Blue Light Effect on Metabolic Changes in Induced Precocious Puberty in Rats
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
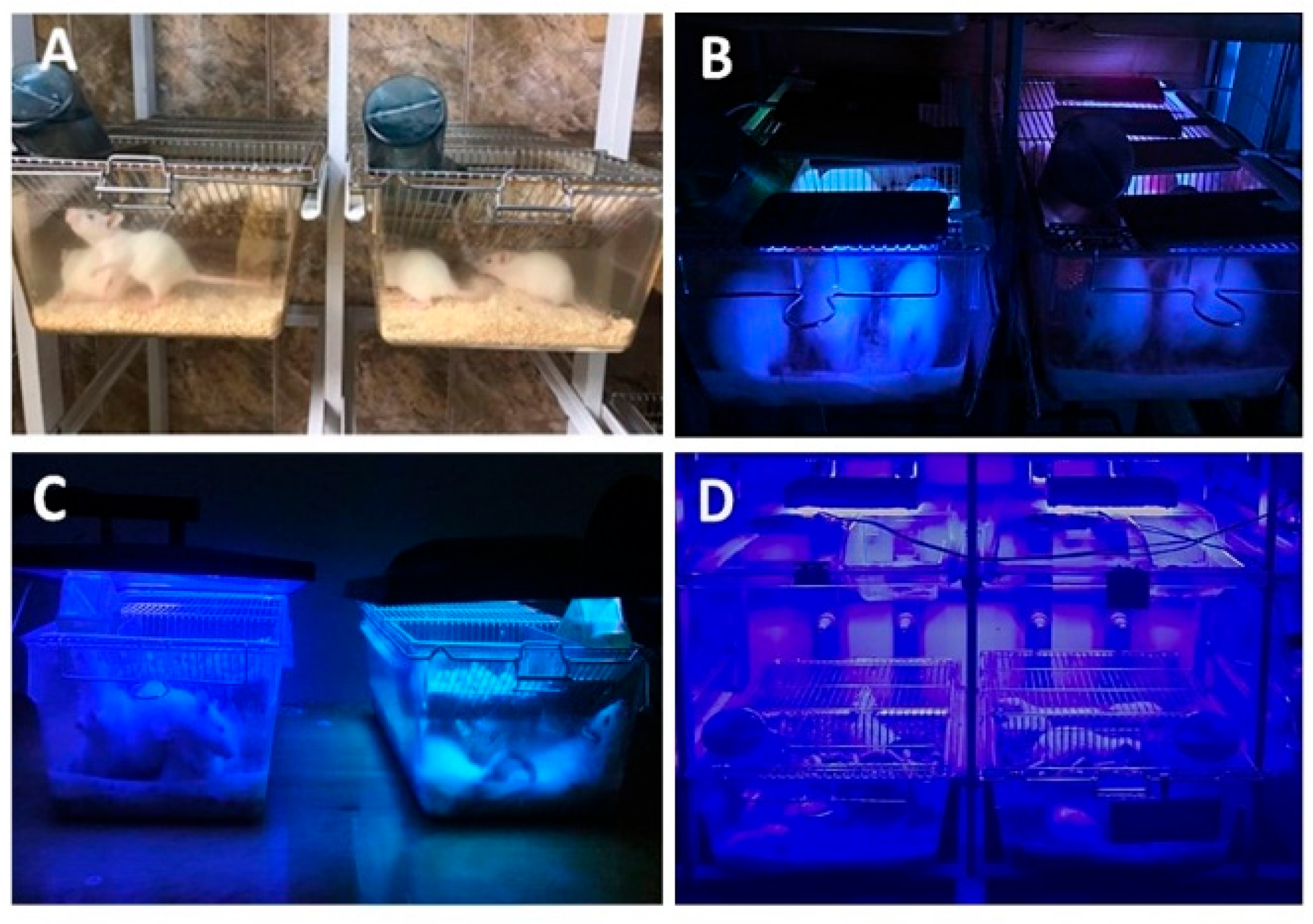
2.2. Experimental Design—LED Exposure Conditions
2.3. Body Weight Measurements
2.4. Sample Collection
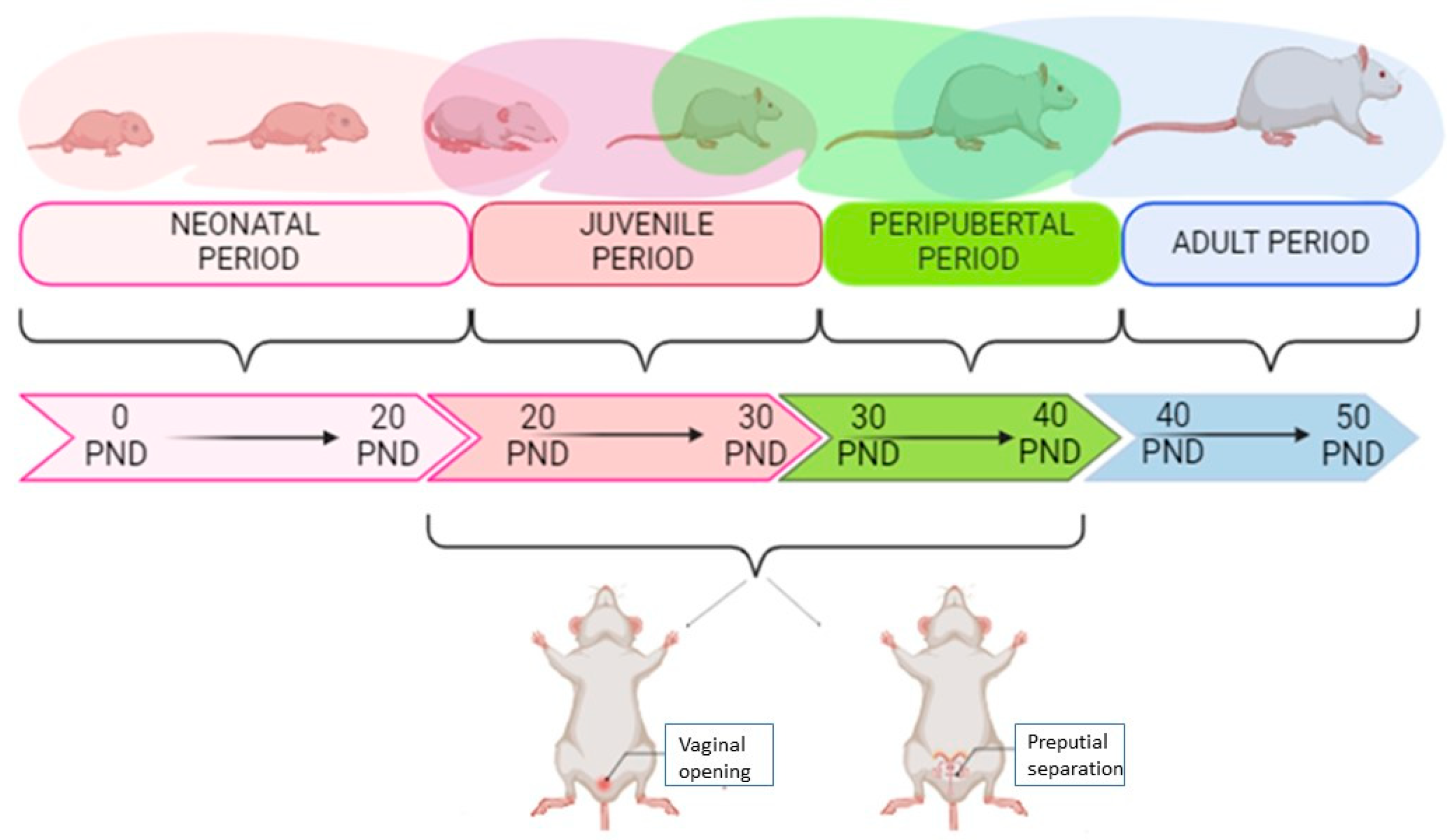
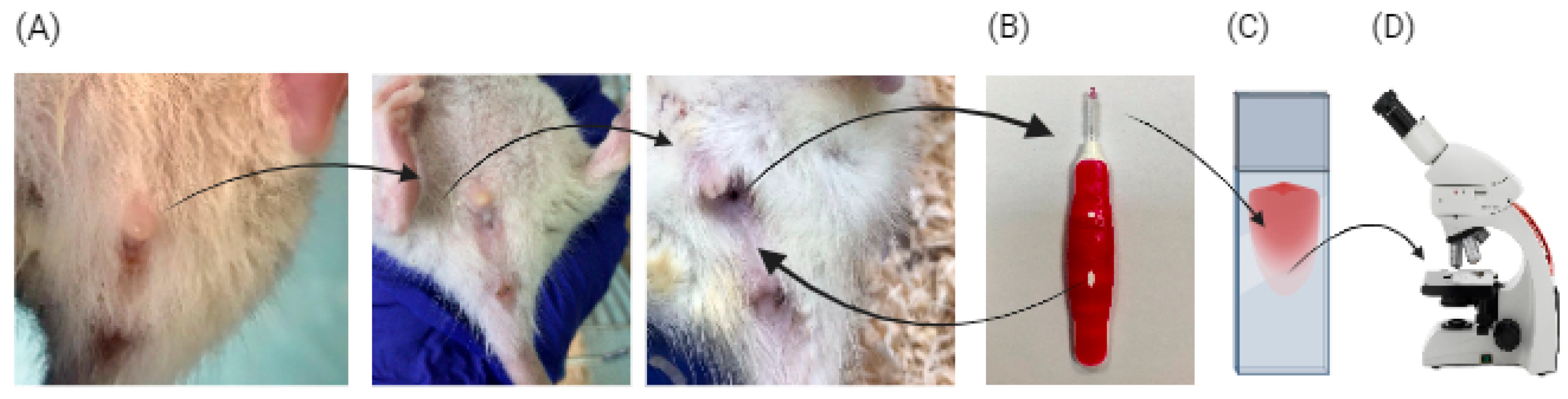
2.5. Puberty Assessment
2.6. Estrous Cycle Assessment
2.7. Assessment of Estrous Cycle
2.8. Development of the Machine Learning Algorithm to Assess Estrous Cycle
2.9. Biochemical Analysis
2.10. Leptin and Insulin Assessment
2.11. Statistical Analysis
3. Results
3.1. Blue Light Exposure Influence on Body Weight
3.2. Estrous Cycle Identification Algorithm
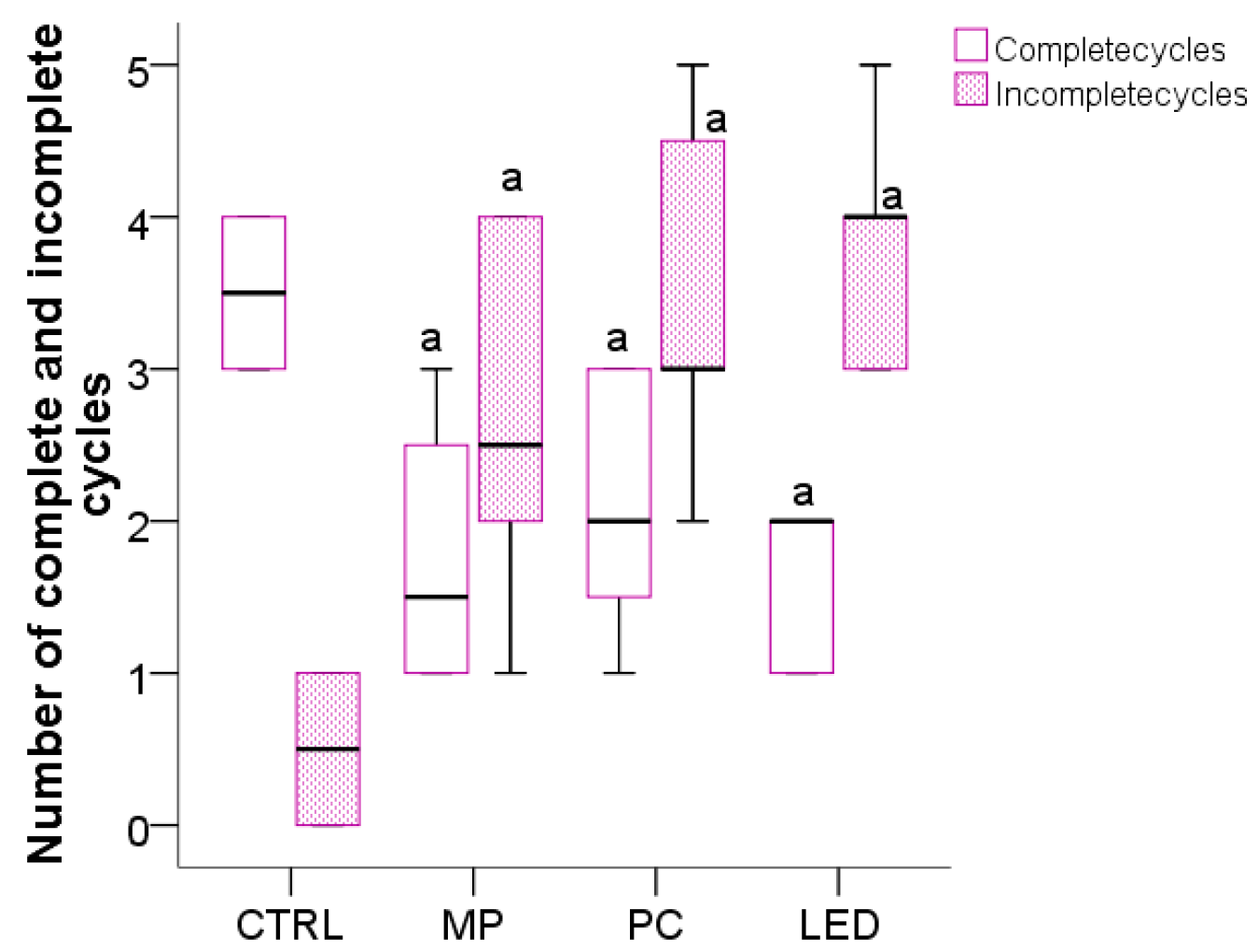
3.3. The Effect of Blue Light on the Cyclicity of the Estrous Cycle and Preputial Separation
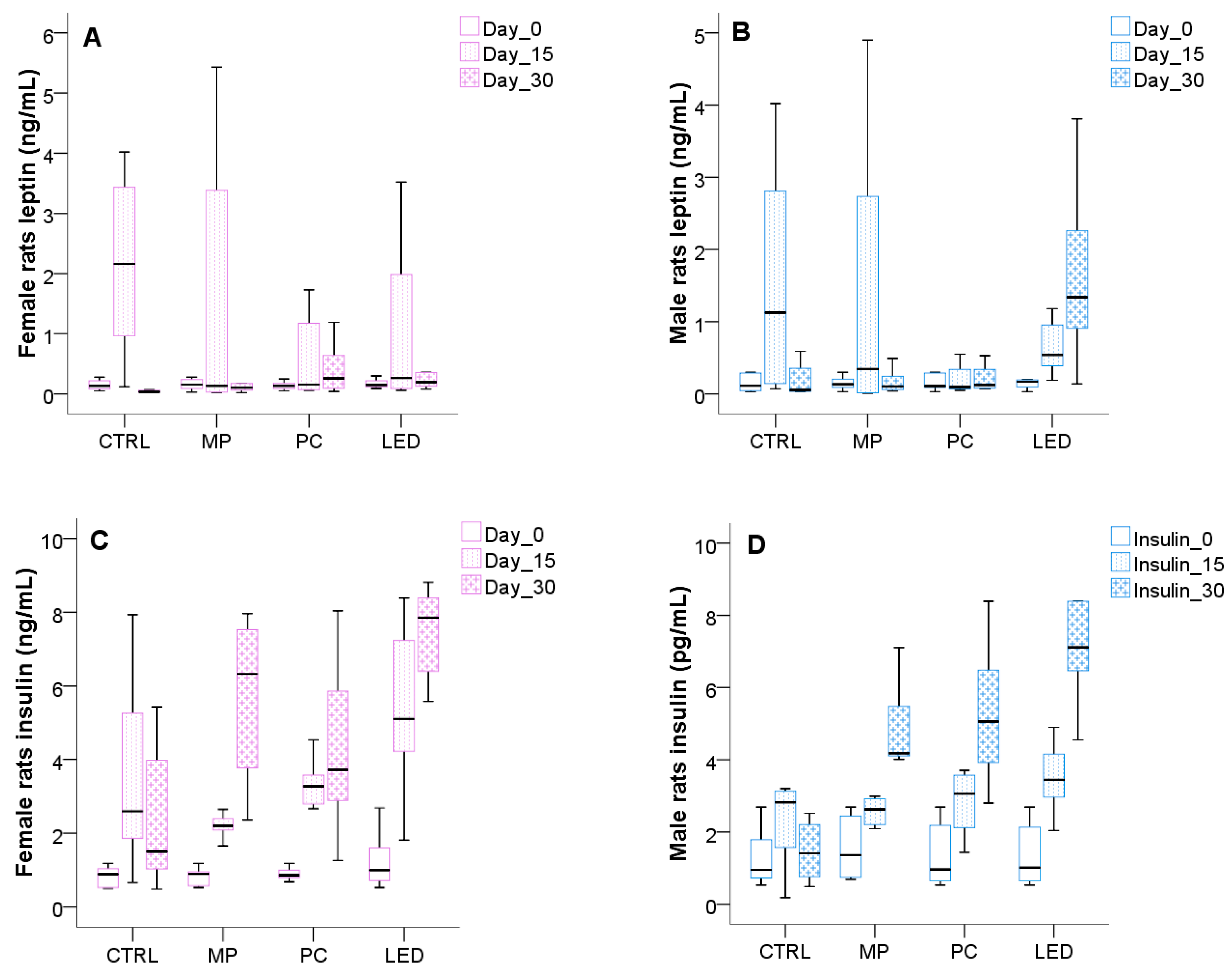
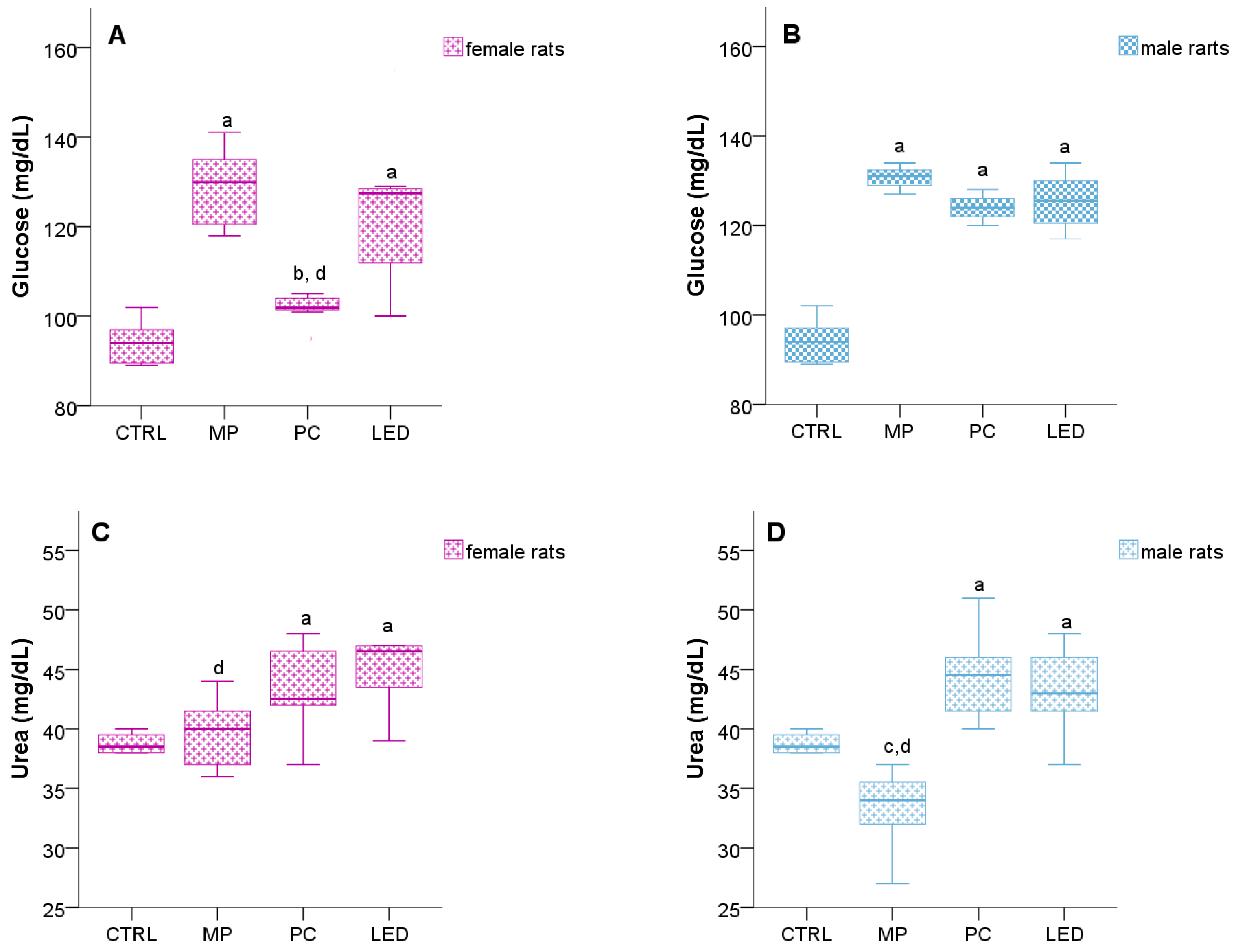
3.4. Light Effects on Metabolic Changes
4. Discussions
5. Study Limitation
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dominoni, D.M.; Borniger, J.C.; Nelson, R.J. Light at night, clocks and health: From humans to wild organisms. Biol. Lett. 2016, 12, 20160015. [Google Scholar] [CrossRef]
- Coomans, C.P.; van den Berg, S.A.A.; Houben, T.; van Klinken, J.B.; van den Berg, R.; Pronk, A.C.M.; Havekes, L.M.; Romijn, J.A.; van Dijk, K.W.; Biermasz, N.R.; et al. Detrimental effects of constant light exposure and high-fat diet on circadian energy metabolism and insulin sensitivity. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2013, 27, 1721–1732. [Google Scholar] [CrossRef]
- Aoyama, S.; Shibata, S. The Role of Circadian Rhythms in Muscular and Osseous Physiology and Their Regulation by Nutrition and Exercise. Front. Neurosci. 2017, 11, 63. [Google Scholar] [CrossRef] [PubMed]
- Chellappa, S.L.; Steiner, R.; Oelhafen, P.; Lang, D.; Götz, T.; Krebs, J.; Cajochen, C. Acute exposure to evening blue-enriched light impacts on human sleep. J. Sleep Res. 2013, 22, 573–580. [Google Scholar] [CrossRef]
- Panda, S.; Sato, T.K.; Castrucci, A.M.; Rollag, M.D.; DeGrip, W.J.; Hogenesch, J.B.; Provencio, I.; Kay, S.A. Melanopsin (Opn4) requirement for normal light-induced circadian phase shifting. Science 2002, 298, 2213–2216. [Google Scholar] [CrossRef]
- West, A.C.; Smith, L.; Ray, D.W.; Loudon, A.S.I.; Brown, T.M.; Bechtold, D.A. Misalignment with the external light environment drives metabolic and cardiac dysfunction. Nat. Commun. 2017, 8, 417. [Google Scholar] [CrossRef]
- Emmer, K.M.; Russart, K.L.G.; Walker, I.I.W.H.; Nelson, R.J.; DeVries, A.C. Effects of light at night on laboratory animals and research outcomes. Behav. Neurosci. 2018, 132, 302–314. [Google Scholar] [CrossRef]
- Wyse, C.A.; Selman, C.; Page, M.M.; Coogan, A.N.; Hazlerigg, D.G. Circadian desynchrony and metabolic dysfunction; did light pollution make us fat? Med. Hypotheses 2011, 77, 1139–1144. [Google Scholar] [CrossRef]
- Tsai, J.W.; Hannibal, J.; Hagiwara, G.; Colas, D.; Ruppert, E.; Ruby, N.F.; Heller, H.C.; Franken, P.; Bourgin, P. Melanopsin as a Sleep Modulator: Circadian Gating of the Direct Effects of Light on Sleep and Altered Sleep Homeostasis in Opn4−/− Mice. PLoS Biol. 2009, 7, e1000125. [Google Scholar] [CrossRef]
- Salgado-Delgado, R.; Angeles-Castellanos, M.; Saderi, N.; Buijs, R.M.; Escobar, C. Food intake during the normal activity phase prevents obesity and circadian desynchrony in a rat model of night work. Endocrinology 2010, 151, 1019–1029. [Google Scholar] [CrossRef]
- Alcocer, A.L. Central Precocious Puberty and Blue Screen Time in Rats—An Endocrinologist’s Thoughts on What This Might Mean for Pediatric Patients. Pediatr. Ann. 2023, 52, e1–e3. [Google Scholar] [CrossRef]
- Chang, A.M.; Aeschbach, D.; Duffy, J.F.; Czeisler, C.A. Evening use of light-emitting eReaders negatively affects sleep, circadian timing, and next-morning alertness. Proc. Natl. Acad. Sci. USA 2015, 112, 1232–1237. [Google Scholar] [CrossRef]
- Fobian, A.D.; Avis, K.; Schwebel, D.C. The Impact of Media Use on Adolescent Sleep Efficiency. J. Dev. Behav. Pediatr. JDBP 2016, 37, 9–14. [Google Scholar] [CrossRef]
- Granich, J.; Rosenberg, M.; Knuiman, M.W.; Timperio, A. Individual, social, and physical environment factors associated with electronic media use among children: Sedentary behavior at home. J. Phys. Act Health 2011, 8, 613–625. [Google Scholar] [CrossRef]
- Zimmet, P.; Alberti, K.G.M.M.; Stern, N.; Bilu, C.; El-Osta, A.; Einat, H.; Kronfeld-Schor, N. The Circadian Syndrome: Is the Metabolic Syndrome and much more! J. Intern. Med. 2019, 286, 181–191. [Google Scholar] [CrossRef]
- Plano, S.A.; Casiraghi, L.P.; García Moro, P.; Paladino, N.; Golombek, D.A.; Chiesa, J.J. Circadian and Metabolic Effects of Light: Implications in Weight Homeostasis and Health. Front. Neurol. 2017, 8, 558. [Google Scholar] [CrossRef]
- Eckel-Mahan, K.L.; Patel, V.R.; de Mateo, S.; Orozco-Solis, R.; Ceglia, N.J.; Sahar, S.; Dilag-Penilla, S.A.; Dyar, K.A.; Baldi, P.; Sassone-Corsi, P. Reprogramming of the circadian clock by nutritional challenge. Cell 2013, 155, 1464–1478. [Google Scholar] [CrossRef]
- Cheuiche, A.V.; da Silveira, L.G.; de Paula, L.C.P.; Lucena, I.R.S.; Silveiro, S.P. Diagnosis and management of precocious sexual maturation: An updated review. Eur. J. Pediatr. 2021, 180, 3073–3087. [Google Scholar] [CrossRef] [PubMed]
- Foster, D.L.; Jackson, L.M.; Padmanabhan, V. Programming of GnRH feedback controls timing puberty and adult reproductive activity. Mol. Cell. Endocrinol. 2006, 254–255, 109–119. [Google Scholar] [CrossRef]
- Parent, A.S.; Rasier, G.; Gerard, A.; Heger, S.; Roth, C.; Mastronardi, C.; Jung, H.; Ojeda, S.R.; Bourguignon, J.P. Early onset of puberty: Tracking genetic and environmental factors. Horm. Res. 2005, 64 (Suppl. 2), 41–47. [Google Scholar] [CrossRef] [PubMed]
- Gamble, J. Puberty: Early starters. Nature 2017, 550, S10–S11. [Google Scholar] [CrossRef]
- MAYO FISKE, V. Effect of light on sexual maturation, estrous cycles, and anterior pituitary of the rat. Endocrinology 1941, 29, 187–196. [Google Scholar] [CrossRef]
- Campbell, C.S.; Schwartz, N.B. The impact of constant light on the estrous cycle of the rat. Endocrinology 1980, 106, 1230–1238. [Google Scholar] [CrossRef]
- Kato, M.; Yano, K.; Matsuo, F.; Saito, H.; Katagiri, T.; Kurumizaka, H.; Yoshimoto, M.; Kasumi, F.; Akiyama, F.; Sakamoto, G.; et al. Identification of Rad51 alteration in patients with bilateral breast cancer. J. Hum. Genet. 2000, 45, 133–137. [Google Scholar] [CrossRef]
- Cora, M.C.; Kooistra, L.; Travlos, G. Vaginal Cytology of the Laboratory Rat and Mouse: Review and Criteria for the Staging of the Estrous Cycle Using Stained Vaginal Smears. Toxicol. Pathol. 2015, 43, 776–793. [Google Scholar] [CrossRef]
- Byers, S.L.; Wiles, M.V.; Dunn, S.L.; Taft, R.A. Mouse estrous cycle identification tool and images. PLoS ONE 2012, 7, e35538. [Google Scholar] [CrossRef]
- Gaytan, F.; Morales, C.; Leon, S.; Heras, V.; Barroso, A.; Avendaño, M.S.; Vazquez, M.J.; Castellano, J.M.; Roa, J.; Tena-Sempere, M. Development and validation of a method for precise dating of female puberty in laboratory rodents: The puberty ovarian maturation score (Pub-Score). Sci. Rep. 2017, 7, 46381. [Google Scholar] [CrossRef]
- Korenbrot, C.; Huhtaniemi, I.; Weiner, R. Preputial seperation as an external sign of pubertal development in the male rat. Biol. Reprod. 1977, 17, 298–303. [Google Scholar] [CrossRef]
- Kılınç Uğurlu, A.; Bideci, A.; Demirel, M.A.; Take Kaplanoğlu, G.; Dayanır, D.; Gülbahar, Ö.; Bulut, T.S.D.; Döğer, E.; Çamurdan, M.O. Effects of Blue Light on Puberty and Ovary in Female Rats. J. Clin. Res. Pediatr. Endocrinol. 2023, 15, 365–374. [Google Scholar] [CrossRef]
- Constantino, D.B.; Tonon, A.C.; de Oliveira, M.A.B.; Amando, G.R.; Freitas, J.J.; Xavier, N.B.; Ribeiro, R.J.; Idiart, M.; Hidalgo, M.P.L. Effects of lighting patterns in pubertal development and metabolism of female wistar rats. Physiol. Behav. 2022, 243, 113641. [Google Scholar] [CrossRef]
- Uğurlu, A.K.; Bideci, A.; Demirel, A.M.; Kaplanoğlu, G.T.; Dayanır, D.; Gülbahar, Ö.; Bulut, T.S.D.; Döğer, E.; Çamurdan, M.O. Is blue light exposure a cause of precocious puberty in male rats? Front. Endocrinol. 2023, 14, 1190445. [Google Scholar] [CrossRef] [PubMed]
- Dauchy, R.T.; Wren-Dail, M.A.; Hoffman, A.E.; Hanifin, J.P.; Warfield, B.; Brainard, G.C.; Hill, S.M.; Belancio, V.P.; Dauchy, E.M.; Blask, D.E. Effects of Daytime Exposure to Light from Blue-Enriched Light-Emitting Diodes on the Nighttime Melatonin Amplitude and Circadian Regulation of Rodent Metabolism and Physiology. Comp. Med. 2016, 66, 373–383. [Google Scholar]
- Pop, R.M.; Gherman, L.M.; Jianu, E.M.; Roșian Ștefan, H.; Onofrei, M.M.; Mocan, L.P.; Chedea, V.S.; Bocsan, I.C.; Apostu, D.; Todea, A.R.; et al. Inflammation and oxidative stress processes in induced precocious puberty in rats. Heliyon 2024, 10, e40962. [Google Scholar] [CrossRef]
- Marcondes, F.K.; Bianchi, F.J.; Tanno, A.P. Determination of the estrous cycle phases of rats: Some helpful considerations. Braz J. Biol. Rev. Brasleira Biol. 2002, 62, 609–614. [Google Scholar] [CrossRef]
- Goldman, J.M.; Murr, A.S.; Cooper, R.L. The rodent estrous cycle: Characterization of vaginal cytology and its utility in toxicological studies. Birth Defects Res. B Dev. Reprod. Toxicol. 2007, 80, 84–97. [Google Scholar] [CrossRef]
- Hassan, E.; Shams, M.Y.; Hikal, N.A.; Elmougy, S. The effect of choosing optimizer algorithms to improve computer vision tasks: A comparative study. Multimed. Tools Appl. 2023, 82, 16591–16633. [Google Scholar] [CrossRef]
- Kang, B.H.; Kim, S.H.; Jung, K.A.; Kim, S.Y.; Chung, S.H.; Park, Y.S.; Yoon, K.L.; Shim, K.S. Comparison of growth and pubertal progression in wild type female rats with different bedding types. Ann. Pediatr. Endocrinol. Metab. 2015, 20, 53. [Google Scholar] [CrossRef] [PubMed]
- Touitou, Y.; Reinberg, A.; Touitou, D. Association between light at night, melatonin secretion, sleep deprivation, and the internal clock: Health impacts and mechanisms of circadian disruption. Life Sci. 2017, 173, 94–106. [Google Scholar] [CrossRef]
- Schwartz, M.W.; Woods, S.C.; Porte, D.; Seeley, R.J.; Baskin, D.G. Central nervous system control of food intake. Nature 2000, 404, 661–671. [Google Scholar] [CrossRef] [PubMed]
- Perreau-Lenz, S.; Kalsbeek, A.; Garidou, M.L.; Wortel, J.; Van Der Vliet, J.; Van Heijningen, C.; Simonneaux, V.; Pévet, P.; Buijs, R.M. Suprachiasmatic control of melatonin synthesis in rats: Inhibitory and stimulatory mechanisms. Eur. J. Neurosci. 2003, 17, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Buijs, R.M.; Chun, S.J.; Niijima, A.; Romijn, H.J.; Nagai, K. Parasympathetic and sympathetic control of the pancreas: A role for the suprachiasmatic nucleus and other hypothalamic centers that are involved in the regulation of food intake. J. Comp. Neurol. 2001, 431, 405–423. [Google Scholar] [CrossRef] [PubMed]
- la Fleur, S.E.; Kalsbeek, A.; Wortel, J.; Buijs, R.M. Polysynaptic neural pathways between the hypothalamus, including the suprachiasmatic nucleus, and the liver. Brain Res. 2000, 871, 50–56. [Google Scholar] [CrossRef]
- Potter, G.D.M.; Skene, D.J.; Arendt, J.; Cade, J.E.; Grant, P.J.; Hardie, L.J. Circadian Rhythm and Sleep Disruption: Causes, Metabolic Consequences, and Countermeasures. Endocr. Rev. 2016, 37, 584–608. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.; Scheer, F.A.J.L. Circadian System and Glucose Metabolism: Implications for Physiology and Disease. Trends Endocrinol. Metab. TEM 2016, 27, 282–293. [Google Scholar] [CrossRef]
- Maury, E.; Hong, H.K.; Bass, J. Circadian disruption in the pathogenesis of metabolic syndrome. Diabetes Metab. 2014, 40, 338–346. [Google Scholar] [CrossRef]
- Schienkiewitz, A.; Truthmann, J.; Ernert, A.; Wiegand, S.; Schwab, K.O.; Scheidt-Nave, C. Age, maturation and serum lipid parameters: Findings from the German Health Survey for Children and Adolescents. BMC Public Health 2019, 19, 1627. [Google Scholar] [CrossRef]
- Jiang, M.; Gao, Y.; Wang, K.; Huang, L. Lipid profile in girls with precocious puberty: A systematic review and meta-analysis. BMC Endocr. Disord. 2023, 23, 225. [Google Scholar] [CrossRef] [PubMed]
- Obradovic, M.; Sudar-Milovanovic, E.; Soskic, S.; Essack, M.; Arya, S.; Stewart, A.J.; Gojobori, T.; Isenovic, E.R. Leptin and Obesity: Role and Clinical Implication. Front. Endocrinol. 2021, 12, 585887. [Google Scholar] [CrossRef] [PubMed]
- Harris, R.B.S. Direct and indirect effects of leptin on adipocyte metabolism. Biochim. Biophys. Acta BBA–Mol. Basis Dis. 2014, 1842, 414–423. [Google Scholar] [CrossRef]
- Minokoshi, Y.; Toda, C.; Okamoto, S. Regulatory role of leptin in glucose and lipid metabolism in skeletal muscle. Indian J. Endocrinol. Metab. 2012, 16 (Suppl. 3), S562–S568. [Google Scholar] [CrossRef]
- Elias, C.F. Leptin action in pubertal development: Recent advances and unanswered questions. Trends Endocrinol. Metab. TEM 2012, 23, 9–15. [Google Scholar] [CrossRef]
- Smith, J.; Waddell, B. Developmental changes in plasma leptin and hypothalamic leptin receptor expression in the rat : Peripubertal changes and the emergence of sex differences. J. Endocrinol. 2003, 176, 313–319. [Google Scholar] [CrossRef]
- Schwenzer, C.; Voelz, C.; Kogel, V.; Schlösser, A.; Herpertz-Dahlmann, B.; Beyer, C.; Seitz, J.; Trinh, S. Fear and food: Anxiety-like behavior and the susceptibility to weight loss in an activity-based anorexia rat model. Clin. Transl. Sci. 2022, 15, 889–898. [Google Scholar] [CrossRef]
- Xie, X.; Yang, H.; An, J.J.; Houtz, J.; Tan, J.W.; Xu, H.; Liao, G.Y.; Xu, Z.X.; Xu, B. Activation of Anxiogenic Circuits Instigates Resistance to Diet-Induced Obesity via Increased Energy Expenditure. Cell Metab. 2019, 29, 917–931.e4. [Google Scholar] [CrossRef]
- Allen, A.A.; Pierce, A.T.; Dauchy, R.T.; Voros, G.B.; Dobek, G.L. Influence of Light Phase Exposure to LED Lighting on Circadian Levels of Neuroendocrine Hormones in Sprague-Dawley Rats. J. Am. Assoc. Lab Anim. Sci. JAALAS 2022, 61, 333–343. [Google Scholar] [CrossRef]
- Masís-Vargas, A.; Hicks, D.; Kalsbeek, A.; Mendoza, J. Blue light at night acutely impairs glucose tolerance and increases sugar intake in the diurnal rodent Arvicanthis ansorgei in a sex-dependent manner. Physiol. Rep. 2019, 7, e14257. [Google Scholar] [CrossRef]
- Nagai, N.; Ayaki, M.; Yanagawa, T.; Hattori, A.; Negishi, K.; Mori, T.; Nakamura, T.J.; Tsubota, K. Suppression of Blue Light at Night Ameliorates Metabolic Abnormalities by Controlling Circadian Rhythms. Investig. Ophthalmol. Vis. Sci. 2019, 60, 3786–3793. [Google Scholar] [CrossRef]
- Seshadri, N.; Doucette, C.A. Circadian Regulation of the Pancreatic Beta Cell. Endocrinology 2021, 162, bqab089. [Google Scholar] [CrossRef]
- Bailey, M.; Silver, R. Sex differences in circadian timing systems: Implications for disease. Front. Neuroendocrinol. 2014, 35, 111–139. [Google Scholar] [CrossRef]
- Tramunt, B.; Smati, S.; Grandgeorge, N.; Lenfant, F.; Arnal, J.F.; Montagner, A.; Gourdy, P. Sex differences in metabolic regulation and diabetes susceptibility. Diabetologia 2020, 63, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Galea, L.A.; Wainwright, S.R.; Roes, M.M.; Duarte-Guterman, P.; Chow, C.; Hamson, D.K. Sex, hormones and neurogenesis in the hippocampus: Hormonal modulation of neurogenesis and potential functional implications. J. Neuroendocrinol. 2013, 25, 1039–1061. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Matsuo, R.; Kurogi, K.; Miyamoto, S.; Morita, T.; Shinozuka, M.; Taniguchi, F.; Ikegami, K.; Yasuo, S. Sex-dependent effects of chronic jet lag on circadian rhythm and metabolism in mice. Biol. Sex Differ. 2024, 15, 102. [Google Scholar] [CrossRef] [PubMed]
- Bedrosian, T.A.; Nelson, R.J. Timing of light exposure affects mood and brain circuits. Transl. Psychiatry 2017, 7, e1017. [Google Scholar] [CrossRef] [PubMed]




| Metrics | Diestrus | Estrus | Metestrus | Proestrus | Mean | |
|---|---|---|---|---|---|---|
| SqueezeNet | Accuracy | 0.9333 | 0.9333 | 0.8947 | 0.6657 | 0.8903 |
| Precision | 0.9032 | 0.8615 | 0.9107 | 0.8696 | 0.8863 | |
| Sensitivity | 0.9333 | 0.9333 | 0.8947 | 0.6667 | 0.9956 | |
| F1 score | 0.9180 | 0.8960 | 0.9027 | 0.7547 | 0.8679 | |
| ResNet50 | Accuracy | 1 | 1 | 0.9825 | 1 | 0.9956 |
| Precision | 0.989 | 1 | 1 | 1 | 0.9973 | |
| Sensitivity | 1 | 1 | 0.9825 | 1 | 0.9956 | |
| F1 score | 0.9945 | 1 | 0.9912 | 1 | 0.9964 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gherman, L.-M.; Jianu, E.-M.; Roșian, Ș.H.; Onofrei, M.M.; Mocan, L.P.; Chedea, V.S.; Bocsan, I.C.; Apostu, D.; Todea, A.R.; Dulf, E.H.; et al. Blue Light Effect on Metabolic Changes in Induced Precocious Puberty in Rats. Biology 2025, 14, 951. https://doi.org/10.3390/biology14080951
Gherman L-M, Jianu E-M, Roșian ȘH, Onofrei MM, Mocan LP, Chedea VS, Bocsan IC, Apostu D, Todea AR, Dulf EH, et al. Blue Light Effect on Metabolic Changes in Induced Precocious Puberty in Rats. Biology. 2025; 14(8):951. https://doi.org/10.3390/biology14080951
Chicago/Turabian StyleGherman, Luciana-Mădălina, Elena-Mihaela Jianu, Ștefan Horia Roșian, Mădălin Mihai Onofrei, Lavinia Patricia Mocan, Veronica Sanda Chedea, Ioana Corina Bocsan, Dragoş Apostu, Andreea Roxana Todea, Eva Henrietta Dulf, and et al. 2025. "Blue Light Effect on Metabolic Changes in Induced Precocious Puberty in Rats" Biology 14, no. 8: 951. https://doi.org/10.3390/biology14080951
APA StyleGherman, L.-M., Jianu, E.-M., Roșian, Ș. H., Onofrei, M. M., Mocan, L. P., Chedea, V. S., Bocsan, I. C., Apostu, D., Todea, A. R., Dulf, E. H., Mogoșan, E. L., Mihu, C. M., Crişan, C. A., Vesa, Ș. C., Buzoianu, A. D., & Pop, R. M. (2025). Blue Light Effect on Metabolic Changes in Induced Precocious Puberty in Rats. Biology, 14(8), 951. https://doi.org/10.3390/biology14080951











