Effects of Hypoxia and Reoxygenation on Hypoxia-Responsive Genes, Physiological and Biochemical Indices in Hybrid Catfish (Pelteobagrus vachelli ♀ × Leiocassis longirostris ♂)
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Hypoxic Stress Treatment
2.3. Temporal Gene Expression Analysis
2.4. Enzyme Activity Assay
2.5. Data Processing
3. Results
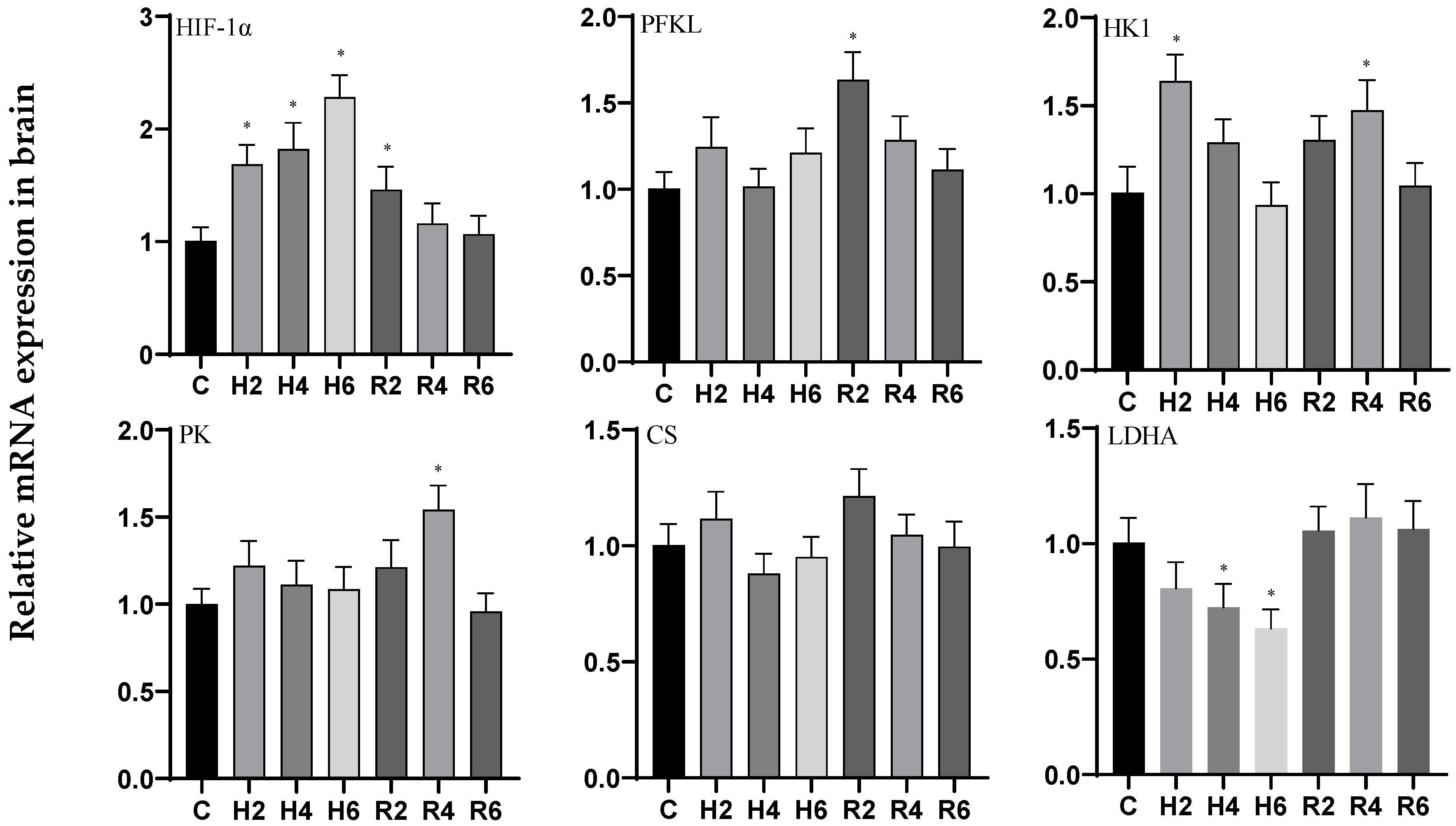
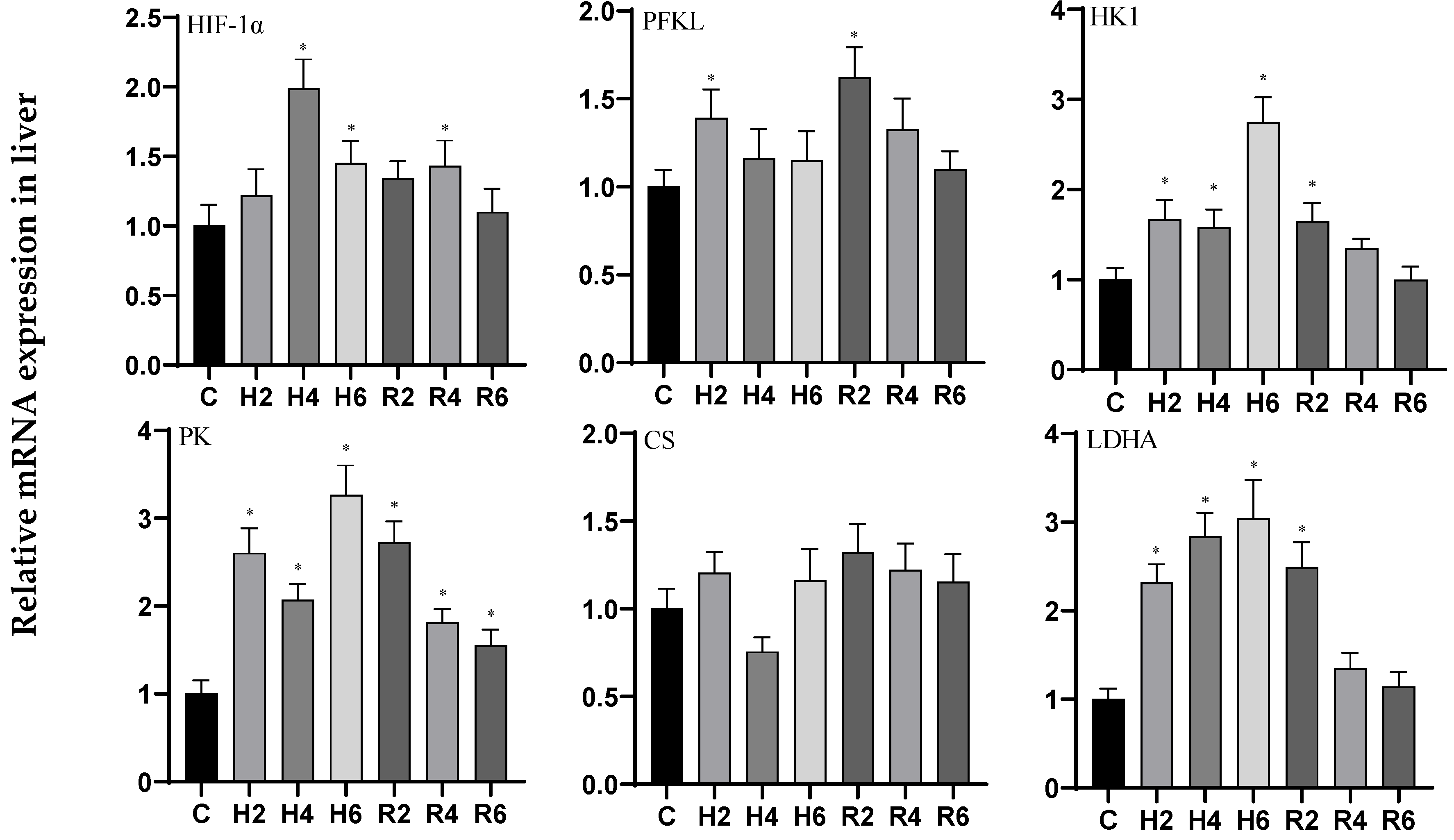
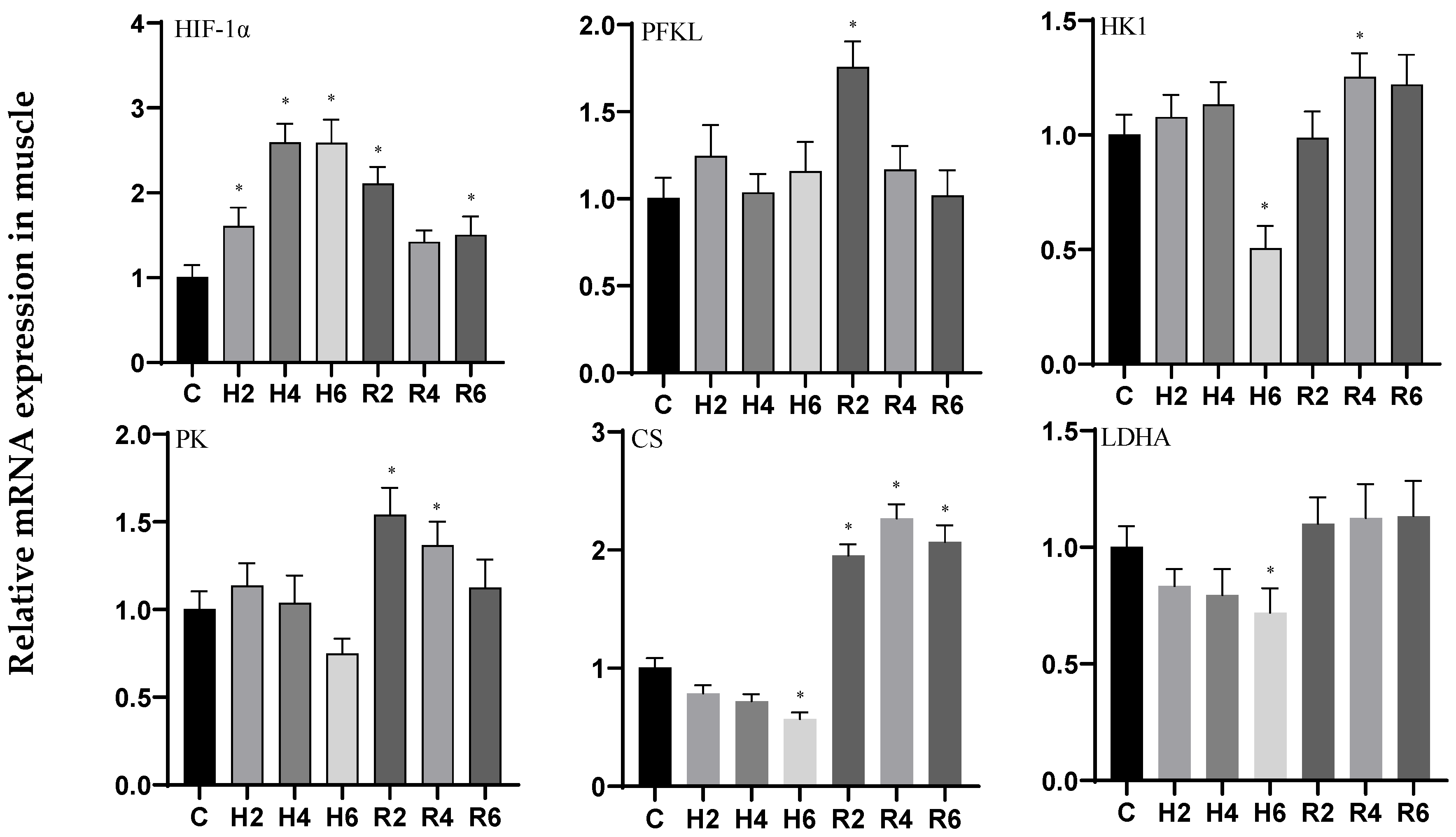
3.1. Effects of Hypoxia and Reoxygenation on Oxygen-Sensing Proteins and Respiratory Metabolism-Related Genes in Hybrid Catfish
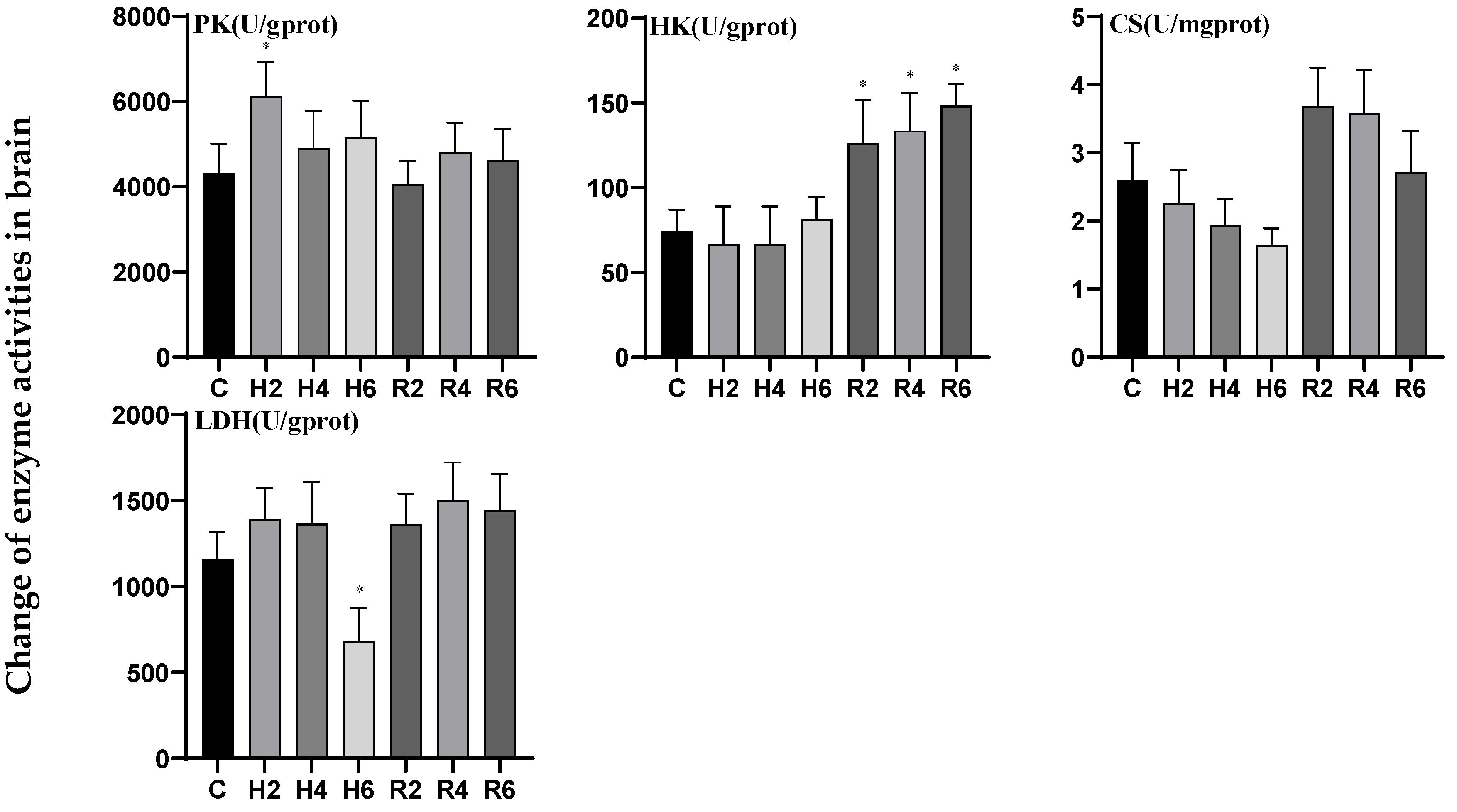
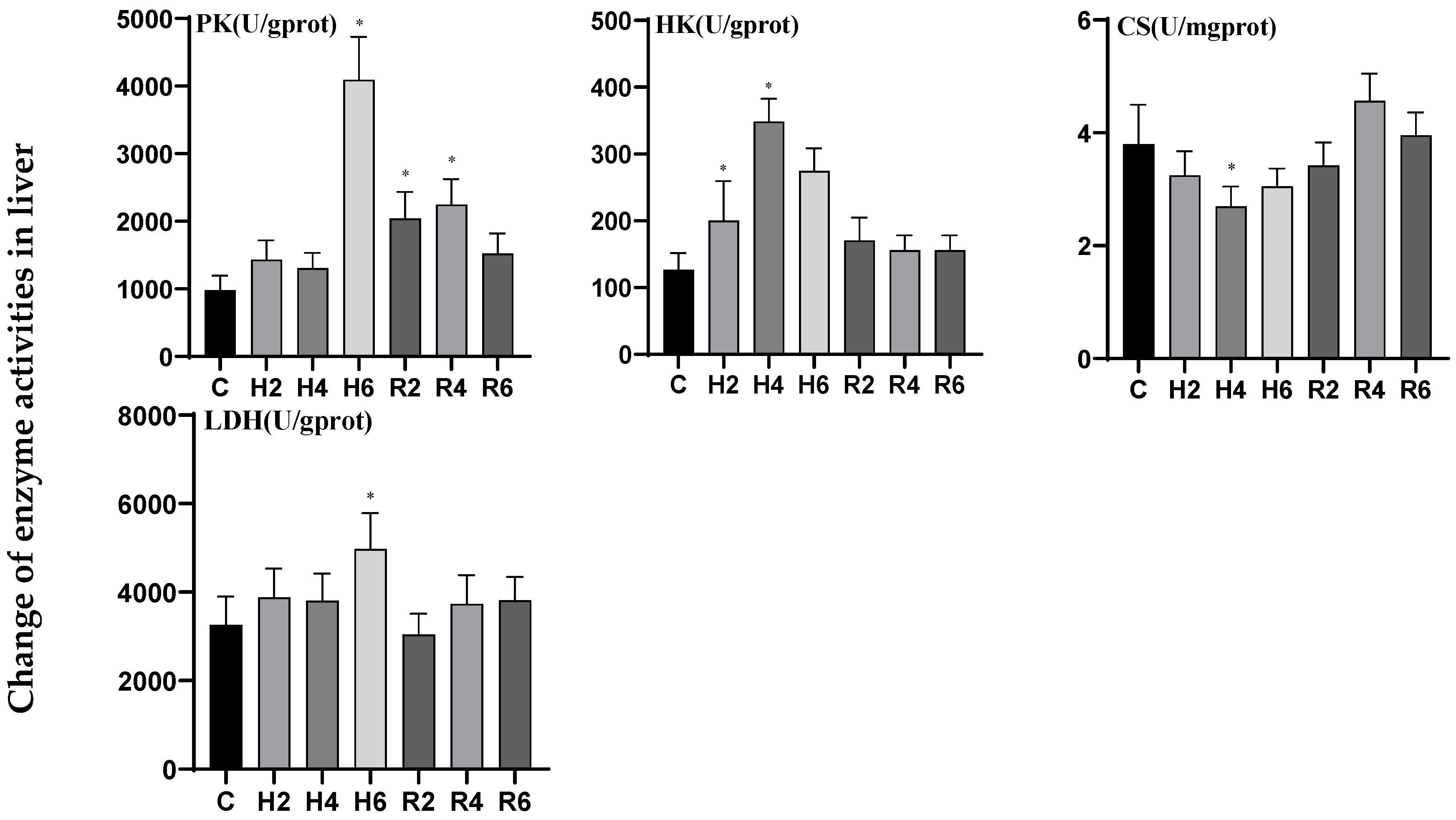
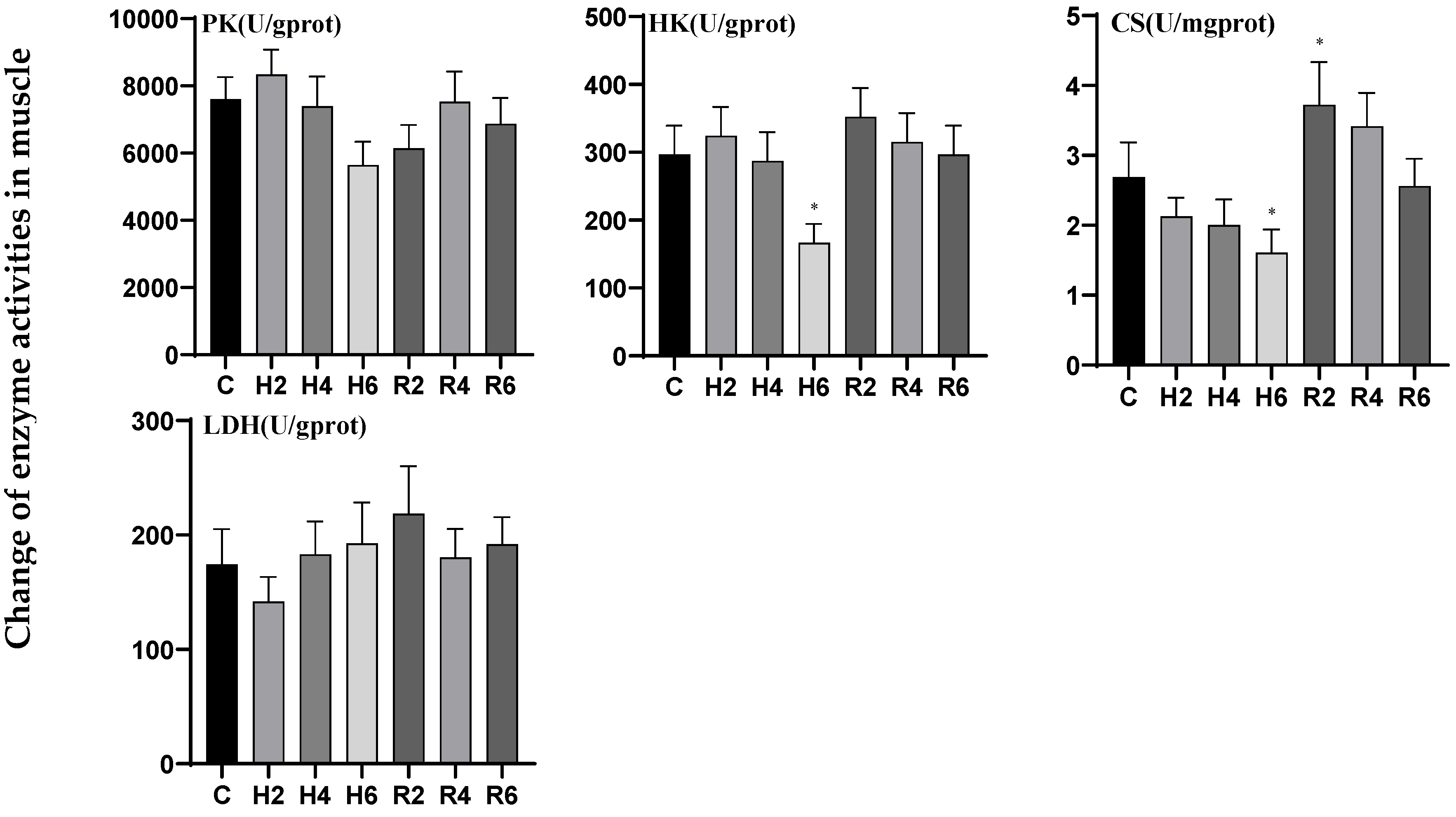
3.2. Effects of Hypoxia and Reoxygenation on Respiratory Metabolism-Related Enzyme Activities in Hybrid Catfish
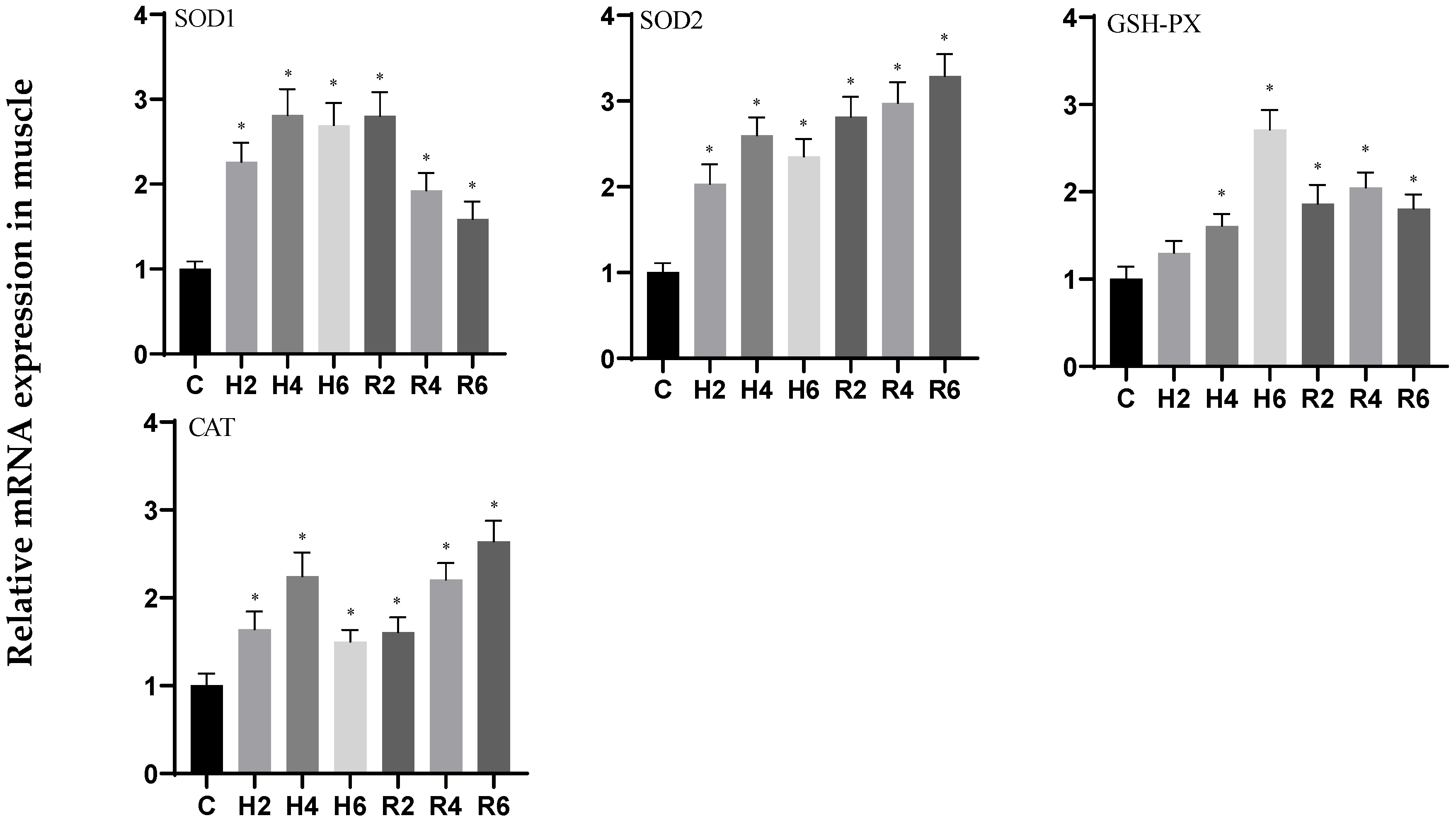
3.3. Effects of Hypoxia and Reoxygenation on Oxidative Stress-Related Genes in Hybrid Catfish
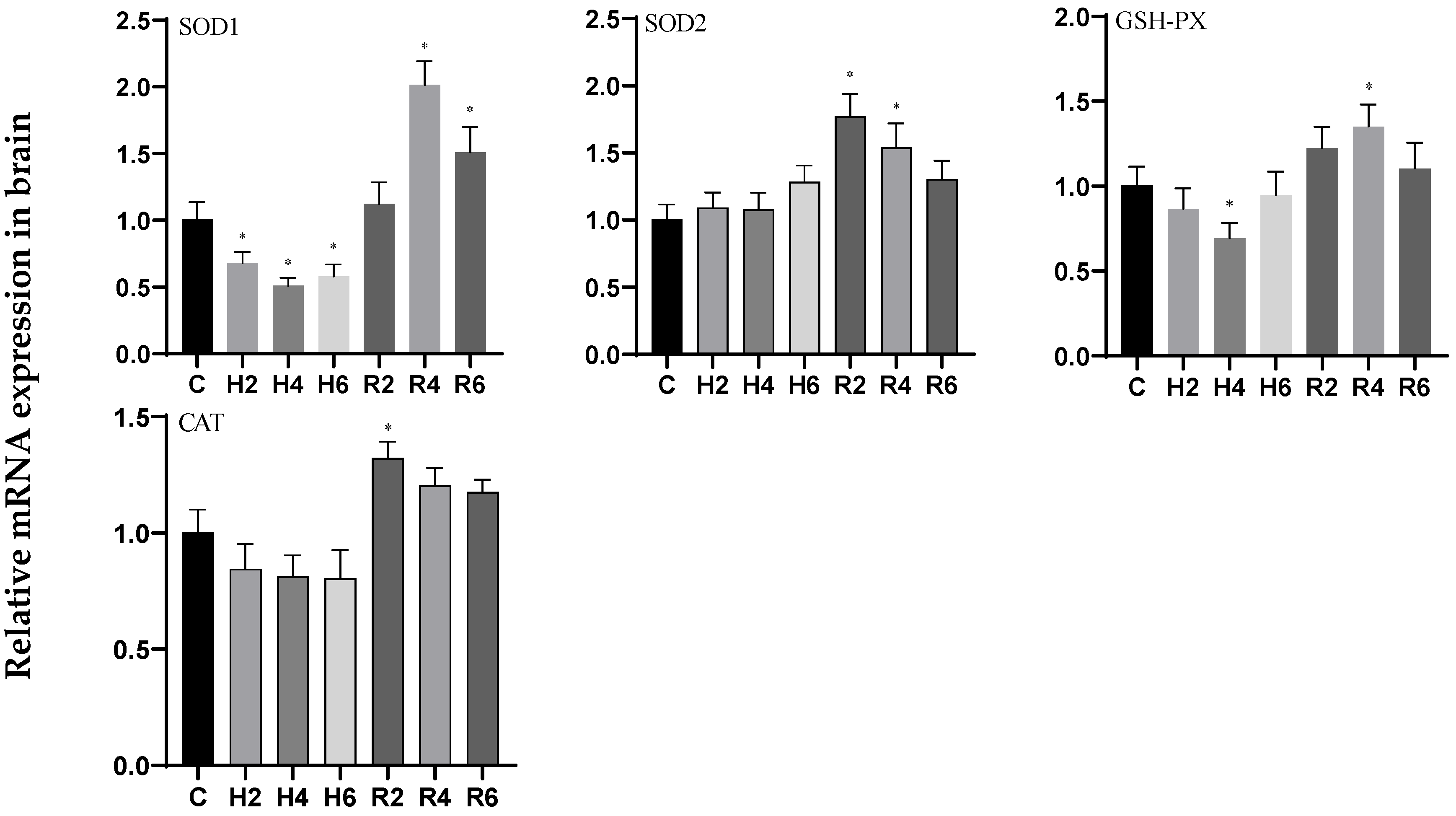
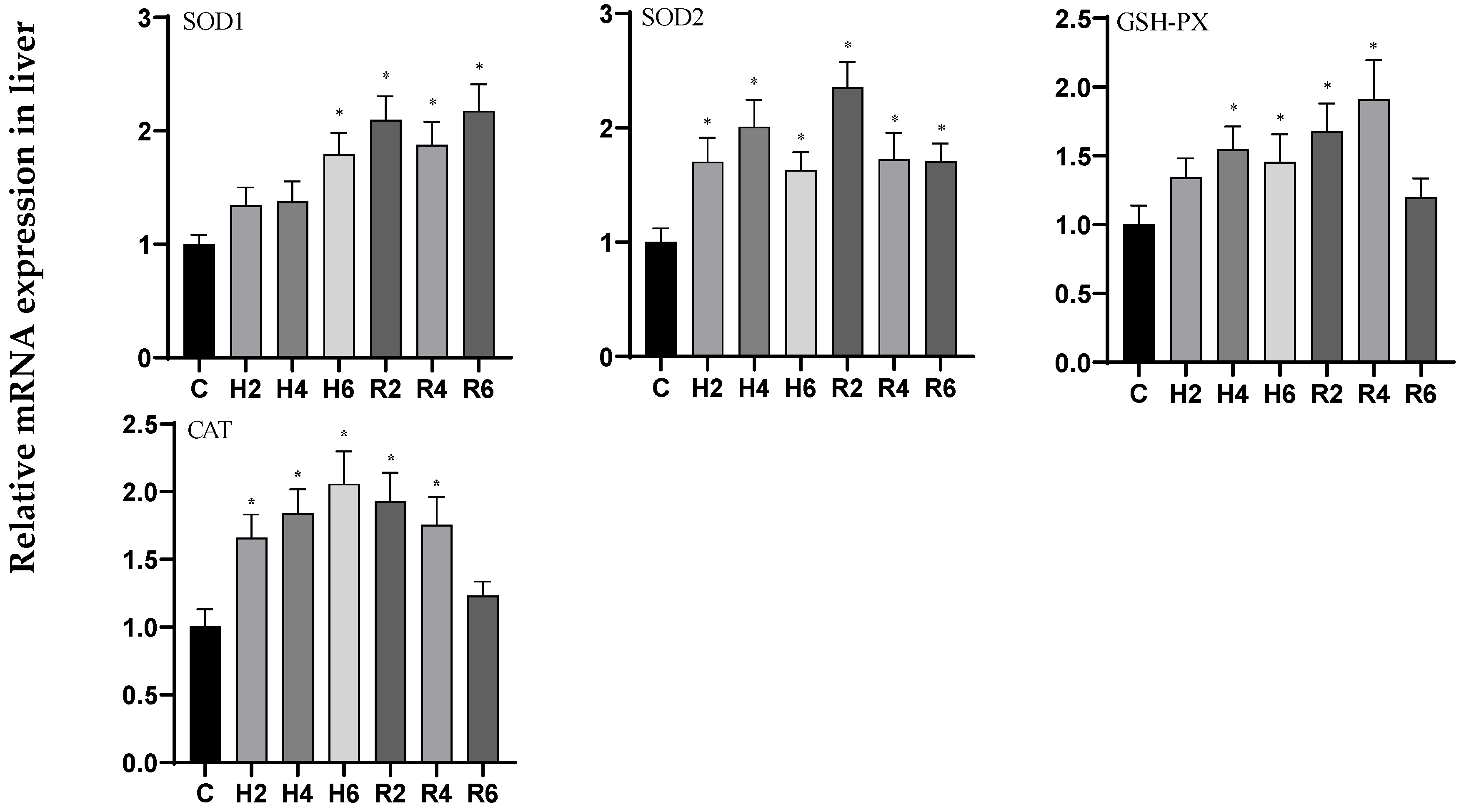
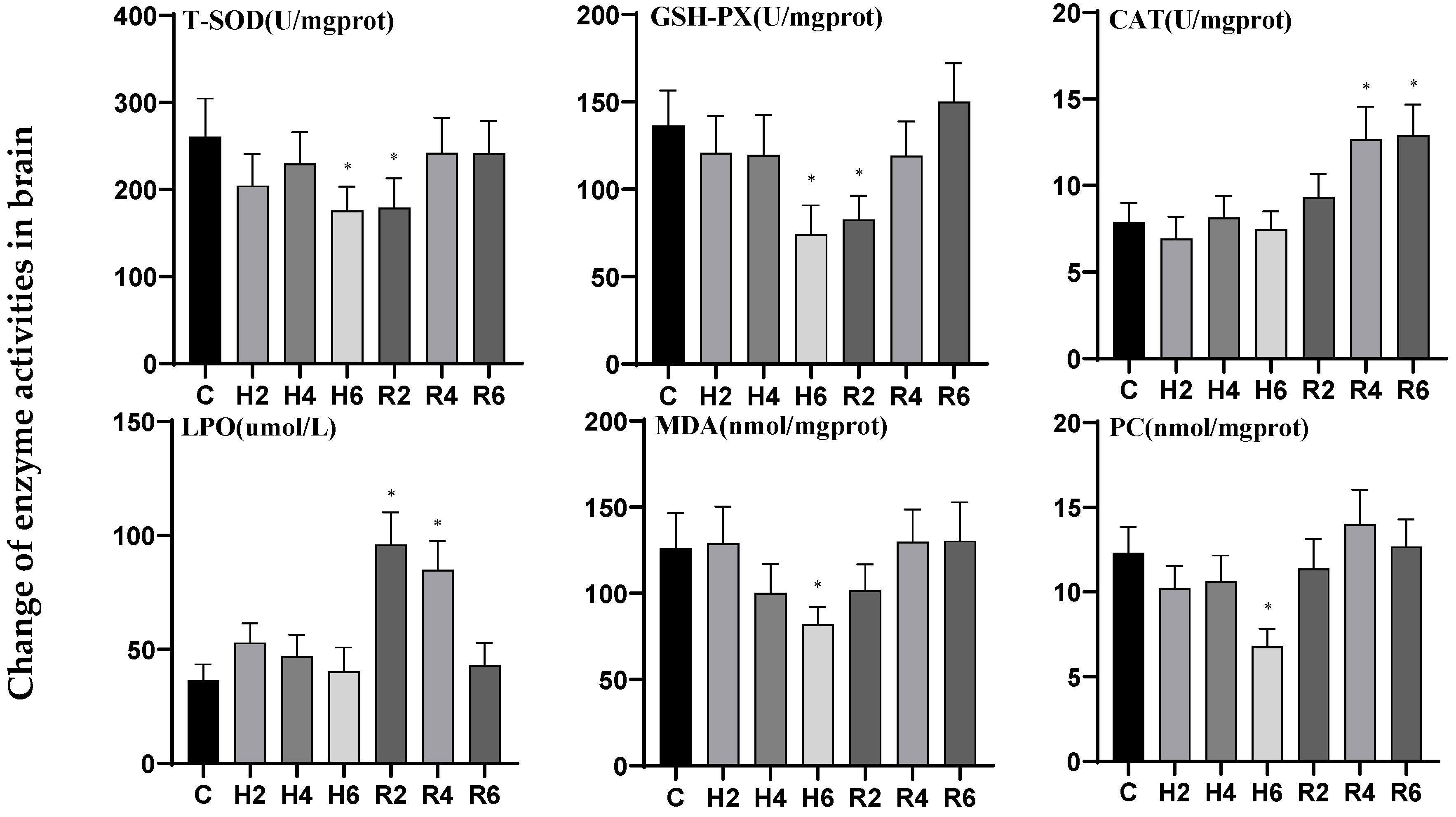
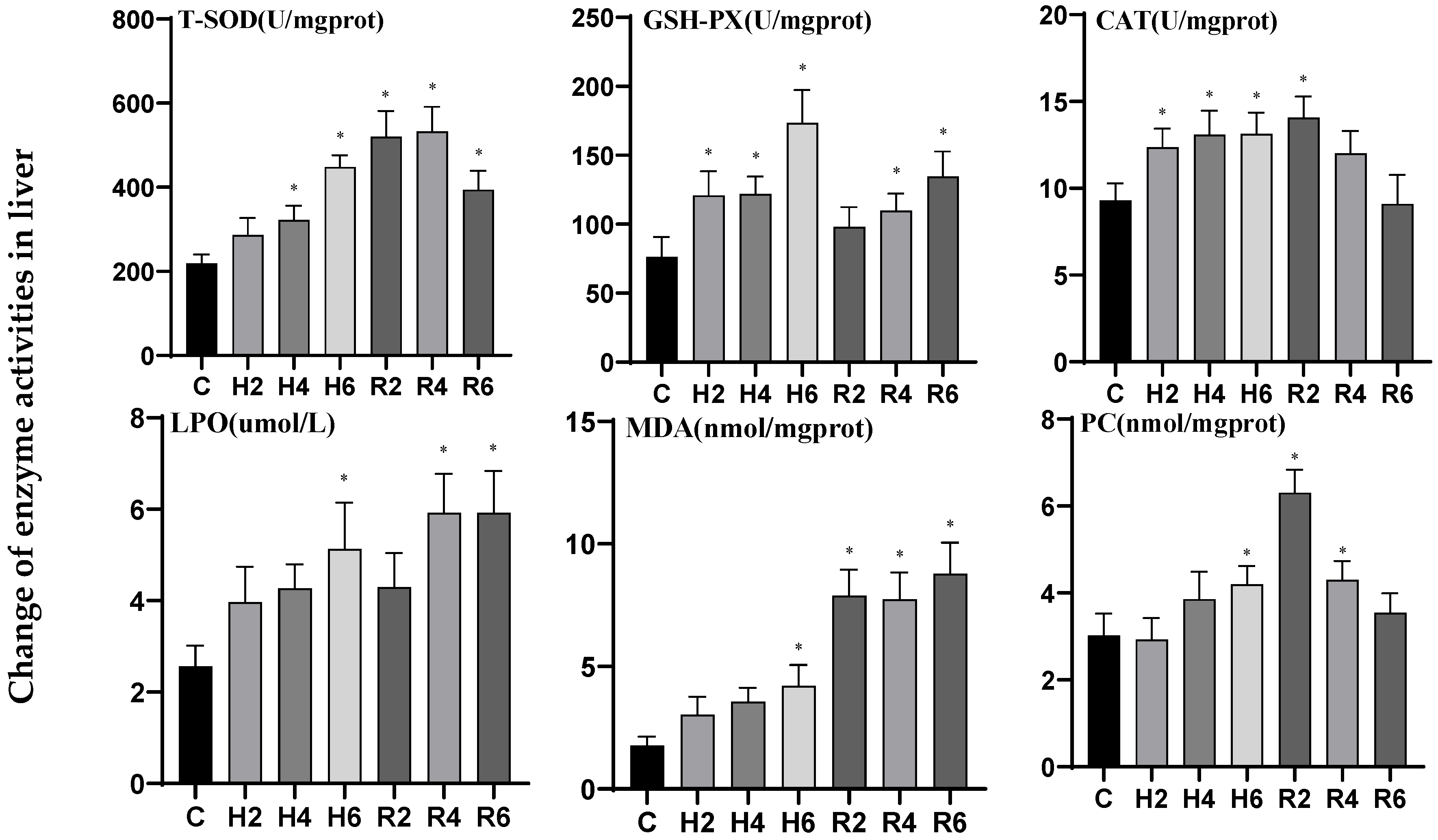
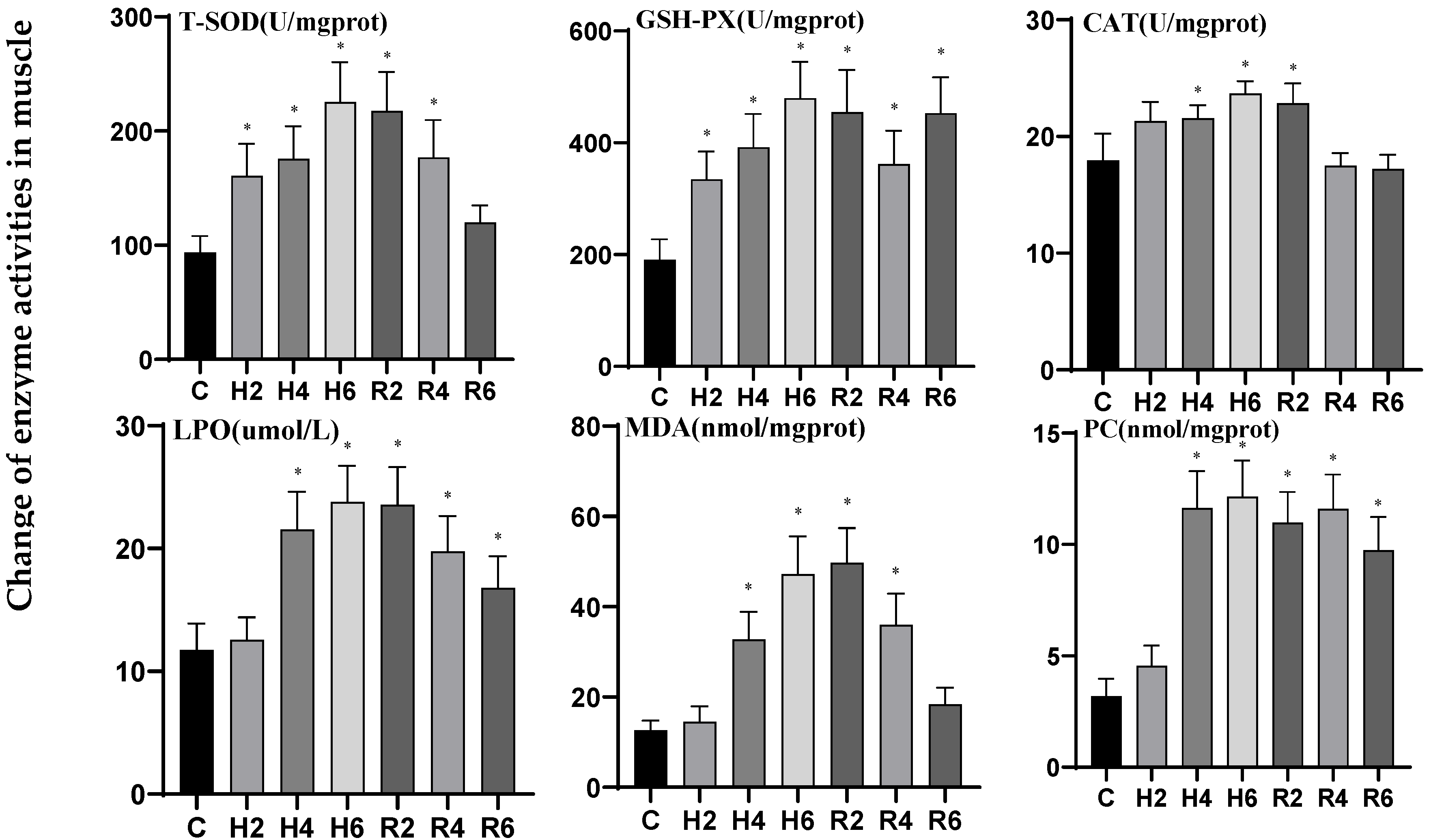
3.4. Effects of Hypoxia and Reoxygenation on Oxidative Stress Enzyme Activities and Parameters in Hybrid Catfish
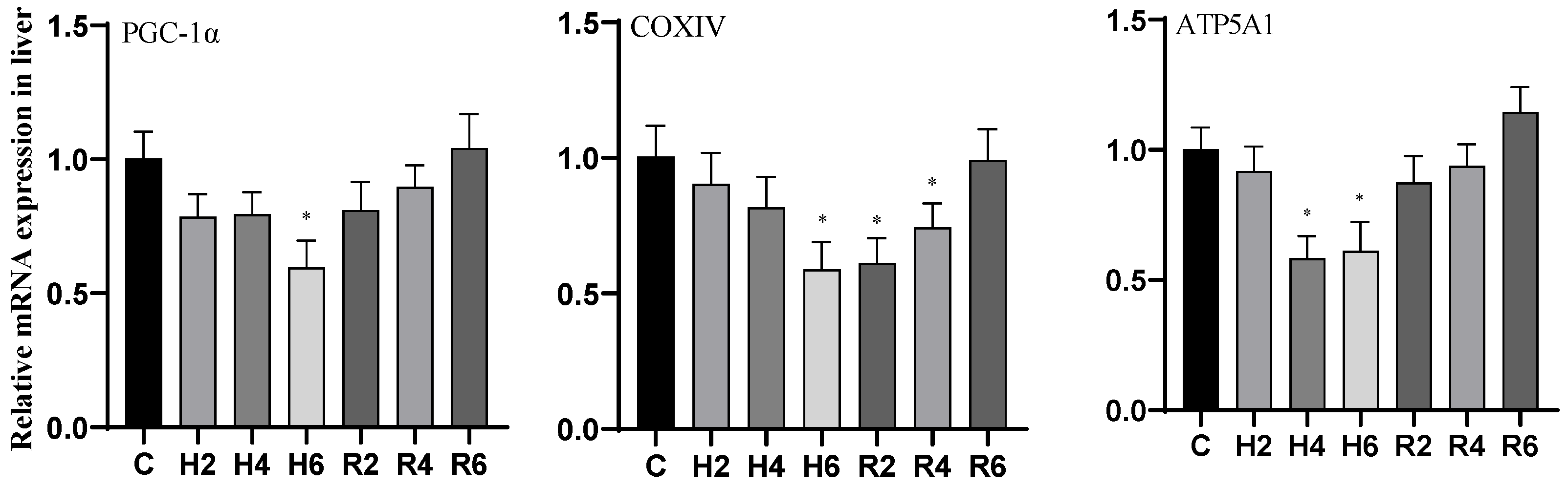
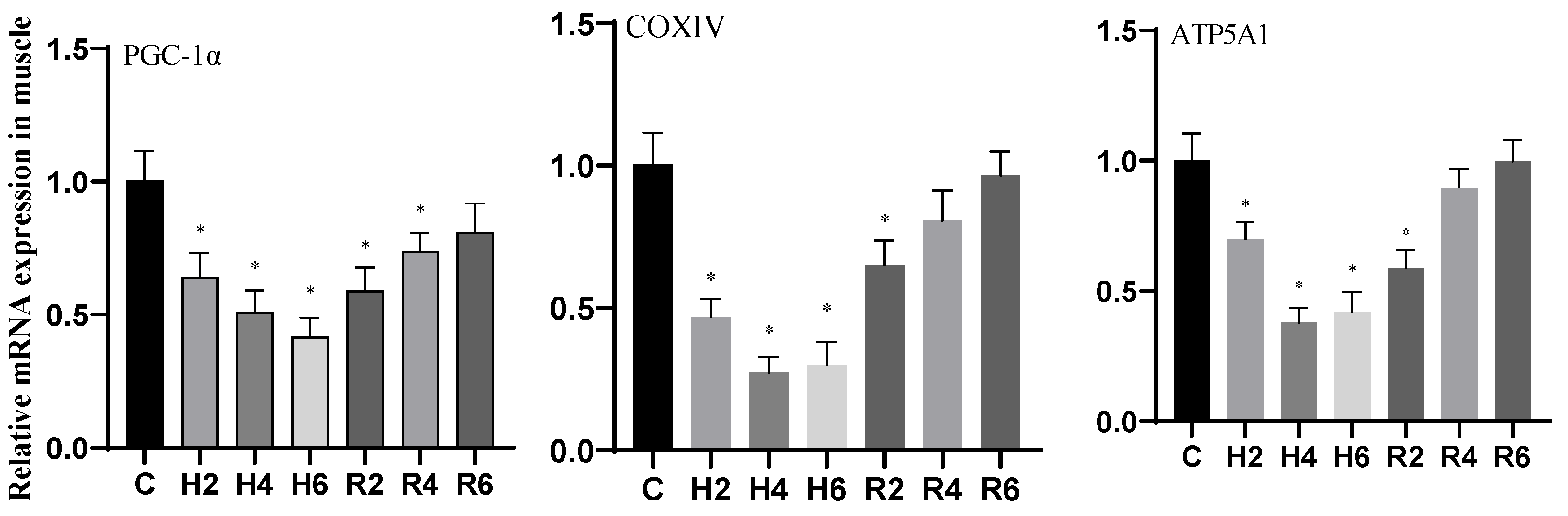
3.5. Effects of Hypoxia and Reoxygenation on Mitochondrial Damage Genes in Hybrid Catfish
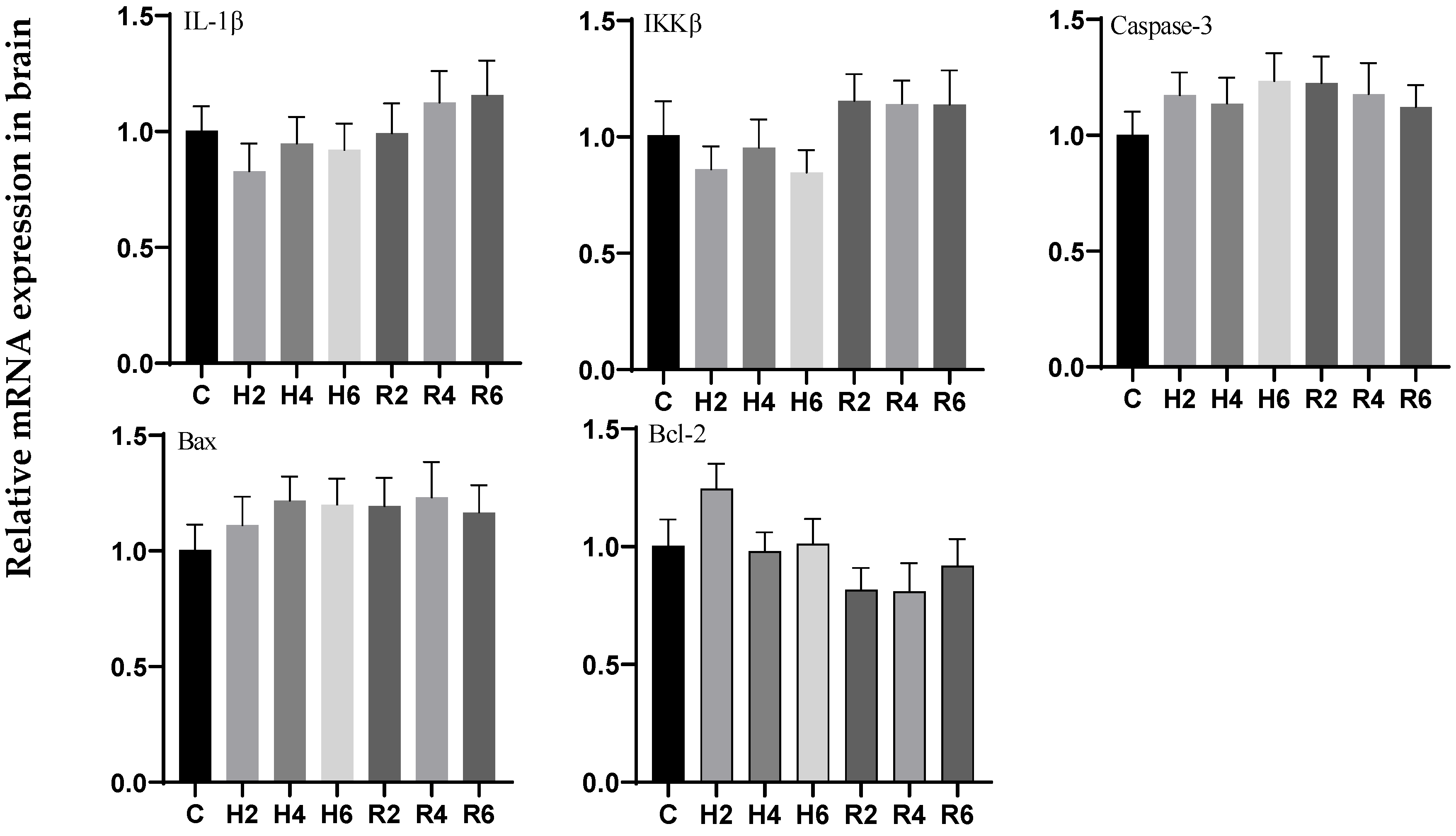
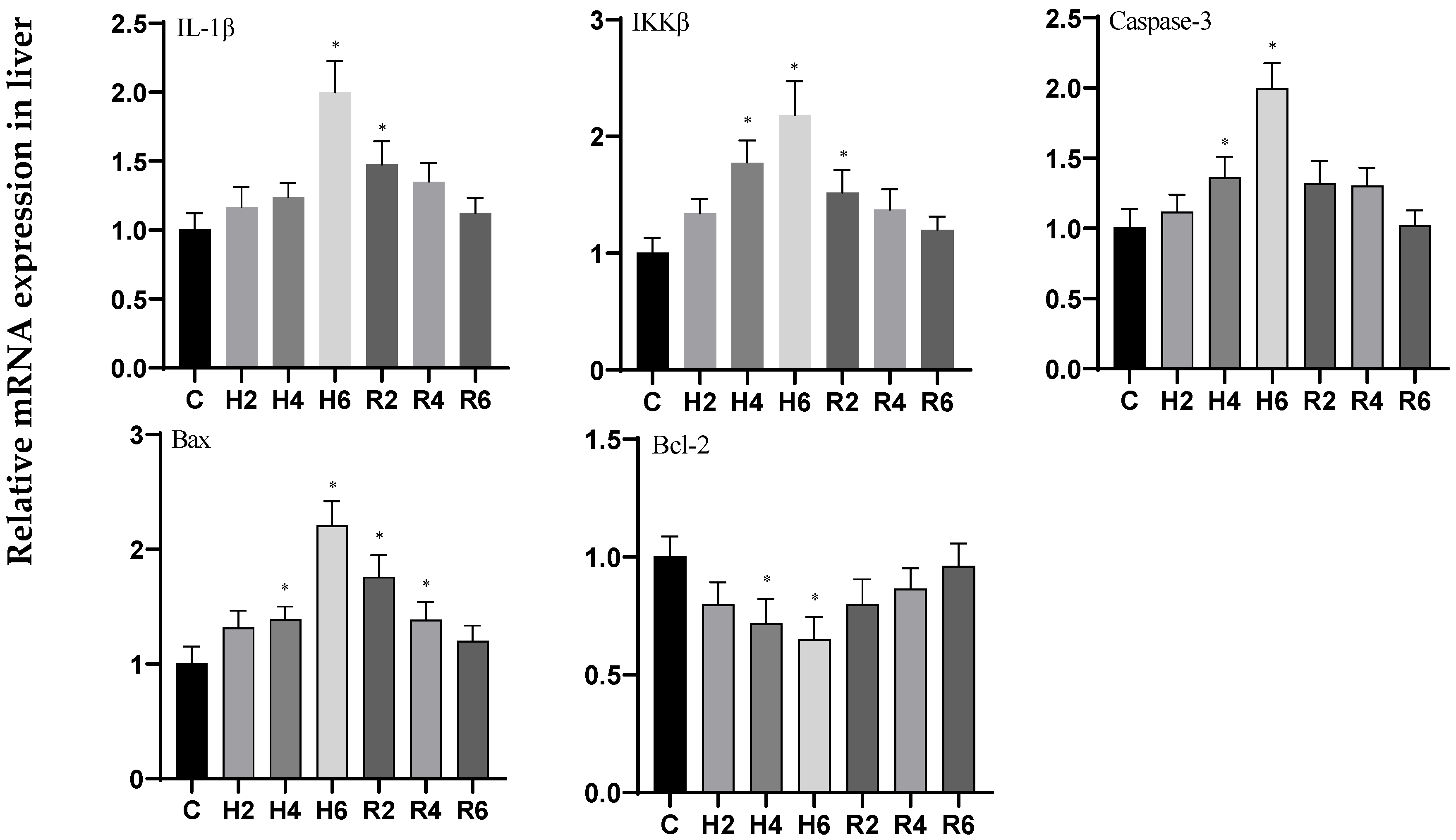
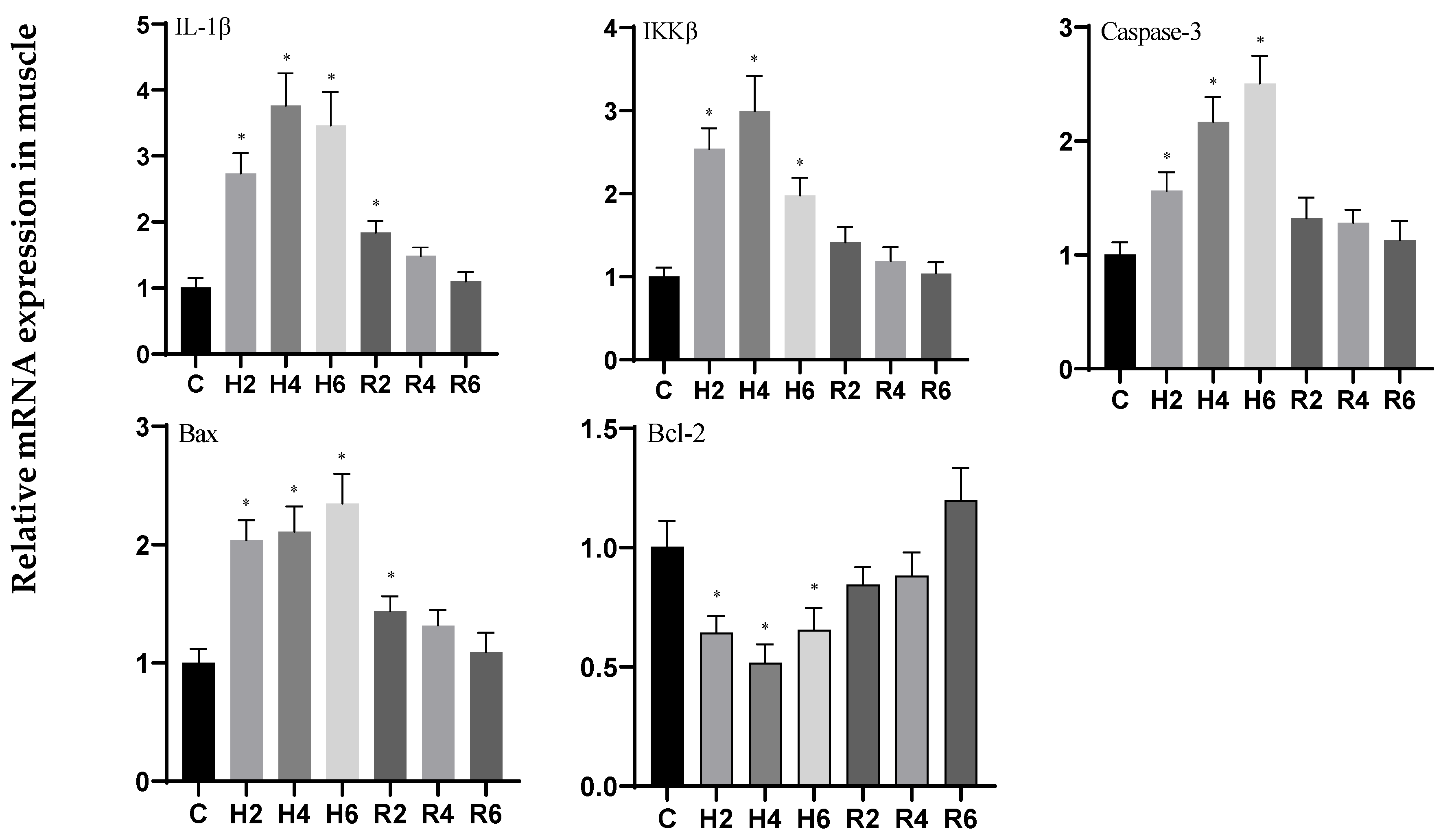
3.6. Effects of Hypoxia and Reoxygenation on Inflammation and Apoptosis-Related Genes in Hybrid Catfish
4. Discussion
4.1. Effects of Hypoxia and Reoxygenation on Oxygen-Sensing Protein HIF-1α and Respiratory Metabolism in Hybrid Catfish
4.2. Effects of Hypoxia and Reoxygenation on Oxidative Stress in Hybrid Catfish
4.3. Effects of Hypoxia and Reoxygenation on Mitochondrial Damage, Inflammation, and Apoptosis in Hybrid Catfish
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hammarlund, E.U.; Flashman, E.; Mohlin, S.; Licausi, F. Oxygen-sensing mechanisms across eukaryotic kingdoms and their roles in complex multicellularity. Science 2020, 370, eaba3512. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.-B.; Zheng, G.-D.; Zhao, X.-Y.; Zhou, S.; Zou, S.-M. Hypoxia tolerance in a selectively bred F4 population of blunt snout bream (Megalobrama amblycephala) under hypoxic stress. Aquaculture 2020, 518, 734484. [Google Scholar] [CrossRef]
- Jenny, J.P.; Francus, P.; Normandeau, A.; Lapointe, F.; Perga, M.E.; Ojala, A.; Schimmelmann, A.; Zolitschka, B. Global spread of hypoxia in freshwater ecosystems during the last three centuries is caused by rising local human pressure. Glob. Change Biol. 2016, 22, 1481–1489. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Pu, D.; Zheng, J.; Li, P.; Lü, H.; Wei, X.; Li, M.; Li, D.; Gao, L. Hypoxia-induced physiological responses in fish: From organism to tissue to molecular levels. Ecotoxicol. Environ. Saf. 2023, 267, 115609. [Google Scholar] [CrossRef] [PubMed]
- Xie, R.; Guo, H.; Luo, Y.; Huang, W.; Ruan, Z.; Liu, W. New Insights into the Mechanism by Which the Pituitary Gland Copes with Hypoxia Stress Based on a Transcriptomic Analysis of Megalobrama amblycephala. Genes 2024, 15, 987. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.-D.; Wang, Z.-H.; Yan, B. Strategies for hypoxia adaptation in fish species: A review. J. Comp. Physiol. B 2013, 183, 1005–1013. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-J.; Si, L.-B. Advances on hypoxia inducible factor-1. Chin. Med. J. 2013, 126, 3567–3571. [Google Scholar] [CrossRef] [PubMed]
- Kumar, H.; Choi, D.-K. Hypoxia inducible factor pathway and physiological adaptation: A cell survival pathway? Mediat. Inflamm. 2015, 2015, 584758. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.; Chao, Y.; Zhao, Y.; Chen, Q.; Zheng, Z.; Xia, M.; Qi, D. Molecular evolution and hypoxia-induced expression of Hif-α gene in Schizothoracinae fishes. Acta Lab. Anim. Sci. Sin. 2019, 27, 433–443. [Google Scholar]
- Zhan, Y.; Qi, X.; Wu, Y.; Gao, D.; Zhao, L.; Cao, S.; Xue, Z.; Wang, W. Hypoxia-inducible factor-1α as a biomarker for individuals under hypoxia duration and pattern in fat greenling Hexagrammos otakii. Aquac. Rep. 2024, 39, 102459. [Google Scholar] [CrossRef]
- Omlin, T.; Weber, J.-M. Hypoxia stimulates lactate disposal in rainbow trout. J. Exp. Biol. 2010, 213, 3802–3809. [Google Scholar] [CrossRef] [PubMed]
- Hoseini, S.M.; Taheri Mirghaed, A.; Pagheh, E.; Hoseinifar, S.H.; Van Doan, H. Anesthesia of rainbow trout with citronellal: Efficacy and biochemical effects. J. Exp. Zool. Part A Ecol. Integr. Physiol. 2022, 337, 227–237. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, S.; Saikia, S. Oxidative stress in fish: A review. J. Sci. Res. 2020, 12, 145–160. [Google Scholar] [CrossRef]
- Birnie-Gauvin, K.; Costantini, D.; Cooke, S.J.; Willmore, W.G. A comparative and evolutionary approach to oxidative stress in fish: A review. Fish Fish. 2017, 18, 928–942. [Google Scholar] [CrossRef]
- Wang, Q.-F.; Shen, W.-L.; Hou, C.-C.; Liu, C.; Wu, X.-F.; Zhu, J.-Q. Physiological responses and changes in gene expression in the large yellow croaker Larimichthys crocea following exposure to hypoxia. Chemosphere 2017, 169, 418–427. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Ling, X.; Zhao, Y.; Fu, S. Hypoxia-induced oxidative stress and apoptosis in gills of scaleless carp (Gymnocypris przewalskii). Fish Physiol. Biochem. 2022, 48, 911–924. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Yan, T.; Wu, H.; Xiao, Q.; Fu, H.; Luo, J.; Zhou, J.; Zhao, L.; Wang, Y.; Yang, S. Acute hypoxic stress: Effect on blood parameters, antioxidant enzymes, and expression of HIF-1alpha and GLUT-1 genes in largemouth bass (Micropterus salmoides). Fish Shellfish Immunol. 2017, 67, 449–458. [Google Scholar] [CrossRef] [PubMed]
- Qian, L. Sex Determination in Ictalurus punctatus and Genetic Identification of Pelteobagrus Vachelli, Leiocassis Longirostris, and their Hybrids. Master’s Thesis, Huazhong Agricultural University, Wuhan, China, 2023. [Google Scholar]
- Bulbul Ali, A.; Mishra, A. Effects of dissolved oxygen concentration on freshwater fish: A review. Int. J. Fish. Aquat. Stud. 2022, 10, 113–127. [Google Scholar] [CrossRef]
- Babin, C.H.; Leiva, F.P.; Verberk, W.C.; Rees, B.B. Evolution of key oxygen-sensing genes is associated with hypoxia tolerance in fishes. Genome Biol. Evol. 2024, 16, evae183. [Google Scholar] [CrossRef] [PubMed]
- Corrado, C.; Fontana, S. Hypoxia and HIF signaling: One axis with divergent effects. Int. J. Mol. Sci. 2020, 21, 5611. [Google Scholar] [CrossRef] [PubMed]
- Qin, H.; Zhang, X.; Xie, T.; Gao, Y.; Li, J.; Jia, Y. Hepatic transcriptomic analysis reveals that Hif1α/ldha signal is involved in the regulation of hypoxia stress in black rockfish Sebastes schlegelii. Comp. Biochem. Physiol. Part D Genom. Proteom. 2023, 47, 101098. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.J.; Gelsleichter, J.; Smith, K.J. Protein expression of hypoxia-inducible factor 1-alpha (HIF-1α) in spot (Leiostomus xanthurus) exposed to constant and diel-cycling hypoxia. J. Exp. Mar. Biol. Ecol. 2012, 424, 1–4. [Google Scholar] [CrossRef]
- Li, M. Study on the Effects of Hypoxic Stress on Glycolipid Metabolism in Nile Tilapia and the Regulatory Role of Salidroside. Master’s Thesis, East China Normal University, Shanghai, China, 2018. [Google Scholar]
- Lynch, E.M.; Hansen, H.; Salay, L.; Cooper, M.; Timr, S.; Kollman, J.M.; Webb, B.A. Structural basis for allosteric regulation of human phosphofructokinase-1. bioRxiv 2024. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.-L.; Ding, L.; Ma, R.-X.; Zhang, Y.; Zhang, Y.-L.; Ni, W.-J.; Tang, T.-T.; Wang, G.-H.; Wang, B.; Lv, L.-L. Activation of HIF-1α C-terminal transactivation domain protects against hypoxia-induced kidney injury through hexokinase 2-mediated mitophagy. Cell Death Dis. 2023, 14, 339. [Google Scholar] [CrossRef] [PubMed]
- Lantushenko, A.; Kohan, A.; Soldatov, A.; Degtyar, I.; Andreeva, A.Y. Expression of pyruvate kinase, malate and octopine dehydrogenase genes in the gills of the Mediterranean mussel Mytilus galloprovincialis (Lamarck, 1819) under conditions of hypoxia and reoxygenation. Biol. Bull. 2024, 51, 1206–1211. [Google Scholar] [CrossRef]
- Dong, M.; Tang, R.; Wang, W.; Xu, J.; Liu, J.; Liang, C.; Hua, J.; Meng, Q.; Yu, X.; Zhang, B. Integrated analysis revealed hypoxia signatures and LDHA related to tumor cell dedifferentiation and unfavorable prognosis in pancreatic adenocarcinoma: Hypoxia in PDAC. Transl. Oncol. 2023, 33, 101692. [Google Scholar] [CrossRef] [PubMed]
- Qi, R.; Zhang, X.; Shao, G.; Qi, R.; Zhang, X.; Shao, G. Advances in adaptive mechanisms to oxygen and research on hypoxic preconditioning. Sci. Technol. Rev. 2020, 38, 86–91. [Google Scholar]
- Wang, J.; Wang, M.; Li, B.; Guo, H.; Zhu, X.; Zhang, L. The combined effect of acute hypoxic stress and feeding status on the metabolism of yellow catfish (Pelteobagrus fulvidraco). Aquaculture 2022, 560, 738605. [Google Scholar] [CrossRef]
- Zhang, G. Molecular Mechanisms of Hypoxia Stress Response in Pelteobagrus Vachelli. Master’s Thesis, Nanjing Normal University, Nanjing, China, 2017. [Google Scholar]
- Sun, J.; Han, G.; Liu, Y.; Li, M.; Yuan, Y.; Fan, Q.; Wang, Y.; Yang, H. Comparison of growth performance and hypoxia tolerance between hybrid yellow catfish and common yellow catfish juveniles. Acta Hydrobiol. Sin. 2019, 43, 1271–1279. [Google Scholar]
- Li, J.; Yang, Z.; Yan, J.; Zhang, K.; Ning, X.; Wang, T.; Ji, J.; Zhang, G.; Yin, S.; Zhao, C. Multi-omics analysis revealed the brain dysfunction induced by energy metabolism in Pelteobagrus vachelli under hypoxia stress. Ecotoxicol. Environ. Saf. 2023, 254, 114749. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, G.; Yin, D.; Li, Y.; Zhang, Y.; Cheng, J.; Zhang, K.; Ji, J.; Wang, T.; Jia, Y. Integrated application of multiomics strategies provides insights into the environmental hypoxia response in Pelteobagrus vachelli muscle. Mol. Cell. Proteom. 2022, 21, 100196. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.L.; Liu, Y.F.; Jiang, T.; Li, Y.Q.; Song, F.B.; Wen, X.; Luo, J. Golden pompano (Trachinotus blochii) adapts to acute hypoxic stress by altering the preferred mode of energy metabolism. Aquaculture 2021, 542, 736842. [Google Scholar] [CrossRef]
- Lushchak, V.I.; Lushchak, L.P.; Mota, A.A.; Hermes-Lima, M. Oxidative stress and antioxidant defenses in goldfish Carassius auratus during anoxia and reoxygenation. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2001, 280, R100–R107. [Google Scholar] [CrossRef] [PubMed]
- Lushchak, V.I. Adaptive response to oxidative stress: Bacteria, fungi, plants and animals. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2011, 153, 175–190. [Google Scholar] [CrossRef] [PubMed]
- Hermes-Lima, M.; Moreira, D.C.; Rivera-Ingraham, G.A.; Giraud-Billoud, M.; Genaro-Mattos, T.C.; Campos, É.G. Preparation for oxidative stress under hypoxia and metabolic depression: Revisiting the proposal two decades later. Free Radic. Biol. Med. 2015, 89, 1122–1143. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Lu, Y.; Sun, M.; Shen, H.; Niu, D. Effects of acute hypoxia and reoxygenation on histological structure, antioxidant response, and apoptosis in razor clam Sinonovacula constricta. Fish Shellfish Immunol. 2024, 145, 109310. [Google Scholar] [CrossRef] [PubMed]
- Shuang, L.; Chen, S.-L.; Ren, C.; Su, X.-L.; Xu, X.-N.; Zheng, G.-D.; Zou, S.-M. Effects of hypoxia and reoxygenation on oxidative stress, histological structure, and apoptosis in a new hypoxia-tolerant variety of blunt snout bream (Megalobrama amblycephala). Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2023, 278, 111358. [Google Scholar] [CrossRef] [PubMed]
- Geihs, M.A.; Vargas, M.A.; Maciel, F.E.; Vakkuri, O.; Meyer-Rochow, V.B.; Allodi, S.; Nery, L.E.M. Effects of hypoxia and reoxygenation on the antioxidant defense system of the locomotor muscle of the crab Neohelice granulata (Decapoda, Varunidae). J. Comp. Physiol. B 2016, 186, 569–579. [Google Scholar] [CrossRef] [PubMed]
- Hermes-Lima, M.; Zenteno-Savın, T. Animal response to drastic changes in oxygen availability and physiological oxidative stress. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2002, 133, 537–556. [Google Scholar] [CrossRef] [PubMed]
- Welker, A.F.; Moreira, D.C.; Campos, É.G.; Hermes-Lima, M. Role of redox metabolism for adaptation of aquatic animals to drastic changes in oxygen availability. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2013, 165, 384–404. [Google Scholar] [CrossRef] [PubMed]
- Lushchak, V.I.; Bagnyukova, T.V. Hypoxia induces oxidative stress in tissues of a goby, the rotan Perccottus glenii. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2007, 148, 390–397. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, Y.; Liang, X.; Yang, Z.; Peng, Y.; Zhang, Y.; Ning, X.; Zhang, K.; Ji, J.; Wang, T. Blood redistribution preferentially protects vital organs under hypoxic stress in Pelteobagrus vachelli. Aquat. Toxicol. 2023, 258, 106498. [Google Scholar] [CrossRef] [PubMed]
- Chapman, C.L.; Hess, H.W.; Worley, M.L. Heterogeneous redistribution of cerebral oxygen delivery to combined thermal and hypoxic exposure. J. Physiol. 2020, 598, 443–445. [Google Scholar] [CrossRef] [PubMed]
- Qi, M.; Hou, Y.; Liu, T.; Miao, Y.; Zhuang, S.; Qu, X.; Liu, Q. Effects of acute hypoxia stress and reoxygenation recovery on oxidative stress and energy metabolism in juvenile Qingtian paddy fish. Freshw. Fish 2020, 50, 92–98. [Google Scholar] [CrossRef]
- Xiong, X.; Huang, G.; Peng, Y.; Liu, X. Effects of hypoxia stress on growth, energy metabolism, and oxidative stress in juvenile mullet (Mugil cephalus). J. Fish. China 2016, 40, 73–82. [Google Scholar]
- Fuhrmann, D.C.; Brüne, B. Mitochondrial composition and function under the control of hypoxia. Redox Biol. 2017, 12, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Cerretelli, P.; Gelfi, C. Energy metabolism in hypoxia: Reinterpreting some features of muscle physiology on molecular grounds. Eur. J. Appl. Physiol. 2011, 111, 421–432. [Google Scholar] [CrossRef] [PubMed]
- Casanova, A.; Wevers, A.; Navarro-Ledesma, S.; Pruimboom, L. Mitochondria: It is all about energy. Front. Physiol. 2023, 14, 1114231. [Google Scholar] [CrossRef] [PubMed]
- McMeekin, L.J.; Fox, S.N.; Boas, S.M.; Cowell, R.M. Dysregulation of PGC-1α-dependent transcriptional programs in neurological and developmental disorders: Therapeutic challenges and opportunities. Cells 2021, 10, 352. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.X.; Thompson, K.; Taylor, R.W.; Oláhová, M. Mitochondrial OXPHOS biogenesis: Co-regulation of protein synthesis, import, and assembly pathways. Int. J. Mol. Sci. 2020, 21, 3820. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Saikia, J.M.; Monte, K.M.A.; Ha, E.; Romaus-Sanjurjo, D.; Sanchez, J.J.; Moore, A.X.; Hernaiz-Llorens, M.; Chavez-Martinez, C.L.; Agba, C.K. Deep scRNA sequencing reveals a broadly applicable Regeneration Classifier and implicates antioxidant response in corticospinal axon regeneration. Neuron 2023, 111, 3953–3969.e3955. [Google Scholar] [CrossRef] [PubMed]
- Wu, N.; Liu, X. The effect of L-arginine supplementation on mitochondrial biogenesis in skeletal muscle induced by endurance exercise in rats. In Proceedings of the Chinese Physiological Society, Annual Meeting of the Exercise Physiology Committee of Chinese Physiological Society and the Academic Seminar on “Exercise and Health”, Guangzhou, China, 8 November 2013; Capital Institute of Physical Education: Beijing, China, 2013; pp. 101–102. [Google Scholar]
- Bo, H.; Peng, P.; Qin, Y.; Zhang, Y. Effects of hypoxia combined with exercise on mitochondrial content in rat skeletal muscle. Chin. J. Pathophysiol. 2014, 30, 1461–1466. [Google Scholar]
- Wu, J.; Ju, L.; Weng, X.; Xu, G.; Lin, W. Research progress on the effect of hypoxia stimulation on PGC-1α expression. Chem. Life 2016, 36, 81–87. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, R.; Yuan, R.; Huo, L.; Gao, H.; Zhuo, Y.; Chen, X.; Zhang, C.; Yang, S. Discovery of New Heterocyclic/Benzofuran Hybrids as Potential Anti-Inflammatory Agents: Design, Synthesis, and Evaluation of the Inhibitory Activity of Their Related Inflammatory Factors Based on NF-κB and MAPK Signaling Pathways. Int. J. Mol. Sci. 2023, 24, 3575. [Google Scholar] [CrossRef] [PubMed]
- Ullah, S.; Feng, F.; Zhao, M.; Zhang, J.; Shao, Q. Comparative Effects of Dietary Supplementations with Microencapsulated Sodium Butyrate, Glycerol Monolaurate and Tributyrin on Growth, Immunity, and Gut Health in Black Sea Bream. Animals 2025, 15, 810. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zeng, Z.; Huang, J.; Guo, Z.; Li, H.; Chen, G. Effects of hypoxia stress on growth, serum biochemistry, and non-specific immune indices of juvenile cobia (Rachycentron canadum). Acta Ocean. Sin 2021, 43, 49–58. [Google Scholar]
- Mu, Y.; Li, W.; Wu, B.; Chen, J.; Chen, X. Transcriptome analysis reveals new insights into immune response to hypoxia challenge of large yellow croaker (Larimichthys crocea). Fish Shellfish Immunol. 2020, 98, 738–747. [Google Scholar] [CrossRef] [PubMed]
- Lai, X.-X.; Zhang, C.-P.; Wu, Y.-X.; Yang, Y.; Zhang, M.-Q.; Qin, W.-J.; Wang, R.-X.; Shu, H. Comparative transcriptome analysis reveals physiological responses in liver tissues of Epinephelus coioides under acute hypoxia stress. Comp. Biochem. Physiol. Part D Genom. Proteom. 2022, 43, 101005. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, Y.; Ganster, R.W.; Gambotto, A.; Shao, L.; Kaizu, T.; Wu, T.; Yagnik, G.P.; Nakao, A.; Tsoulfas, G.; Ishikawa, T. Role of NF-κB on liver cold ischemia-reperfusion injury. Am. J. Physiol.-Gastrointest. Liver Physiol. 2002, 283, G1175–G1184. [Google Scholar] [CrossRef] [PubMed]
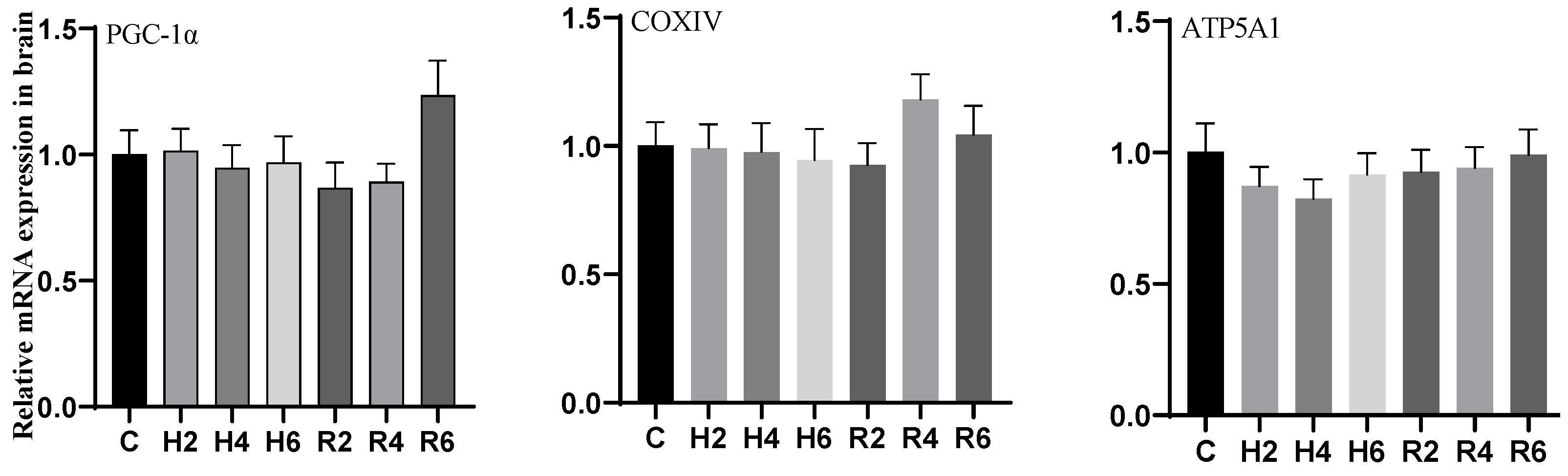
| Gene | Primer Sequence (5′-3′) | Product Size (bp) | Accession No. Or Publication | Amplification Efficiency |
|---|---|---|---|---|
| HIF-1α | Forward: CTGGAAAGAGGGCTAAGGTG | 151 | XP_027023235.1 | 98.26 |
| Reverse: AGTGACGGTCCTGAATAGGG | ||||
| PFKL | Forward: TTGTGGATACCTGGCAACCA | 188 | XP_027018682.1 | 98.81 |
| Reverse: AGTCGGTGCTGTATTGTGGA | ||||
| PK | Forward: GGCTAATGCTGTTCTGGATG | 166 | XP_027029118.1 | 97.74 |
| Reverse: GGGTCGGTAGAGTAGGCTGT | ||||
| HK1 | Forward: CGAGACTGTGGACGGAGACG | 141 | XP_027030735.1 | 98.12 |
| Reverse: TTTGCCAGGGTTGAGGGAGA | ||||
| CS | Forward: GATGGGTGAAGTGGGGAAGA | 154 | XP_027016864.1 | 98.63 |
| Reverse: GGTGTTGAGCAGGAAGTTGG | ||||
| LDHA | Forward: ATCTGGACTCTGCTCGGTTC | 112 | XP_027028964.1 | 98.73 |
| Reverse: CATACAGGCACGCTTGAGTC | ||||
| SOD1 | Forward: GTAATGTGACTGCCGGTTCC | 106 | AOQ25512.1 | 97.72 |
| Reverse: TGAATCACCATGGTCCTCCC | ||||
| SOD2 | Forward: CCAAAGGTGACGTGACAACA | 153 | AOQ25511.1 | 97.95 |
| Reverse: AATCACGCTTAATGGCCTCC | ||||
| GSH-PX | Forward: GAATGGGAAAGACGCTCACC | 118 | XP_026771571.1 | 98.25 |
| Reverse: GCACACAGGACTCCAGATGA | ||||
| CAT | Forward: TCCCACACCTTCAAGCTGAT | 156 | XP_027019602.1 | 98.17 |
| Reverse: GGAGTTGTACAGGTCACGGA | ||||
| Bcl-2 | Forward: CTTGTACCGACCCGACTTTG | 122 | XM_027142465.2 | 97.88 |
| Reverse: GTCCCCAGTTGATCCCGTC | ||||
| Bax | Forward: TGGAGATGAACTGGACAGCA | 128 | XP_027009035.1 | 97.52 |
| Reverse: TGCCCCAGTTAAACTTCCCA | ||||
| IL-1β | Forward: TTAGGCATAGAGGAGGTAAAAGAC | 123 | XP_027008231.1 | 97.92 |
| Reverse: TTCACCGACTCGAAGGTGTT | ||||
| IKKβ | Forward: GCGAGAGATGGAGCAAACTG | 183 | XM_047823116.1 | 98.59 |
| Reverse: TTCTCTCTCAGCTTGCGGAA | ||||
| Caspase 3 | Forward: AGACCTGGACCCTGGTATTG | 138 | XP_026990614.1 | 97.64 |
| GAGTAGTAACCTGGTGCTGTAGAA | ||||
| PGC-1α | Forward: TTGACCACCACAGGCATAGT | 112 | XM_027150337.2 | 98.87 |
| Reverse: GTCCTCTCTTCTGGTGGCAT | ||||
| COXIV | Forward: ACTGAATCCGGAGCAGAAGT | 165 | XM_027177342.2 | 98.45 |
| Reverse: AACAACATTCCAGCGACCAC | ||||
| ATP5A1 | Forward: ACTGATACCGGTGCCATGAA | 137 | XM_027133276.2 | 97.29 |
| Reverse: ATAAGTGCGTGTTTGCCGTT | ||||
| β-actin | Forward: CACTGCTGCCTCTTCCTC | 181 | XM_027148463.2 | 98.62 |
| Reverse: ATCCACATCGCACTTCAT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, J.; Zhang, F.; Liang, F.; Zhao, C.; Yin, S.; Zhang, G. Effects of Hypoxia and Reoxygenation on Hypoxia-Responsive Genes, Physiological and Biochemical Indices in Hybrid Catfish (Pelteobagrus vachelli ♀ × Leiocassis longirostris ♂). Biology 2025, 14, 915. https://doi.org/10.3390/biology14080915
Yan J, Zhang F, Liang F, Zhao C, Yin S, Zhang G. Effects of Hypoxia and Reoxygenation on Hypoxia-Responsive Genes, Physiological and Biochemical Indices in Hybrid Catfish (Pelteobagrus vachelli ♀ × Leiocassis longirostris ♂). Biology. 2025; 14(8):915. https://doi.org/10.3390/biology14080915
Chicago/Turabian StyleYan, Jie, Faling Zhang, Fenfei Liang, Cheng Zhao, Shaowu Yin, and Guosong Zhang. 2025. "Effects of Hypoxia and Reoxygenation on Hypoxia-Responsive Genes, Physiological and Biochemical Indices in Hybrid Catfish (Pelteobagrus vachelli ♀ × Leiocassis longirostris ♂)" Biology 14, no. 8: 915. https://doi.org/10.3390/biology14080915
APA StyleYan, J., Zhang, F., Liang, F., Zhao, C., Yin, S., & Zhang, G. (2025). Effects of Hypoxia and Reoxygenation on Hypoxia-Responsive Genes, Physiological and Biochemical Indices in Hybrid Catfish (Pelteobagrus vachelli ♀ × Leiocassis longirostris ♂). Biology, 14(8), 915. https://doi.org/10.3390/biology14080915






