In Vivo Shadows and In Vitro Light: The Early Embryological Journey Amid Endometriosis
Simple Summary
Abstract
1. Background
2. The In Vivo Impact of Endometriosis on Fertility
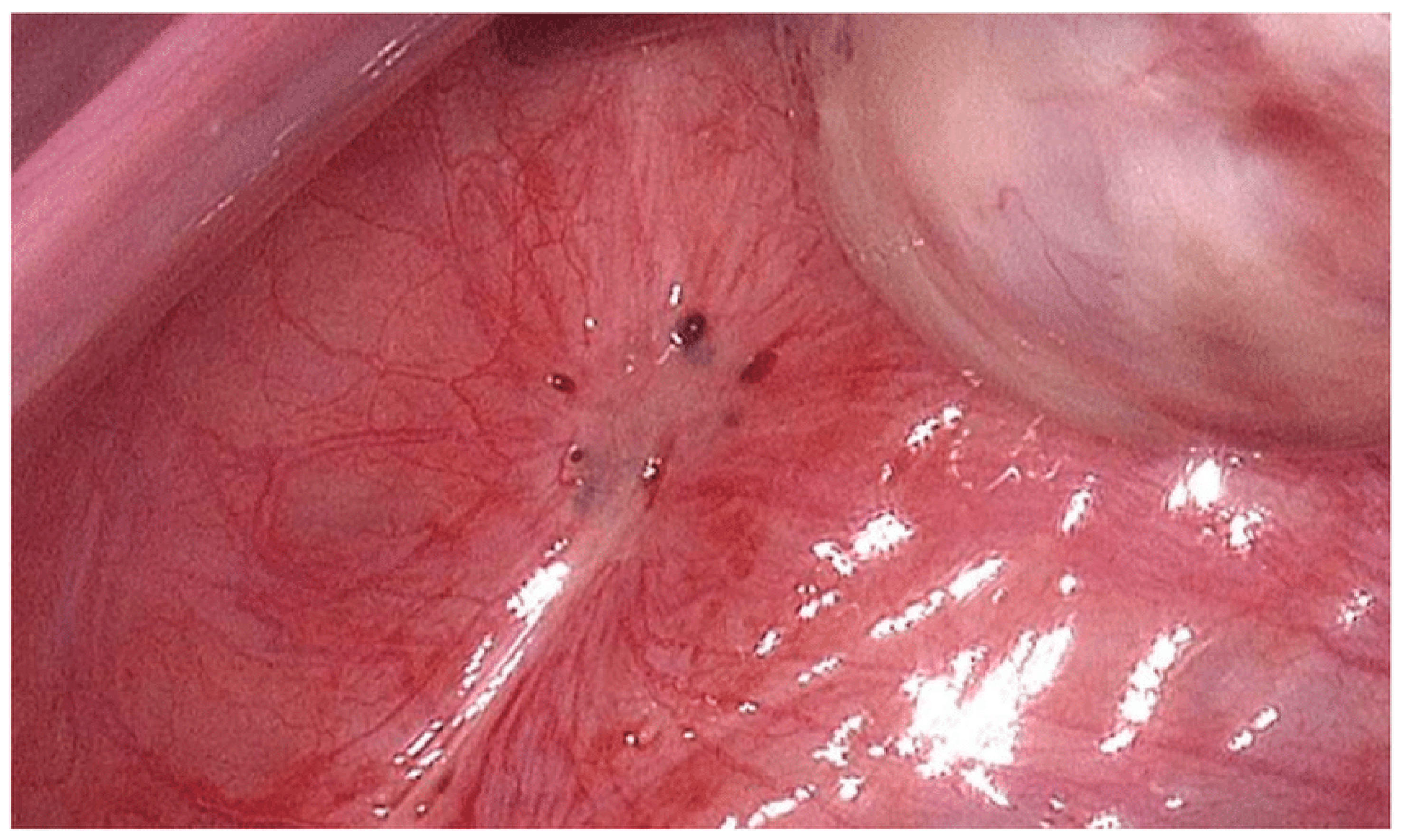
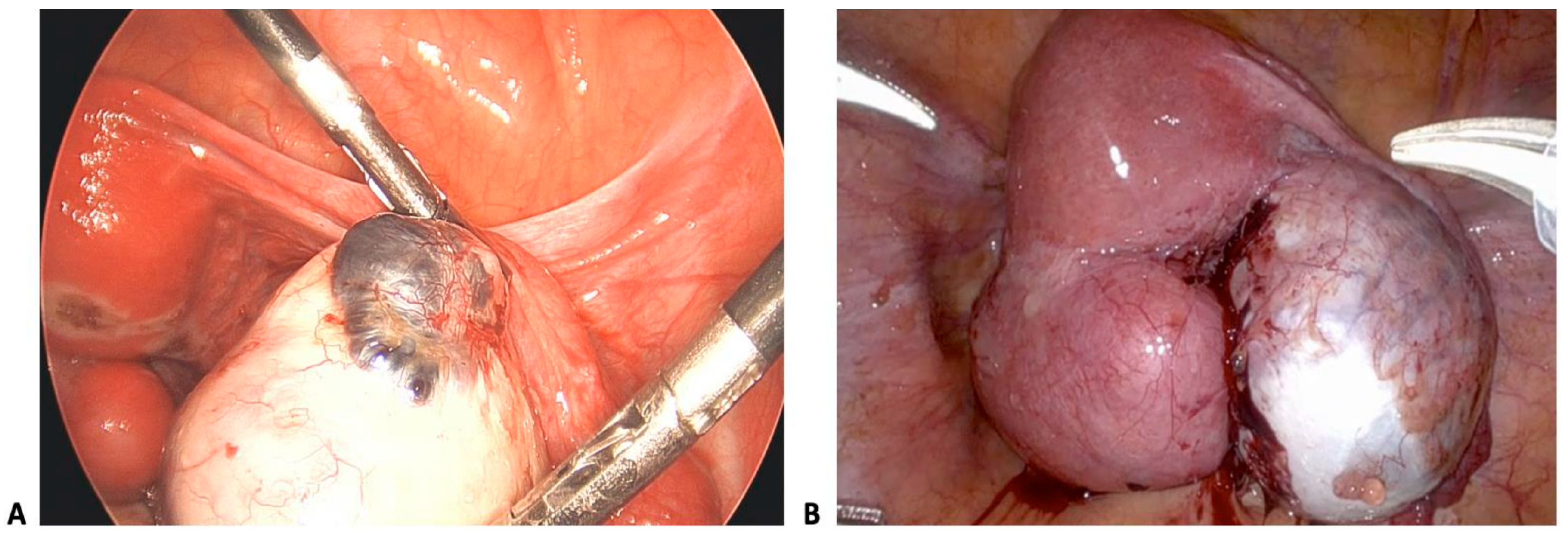
2.1. Pathophysiology and Classification
2.2. Reproductive Consequences
2.3. Immune Dysregulation and Inflammation
2.4. Cellular Actors and Pathways
2.5. Oxidative Stress and Free Radicals
2.6. Cellular Damage: Mechanisms of ROS Damage
2.7. In Vivo Summary
3. The In Vitro Alternative: Controlled Rescue
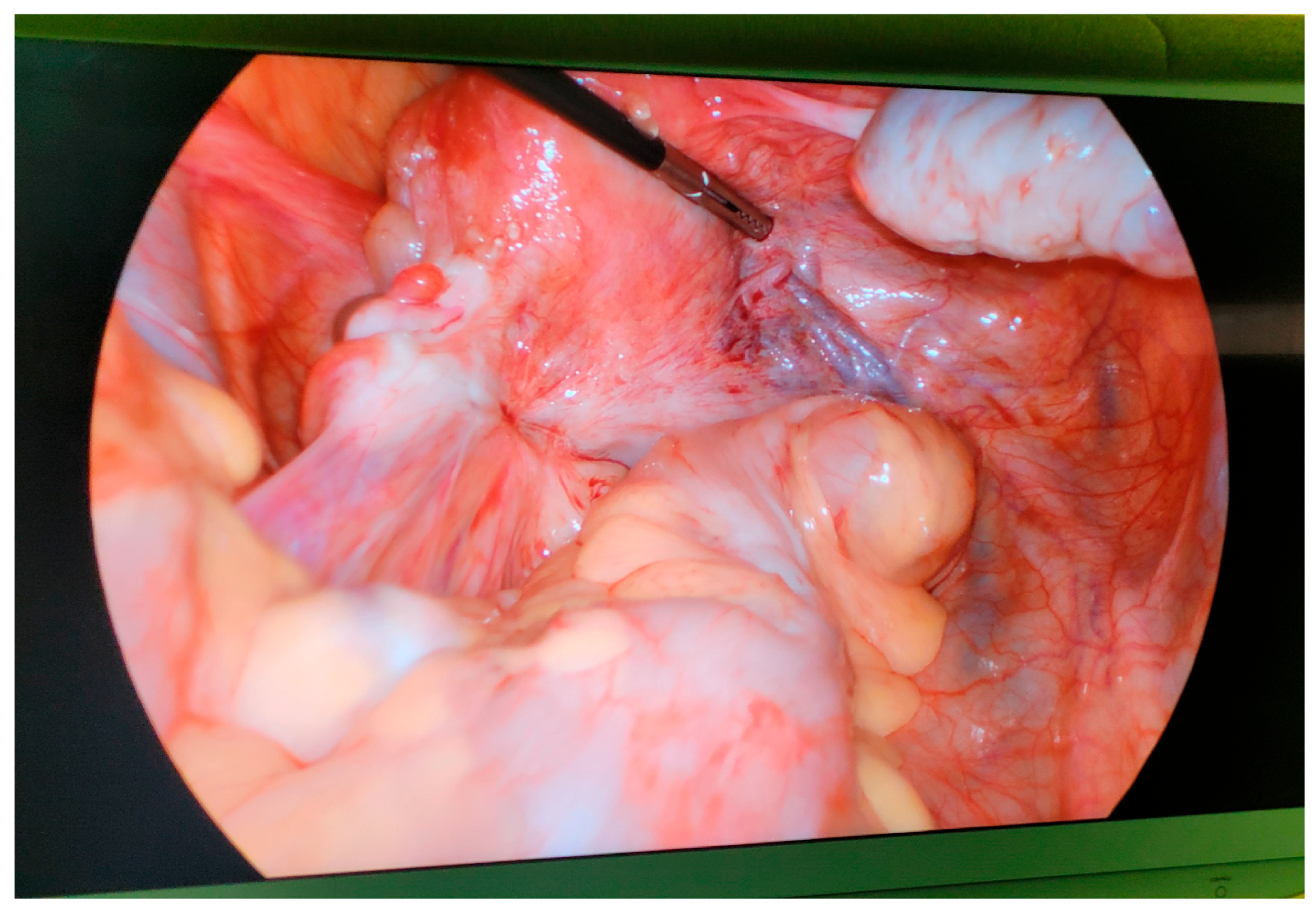
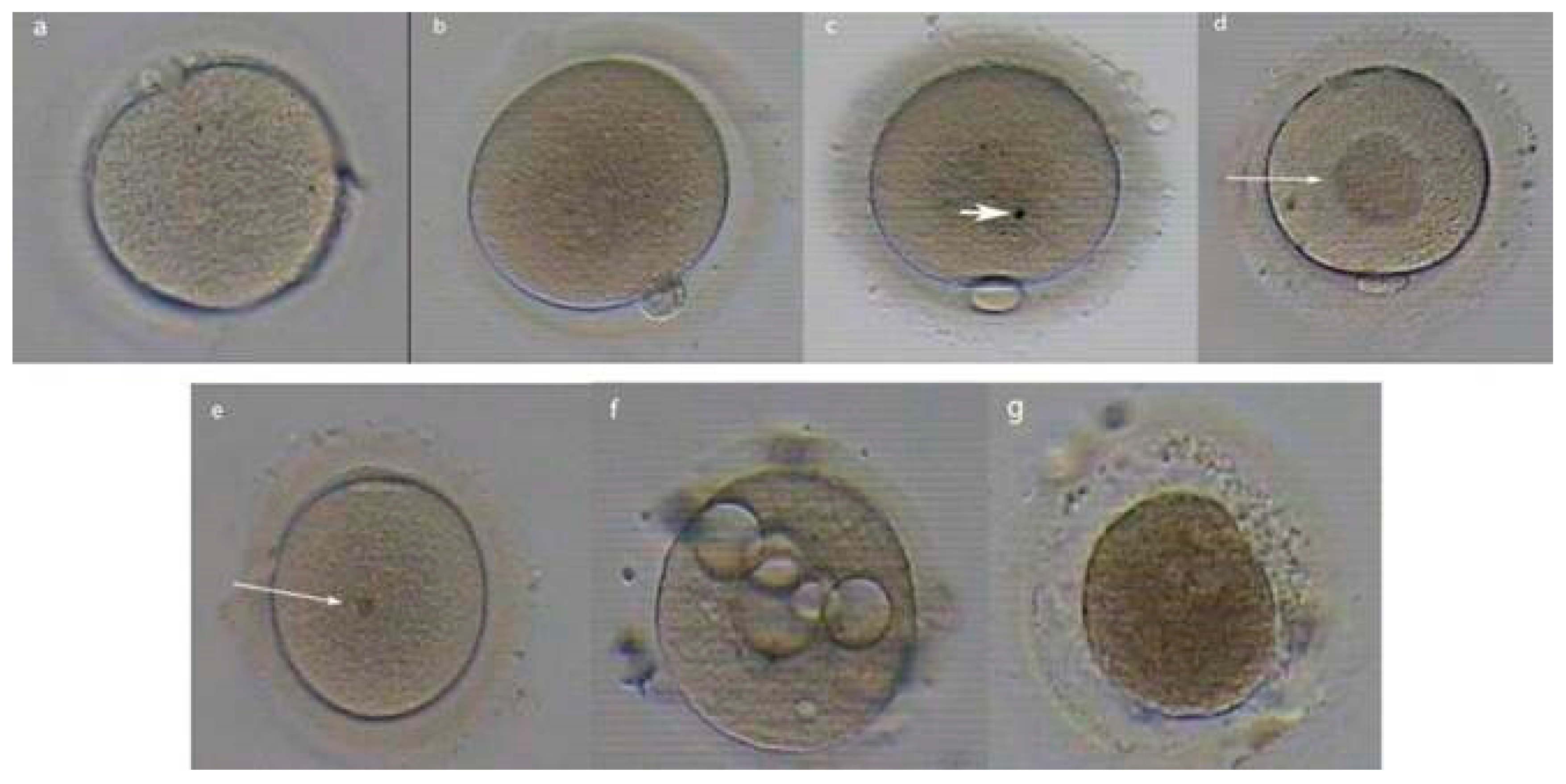
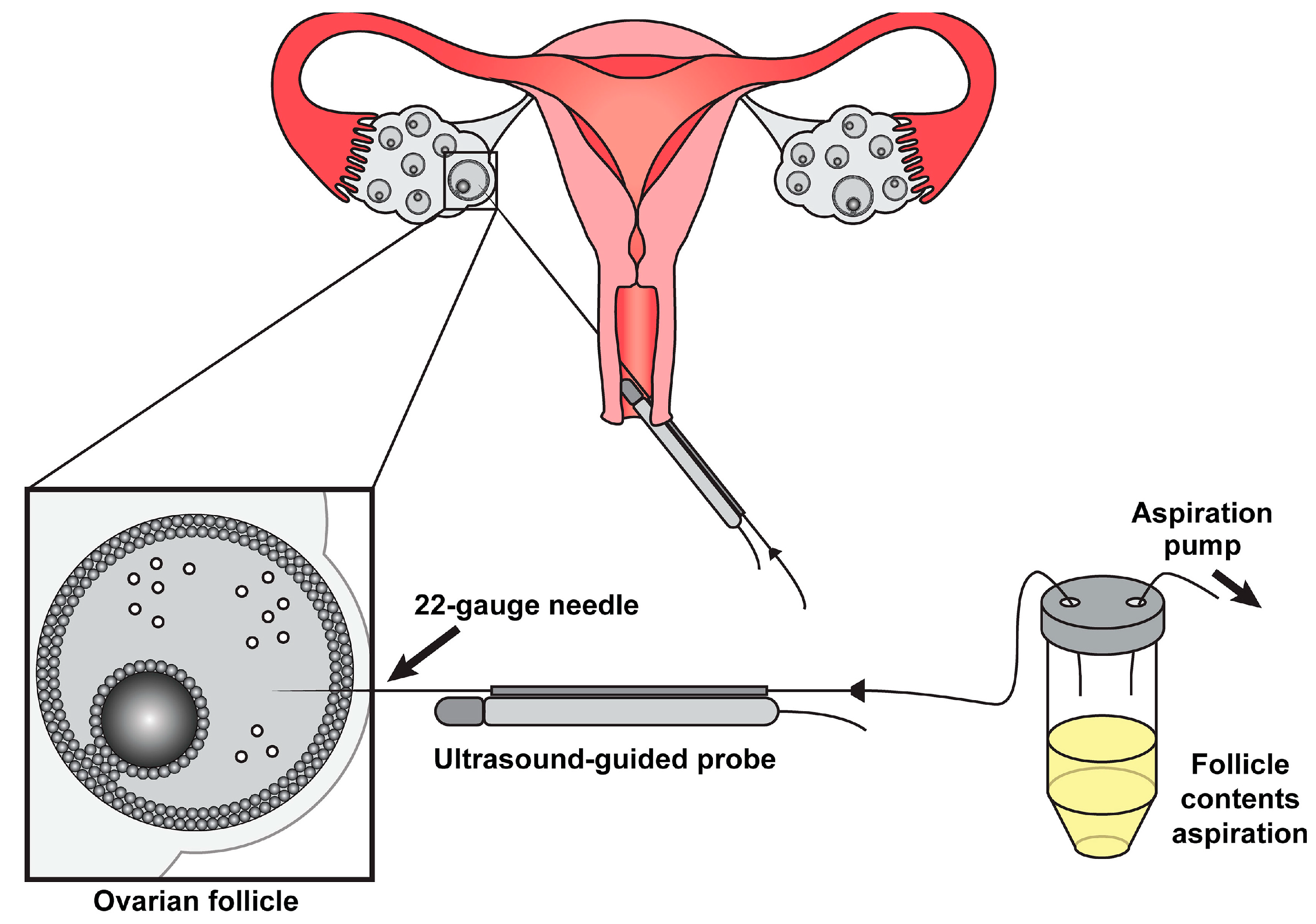
3.1. Liberating the Oocyte
3.2. IVF Efficacy
3.3. Conflicting Evidence: The IVF Outcome Debate
3.4. IVF Conclusion
4. Embryo Morphokinetics: Time-Lapse and Timing in Endometriosis
4.1. Introduction to Time-Lapse Imaging in Embryo Selection
4.2. Morphokinetic Profiles in Endometriosis: Altered Dynamics?
4.3. Clinical Implications and Unanswered Questions
4.4. TLM Summary
5. Oocyte Donation Models: Disentangling Nature from Nurture
6. Limitations, Gaps, and Future Horizons in Endometriosis-Related Infertility
6.1. What We Do Not Yet Know
6.2. Emerging Diagnostic and Therapeutic Innovations
6.3. Omics and Molecular Breakthroughs
7. Conclusions: Navigating Endometriosis-Related Infertility
- Personalized ART strategies: Tailoring protocols based on the endometriosis subtype and severity may optimize outcomes.
- Oocyte quality as a priority: Emerging data emphasize cytoplasmic competence over uterine factors. This necessitates focusing on oxidative stress mitigation and advanced embryo selection tools, such as leveraging AI. These strategies would facilitate better IVF outcomes than what is currently in place.
- Patient-centered care: Patients need transparent counseling about the variability in IVF success rates and related innovative options. This is critical to managing expectations.
The Road Ahead
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| IVF | In Vitro Fertilization |
| AI | Artificial Intelligence |
| ART | Assisted Reproductive Technology |
| rASRM | Revised American Society for Reproductive Medicine Classification of Endometriosis |
| GnRH | Gonadotropin-Releasing Hormone |
| AFC | Antral Follicle Count |
| AMH | Anti-Müllerian Hormone |
| IL-6 | Interleukin-6 |
| TNF | Tumor Necrosis Factor |
| PGE2 | Prostaglandin E2 |
| ROS | Reactive Oxygen Species |
| COC | Cumulus–Oocyte Complex |
| TLM | Time-Lapse Microscopy |
| ERA | Endometrial Receptivity Array |
| BCL 6 | B-cell Lymphoma 6 |
| mtDNA | Mitochondrial Deoxyribonucleic Acid |
| Fe2+ | Free Iron |
| H2O2 | Hydrogen Peroxide |
| ·OH | Hydroxyl Radical |
| PUFAs | Polyunsaturated Fatty Acids |
| H4K16 | Acetylation at Lysine 16 of Histone H4 |
| SOD2 | Superoxide Dismutase 2 |
| PRDX1/2 | Peroxiredoxin ½ |
| DNMT3A | DNA Methyltransferase 3 Alpha |
| NRF2 | Nuclear Factor Erythroid 2-Related Factor 2 |
| KEAP1 | Kelch-Like ECH-Associated Protein 1 |
| HIF-1α | Hypoxia-Inducible Factor 1-Alpha |
| CD 68+ | Cluster of Differentiation 68 |
| FAS | FS7-associated cell surface antigen |
| FASL | FS7-associated cell surface antigen Ligand |
| STAT3 | Signal Transducer and Activator of Transcription 3 |
| MAC | Membrane Attack Complex |
| NK | Natural Killer |
| uNK | Uterine Natural Killer |
| TLR4 | Toll-Like Receptor 4 |
| NF-κB | Nuclear Factor Kappa B |
| PGT-A | Preimplantation Genetic Testing for Aneuploidy |
| PVS | Perivitelline Space |
References
- Kedzia, M.; Basta, P.; Czajkowski, K.; Gogacz, M.; Spaczynski, R.; Stojko, R.; Szaflik, T.; Szubert, M.; Szyllo, K.; Zaborowski, M.; et al. Guidelines of the Polish Society of Gynecologists and Obstetricians on the management of women with endometriosis. Ginekol. Pol. 2024, 95, 729–758. [Google Scholar] [CrossRef] [PubMed]
- American Society for Reproductive Medicine. Revised American Society for Reproductive Medicine classification of endometriosis: 1996. Fertil. Steril. 1997, 67, 817–821. [Google Scholar] [CrossRef]
- American Fertility Society. Classification of endometriosis. Fertil. Steril. 1979, 32, 633. [Google Scholar] [CrossRef]
- Ibrahim, M.G.; Krentel, H.; Kiesel, L.; Schäfer, S. Counseling endometriosis patients in fertility clinics: A guidelines-based approach. EC Gynaecol. Spec. Issue 2020, 1–10. [Google Scholar]
- Dunselman, G.A.J.; Vermeulen, N.; Becker, C.; Calhaz-Jorge, C.; D’Hooghe, T.; De Bie, B.; Heikinheimo, O.; Horne, A.W.; Kiesel, L.; Nap, A.; et al. ESHRE guideline: Management of women with endometriosis. Hum. Reprod. 2014, 29, 400–412. [Google Scholar] [CrossRef]
- Becker, C.M.; Bokor, A.; Heikinheimo, O.; Horne, A.; Jansen, F.; Kiesel, L.; King, K.; Kvaskoff, M.; Nap, A.; Petersen, K.; et al. ESHRE guideline: Endometriosis. Hum. Reprod. Open 2022, 2022, hoac009. [Google Scholar] [CrossRef]
- Adamson, G.D.; Pasta, D.J. Endometriosis fertility index: The new, validated endometriosis staging system. Fertil. Steril. 2010, 94, 1609–1615. [Google Scholar] [CrossRef] [PubMed]
- Capezzuoli, T.; Clemenza, S.; Sorbi, F.; Campana, D.; Vannuccini, S.; Chapron, C.; Petraglia, F. Classification/staging systems for endometriosis: The state of the art. Gynecol. Reprod. Endocrinol. Metab. 2020, 1, 14–22. [Google Scholar] [CrossRef]
- Vermeulen, N.; Abrao, M.S.; Einarsson, J.I.; Horne, A.W.; Johnson, N.P.; Lee, T.T.; Missmer, S.; Petrozza, J.; Tomassetti, C.; Zondervan, K.T.; et al. Endometriosis classification, staging and reporting systems: A review on the road to a universally accepted endometriosis classification. J. Minim. Invasive Gynecol. 2021, 28, 1822–1848. [Google Scholar] [CrossRef] [PubMed]
- Johnson, N.P.; Hummelshoj, L.; Adamson, G.D.; Keckstein, J.; Taylor, H.S.; Abrao, M.S.; Bush, D.; Kiesel, L.; Tamimi, R.; Sharpe-Timms, K.L.; et al. World Endometriosis Society consensus on the classification of endometriosis. Hum. Reprod. 2016, 32, 315–324. [Google Scholar] [CrossRef]
- Adamson, G.D. Endometriosis classification: An update. Curr. Opin. Obstet. Gynecol. 2011, 23, 213–220. [Google Scholar] [CrossRef]
- Lee, S.Y.; Koo, Y.J.; Lee, D.H. Classification of endometriosis. Yeungnam Univ. J. Med. 2021, 38, 10–18. [Google Scholar] [CrossRef]
- Keckstein, J. The #Enzian classification for the diagnosis and surgery of endometriosis: A narrative review. Gynecol. Pelvic Med. 2021, 4, 40. [Google Scholar] [CrossRef]
- Tomassetti, C.; Johnson, N.P.; Petrozza, J.; Abrao, M.S.; Einarsson, J.I.; Horne, A.W.; Lee, T.T.M.; Missmer, S.; Vermeulen, N.; Zondervan, K.T.; et al. An international terminology for endometriosis, 2021. Hum. Reprod. Open 2021, 2021, hoab029. [Google Scholar] [CrossRef] [PubMed]
- Imperiale, L.; Nisolle, M.; Noël, J.C.; Fastrez, M. Three types of endometriosis: Pathogenesis, diagnosis, and treatment. State of the art. J. Clin. Med. 2023, 12, 994. [Google Scholar] [CrossRef]
- Lousse, J.C.; Van Langendonckt, A.; Defrère, S.; Gonzalez Ramos, R.; Colette, S.; Donnez, J. Peritoneal endometriosis is an inflammatory disease. Fertil. Steril. 2008, 90, 911–915. [Google Scholar] [CrossRef]
- Hoyle, A.T.; Puckett, Y. Endometrioma; StatPearls: Treasure Island, FL, USA, 2023. [Google Scholar] [PubMed]
- Brosens, I.A.; Puttemans, P.; Campo, R.; Gordts, S.; Gordts, S. Endometriomas—More careful examination in vivo and communication with the pathologist. Fertil. Steril. 2007, 88, 534. [Google Scholar] [CrossRef] [PubMed]
- Chatman, D.L.; Zbella, E.A. Pelvic peritoneal defects and endometriosis: Further observations. Fertil. Steril. 1986, 45, 711–714. [Google Scholar] [CrossRef] [PubMed]
- Tosti, C.; Pinzauti, S.; Santulli, P.; Chapron, C.; Petraglia, F. Pathogenetic mechanisms of deep infiltrating endometriosis. Reprod. Sci. 2015, 22, 1053–1059. [Google Scholar] [CrossRef]
- Koninckx, P.R.; Ussia, A.; Adamyan, L.; Wattiez, A.; Donnez, J. Deep endometriosis: Definition, diagnosis, and treatment. Fertil. Steril. 2012, 98, 564–571. [Google Scholar] [CrossRef]
- Ochoa Bernal, M.A.; Fazleabas, A.T. The known, the unknown and the future of the pathophysiology of endometriosis. Int. J. Mol. Sci. 2024, 25, 5815. [Google Scholar] [CrossRef]
- Lati, S.; Saridogan, E. Endometriosis, oocyte, and embryo quality. J. Clin. Med. 2023, 12, 4186. [Google Scholar] [CrossRef] [PubMed]
- Goud, P.T.; Goud, A.P.; Joshi, N.; Puscheck, E.; Diamond, M.P.; Abu-Soud, H.M. Dynamics of nitric oxide, altered follicular microenvironment, and oocyte quality in women with endometriosis. Fertil. Steril. 2014, 102, 151–159.e5. [Google Scholar] [CrossRef] [PubMed]
- Máté, G.; Bernstein, L.R.; Török, A.L. Endometriosis is a cause of infertility: Does reactive oxygen damage to gametes and embryos play a key role in the pathogenesis of infertility caused by endometriosis? Front. Endocrinol. 2018, 9, 725. [Google Scholar] [CrossRef]
- Carrillo, L.; Seidman, D.S.; Cittadini, E.; Meirow, D. The role of fertility preservation in patients with endometriosis. J. Assist. Reprod. Genet. 2016, 33, 317–323. [Google Scholar] [CrossRef]
- Simopoulou, M.; Rapani, A.; Grigoriadis, S.; Pantou, A.; Tsioulou, P.; Maziotis, E.; Tzanakaki, D.; Triantafyllidou, O.; Kalampokas, T.; Siristatidis, C.; et al. Getting to know endometriosis-related infertility better: A review on how endometriosis affects oocyte quality and embryo development. Biomedicines 2021, 9, 273. [Google Scholar] [CrossRef]
- Ferrero, H.; Corachán, A.; Aguilar, A.; Quiñonero, A.; Carbajo-García, M.C.; Alamá, P.; Tejera, A.; Taboas, E.; Muñoz, E.; Pellicer, A.; et al. Single-cell RNA sequencing of oocytes from ovarian endometriosis patients reveals a differential transcriptomic profile associated with lower quality. Hum. Reprod. 2019, 34, 1302–1312. [Google Scholar] [CrossRef]
- Da Broi, M.G.; Navarro, P.A. Oxidative stress and oocyte quality: Ethiopathogenic mechanisms of minimal/mild endometriosis-related infertility. Cell Tissue Res. 2016, 364, 1–7. [Google Scholar] [CrossRef]
- Kasapoglu, I.; Kuspinar, G.; Saribal, S.; Turk, P.; Avcı, B.; Uncu, G. Detrimental effects of endometriosis on oocyte morphology in intracytoplasmic sperm injection cycles: A retrospective cohort study. Gynecol. Endocrinol. 2017, 34, 206–211. [Google Scholar] [CrossRef] [PubMed]
- Balaban, B.; Urman, B. Effect of oocyte morphology on embryo development and implantation. Reprod. Biomed. Online 2006, 12, 608–615. [Google Scholar] [CrossRef]
- Alpha Scientists in Reproductive Medicine and ESHRE Special Interest Group of Embryology. The Istanbul consensus workshop on embryo assessment: Proceedings of an expert meeting. Hum. Reprod. 2011, 26, 1270–1283. [Google Scholar] [CrossRef]
- Robin, C.; Uk, A.; Decanter, C.; Behal, H.; Collinet, P.; Rubod, C.; Barbotin, A.-L.; Robin, G. Impact of endometriosis on oocyte morphology in IVF-ICSI: Retrospective study of a cohort of more than 6000 mature oocytes. Reprod. Biol. Endocrinol. 2021, 19, 160. [Google Scholar] [CrossRef]
- Metzemaekers, J.; Lust, E.; Rhemrev, J.; Van Geloven, N.; Twijnstra, A.; Van DerWesterlaken, L.; Jansen, F. Prognosis in fertilisation rate and outcome in IVF cycles in patients with and without endometriosis: A population-based comparative cohort study with controls. Hum. Reprod. Open 2021, 13, 27–34. [Google Scholar] [CrossRef]
- Hamdan, M.; Dunselman, G.; Li, T.C.; Cheong, Y. The impact of endometrioma on IVF/ICSI outcomes: A systematic review and meta-analysis. Hum. Reprod. Update 2015, 21, 809–825. [Google Scholar] [CrossRef] [PubMed]
- Invernici, D.; Reschini, M.; Benaglia, L.; Somigliana, E.; Galati, G.; La Vecchia, I.; Vercellini, P. The impact of endometriosis on IVF efficacy: Qualitative and quantitative assessment of ovarian response and embryo development. Reprod. Biomed. Online 2022, 45, 275–281. [Google Scholar] [CrossRef]
- Dongye, H.; Ji, X.; Ma, X.; Song, J.; Yan, L. The Impact of Endometriosis on Embryo Quality in in-vitro Fertilization/ intracytoplasmic Sperm Injection: A Systematic Review and Meta-Analysis. Front. Med. 2021, 8, 669342. [Google Scholar] [CrossRef]
- Magli, M.C.; Jones, G.M.; Lundin, K.; Abbeel, E.v.D. Preface. Hum. Reprod. 2012, 27, i1. [Google Scholar] [CrossRef]
- Matsuzaki, S.; Schubert, B. Oxidative stress status in normal ovarian cortex surrounding ovarian endometriosis. Fertil. Steril. 2010, 93, 2431–2432. [Google Scholar] [CrossRef] [PubMed]
- Prieto, L.; Quesada, J.F.; Cambero, O.; Pacheco, A.; Pellicer, A.; Codoceo, R.; Garcia-Velasco, J.A. Analysis of follicular fluid and serum markers of oxidative stress in women with infertility related to endometriosis. Fertil. Steril. 2012, 98, 126–130. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, A.M.; Papaleo, E.; Corti, L.; Santambrogio, P.; Levi, S.; Viganò, P.; Candiani, M.; Panina-Bordignon, P. Iron availability is increased in individual human ovarian follicles in close proximity to an endometrioma compared with distal ones. Hum. Reprod. 2014, 29, 577–583. [Google Scholar] [CrossRef]
- Sung, L.; Mukherjee, T.; Takeshige, T.; Bustillo, M.; Copperman, A.B. Endometriosis is not detrimental to embryo implantation in oocyte recipients. J. Assist. Reprod. Genet. 1997, 14, 152–156. [Google Scholar] [CrossRef] [PubMed]
- Simón, C.; Gutiérrez, A.; Vidal, A.; de los Santos, M.J.; Tarín, J.J.; Remohí, J.; Pellicer, A. Outcome of patients with endometriosis in assisted reproduction: Results from in-vitro fertilization and oocyte donation. Hum. Reprod. 1994, 9, 725–729. [Google Scholar] [CrossRef]
- Magli, M.C.; Gianaroli, L.; Ferraretti, A.P.; Lappi, M.; Ruberti, A.; Farfalli, V. Embryo morphology and development are dependent on the chromosomal complement. Fertil. Steril. 2007, 87, 534–541. [Google Scholar] [CrossRef]
- Schippert, C.; Witte, Y.; Bartels, J.; Garcia-Rocha, G.J.; Jentschke, M.; Hillemanns, P.; Kundu, S. Reproductive capacity and recurrence of disease after surgery for moderate and severe endometriosis—A retrospective single center analysis. BMC Women’s Health 2020, 20, 144. [Google Scholar] [CrossRef]
- Yang, C.; Geng, Y.; Li, Y.; Chen, C.; Gao, Y. Impact of ovarian endometrioma on ovarian responsiveness and IVF: A systematic review and meta-analysis. Reprod. Biomed. Online 2015, 31, 9–19. [Google Scholar] [CrossRef]
- Filippi, F.; Benaglia, L.; Paffoni, A.; Restelli, L.; Vercellini, P.; Somigliana, E.; Fedele, L. Ovarian endometriomas and oocyte quality: Insights from in vitro fertilization cycles. Fertil. Steril. 2014, 101, 988–993.e1. [Google Scholar] [CrossRef] [PubMed]
- Demirel, C.; Bastu, E.; Aydogdu, S.; Donmez, E.; Benli, H.; Tuysuz, G.; Keskin, G.; Buyru, F. The Presence of Endometrioma Does Not Impair Time-Lapse Morphokinetic Parameters and Quality of Embryos: A Study On Sibling Oocytes. Reprod. Sci. 2016, 23, 1053–1057. [Google Scholar] [CrossRef]
- Seraji, S.; Ali, A.; Demirel, E.; Akerman, M.; Nezhat, C.; Nezhat, F.R. Association between ovarian endometriomas and stage of endometriosis. J. Clin. Med. 2024, 13, 4530. [Google Scholar] [CrossRef]
- Esmaeilzadeh, S.; Ghorbani, M.; Abdolahzadeh, M.; Chehrazi, M.; Jorsaraei, S.G.; Mirabi, P. Stages of endometriosis: Does it affect oocyte quality, embryo development and fertilization rate? JBRA Assist. Reprod. 2022, 26, 620. [Google Scholar] [CrossRef]
- Paffoni, A.; Bolis, V.; Ferrari, S.; Benaglia, L.; Vercellini, P.; Somigliana, E. The Gametotoxic Effects of the Endometrioma Content: Insights From a Parthenogenetic Human Model. Reprod. Sci. 2019, 26, 573–579. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, A.M.; Pagliardini, L.; Cermisoni, G.C.; Privitera, L.; Makieva, S.; Alteri, A.; Corti, L.; Rabellotti, E.; Candiani, M.; Viganò, P. Does Endometriosis Influence the Embryo Quality and/or Development? Insights from a Large Retrospective Matched Cohort Study. Diagnostics 2020, 10, 83. [Google Scholar] [CrossRef]
- Jozwik, M.; Miłobędzka, M.; Wojtkiewicz, J.; Neymeyer, J.; Jakimiuk, A.; Jozwik, M. Deep Infiltrating Endometriosis of the Left Ureter Managed with Laparoscopic Ureterolysis Combined with Allium Ureteral Self-Expandable Stent: A Case Report. J. Clin. Med. 2024, 13, 6769. [Google Scholar] [CrossRef]
- Ducreux, B.; Patrat, C.; Trasler, J.; Fauque, P. Transcriptomic integrity of human oocytes used in ARTs: Technical and intrinsic factor effects. Hum. Reprod. Update 2024, 30, 26–47. [Google Scholar] [CrossRef]
- Alshehre, S.M.; Narice, B.F.; Fenwick, M.A.; Metwally, M. The impact of endometrioma on in vitro fertilisation/intra-cytoplasmic injection IVF/ICSI reproductive outcomes: A systematic review and meta-analysis. Arch. Gynecol. Obstet. 2020, 303, 3–16. [Google Scholar] [CrossRef]
- Juneau, C.; Kraus, E.; Werner, M.; Franasiak, J.; Morin, S.; Patounakis, G.; Molinaro, T.; de Ziegler, D.; Scott, R.T. Patients with endometriosis have aneuploidy rates equivalent to their age-matched peers in the in vitro fertilization population. Fertil. Steril. 2017, 108, 284–288. [Google Scholar] [CrossRef]
- Bishop, L.A.; Gunn, J.; Jahandideh, S.; Devine, K.; Decherney, A.H.; Hill, M.J. Endometriosis does not impact live-birth rates in frozen embryo transfers of euploid blastocysts. Fertil. Steril. 2021, 115, 416–422. [Google Scholar] [CrossRef] [PubMed]
- Guerin, P.; El Mouatassim, S.; Menezo, Y. Oxidative stress and protection against reactive oxygen species in the pre-implantation embryo and its surroundings. Hum. Reprod. Update 2001, 7, 175–189. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, L.F.P.; Samadder, A.N.; Agarwal, A.; Fernandes, L.F.C.; Abrão, M.S. Oxidative Stress Biomarkers in Patients with Endometriosis: Systematic Review. Arch. Gynecol. Obstet. 2012, 286, 1033–1040. [Google Scholar] [CrossRef] [PubMed]
- Ngô, C.; Chéreau, C.; Nicco, C.; Weill, B.; Chapron, C.; Batteux, F. Reactive Oxygen Species Controls Endometriosis Progression. Am. J. Pathol. 2009, 175, 225–234. [Google Scholar] [CrossRef]
- Scutiero, G.; Iannone, P.; Bernardi, G.; Bonaccorsi, G.; Spadaro, S.; Volta, C.A.; Greco, P.; Nappi, L. Oxidative stress and endometriosis: A systematic review of the literature. Oxidative Med. Cell. Longev. 2017, 2017, 7265238. [Google Scholar] [CrossRef]
- Huang, L.; Shi, L.; Li, M.; Yin, X.; Ji, X. Oxidative stress in endometriosis: Sources, mechanisms and therapeutic potential of antioxidants. Int. J. Mol. Med. 2025, 55, 72. [Google Scholar] [CrossRef]
- Alizadeh, M.; Mahjoub, S.; Esmaelzadeh, S.; Hajian, K.; Basirat, Z.; Ghasemi, M. Evaluation of Oxidative Stress in Endometriosis: A Case-Control Study. Casp. J. Intern. Med. 2015, 6, 25–29. [Google Scholar] [PubMed] [PubMed Central]
- Defrère, S.; Lousse, J.C.; Gonzalez-Ramos, R.; Colette, S.; Donnez, J.V.; Van Langendonckt, A.J. Potential involvement of iron in the pathogenesis of peritoneal endometriosis. Mol. Hum. Reprod. 2008, 14, 377–385. [Google Scholar] [CrossRef]
- Younis, J.S.; Taylor, H.S. The impact of ovarian endometrioma and endometriotic cystectomy on anti-Müllerian hormone, and antral follicle count: A contemporary critical appraisal of systematic reviews. Front. Endocrinol. 2024, 15, 1397279. [Google Scholar] [CrossRef] [PubMed]
- Szczepańska, M.; Koźlik, J.; Skrzypczak, J.; Mikołajczyk, M. Oxidative Stress May Be a Piece in the Endometriosis Puzzle. Fertil. Steril. 2003, 79, 1288–1293. [Google Scholar] [CrossRef]
- Agarwal, A.; Gupta, S.; Sharma, R.K. Role of Oxidative Stress in Female Reproduction. Reprod. Biol. Endocrinol. RBE 2005, 3, 28–48. [Google Scholar] [CrossRef] [PubMed]
- Amreen, S.; Kumar, P.; Gupta, P.; Rao, P. Evaluation of Oxidative Stress and Severity of Endometriosis. J. Hum. Reprod. Sci. 2019, 12, 40–46. [Google Scholar] [CrossRef]
- Richards, J.S.; Pangas, S.A. New insights into ovarian function. In Fertility Control; Springer: Berlin/Heidelberg, Germany, 2010; pp. 3–27. [Google Scholar] [CrossRef]
- Sugino, N. Reactive oxygen species in ovarian physiology. Reprod. Med. Biol. 2005, 4, 31–44. [Google Scholar] [CrossRef]
- Turrens, J.F. Mitochondrial formation of reactive oxygen species. J. Physiol. 2003, 552, 335–344. [Google Scholar] [CrossRef]
- Luberda, Z. The role of glutathione in mammalian gametes. Reprod. Biol. 2005, 5, 5–17. [Google Scholar] [PubMed]
- Shaeib, F.; Khan, S.N.; Ali, I.; Thakur, M.; Saed, M.G.; Dai, J.; Awonuga, A.O.; Banerjee, J.; Abu-Soud, H.M. The defensive role of cumulus cells against reactive oxygen species insultin metaphase II mouse oocytes. Reprod. Sci. 2016, 23, 498–507. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Wang, L. Oxidative stress in oocytes and embryo development: Implications for in vitro systems. Antioxid. Redox Signal. 2021, 34, 1394–1406. [Google Scholar] [CrossRef] [PubMed]
- Lim, M.; Brown, H.M.; Kind, K.L.; Thompson, J.G.; Dunning, K.R. Hemoglobin: Potential roles in the oocyte and early embryo. Biol. Reprod. 2019, 101, 262–270. [Google Scholar] [CrossRef]
- Attaran, M.; Pasqualotto, E.; Falcone, T.; Goldberg, J.M.; Miller, K.F.; Agarwal, A.; Sharma, R.K. The effect of follicular fluid reactive oxygen species on the outcome of in vitro fertilization. Int. J. Fertil. Women’s Med. 2000, 45, 314–320. [Google Scholar] [PubMed]
- Devine, P.J.; Perreault, S.D.; Luderer, U. Roles of reactive oxygen species and antioxidants in ovarian toxicity. Biol. Reprod. 2012, 86, 1–10. [Google Scholar] [CrossRef]
- Behrman, H.R.; Kodaman, P.H.; Preston, S.L.; Gao, S. Oxidative stress and the ovary. J. Soc. Gynecol. Investig. 2001, 8 (Suppl. S1), S40–S42. [Google Scholar] [CrossRef]
- Shkolnik, K.; Tadmor, A.; Ben-Dor, S.; Nevo, N.; Galiani, D.; Dekel, N. Reactive oxygen species are indispensable in ovulation. Proc. Natl. Acad. Sci. USA 2011, 108, 1462–1467. [Google Scholar] [CrossRef]
- Ng, K.Y.; Mingels, R.; Morgan, H.; Macklon, N.; Cheong, Y. In vivo oxygen, temperature and pH dynamics in the female reproductive tract and their importance in human conception: A systematic review. Hum. Reprod. Update 2018, 24, 15–34. [Google Scholar] [CrossRef]
- Ufer, C.; Wang, C.C.; Borchert, A.; Heydeck, D.; Kuhn, H. Redox control in mammalian embryo development. Antioxid. Redox Signal. 2010, 13, 833–875. [Google Scholar] [CrossRef]
- Rahman, I.; Marwick, J.; Kirkham, P. Redox modulation of chromatin remodeling: Impact on histone acetylation and deacetylation, NF-κB and pro-inflammatory gene expression. Biochem. Pharmacol. 2004, 68, 1255–1267. [Google Scholar] [CrossRef]
- Weitzman, S.A.; Turk, P.W.; Milkowski, D.H.; Kozlowski, K. Free radical adducts induce alterations in DNA cytosine methylation. Proc. Natl. Acad. Sci. USA 1994, 91, 1261–1264. [Google Scholar] [CrossRef]
- Malvasi, A.; Baldini, D. (Eds.) Pick up and Oocyte Management; Springer: Berlin/Heidelberg, Germany, 2019. [Google Scholar]
- Lane, M.; Gardner, D.K. Embryo culture medium: Which is the best? Best Pract. Res. Clin. Obstet. Gynaecol. 2007, 21, 83–100. [Google Scholar] [CrossRef]
- Nazari, S.; Khalili, M.A.; Esmaielzadeh, F.; Mohsenzadeh, M. Maturation capacity, morphology and morphometric assessment of human immature oocytes after vitrification and in vitro maturation. Iran. J. Reprod. Med. 2011, 9, 209–216. [Google Scholar]
- Lin, H.; Chen, H.; Chang, Y.; Au, K.; Tzeng, R.; Huang, H. Chronic niche inflammation in endometriosis-associated infertility: Current understanding and future therapeutic strategies. Int. J. Mol. Sci. 2018, 19, 2385. [Google Scholar] [CrossRef]
- Hever, A.; Roth, R.B.; Hevezi, P.; Marin, M.E.; Acosta, J.A.; Acosta, H.; Rojas, J.; Herrera, R.; Grigoriadis, D.; White, E.; et al. Human endometriosis is associated with plasma cells and overexpression of B lymphocyte stimulator. Proc. Natl. Acad. Sci. USA 2007, 104, 12451–12456. [Google Scholar] [CrossRef] [PubMed]
- Mohammed Rasheed, H.A.; Hamid, P. Inflammation to infertility: Panoramic view on endometriosis. Cureus 2020, 12, e11516. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.E.; Ahn, S.; Monsanto, S.P.; Khalaj, K.; Koti, M.; Tayade, C. Implications of immune dysfunction on endometriosis associated infertility. Oncotarget 2016, 7, 7138–7147. [Google Scholar] [CrossRef] [PubMed]
- de Ziegler, D.; Borghese, B.; Chapron, C. Endometriosis and infertility: Pathophysiology and management. Lancet 2010, 376, 730–738. [Google Scholar] [CrossRef]
- Wyse, B.A.; Salehi, R.; Russell, S.J.; Mereniuk, T.R.; Schultz, G.A.; Leader, A. Obesity and PCOS radically alters the snRNA composition of follicular fluid extracellular vesicles. Front. Endocrinol. 2023, 14, 1205385. [Google Scholar] [CrossRef]
- Ali, I.; Padhiar, A.A.; Wang, T.; He, L.; Chen, M.; Wu, S.; Zhou, Y.; Zhou, G. Stem cell-based therapeutic strategies for premature ovarian insufficiency and infertility: A focus on aging. Cells 2022, 11, 3713. [Google Scholar] [CrossRef]
- Jiang, Z.; Shen, H. Mitochondria: Emerging therapeutic strategies for oocyte rescue. Reprod. Sci. 2022, 29, 711–722. [Google Scholar] [CrossRef] [PubMed]
| Process | ROS Target | Downstream Effect |
|---|---|---|
| Meiosis | SOD2 (Superoxide Dismutase 2) | Superoxide increases in metaphase II oocytes, impacting oocyte quality and fostering pertinent aging |
| Embryogenesis | PRDX (peroxiredoxin) ½ | Loss of redox homeostasis |
| Imprinting | DNMT3A (DNA methyltransferase 3 alpha) | Abnormal methylation patterns |
| Parameter | Mild–Moderate Endometriosis | Severe Endometriosis (DIE/Endometriomas) | Non-Endometriosis Controls |
|---|---|---|---|
| Live Birth Rate Per Cycle | 22–28% | 12–18% | 30–35% |
| Oocytes Retrieved | Slightly reduced (8–12) | Significantly reduced (4–8) | 10–14 |
| Fertilization Rate | 70% | 60–65% | 75–80% |
| Blastocyst Formation | 35–45% | 25–35% | 50–55% |
| Euploid Embryo Rate | Comparable to controls | Comparable to controls | 50–60% (age-matched) |
| Implantation Rate | 20–25% | 12–18% | 25–30% |
| Optimal Protocol | Antagonist + standard FSH | Long GnRH agonist + high FSH | Standard protocols |
| Adjuvant Therapies | Optimal antioxidants | GnRH agonists (3–6 months) + antioxidants | Rarely needed |
| Cost Per Live Birth | USD 35,000 | USD 45,000 | USD 28,000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mrugacz, G.; Mospinek, A.; Modrzyńska-Olejniczak, M.; Byczkowski, B.; Radaj, E.; Olcha, P. In Vivo Shadows and In Vitro Light: The Early Embryological Journey Amid Endometriosis. Biology 2025, 14, 957. https://doi.org/10.3390/biology14080957
Mrugacz G, Mospinek A, Modrzyńska-Olejniczak M, Byczkowski B, Radaj E, Olcha P. In Vivo Shadows and In Vitro Light: The Early Embryological Journey Amid Endometriosis. Biology. 2025; 14(8):957. https://doi.org/10.3390/biology14080957
Chicago/Turabian StyleMrugacz, Grzegorz, Aleksandra Mospinek, Maria Modrzyńska-Olejniczak, Bartłomiej Byczkowski, Ewelina Radaj, and Piotr Olcha. 2025. "In Vivo Shadows and In Vitro Light: The Early Embryological Journey Amid Endometriosis" Biology 14, no. 8: 957. https://doi.org/10.3390/biology14080957
APA StyleMrugacz, G., Mospinek, A., Modrzyńska-Olejniczak, M., Byczkowski, B., Radaj, E., & Olcha, P. (2025). In Vivo Shadows and In Vitro Light: The Early Embryological Journey Amid Endometriosis. Biology, 14(8), 957. https://doi.org/10.3390/biology14080957








