; Kusunoki, T.; Ikeda, K.; Hino, O. Overexpression of GLUT-1 in the invasion front is associated with depth of oral squamous cell carcinoma and prognosis. J. Oral Pathol. Med. 2010, 39, 74–78. [Google Scholar] [CrossRef]
- Zhu, J.; Wu, Y.-N.; Zhang, W.; Zhang, X.-M.; Ding, X.; Li, H.-Q.; Geng, M.; Xie, Z.-Q.; Wu, H.-M. Monocarboxylate transporter 4 facilitates cell proliferation and migration and is associated with poor prognosis in oral squamous cell carcinoma patients. PLoS ONE 2014, 9, e87904. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Q.; Niu, L.; Xu, L.; Guo, Y.; Wang, L.; Guo, C. Suppression of G6PD induces the expression and bisecting GlcNAc-branched N-glycosylation of E-Cadherin to block epithelial-mesenchymal transition and lymphatic metastasis. Br. J. Cancer 2020, 123, 1315–1325. [Google Scholar] [CrossRef]
- Mele, L.; la Noce, M.; Paino, F.; Regad, T.; Wagner, S.; Liccardo, D.; Papaccio, G.; Lombardi, A.; Caraglia, M.; Tirino, V. Glucose-6-phosphate dehydrogenase blockade potentiates tyrosine kinase inhibitor effect on breast cancer cells through autophagy perturbation. J. Exp. Clin. Cancer Res. 2019, 38, 160. [Google Scholar] [CrossRef]
- Cao, S.S.; Kaufman, R.J. Endoplasmic reticulum stress and oxidative stress in cell fate decision and human disease. Antioxid. Redox Signal. 2014, 21, 396–413. [Google Scholar] [CrossRef]
- Mele, L.; Paino, F.; Papaccio, F.; Regad, T.; Boocock, D.; Stiuso, P.; Lombardi, A.; Liccardo, D.; Aquino, G.; Barbieri, A. A new inhibitor of glucose-6-phosphate dehydrogenase blocks pentose phosphate pathway and suppresses malignant proliferation and metastasis in vivo. Cell Death Dis. 2018, 9, 572. [Google Scholar] [CrossRef]
- Wei, Z.; Liu, X.; Cheng, C.; Yu, W.; Yi, P. Metabolism of amino acids in cancer. Front. Cell Dev. Biol. 2021, 8, 603837. [Google Scholar] [CrossRef] [PubMed]
- Sridharan, G.; Ramani, P.; Patankar, S.; Vijayaraghavan, R. Evaluation of salivary metabolomics in oral leukoplakia and oral squamous cell carcinoma. J. Oral Pathol. Med. 2019, 48, 299–306. [Google Scholar] [CrossRef]
- Yang, X.-H.; Zhang, X.-X.; Jing, Y.; Ding, L.; Fu, Y.; Wang, S.; Hu, S.-q.; Zhang, L.; Huang, X.-F.; Ni, Y.-H. Amino acids signatures of distance-related surgical margins of oral squamous cell carcinoma. EBioMedicine 2019, 48, 81–91. [Google Scholar] [CrossRef] [PubMed]
- de Sá Alves, M.; de Sá Rodrigues, N.; Bandeira, C.M.; Chagas, J.F.S.; Pascoal, M.B.N.; Nepomuceno, G.L.J.T.; da Silva Martinho, H.; Alves, M.G.O.; Mendes, M.A.; Dias, M. Identification of possible salivary metabolic biomarkers and altered metabolic pathways in South American patients diagnosed with oral squamous cell carcinoma. Metabolites 2021, 11, 650. [Google Scholar] [CrossRef]
- Cristaldi, M.; Mauceri, R.; Di Fede, O.; Giuliana, G.; Campisi, G.; Panzarella, V. Salivary biomarkers for oral squamous cell carcinoma diagnosis and follow-up: Current status and perspectives. Front. Physiol. 2019, 10, 1476. [Google Scholar] [CrossRef] [PubMed]
- Nocini, R.; Vianini, M.; Girolami, I.; Calabrese, L.; Scarpa, A.; Martini, M.; Morbini, P.; Marletta, S.; Brunelli, M.; Molteni, G. PD-L1 in oral squamous cell carcinoma: A key biomarker from the laboratory to the bedside. Clin. Exp. Dent. Res. 2022, 8, 690–698. [Google Scholar] [CrossRef]
- Smyth, M.J.; Dunn, G.P.; Schreiber, R.D. Cancer immunosurveillance and immunoediting: The roles of immunity in suppressing tumor development and shaping tumor immunogenicity. Adv. Immunol. 2006, 90, 1–50. [Google Scholar] [CrossRef]
- Marcus, A.; Gowen, B.G.; Thompson, T.W.; Iannello, A.; Ardolino, M.; Deng, W.; Wang, L.; Shifrin, N.; Raulet, D.H. Recognition of tumors by the innate immune system and natural killer cells. Adv. Immunol. 2014, 122, 91–128. [Google Scholar] [CrossRef]
- Vinay, D.S.; Ryan, E.P.; Pawelec, G.; Talib, W.H.; Stagg, J.; Elkord, E.; Lichtor, T.; Decker, W.K.; Whelan, R.L.; Kumara, H.S. Immune evasion in cancer: Mechanistic basis and therapeutic strategies. Semin. Cancer Biol. 2015, 35, S185–S198. [Google Scholar] [CrossRef]
- Bridge, J.A.; Lee, J.C.; Daud, A.; Wells, J.W.; Bluestone, J.A. Cytokines, chemokines, and other biomarkers of response for checkpoint inhibitor therapy in skin cancer. Front. Med. 2018, 5, 351. [Google Scholar] [CrossRef]
- Sarhadi, V.K.; Armengol, G. Molecular biomarkers in cancer. Biomolecules 2022, 12, 1021. [Google Scholar] [CrossRef]
- Robert, C. A decade of immune-checkpoint inhibitors in cancer therapy. Nat. Commun. 2020, 11, 3801. [Google Scholar] [CrossRef]
- Sahibzada, H.A.; Khurshid, Z.; Sannam Khan, R.; Naseem, M.; Mahmood Siddique, K.; Mali, M.; Zafar, M.S. Salivary IL-8, IL-6 and TNF-α as potential diagnostic biomarkers for oral cancer. Diagnostics 2017, 7, 21. [Google Scholar] [CrossRef]
- Duffy, S.A.; Taylor, J.M.; Terrell, J.E.; Islam, M.; Li, Y.; Fowler, K.E.; Wolf, G.T.; Teknos, T.N. Interleukin-6 predicts recurrence and survival among head and neck cancer patients. Cancer Interdiscip. Int. J. Am. Cancer Soc. 2008, 113, 750–757. [Google Scholar] [CrossRef] [PubMed]
- Benito-Ramal, E.; Egido-Moreno, S.; González-Navarro, B.; Jané-Salas, E.; Roselló-Llabrés, X.; López-López, J. Role of selected salivary inflammatory cytokines in the diagnosis and prognosis of oral squamous cell carcinoma. A Systematic Review and Meta-analysis. Med. Oral Patol. Oral Y Cir. Bucal 2023, 28, e474. [Google Scholar] [CrossRef] [PubMed]
- Landskron, G.; De la Fuente, M.; Thuwajit, P.; Thuwajit, C.; Hermoso, M.A. Chronic inflammation and cytokines in the tumor microenvironment. J. Immunol. Res. 2014, 2014, 149185. [Google Scholar] [CrossRef]
- Roi, A.; Roi, C.I.; Negruțiu, M.L.; Riviș, M.; Sinescu, C.; Rusu, L.-C. The challenges of OSCC diagnosis: Salivary Cytokines as potential biomarkers. J. Clin. Med. 2020, 9, 2866. [Google Scholar] [CrossRef] [PubMed]
- Dikova, V.; Jantus-Lewintre, E.; Bagan, J. Potential non-invasive biomarkers for early diagnosis of oral squamous cell carcinoma. J. Clin. Med. 2021, 10, 1658. [Google Scholar] [CrossRef] [PubMed]
- Aziz, S.; Ahmed, S.S.; Ali, A.; Khan, F.A.; Zulfiqar, G.; Iqbal, J.; Khan, A.A.; Shoaib, M. Salivary immunosuppressive cytokines IL-10 and IL-13 are significantly elevated in oral squamous cell carcinoma patients. Cancer Investig. 2015, 33, 318–328. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Luo, F.; Deng, M.; Zhang, J. The relationship between salivary cytokines and oral cancer and their diagnostic capability for oral cancer: A systematic review and network meta-analysis. BMC Oral Health 2024, 24, 1044. [Google Scholar] [CrossRef]
- Hsing, E.-W.; Shiah, S.-G.; Peng, H.-Y.; Chen, Y.-W.; Chuu, C.-P.; Hsiao, J.-R.; Lyu, P.-C.; Chang, J.-Y. TNF-α-induced miR-450a mediates TMEM182 expression to promote oral squamous cell carcinoma motility. PLoS ONE 2019, 14, e0213463. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhu, M.; Wang, W.; Chen, D.; Chen, S.; Zheng, H. TNF-α promotes tumor lymph angiogenesis in head and neck squamous cell carcinoma through regulation of ERK3. Transl. Cancer Res. 2019, 8, 2439. [Google Scholar] [CrossRef]
- Espinosa-Cotton, M.; Fertig, E.J.; Stabile, L.P.; Gaither-Davis, A.; Bauman, J.E.; Schmitz, S.; Gibson-Corley, K.N.; Cheng, Y.; Jensen, I.J.; Badovinac, V.P. A preliminary analysis of interleukin-1 ligands as potential predictive biomarkers of response to cetuximab. Biomark. Res. 2019, 7, 14. [Google Scholar] [CrossRef]
- Lacy, P. Editorial: Secretion of cytokines and chemokines by innate immune cells. Front. Immunol. 2015, 6, 190. [Google Scholar] [CrossRef]
- Chow, M.T.; Luster, A.D. Chemokines in cancer. Cancer Immunol. Res. 2014, 2, 1125–1131. [Google Scholar] [CrossRef]
- Oliveira-Neto, H.H.; de Souza, P.P.C.; Da Silva, M.R.B.; Mendonça, E.F.; Silva, T.A.; Batista, A.C. The expression of chemokines CCL19, CCL21 and their receptor CCR7 in oral squamous cell carcinoma and its relevance to cervical lymph node metastasis. Tumor Biol. 2013, 34, 65–70. [Google Scholar] [CrossRef]
- Zhang, A.; Sun, H.; Wang, P.; Wang, X. Salivary proteomics in biomedical research. Clin. Chim. Acta 2013, 415, 261–265. [Google Scholar] [CrossRef]
- Yuvaraj, S.; Griffin, A.C.; Sundaram, K.; Kirkwood, K.L.; Norris, J.S.; Reddy, S.V. A novel function of CXCL13 to stimulate RANK ligand expression in oral squamous cell carcinoma cells. Mol. Cancer Res. 2009, 7, 1399–1407. [Google Scholar] [CrossRef]
- Eain, H.S.; Kawai, H.; Nakayama, M.; Oo, M.W.; Ohara, T.; Fukuhara, Y.; Takabatake, K.; Shan, Q.; Soe, Y.; Ono, K. Double-faced CX3CL1 enhances lymphangiogenesis-dependent metastasis in an aggressive subclone of oral squamous cell carcinoma. JCI Insight 2024, 9, 174618. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.Y.; Peng, P.W.; Renn, T.Y.; Lee, C.J.; Chang, T.M.; Wei, A.I.C.; Liu, J.F.J.J.o.C.; Medicine, M. CX3CL1 induces cell migration and invasion through ICAM-1 expression in oral squamous cell carcinoma cells. J. Cell. Mol. Med. 2023, 27, 1509–1522. [Google Scholar] [CrossRef]
- Tojo, S.; Nakashiro, K.i.; Kuribayashi, N.; Uchida, D. Serum CXCL13 as a Novel Biomarker in Oral Squamous Cell Carcinoma. Cancer Med. 2024, 13, e70263. [Google Scholar] [CrossRef] [PubMed]
- Mehta, M.K.; Gupta, S.; Fatima, T.; Selvam, R.; Chandra, S.; Singh, D.; Sivakumar, N. An Increase in the Chemokine Mediators (CCL28 and CCR10) Associated with the Progression of Oral Squamous Cell Carcinoma: A Cross-Sectional Investigation. Indian J. Otolaryngol. Head Neck Surg. 2024, 76, 5717–5724. [Google Scholar] [CrossRef] [PubMed]
- Whiteside, T. The tumor microenvironment and its role in promoting tumor growth. Oncogene 2008, 27, 5904–5912. [Google Scholar] [CrossRef] [PubMed]
- Tampa, M.; Mitran, M.I.; Mitran, C.I.; Sarbu, M.I.; Matei, C.; Nicolae, I.; Caruntu, A.; Tocut, S.M.; Popa, M.I.; Caruntu, C. Mediators of inflammation–a potential source of biomarkers in oral squamous cell carcinoma. J. Immunol. Res. 2018, 2018, 1061780. [Google Scholar] [CrossRef]
- Miguel, A.F.; Mello, F.W.; Melo, G.; Rivero, E.R. Association between immunohistochemical expression of matrix metalloproteinases and metastasis in oral squamous cell carcinoma: Systematic review and meta-analysis. Head Neck 2020, 42, 569–584. [Google Scholar] [CrossRef]
- Hsiao, Y.-C.; Lin, S.-Y.; Chien, K.-Y.; Chen, S.-F.; Wu, C.-C.; Chang, Y.-T.; Chi, L.-M.; Chu, L.J.; Chiang, W.-F.; Chien, C.-Y. An immuno-MALDI mass spectrometry assay for the oral cancer biomarker, matrix metalloproteinase-1, in dried saliva spot samples. Anal. Chim. Acta 2020, 1100, 118–130. [Google Scholar] [CrossRef]
- Mukherjee, G.; Bag, S.; Chakraborty, P.; Dey, D.; Roy, S.; Jain, P.; Roy, P.; Soong, R.; Majumder, P.P.; Dutt, S. Density of CD3+ and CD8+ cells in gingivo-buccal oral squamous cell carcinoma is associated with lymph node metastases and survival. PLoS ONE 2020, 15, e0242058. [Google Scholar] [CrossRef]
- Lao, X.M.; Liang, Y.J.; Su, Y.X.; Zhang, S.E.; Zhou, X.; Liao, G.Q. Distribution and significance of interstitial fibrosis and stroma-infiltrating B cells in tongue squamous cell carcinoma. Oncol. Lett. 2016, 11, 2027–2034. [Google Scholar] [CrossRef]
- Mullen, A.C.; High, F.A.; Hutchins, A.S.; Lee, H.W.; Villarino, A.V.; Livingston, D.M.; Kung, A.L.; Cereb, N.; Yao, T.-P.; Yang, S.Y. Role of T-bet in commitment of TH1 cells before IL-12-dependent selection. Science 2001, 292, 1907–1910. [Google Scholar] [CrossRef] [PubMed]
- Goswami, K.K.; Ghosh, T.; Ghosh, S.; Sarkar, M.; Bose, A.; Baral, R. Tumor promoting role of anti-tumor macrophages in tumor microenvironment. Cell. Immunol. 2017, 316, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Pennock, N.D.; White, J.T.; Cross, E.W.; Cheney, E.E.; Tamburini, B.A.; Kedl, R.M. T cell responses: Naive to memory and everything in between. Adv. Physiol. Educ. 2013, 37, 273–283. [Google Scholar] [CrossRef]
- Chaudhary, B.; Elkord, E. Regulatory T cells in the tumor microenvironment and cancer progression: Role and therapeutic targeting. Vaccines 2016, 4, 28. [Google Scholar] [CrossRef]
- Mittrücker, H.-W.; Visekruna, A.; Huber, M. Heterogeneity in the differentiation and function of CD8+ T cells. Arch. Immunol. Ther. Exp. 2014, 62, 449–458. [Google Scholar] [CrossRef] [PubMed]
- Dyck, L.; Mills, K.H. Immune checkpoints and their inhibition in cancer and infectious diseases. Eur. J. Immunol. 2017, 47, 765–779. [Google Scholar] [CrossRef]
- Chakravarthy, A.; Khan, L.; Bensler, N.P.; Bose, P.; De Carvalho, D.D. TGF-β-associated extracellular matrix genes link cancer-associated fibroblasts to immune evasion and immunotherapy failure. Nat. Commun. 2018, 9, 4692. [Google Scholar] [CrossRef]
- Kellermann, M.; Sobral, L.; Silva, S.d.; Zecchin, K.; Graner, E.; Lopes, M.; Nishimoto, I.; Kowalski, L.; Coletta, R. Myofibroblasts in the stroma of oral squamous cell carcinoma are associated with poor prognosis. Histopathology 2007, 51, 849–853. [Google Scholar] [CrossRef]
- Eltohami, Y.I.; Kao, H.-K.; Lao, W.W.-K.; Huang, Y.; Abdelrahman, M.; Liao, C.-T.; Yen, T.-C.; Chang, K.-P. The prediction value of the systemic inflammation score for oral cavity squamous cell carcinoma. Otolaryngol.–Head Neck Surg. 2018, 158, 1042–1050. [Google Scholar] [CrossRef]
- Dourado, M.R.; Guerra, E.N.; Salo, T.; Lambert, D.W.; Coletta, R.D. Prognostic value of the immunohistochemical detection of cancer-associated fibroblasts in oral cancer: A systematic review and meta-analysis. J. Oral Pathol. Med. 2018, 47, 443–453. [Google Scholar] [CrossRef]
- Zhang, D.; Song, Y.; Li, D.; Liu, X.; Pan, Y.; Ding, L.; Shi, G.; Wang, Y.; Ni, Y.; Hou, Y. Cancer-associated fibroblasts promote tumor progression by lncRNA-mediated RUNX2/GDF10 signaling in oral squamous cell carcinoma. Mol. Oncol. 2022, 16, 780–794. [Google Scholar] [CrossRef] [PubMed]
- Pang, X.; Fan, H.-y.; Tang, Y.-l.; Wang, S.-s.; Cao, M.-x.; Wang, H.-f.; Dai, L.-l.; Wang, K.; Yu, X.-h.; Wu, J.-b. Myeloid derived suppressor cells contribute to the malignant progression of oral squamous cell carcinoma. PLoS ONE 2020, 15, e0229089. [Google Scholar] [CrossRef] [PubMed]
- Vitale, I.; Manic, G.; Coussens, L.M.; Kroemer, G.; Galluzzi, L. Macrophages and metabolism in the tumor microenvironment. Cell Metab. 2019, 30, 36–50. [Google Scholar] [CrossRef]
- Boutilier, A.J.; Elsawa, S.F. Macrophage polarization states in the tumor microenvironment. Int. J. Mol. Sci. 2021, 22, 6995. [Google Scholar] [CrossRef] [PubMed]
- Umemura, N.; Sugimoto, M.; Kitoh, Y.; Saio, M.; Sakagami, H. Metabolomic profiling of tumor-infiltrating macrophages during tumor growth. Cancer Immunol. Immunother. 2020, 69, 2357–2369. [Google Scholar] [CrossRef]
- Dan, H.; Liu, S.; Liu, J.; Liu, D.; Yin, F.; Wei, Z.; Wang, J.; Zhou, Y.; Jiang, L.; Ji, N. RACK1 promotes cancer progression by increasing the M2/M1 macrophage ratio via the NF-κB pathway in oral squamous cell carcinoma. Mol. Oncol. 2020, 14, 795–807. [Google Scholar] [CrossRef]
- Ai, Y.; Liu, S.; Luo, H.; Wu, S.; Wei, H.; Tang, Z.; Li, X.; Zou, C. lncRNA DCST1-AS1 facilitates oral squamous cell carcinoma by promoting M2 macrophage polarization through activating NF-κB signaling. J. Immunol. Res. 2021, 2021, 5524231. [Google Scholar] [CrossRef]
- Mori, K.; Hiroi, M.; Shimada, J.; Ohmori, Y. Infiltration of m2 tumor-associated macrophages in oral squamous cell carcinoma correlates with tumor malignancy. Cancers 2011, 3, 3726–3739. [Google Scholar] [CrossRef]
- Xue, Y.; Song, X.; Fan, S.; Deng, R. The role of tumor-associated macrophages in oral squamous cell carcinoma. Front. Physiol. 2022, 13, 959747. [Google Scholar] [CrossRef]
- Chaudhari, N.; Prakash, N.; Pradeep, G.L.; Mahajan, A.; Lunawat, S.; Salunkhe, V. Evaluation of density of tumor-associated macrophages using CD163 in histological grades of oral squamous cell carcinoma, an immunohistochemical study. J. Oral Maxillofac. Pathol. JOMFP 2020, 24, 577. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, D.; Watts, D.; Gaete, D.; Sormendi, S.; Wielockx, B. Hypoxia pathway proteins and their impact on the blood vasculature. Int. J. Mol. Sci. 2021, 22, 9191. [Google Scholar] [CrossRef] [PubMed]
- Corry, J.; Peters, L.J.; Rischin, D. Optimising the therapeutic ratio in head and neck cancer. Lancet Oncol. 2010, 11, 287–291. [Google Scholar] [CrossRef] [PubMed]
- Eckert, A.W.; Schütze, A.; Lautner, M.H.; Taubert, H.; Schubert, J.; Bilkenroth, U. HIF-1α is a prognostic marker in oral squamous cell carcinomas. Int. J. Biol. Markers 2010, 25, 87–92. [Google Scholar] [CrossRef]
- Roh, J.-L.; Cho, K.-J.; Kwon, G.Y.; Ryu, C.H.; Chang, H.W.; Choi, S.-H.; Nam, S.Y.; Kim, S.Y. The prognostic value of hypoxia markers in T2-staged oral tongue cancer. Oral Oncol. 2009, 45, 63–68. [Google Scholar] [CrossRef]
- Winter, S.C.; Shah, K.A.; Han, C.; Campo, L.; Turley, H.; Leek, R.; Corbridge, R.J.; Cox, G.J.; Harris, A.L. The relation between hypoxia-inducible factor (HIF)-1alpha and HIF-2alpha expression with anemia and outcome in surgically treated head and neck cancer. Cancer 2006, 107, 757–766. [Google Scholar] [CrossRef]
- Uehara, M.; Sano, K.; Ikeda, H.; Nonaka, M.; Asahina, I. Hypoxia-inducible factor 1 alpha in oral squamous cell carcinoma and its relation to prognosis. Oral Oncol. 2009, 45, 241–246. [Google Scholar] [CrossRef]
- Eltzschig, H.K.; Carmeliet, P. Hypoxia and inflammation. N. Engl. J. Med. 2011, 364, 656–665. [Google Scholar] [CrossRef]
- Hasegawa, T.; Iga, T.; Takeda, D.; Amano, R.; Saito, I.; Kakei, Y.; Kusumoto, J.; Kimoto, A.; Sakakibara, A.; Akashi, M. Neutrophil-lymphocyte ratio associated with poor prognosis in oral cancer: A retrospective study. BMC Cancer 2020, 20, 568. [Google Scholar] [CrossRef]
- Grimm, M.; Rieth, J.; Hoefert, S.; Krimmel, M.; Rieth, S.; Teriete, P.; Kluba, S.; Biegner, T.; Munz, A.; Reinert, S. Standardized pretreatment inflammatory laboratory markers and calculated ratios in patients with oral squamous cell carcinoma. Eur. Arch. Oto-Rhino-Laryngol. 2016, 273, 3371–3384. [Google Scholar] [CrossRef]
- Baldwin, A.S. Control of oncogenesis and cancer therapy resistance by the transcription factor NF-κB. J. Clin. Investig. 2001, 107, 241–246. [Google Scholar] [CrossRef]
- Byatnal, A.A.; Byatnal, A.; Sen, S.; Guddattu, V.; Solomon, M.C. Cyclooxygenase-2–an imperative prognostic biomarker in oral squamous cell carcinoma-an immunohistochemical study. Pathol. Oncol. Res. 2015, 21, 1123–1131. [Google Scholar] [CrossRef]
- Thomas, N.; Krishnapillai, R.; Bindhu, P.R.; Thomas, P. Immunohistochemical expression of cyclooxygenase-2 in oral squamous cell carcinoma. Indian J. Dent. Res. 2019, 30, 102. [Google Scholar] [PubMed]
- Mitra, A.; Kumar, A.; Amdare, N.P.; Pathak, R. Current Landscape of Cancer Immunotherapy: Harnessing the Immune Arsenal to Overcome Immune Evasion. Biology 2024, 13, 307. [Google Scholar] [CrossRef] [PubMed]
- Kujan, O.; van Schaijik, B.; Farah, C.S. Immune checkpoint inhibitors in oral cavity squamous cell carcinoma and oral potentially malignant disorders: A systematic review. Cancers 2020, 12, 1937. [Google Scholar] [CrossRef]
- Marin-Acevedo, J.A.; Dholaria, B.; Soyano, A.E.; Knutson, K.L.; Chumsri, S.; Lou, Y. Next generation of immune checkpoint therapy in cancer: New developments and challenges. J. Hematol. Oncol. 2018, 11, 39. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.B.; Fan, Z.Z.; Anton, D.; Vollenhoven, A.V.; Ni, Z.H.; Chen, X.F.; Lefvert, A.K. CTLA4 is expressed on mature dendritic cells derived from human monocytes and influences their maturation and antigen presentation. BMC Immunol. 2011, 12, 21. [Google Scholar] [CrossRef]
- Jie, H.-B.; Schuler, P.J.; Lee, S.C.; Srivastava, R.M.; Argiris, A.; Ferrone, S.; Whiteside, T.L.; Ferris, R.L. CTLA-4+ regulatory T cells increased in cetuximab-treated head and neck cancer patients suppress NK cell cytotoxicity and correlate with poor prognosis. Cancer Res. 2015, 75, 2200–2210. [Google Scholar] [CrossRef]
- Wing, J.B.; Ise, W.; Kurosaki, T.; Sakaguchi, S. Regulatory T cells control antigen-specific expansion of Tfh cell number and humoral immune responses via the coreceptor CTLA-4. Immunity 2014, 41, 1013–1025. [Google Scholar] [CrossRef]
- Blank, C.U.; Enk, A. Therapeutic use of anti-CTLA-4 antibodies. Int. Immunol. 2014, 27, 3–10. [Google Scholar] [CrossRef]
- Camacho, L.H.; Antonia, S.; Sosman, J.; Kirkwood, J.M.; Gajewski, T.F.; Redman, B.; Pavlov, D.; Bulanhagui, C.; Bozon, V.A.; Gomez-Navarro, J.; et al. Phase I/II trial of tremelimumab in patients with metastatic melanoma. J. Clin. Oncol. 2009, 27, 1075–1081. [Google Scholar] [CrossRef] [PubMed]
- Hamid, O.; Schmidt, H.; Nissan, A.; Ridolfi, L.; Aamdal, S.; Hansson, J.; Guida, M.; Hyams, D.M.; Gómez, H.; Bastholt, L.; et al. A prospective phase II trial exploring the association between tumor microenvironment biomarkers and clinical activity of ipilimumab in advanced melanoma. J. Transl. Med. 2011, 9, 204. [Google Scholar] [CrossRef] [PubMed]
- Ferris, R.L.; Gooding, W.E.; Chiosea, S.I.; Duvvuri, U.; Kim, S.; Kubik, M.; Sridharan, S.; Fenton, M.J.; Skinner, H.D.; Kelly, Z.R. Neoadjuvant nivolumab alone or in combination with relatlimab or ipilimumab in resectable head and neck squamous cell carcinoma (HNSCC). J. Clin. Oncol. 2023, 41, 6018. [Google Scholar] [CrossRef]
- Keir, M.E.; Butte, M.J.; Freeman, G.J.; Sharpe, A.H. PD-1 and its ligands in tolerance and immunity. Annu. Rev. Immunol. 2008, 26, 677–704. [Google Scholar] [CrossRef]
- Chen, M.; Zhao, H. Next-generation sequencing in liquid biopsy: Cancer screening and early detection. Hum. Genom. 2019, 13, 34. [Google Scholar] [CrossRef]
- Kaur, J.; Jacobs, R.; Huang, Y.; Salvo, N.; Politis, C. Salivary biomarkers for oral cancer and pre-cancer screening: A review. Clin. Oral Investig. 2018, 22, 633–640. [Google Scholar] [CrossRef]
- Bennett, F.; Luxenberg, D.; Ling, V.; Wang, I.M.; Marquette, K.; Lowe, D.; Khan, N.; Veldman, G.; Jacobs, K.A.; Valge-Archer, V.E.; et al. Program death-1 engagement upon TCR activation has distinct effects on costimulation and cytokine-driven proliferation: Attenuation of ICOS, IL-4, and IL-21, but not CD28, IL-7, and IL-15 responses. J. Immunol. 2003, 170, 711–718. [Google Scholar] [CrossRef]
- Bai, R.; Chen, N.; Li, L.; Du, N.; Bai, L.; Lv, Z.; Tian, H.; Cui, J. Mechanisms of cancer resistance to immunotherapy. Front. Oncol. 2020, 10, 1290. [Google Scholar] [CrossRef]
- Meci, A.; Goyal, N.; Slonimsky, G. Mechanisms of resistance and therapeutic perspectives in immunotherapy for advanced head and neck cancers. Cancers 2024, 16, 703. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Wang, Z.; Xu, M.; Li, B.; Huang, Z.; Qin, S.; Nice, E.C.; Tang, J.; Huang, C. Oral squamous cell carcinomas: State of the field and emerging directions. Int. J. Oral Sci. 2023, 15, 44. [Google Scholar] [CrossRef] [PubMed]
- Venkatesiah, S.S.; Augustine, D.; Mishra, D.; Gujjar, N.; Haragannavar, V.C.; Awan, K.H.; Patil, S. Immunology of oral squamous cell carcinoma—A comprehensive insight with recent concepts. Life 2022, 12, 1807. [Google Scholar] [CrossRef]
- Chaudhary, A.; Bag, S.; Arora, N.; Radhakrishnan, V.S.; Mishra, D.; Mukherjee, G. Hypoxic transformation of immune cell metabolism within the microenvironment of oral cancers. Front. Oral Health 2020, 1, 585710. [Google Scholar] [CrossRef]
- Yi, M.; Zheng, X.; Niu, M.; Zhu, S.; Ge, H.; Wu, K. Combination strategies with PD-1/PD-L1 blockade: Current advances and future directions. Mol. Cancer 2022, 21, 28. [Google Scholar] [CrossRef] [PubMed]
- Codony, V.L.; Tavassoli, M. Hypoxia-induced therapy resistance: Available hypoxia-targeting strategies and current advances in head and neck cancer. Transl. Oncol. 2021, 14, 101017. [Google Scholar] [CrossRef] [PubMed]
- Lacerna, L.V.; Hester, J.; Pineda, A.A.; Burgstaler, E.; Klein, H.G. Clinical/technical challenges in adoptive cellular immunotherapy (ACI): The role of cytapheresis. In Adoptive Cellular Immunotherapy of Cancer; CRC Press: Boca Raton, FL, USA, 2021; pp. 175–189. [Google Scholar]
- Rohaan, M.W.; Wilgenhof, S.; Haanen, J.B. Adoptive cellular therapies: The current landscape. Virchows Arch. 2019, 474, 449–461. [Google Scholar] [CrossRef]
- Ying, Z.; Huang, X.F.; Xiang, X.; Liu, Y.; Kang, X.; Song, Y.; Guo, X.; Liu, H.; Ding, N.; Zhang, T. A safe and potent anti-CD19 CAR T cell therapy. Nat. Med. 2019, 25, 947–953. [Google Scholar] [CrossRef] [PubMed]
- Gorchakov, A.A.; Kulemzin, S.V.; Kochneva, G.V.; Taranin, A.V. Challenges and prospects of chimeric antigen receptor T-cell therapy for metastatic prostate cancer. Eur. Urol. 2020, 77, 299–308. [Google Scholar] [CrossRef]
- Mei, Z.; Zhang, K.; Lam, A.K.; Huang, J.; Qiu, F.; Qiao, B.; Zhang, Y. MUC1 as a target for CAR-T therapy in head and neck squamous cell carinoma. Cancer Med. 2020, 9, 640–652. [Google Scholar] [CrossRef]
- Summers, S.E.; Salih, V.; Foey, A.D. ErbB-and MUC1-targetted CAR-T cell immunotherapy of oral squamous cell carcinoma. Front. Dent. Med. 2023, 4, 1116402. [Google Scholar] [CrossRef]
- Yuan, Y.; Li, H.; Pu, W.; Chen, L.; Guo, D.; Jiang, H.; He, B.; Qin, S.; Wang, K.; Li, N. Cancer metabolism and tumor microenvironment: Fostering each other? Sci. China Life Sci. 2022, 65, 236–279. [Google Scholar] [CrossRef]
- Lu, C.; Yang, D.; Klement, J.D.; Colson, Y.L.; Oberlies, N.H.; Pearce, C.J.; Colby, A.H.; Grinstaff, M.W.; Ding, H.-F.; Shi, H. G6PD functions as a metabolic checkpoint to regulate granzyme B expression in tumor-specific cytotoxic T lymphocytes. J. Immunother. Cancer 2022, 10, e003543. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.; Tong, Y.; Jiang, X.; Meng, Y.; Jiang, H.; Du, L.; Wu, Q.; Li, S.; Luo, S.; Li, M. Aerobic glycolysis promotes tumor immune evasion by hexokinase2-mediated phosphorylation of IκBα. Cell Metab. 2022, 34, 1312–1324.e6. [Google Scholar] [CrossRef]
- Brand, A.; Singer, K.; Koehl, G.E.; Kolitzus, M.; Schoenhammer, G.; Thiel, A.; Matos, C.; Bruss, C.; Klobuch, S.; Peter, K. LDHA-associated lactic acid production blunts tumor immunosurveillance by T and NK cells. Cell Metab. 2016, 24, 657–671. [Google Scholar] [CrossRef]
- Dodard, G.; Tata, A.; Erick, T.K.; Jaime, D.; Miah, S.S.; Quatrini, L.; Escalière, B.; Ugolini, S.; Vivier, E.; Brossay, L. Inflammation-induced lactate leads to rapid loss of hepatic tissue-resident NK cells. Cell Rep. 2020, 32, 107855. [Google Scholar] [CrossRef]
- Liu, N.; Luo, J.; Kuang, D.; Xu, S.; Duan, Y.; Xia, Y.; Wei, Z.; Xie, X.; Yin, B.; Chen, F. Lactate inhibits ATP6V0d2 expression in tumor-associated macrophages to promote HIF-2α–mediated tumor progression. J. Clin. Investig. 2019, 129, 631–646. [Google Scholar] [CrossRef]
- Carr, E.L.; Kelman, A.; Wu, G.S.; Gopaul, R.; Senkevitch, E.; Aghvanyan, A.; Turay, A.M.; Frauwirth, K.A. Glutamine uptake and metabolism are coordinately regulated by ERK/MAPK during T lymphocyte activation. J. Immunol. 2010, 185, 1037–1044. [Google Scholar] [CrossRef]
- Keshet, R.; Lee, J.S.; Adler, L.; Iraqi, M.; Ariav, Y.; Lim, L.Q.J.; Lerner, S.; Rabinovich, S.; Oren, R.; Katzir, R. Targeting purine synthesis in ASS1-expressing tumors enhances the response to immune checkpoint inhibitors. Nat. Cancer 2020, 1, 894–908. [Google Scholar] [CrossRef]
- Li, L.; Zeng, X.; Chao, Z.; Luo, J.; Guan, W.; Zhang, Q.; Ge, Y.; Wang, Y.; Xiong, Z.; Ma, S. Targeting Alpha-Ketoglutarate Disruption Overcomes Immunoevasion and Improves PD-1 Blockade Immunotherapy in Renal Cell Carcinoma. Adv. Sci. 2023, 10, 2301975. [Google Scholar] [CrossRef]
- Wu, J.-Y.; Huang, T.-W.; Hsieh, Y.-T.; Wang, Y.-F.; Yen, C.-C.; Lee, G.-L.; Yeh, C.-C.; Peng, Y.-J.; Kuo, Y.-Y.; Wen, H.-T. Cancer-derived succinate promotes macrophage polarization and cancer metastasis via succinate receptor. Mol. Cell 2020, 77, 213–227.e5. [Google Scholar] [CrossRef] [PubMed]
- Manzo, T.; Prentice, B.M.; Anderson, K.G.; Raman, A.; Schalck, A.; Codreanu, G.S.; Nava Lauson, C.B.; Tiberti, S.; Raimondi, A.; Jones, M.A. Accumulation of long-chain fatty acids in the tumor microenvironment drives dysfunction in intrapancreatic CD8+ T cells. J. Exp. Med. 2020, 217, e20191920. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.A.; Wei, J.; Nguyen, T.-L.M.; Shi, H.; Su, W.; Palacios, G.; Dhungana, Y.; Chapman, N.M.; Long, L.; Saravia, J. Lipid signalling enforces functional specialization of Treg cells in tumours. Nature 2021, 591, 306–311. [Google Scholar] [CrossRef]
- Cheng, J.; Yan, J.; Liu, Y.; Shi, J.; Wang, H.; Zhou, H.; Zhou, Y.; Zhang, T.; Zhao, L.; Meng, X. Cancer-cell-derived fumarate suppresses the anti-tumor capacity of CD8+ T cells in the tumor microenvironment. Cell Metab. 2023, 35, 961–978.e10. [Google Scholar] [CrossRef]
- Davel, L.E.; Jasnis, M.a.A.; de la Torre, E.; Gotoh, T.; Diament, M.; Magenta, G.; de Lustig, E.S.; Sales, M.a.E. Arginine metabolic pathways involved in the modulation of tumor-induced angiogenesis by macrophages. FEBS Lett. 2002, 532, 216–220. [Google Scholar] [CrossRef]
- Püschel, F.; Favaro, F.; Redondo-Pedraza, J.; Lucendo, E.; Iurlaro, R.; Marchetti, S.; Majem, B.; Eldering, E.; Nadal, E.; Ricci, J.-E. Starvation and antimetabolic therapy promote cytokine release and recruitment of immune cells. Proc. Natl. Acad. Sci. USA 2020, 117, 9932–9941. [Google Scholar] [CrossRef] [PubMed]
- Opitz, C.A.; Litzenburger, U.M.; Sahm, F.; Ott, M.; Tritschler, I.; Trump, S.; Schumacher, T.; Jestaedt, L.; Schrenk, D.; Weller, M. An endogenous tumour-promoting ligand of the human aryl hydrocarbon receptor. Nature 2011, 478, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liang, X.; Dong, W.; Fang, Y.; Lv, J.; Zhang, T.; Fiskesund, R.; Xie, J.; Liu, J.; Yin, X. Tumor-repopulating cells induce PD-1 expression in CD8+ T cells by transferring kynurenine and AhR activation. Cancer Cell 2018, 33, 480–494.e7. [Google Scholar] [CrossRef] [PubMed]
- Mezrich, J.D.; Fechner, J.H.; Zhang, X.; Johnson, B.P.; Burlingham, W.J.; Bradfield, C.A. An interaction between kynurenine and the aryl hydrocarbon receptor can generate regulatory T cells. J. Immunol. 2010, 185, 3190–3198. [Google Scholar] [CrossRef]
- Qin, W.-H.; Yang, Z.-S.; Li, M.; Chen, Y.; Zhao, X.-F.; Qin, Y.-Y.; Song, J.-Q.; Wang, B.-B.; Yuan, B.; Cui, X.-L. High serum levels of cholesterol increase antitumor functions of nature killer cells and reduce growth of liver tumors in mice. Gastroenterology 2020, 158, 1713–1727. [Google Scholar] [CrossRef]
- Polz, A.; Morshed, K.; Drop, B.; Polz-Dacewicz, M. Could MMP3 and MMP9 serve as biomarkers in EBV-related oropharyngeal cancer. Int. J. Mol. Sci. 2024, 25, 2561. [Google Scholar] [CrossRef]
- Lechner, M.; Liu, J.; Masterson, L.; Fenton, T.R. HPV-associated oropharyngeal cancer: Epidemiology, molecular biology and clinical management. Nat. Rev. Clin. Oncol. 2022, 19, 306–327. [Google Scholar] [CrossRef]
- Chang, E.T.; Ye, W.; Zeng, Y.-X.; Adami, H.-O. The evolving epidemiology of nasopharyngeal carcinoma. Cancer Epidemiol. Biomark. Prev. 2021, 30, 1035–1047. [Google Scholar] [CrossRef]
- Smith, C.D.; McMahon, A.D.; Purkayastha, M.; Creaney, G.; Clements, K.; Inman, G.J.; Bhatti, L.A.; Douglas, C.M.; Paterson, C.; Conway, D.I. Head and neck cancer incidence is rising but the sociodemographic profile is unchanging: A population epidemiological study (2001–2020). BJC Rep. 2024, 2, 71. [Google Scholar] [CrossRef]
- Vossen, D.M.; Verhagen, C.V.; Verheij, M.; Wessels, L.F.; Vens, C.; van den Brekel, M.W. Comparative genomic analysis of oral versus laryngeal and pharyngeal cancer. J. Oral Oncol. 2018, 81, 35–44. [Google Scholar] [CrossRef]
- Farah, C.S. Molecular landscape of head and neck cancer and implications for therapy. Ann. Transl. Med. 2021, 9, 915. [Google Scholar] [CrossRef]
- Arriola Benítez, P.C.; Fusco, M.; Amorin, R.; Picón, C.R.; Piccioni, F.; Victoria, L.; Rizzo, M.M.; Malvicini, M. Unraveling the Role of Tumor-Infiltrating Immune Cells in Head and Neck Squamous Cell Carcinoma: Implications for Antitumor Immune Responses and Immunotherapy. Int. J. Mol. Sci. 2025, 26, 6337. [Google Scholar] [CrossRef]
- Ali, A.; Bari, M.F.; Arshad, S.; Wahid, M.; Safdar, J.; Anwar, K.; Farooqui, W.A. Tissue-resident memory T-cell expressions and their prognostic role in head and neck squamous cell carcinoma: A systematic review and meta-analysis. BMC Cancer 2025, 25, 356. [Google Scholar] [CrossRef] [PubMed]
- Chuchueva, N.; Carta, F.; Nguyen, H.N.; Luevano, J.; Lewis, I.A.; Rios-Castillo, I.; Fanos, V.; King, E.; Swistushkin, V.; Reshetov, I. Metabolomics of head and neck cancer in biofluids: An integrative systematic review. Metabolomics 2023, 19, 77. [Google Scholar] [CrossRef] [PubMed]
- Jung, M.; Pützer, S.; Gevensleben, H.; Meller, S.; Kristiansen, G.; Dietrich, D. Diagnostic and prognostic value of SHOX2 and SEPT9 DNA methylation and cytology in benign, paramalignant, and malignant ascites. Clin. Epigenet. 2016, 8, 24. [Google Scholar] [CrossRef] [PubMed]
- Niklander, S.E. Inflammatory mediators in oral cancer: Pathogenic mechanisms and diagnostic potential. Front. Oral Health 2021, 2, 642238. [Google Scholar] [CrossRef]
- Li, J.; Li, S.; Shu, M.; Hu, W. Unravelling the heterogeneity of oral squamous cell carcinoma by integrative analysis of single-cell and bulk transcriptome data. J. Cell. Mol. Med. 2024, 28, e18108. [Google Scholar] [CrossRef]
- Veeraraghavan, V.P.; Doni, B.R.; Dasari, A.K.; Patil, C.; Rao, K.A.; Patil, S.R. Deciphering Genomic Complexity: Understanding Intratumor Heterogeneity, Clonal Evolution, and Therapeutic Vulnerabilities in Oral Squamous Cell Carcinoma. Oral Oncol. Rep. 2024, 10, 100469. [Google Scholar] [CrossRef]
- Watanabe, K.; Nakamura, Y.; Low, S.-K. Clinical implementation and current advancement of blood liquid biopsy in cancer. J. Hum. Genet. 2021, 66, 909–926. [Google Scholar] [CrossRef]
- Ribeiro, I.P.; Esteves, L.; Santos, A.; Barroso, L.; Marques, F.; Caramelo, F.; Melo, J.B.; Carreira, I.M. A seven-gene signature to predict the prognosis of oral squamous cell carcinoma. Oncogene 2021, 40, 3859–3869. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Chen, X.; Chen, H.; Zhou, M.; Liu, Y.; Hou, Y.; Nie, M.; Liu, X. Identification and validation of potential novel biomarkers for oral squamous cell carcinoma. Bioengineered 2021, 12, 8845–8862. [Google Scholar] [CrossRef]
- Yang, W.; Zhou, W.; Zhao, X.; Wang, X.; Duan, L.; Li, Y.; Niu, L.; Chen, J.; Zhang, Y.; Han, Y. Prognostic biomarkers and therapeutic targets in oral squamous cell carcinoma: A study based on cross-database analysis. Hereditas 2021, 158, 15. [Google Scholar] [CrossRef] [PubMed]
- Arora, R.; Cao, C.; Kumar, M.; Sinha, S.; Chanda, A.; McNeil, R.; Samuel, D.; Arora, R.K.; Matthews, T.W.; Chandarana, S. Spatial transcriptomics reveals distinct and conserved tumor core and edge architectures that predict survival and targeted therapy response. Nat. Commun. 2023, 14, 5029. [Google Scholar] [CrossRef]
- Adeoye, J.; Su, Y.X. Artificial intelligence in salivary biomarker discovery and validation for oral diseases. Oral Dis. 2024, 30, 23–37. [Google Scholar] [CrossRef] [PubMed]
- Vinay, V.; Jodalli, P.; Chavan, M.S.; Buddhikot, C.S.; Luke, A.M.; Ingafou, M.S.H.; Reda, R.; Pawar, A.M.; Testarelli, L. Artificial Intelligence in Oral Cancer: A Comprehensive Scoping Review of Diagnostic and Prognostic Applications. Diagnostics 2025, 15, 280. [Google Scholar] [CrossRef]
- Shulman, E.D.; Campagnolo, E.M.; Lodha, R.; Stemmer, A.; Cantore, T.; Ru, B.; Wang, A.; Hu, T.; Nasrallah, M.; Hoang, D.-T. Path2Space: An AI approach for cancer biomarker discovery via histopathology inferred spatial transcriptomics. Cancer Res. 2025, 85, 6353. [Google Scholar] [CrossRef]
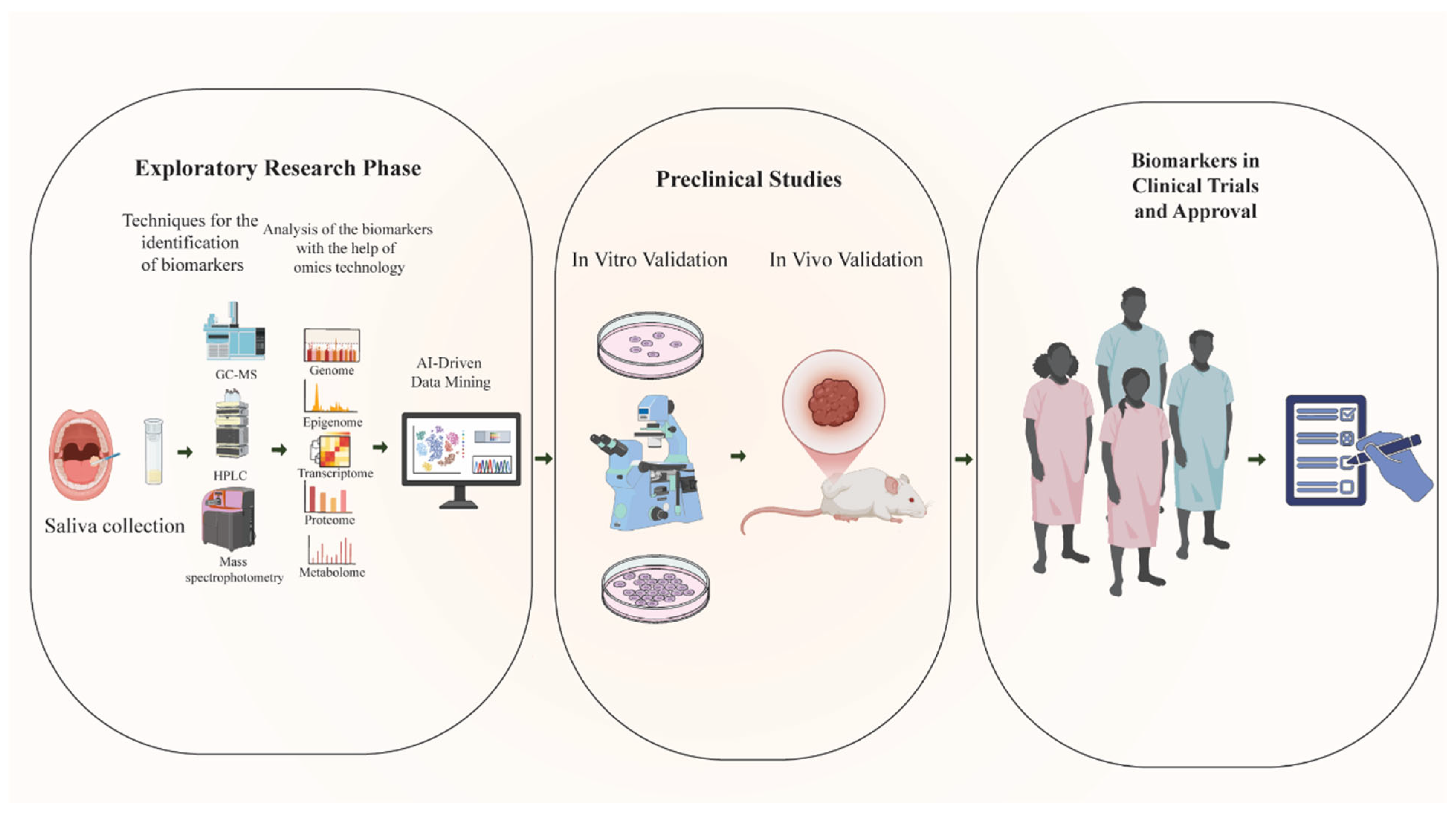

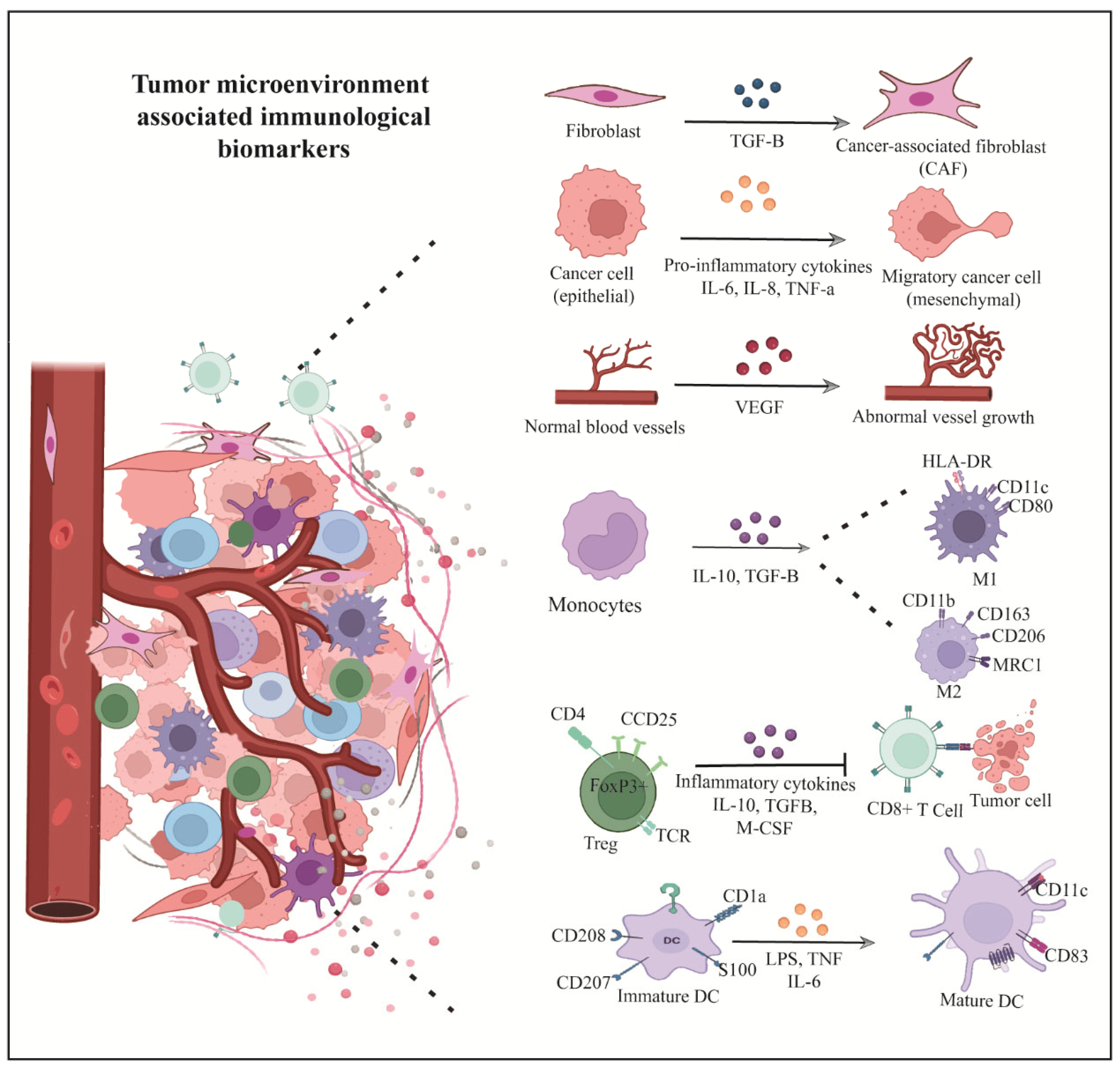

| Metabolic Pathway/Biomarker | Key Alterations in OSCC | Diagnostic Methods/Biomarkers | Clinical/Diagnostic Performance | Therapeutic Targets/Implications |
|---|---|---|---|---|
| Amino Acid Metabolism | ↓ Aspartate, glutamate, arginine; ↑ asparagine, glutamine, cysteine; tryptophan metabolism via kynurenine pathway enhances Treg function | Plasma free amino acid profiling (HPLC, UHPLC/Q-Orbitrap HRMS); urine metabolite analysis; Mendelian randomization studies | Plasma panel (decanoylcarnitine, cysteine, cholic acid): AUC 0.998; urine markers: sensitivity 94.4%, specificity 91.4% | SLC1A5/ASCT2 (glutamine transporter) inhibition; targeting tryptophan metabolism |
| Lipid/Choline Metabolism | ↑ Phosphatidylcholine, phosphatidylethanolamine; ↓ free choline; ↑ trimethylamine N-oxide; ↑ FA uptake (CD36, FABP5); ↑ FASN | NMR, UHPLC/Q-Orbitrap HRMS, salivary metabolomics | Salivary propionylcholine: sensitivity 76.9%, specificity 96.7%; multi-marker AUC 0.997 | FASN, S1PR2, CD36, FABP5, SREBP1, ACC inhibitors |
| Glycolysis (Warburg Effect) | ↑ Glycolysis/lactate production; ↑ HK-II, GLUT-1/3, MCT-4 expression; ↑ serum pyruvic acid | Serum pyruvic acid measurement; enzyme/transporter expression analysis | Serum pyruvic acid: OSCC 2.65 mg% vs. controls 0.95 mg% (p < 0.0001) | HK-II inhibition (2-DG), GLUT-1/3, MCT-4 inhibitors |
| Pentose Phosphate Pathway (PPP) | ↑ G6PD expression; ↑ NADPH and ribose-5-phosphate production; supports redox and nucleotide synthesis | G6PD expression analysis | G6PD correlates with advanced disease, poor prognosis | G6PD inhibition (e.g., polydatin); PPP inhibition restores chemosensitivity |
| Salivary Metabolites | ↑ Glutamate, histidine, sialic acid, TMAO, malic acid, methionine, inosine, guanine, spermidine, pipercolate; ↓ urea | CE-MS, CPSI-MS, machine learning panels, salivary metabolomics | Multi-marker panels: up to 100% sensitivity, AUC 0.997; decanoylcarnitine: AUC >0.95, 97.3% | Non-invasive diagnosis, early detection, large-scale screening |
| Clinical Trial Name/Number | Cancer Type | Treatment Regimen | Clinical Phase/Approval | Key Findings/Focus |
|---|---|---|---|---|
| KEYNOTE-048 NCT02358031 | Recurrent/metastatic HNSCC (incl. OSCC) | Pembrolizumab ± chemotherapy | Phase III FDA-approved | Improved overall survival (OS), especially in PD-L1+ patients; fewer severe AEs than chemotherapy. |
| NBTXR3 NCT03589339 | Locally advanced HNSCC (incl. OSCC) | NBTXR3 + radiotherapy + anti-PD-1 | Phase I | 40% ORR, 75% disease control; effective in anti-PD-1-resistant cases; safe and durable responses. |
| TACTI-003 NCT04811027 | Unresectable recurrent/metastatic HNSCC | Eftilagimod alpha + pembrolizumab | Phase IIb | Dual immunotherapy (LAG-3 + PD-1); evaluating efficacy in PD-L1-low tumors. |
| ONC-392 NCT04140526 | Advanced solid tumors (incl. OSCC) | Anti-CTLA-4 (ONC-392) ± pembrolizumab | Phase I/II | Novel CTLA-4 inhibitor with reduced toxicity; under evaluation for advanced/refractory disease. |
| AGEN1181 NCT03860272 | Advanced solid tumors (incl. OSCC/HNSCC) | Fc-enhanced anti-CTLA-4 (AGEN1181) ± AGEN2034 (PD-1) | Phase I/II | Engineered CTLA-4 therapy aiming for broader immune activation and better safety profile. |
| PRGN-2009 NCT04432597 | HPV-positive HNSCC (incl. OSCC) | Therapeutic HPV vaccine ± M7824 | Phase I/II | Boosting HPV-specific T cell immunity; promising for virus-driven oral cancers. |
| TG4050 NCT03839524 | Resected HNSCC (incl. OSCC) | Personalized mRNA neoantigen vaccine | Phase I/II | Individualized vaccine for post-surgical patients to prevent recurrence; early positive immune response. |
| Ipilimumab/Tremelimumab (Various Trials) | HNSCC (incl. OSCC) | Anti-CTLA-4 ± anti-PD-1 (e.g., nivolumab) | Phase I/II | Combination checkpoint blockade; under study for synergy and durable responses. |
| Metabolic Pathway | Tumor-Driven Mechanisms | Immune Consequences | Therapeutic Strategies |
|---|---|---|---|
| Glycolysis |
|
|
|
| TCA Cycle |
|
|
|
| Amino Acid Metabolism |
|
|
|
| Lipid Metabolism |
|
|
|
| Bidirectional Interactions |
|
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rajneesh; Tiwari, R.; Singh, V.K.; Kumar, A.; Mehrotra, S.; Gautam, V.; Neville, J.F.; Bansal, V.; Pathak, R.; Singh, A.K.; et al. Exploring Metabolic and Immunological Biomarkers for Oral Squamous Cell Carcinoma: Potential Targets for Precision Therapy. Biology 2025, 14, 1109. https://doi.org/10.3390/biology14091109
Rajneesh, Tiwari R, Singh VK, Kumar A, Mehrotra S, Gautam V, Neville JF, Bansal V, Pathak R, Singh AK, et al. Exploring Metabolic and Immunological Biomarkers for Oral Squamous Cell Carcinoma: Potential Targets for Precision Therapy. Biology. 2025; 14(9):1109. https://doi.org/10.3390/biology14091109
Chicago/Turabian StyleRajneesh, Rahul Tiwari, Vishal Kumar Singh, Awnish Kumar, Sanjana Mehrotra, Vibhav Gautam, J. F. Neville, Vyomika Bansal, Rajiv Pathak, Akhilesh Kumar Singh, and et al. 2025. "Exploring Metabolic and Immunological Biomarkers for Oral Squamous Cell Carcinoma: Potential Targets for Precision Therapy" Biology 14, no. 9: 1109. https://doi.org/10.3390/biology14091109
APA StyleRajneesh, Tiwari, R., Singh, V. K., Kumar, A., Mehrotra, S., Gautam, V., Neville, J. F., Bansal, V., Pathak, R., Singh, A. K., & Kumar, R. (2025). Exploring Metabolic and Immunological Biomarkers for Oral Squamous Cell Carcinoma: Potential Targets for Precision Therapy. Biology, 14(9), 1109. https://doi.org/10.3390/biology14091109








