Mechanistic Study on the Inhibitory Effect of Dandelion Extract on Breast Cancer Cell Proliferation and Its Induction of Apoptosis
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Chemicals and Reagents
2.3. Preparation of Crude Extracts from Dandelion Roots and Leaves
2.4. Isolation of Ethanol Extract from Dandelion Root
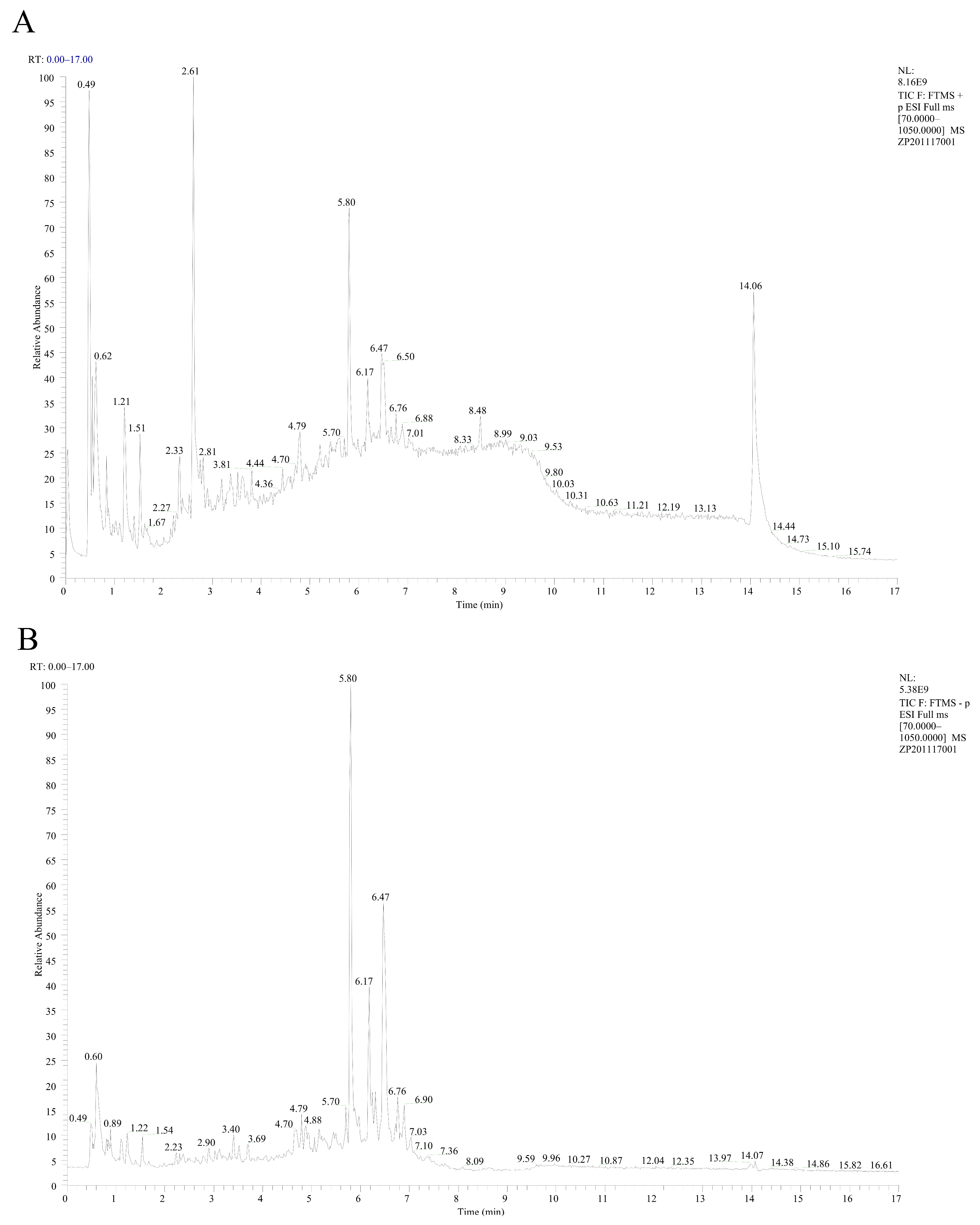
2.5. LC-MS Analysis
2.6. Cell Culture of MDA-MB-231
2.7. MTT Assay for Cell Viability
2.8. Hoechst Staining
2.9. Detection of Apoptosis and Cell Cycle in MDA-MB-231 Cells by Annexin V/PI Dual Staining
2.10. Preparation of Samples for Proteomic Analysis
2.11. Protein Identification and Quantification
2.12. Molecular Docking
2.13. Data Analysis
3. Results
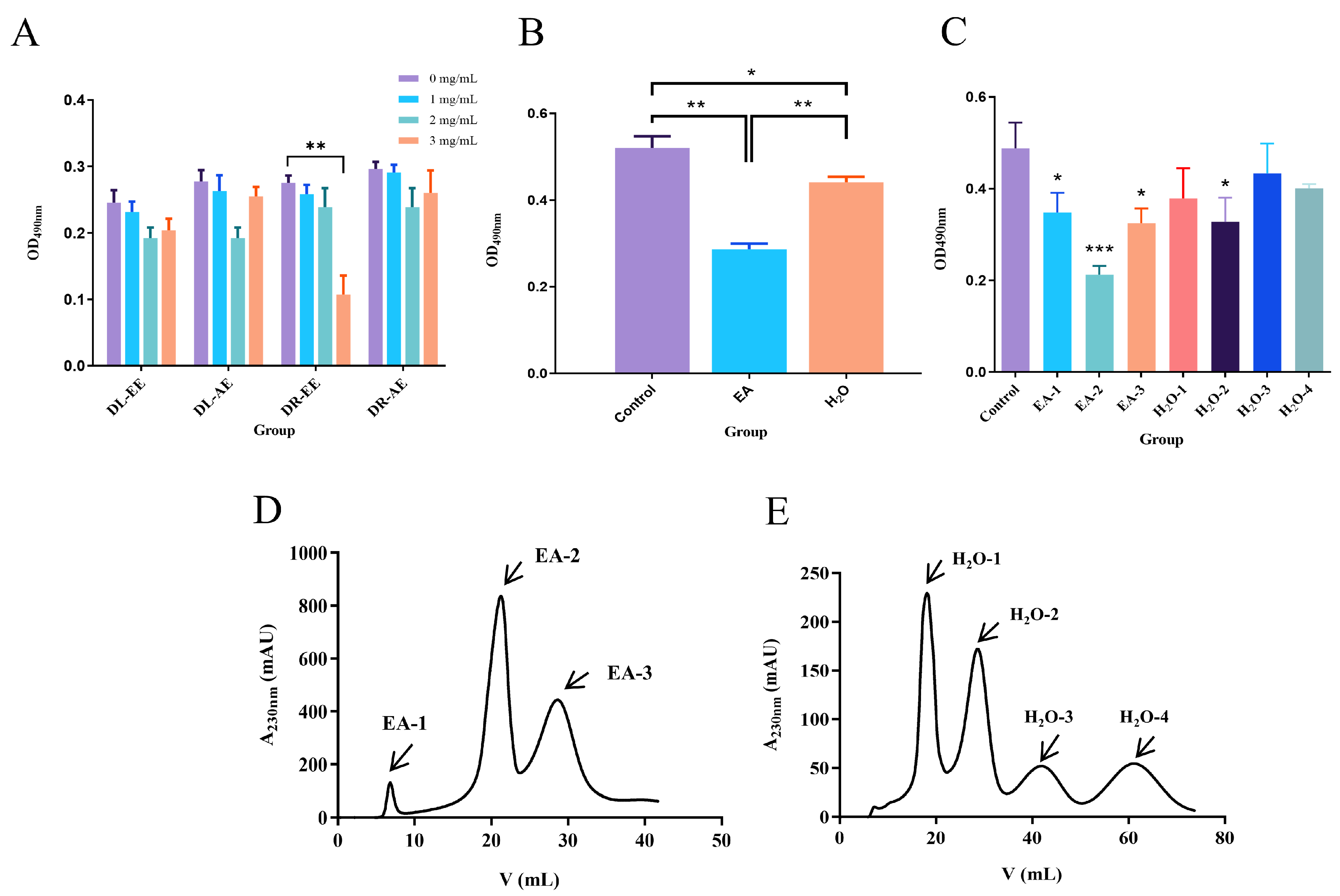
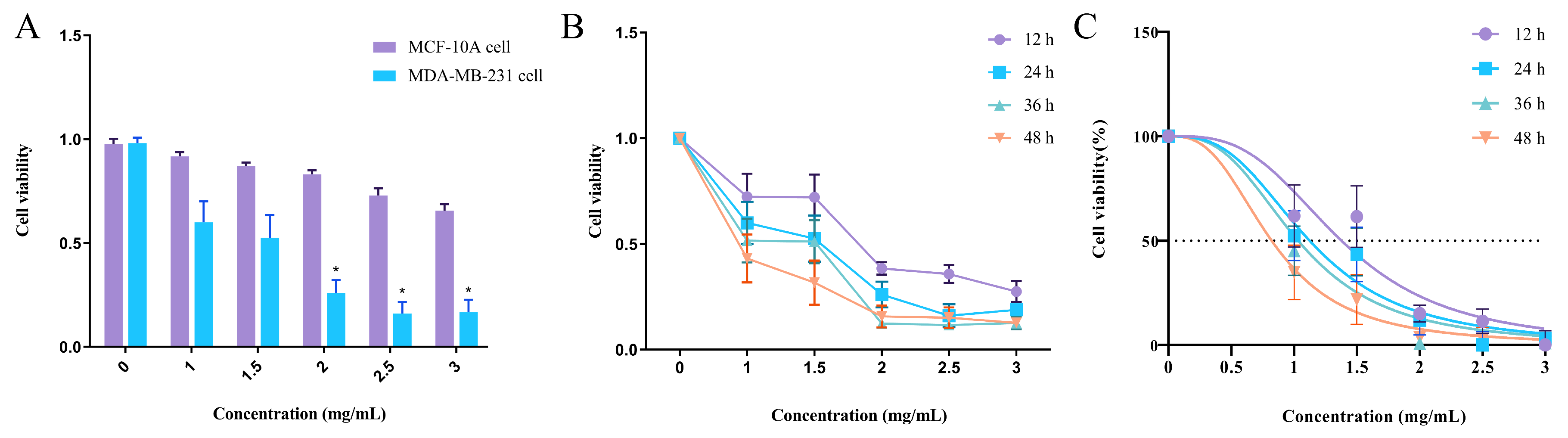
3.1. Effect of Dandelion Extract on the Viability of Human Breast Cancer MDA-MB-231 Cells
3.2. Identification of EA-2 Compound Components
3.3. Effect of EA-2 on the Viability of Normal Mammary Epithelial Cells
3.4. Concentration–Time Effect of EA-2 on MDA-MB-231 Cell Growth
3.5. EA-2 Inhibits the Proliferation of MDA-MB-231 Breast Cancer Cells In Vitro
3.6. Effect of EA-2 on the Morphology of MDA-MB-231 Breast Cancer Cells
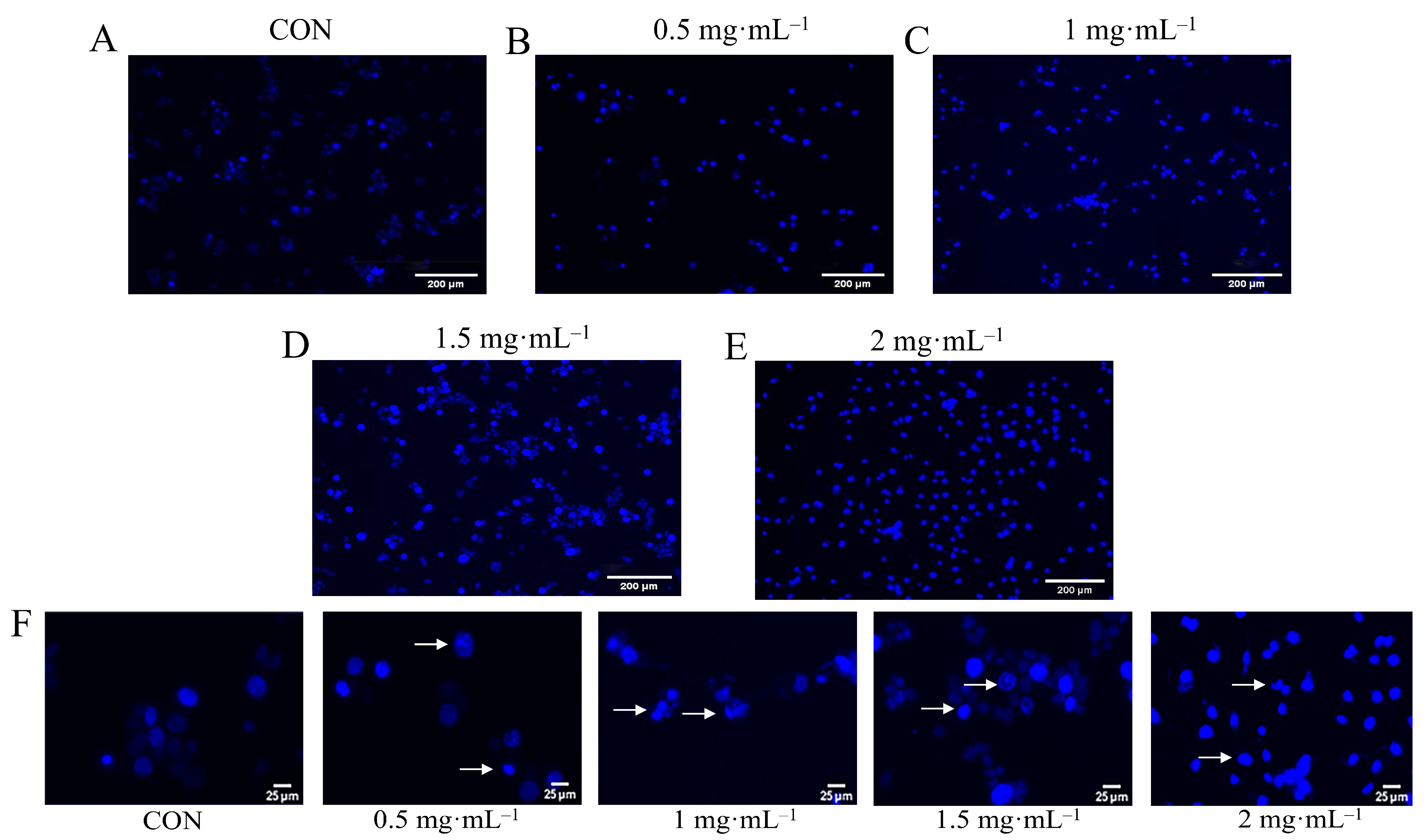
3.7. Hoechst Staining Observation of EA-2-Induced Apoptosis in MDA-MB-231 Cells
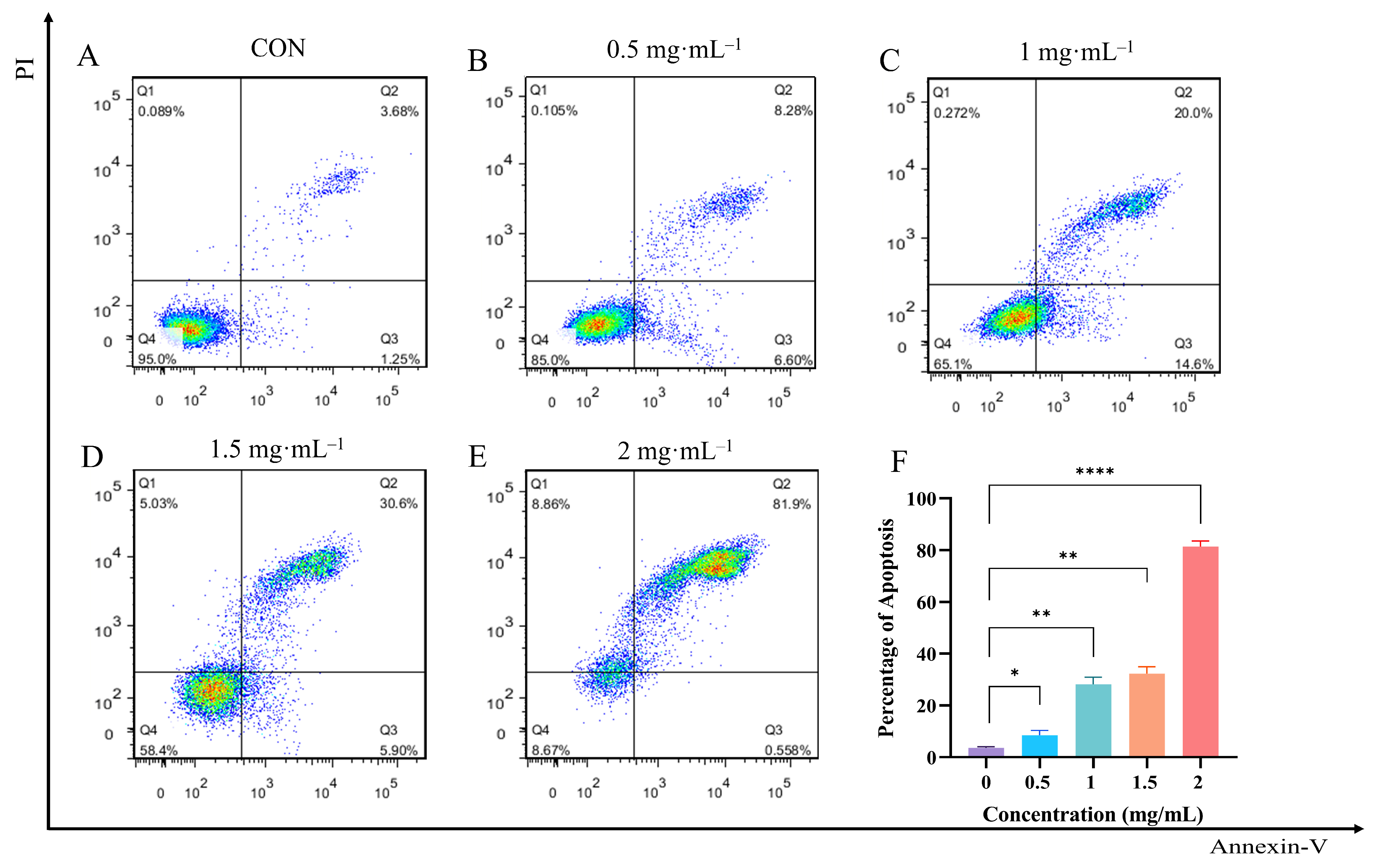
3.8. Effect of EA-2 on Apoptosis Rate of MDA-MB-231 Cells
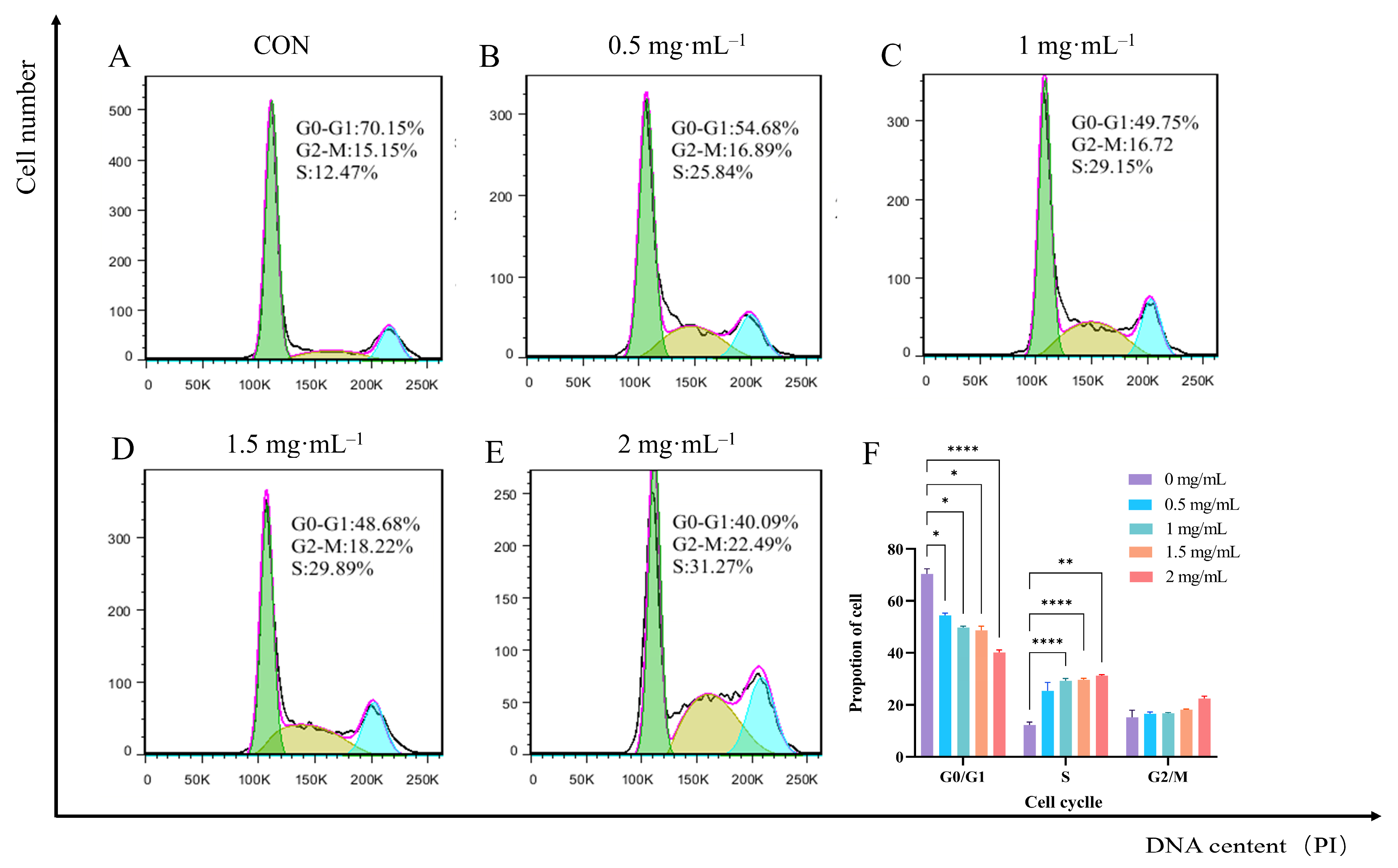
3.9. Effect of EA-2 on Cell Cycle Distribution of MDA-MB-231 Cells
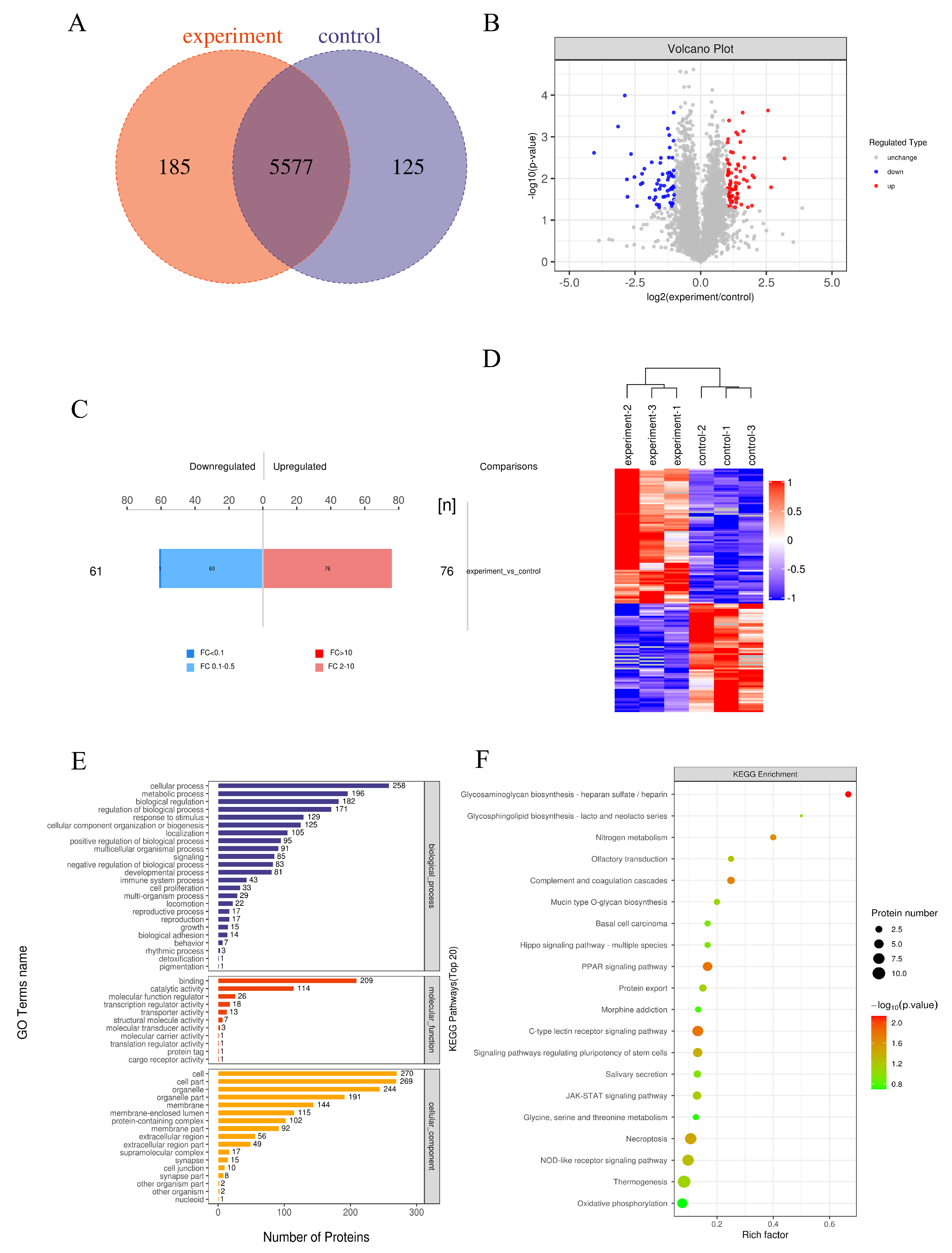
3.10. Differential Protein Identification and Quantification Analysis
3.11. Validation Analysis of Interactions Between Major Active Compounds and Potential Targets
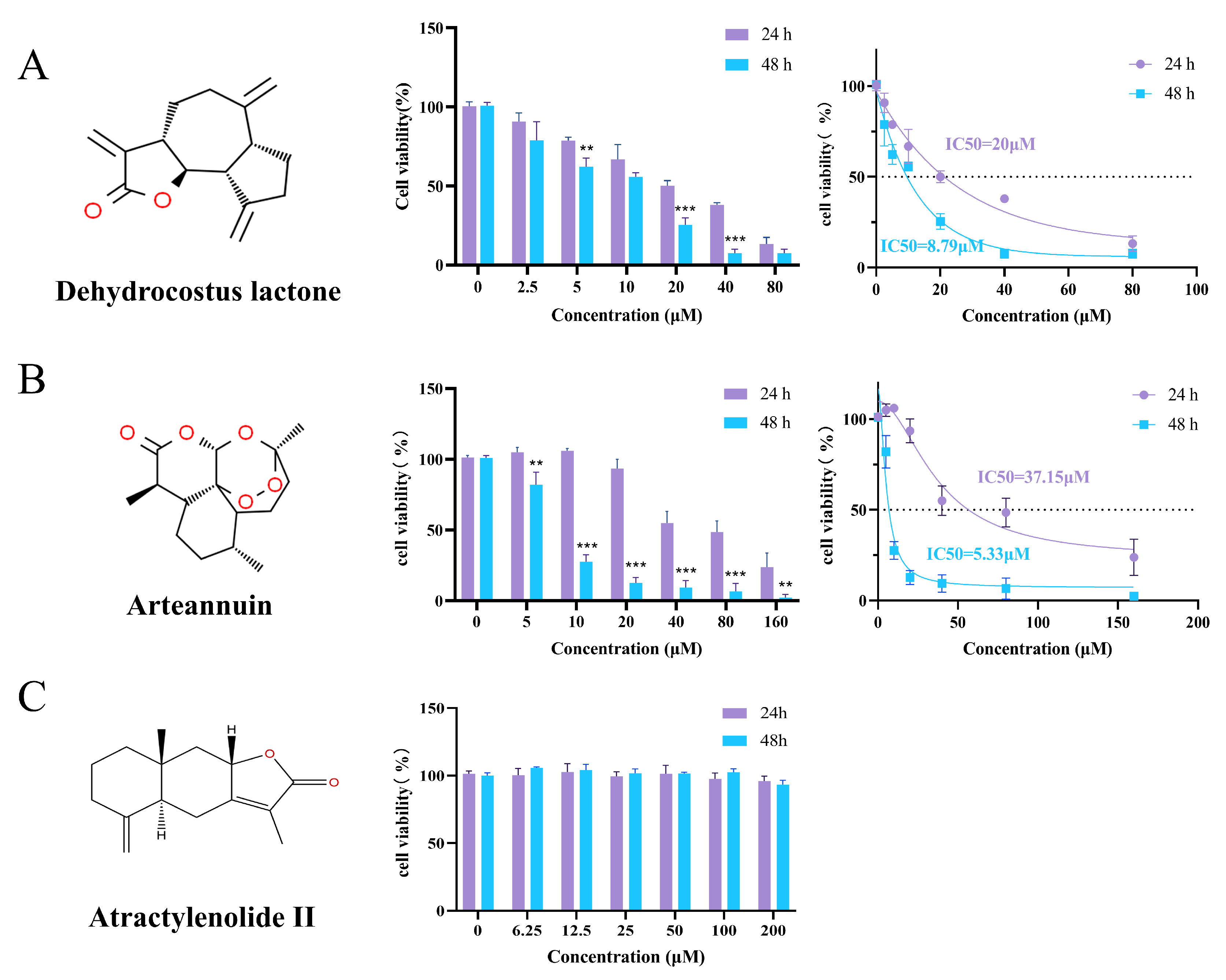
3.12. MTT Evaluation of Bioactive Compounds on MDA-MB-231 Breast Cancer Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| LC-MS | Liquid chromatography–tandem mass spectrometry |
| TNBC | Triple-negative breast cancer |
| ER | Estrogen receptor |
| PR | Progesterone receptor |
| HER2 | Human epidermal growth factor receptor 2 |
| CDKs | Cyclin-dependent kinases |
| PCD | Programmed cell death |
| FBS | Fetal bovine serum |
| ESI | Electrospray ionization |
| TIC | Total ion chromatograms |
| HRMS | Human epidermal growth factor receptor 2 |
| IC50 | Half-maximal inhibitory concentration |
| PI | Propidium iodide |
| LFQ | Label-Free Quantification |
| DR-AE | Dandelion root aqueous extract |
| DR-EE | Dandelion root ethanol extract |
| DL-AE | Dandelion leaf aqueous extract |
| DL-EE | Dandelion leaf ethanol extract |
| EA | Ethyl acetate |
| EA-2 | The second subfraction of the ethyl acetate extract |
| FDR | False discovery rate |
| DEPs | Differentially expressed proteins |
| GO | Gene Ontology |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| LogP | Partition coefficients |
| EMT | Epithelial–mesenchymal transition |
| ROS | Reactive oxygen species |
| UPLC | Ultra-performance liquid chromatography |
| SIM | Selected ion monitoring |
| SDT | Lysis buffer composed of SDS, DTT, and Tris-HCl |
| SDS | Sodium dodecyl sulfate |
| DTT | Dithiothreitol |
| BCA | Bicinchoninic Acid Assay |
| FASP | Filter-aided sample preparation |
| IAA | Iodoacetamide |
| DDA | Data-dependent acquisition |
| HCD | Higher-energy collisional dissociation |
| FC | Fold change |
| KAAS | KEGG Automatic Annotation Server |
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Zheng, R.; Baade, P.D.; Zhang, S.; Zeng, H.; Bray, F.; Jemal, A.; Yu, X.Q.; He, J. Cancer statistics in China, 2015. CA A Cancer J. Clin. 2016, 66, 115–132. [Google Scholar] [CrossRef] [PubMed]
- Zheng, R.; Zhang, S.; Zeng, H.; Wang, S.; Sun, K.; Chen, R.; Li, L.; Wei, W.; He, J. Cancer incidence and mortality in China, 2016. J. Natl. Cancer Cent. 2022, 2, 1–9. [Google Scholar] [CrossRef] [PubMed]
- McDonald, E.S.; Clark, A.S.; Tchou, J.; Zhang, P.; Freedman, G.M. Clinical Diagnosis and Management of Breast Cancer. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2016, 57 (Suppl. 1), 9S–16S. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.L.; Schwettmann, B.; McArthur, H.L.; Chan, I.S. Antibody-drug conjugates in breast cancer: Overcoming resistance and boosting immune response. J. Clin. Investig. 2023, 133, e172156. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Qiu, J.; Pan, C.; Tang, Y.; Chen, M.; Song, H.; Yang, J.; Hao, X. Potential roles and molecular mechanisms of bioactive ingredients in Curcumae Rhizoma against breast cancer. Phytomed. Int. J. Phytother. Phytopharm. 2023, 114, 154810. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Fu, X.; Shao, J.; Tang, Y.; Yu, M.; Li, L.; Huang, L.; Tang, K. Transcriptional regulatory network of high-value active ingredients in medicinal plants. Trends Plant Sci. 2023, 28, 429–446. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Yuan, X.; Mu, J.; Zou, Y.; Xu, L.; Chen, J.; Zhu, X.; Li, B.; Zeng, Z.; Wu, X.; et al. Quercetin induces MGMT(+) glioblastoma cells apoptosis via dual inhibition of Wnt3a/β-Catenin and Akt/NF-κB signaling pathways. Phytomed. Int. J. Phytother. Phytopharm. 2023, 118, 154933. [Google Scholar] [CrossRef] [PubMed]
- Cavalu, S.; Abdelhamid, A.M.; Saber, S.; Elmorsy, E.A.; Hamad, R.S.; Abdel-Reheim, M.A.; Yahya, G.; Salama, M.M. Cell cycle machinery in oncology: A comprehensive review of therapeutic targets. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2024, 38, e23734. [Google Scholar] [CrossRef] [PubMed]
- Malumbres, M.; Barbacid, M. Cell cycle, CDKs and cancer: A changing paradigm. Nat. Rev. Cancer 2009, 9, 153–166. [Google Scholar] [CrossRef] [PubMed]
- Vousden, K.H.; Prives, C. Blinded by the Light: The Growing Complexity of p53. Cell 2009, 137, 413–431. [Google Scholar] [CrossRef] [PubMed]
- Ahagh, M.H.; Dehghan, G.; Mehdipour, M.; Teimuri-Mofrad, R.; Payami, E.; Sheibani, N.; Ghaffari, M.; Asadi, M. Synthesis, characterization, anti-proliferative properties and DNA binding of benzochromene derivatives: Increased Bax/Bcl-2 ratio and caspase-dependent apoptosis in colorectal cancer cell line. Bioorg. Chem. 2019, 93, 103329. [Google Scholar] [CrossRef] [PubMed]
- Fan, M.; Zhang, X.; Song, H.; Zhang, Y. Dandelion (Taraxacum Genus): A Review of Chemical Constituents and Pharmacological Effects. Molecules 2023, 28, 5022. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yang, X.; Gan, J.; Chen, S.; Xiao, Z.X.; Cao, Y. CB-Dock2: Improved protein-ligand blind docking by integrating cavity detection, docking and homologous template fitting. Nucleic Acids Res. 2022, 50, W159–W164. [Google Scholar] [CrossRef] [PubMed]
- Abderrazak, A.; Couchie, D.; Mahmood, D.F.; Elhage, R.; Vindis, C.; Laffargue, M.; Matéo, V.; Büchele, B.; Ayala, M.R.; El Gaafary, M.; et al. Anti-inflammatory and antiatherogenic effects of the NLRP3 inflammasome inhibitor arglabin in ApoE2.Ki mice fed a high-fat diet. Circulation 2015, 131, 1061–1070. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.X.; Jiang, F.J.; Liu, G.; Wang, Y.Y.; Gao, Z.Q.; Jin, S.H.; Nie, Y.J.; Chen, D.; Chen, J.L.; Pang, Q.F. Dehydrocostus Lactone Attenuates Methicillin-Resistant Staphylococcus aureus-Induced Inflammation and Acute Lung Injury via Modulating Macrophage Polarization. Int. J. Mol. Sci. 2021, 22, 9754. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Peng, Z.; Su, C. Potential anti-cancer activities and mechanisms of costunolide and dehydrocostuslactone. Int. J. Mol. Sci. 2015, 16, 10888–10906. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Shen, J.; Yuan, M.; Li, H.; Li, Y.; Zheng, S.; Han, B.; Zhang, C.; Liu, S.; Sun, Q.; et al. Dehydrocostus lactone suppresses gastric cancer progression by targeting ACLY to inhibit fatty acid synthesis and autophagic flux. J. Adv. Res. 2025, 67, 331–348. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Nasser, M.I.; Adlat, S.; Ming Jiang, M.; Jiang, N.; Gao, L. Atractylenolide II Induces Apoptosis of Prostate Cancer Cells through Regulation of AR and JAK2/STAT3 Signaling Pathways. Molecules 2018, 23, 3298. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Song, L.; Zuo, Z.; Chen, Z.; Wang, Y.; Cai, H.; Gu, Y.; Lv, Z.; Guan, J.; Chen, R.; et al. Parthenolide attenuates hypoxia-induced pulmonary hypertension through inhibiting STAT3 signaling. Phytomed. Int. J. Phytother. Phytopharm. 2024, 134, 155976. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Ding, C.; Rasul, A.; Yi, F.; Li, T.; Gao, H.; Gao, R.; Zhong, L.; Zhang, K.; Fang, X.; et al. Isoalantolactone induces reactive oxygen species mediated apoptosis in pancreatic carcinoma PANC-1 cells. Int. J. Biol. Sci. 2012, 8, 533–547. [Google Scholar] [CrossRef] [PubMed]
- Tu, Y. Artemisinin-A Gift from Traditional Chinese Medicine to the World (Nobel Lecture). Angew. Chem. 2016, 55, 10210–10226. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Hu, Q.; Wen, T.; Luo, L.; Liu, L.; Wang, L.; Shen, X. Arteannuin B, a sesquiterpene lactone from Artemisia annua, attenuates inflammatory response by inhibiting the ubiquitin-conjugating enzyme UBE2D3-mediated NF-κB activation. Phytomed. Int. J. Phytother. Phytopharm. 2024, 124, 155263. [Google Scholar] [CrossRef] [PubMed]
- Gao, T.; Yu, C.; Shi, X.; Hu, Y.; Chang, Y.; Zhang, J.; Wang, Y.; Zhai, Z.; Jia, X.; Mao, Y. Artemisinic acid attenuates osteoclast formation and titanium particle-induced osteolysis via inhibition of RANKL-induced ROS accumulation and MAPK and NF-κB signaling pathways. Front. Pharmacol. 2024, 15, 1345380. [Google Scholar] [CrossRef] [PubMed]
- Augustin, Y.; Staines, H.M.; Krishna, S. Artemisinins as a novel anti-cancer therapy: Targeting a global cancer pandemic through drug repurposing. Pharmacol. Ther. 2020, 216, 107706. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Yu, H.; Xia, J.; Liu, L.; Liu, G.J.; Sang, H.; Peinemann, F. Topical azelaic acid, salicylic acid, nicotinamide, sulphur, zinc and fruit acid (alpha-hydroxy acid) for acne. Cochrane Database Syst. Rev. 2020, 5, CD011368. [Google Scholar] [CrossRef] [PubMed]
- Searle, T.; Ali, F.R.; Al-Niaimi, F. The versatility of azelaic acid in dermatology. J. Dermatol. Treat. 2022, 33, 722–732. [Google Scholar] [CrossRef] [PubMed]
- Zakeri Siavashani, A.; Mohammadi, J.; Maniura-Weber, K.; Senturk, B.; Nourmohammadi, J.; Sadeghi, B.; Huber, L.; Rottmar, M. Silk based scaffolds with immunomodulatory capacity: Anti-inflammatory effects of nicotinic acid. Biomater. Sci. 2019, 8, 148–162. [Google Scholar] [CrossRef] [PubMed]
- Ji, C.; Shinohara, M.; Kuhlenkamp, J.; Chan, C.; Kaplowitz, N. Mechanisms of protection by the betaine-homocysteine methyltransferase/betaine system in HepG2 cells and primary mouse hepatocytes. Hepatology 2007, 46, 1586–1596. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; He, F.; Wu, C.; Li, P.; Li, N.; Deng, J.; Zhu, G.; Ren, W.; Peng, Y. Betaine in Inflammation: Mechanistic Aspects and Applications. Front. Immunol. 2018, 9, 1070. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Qi, Y.; Zhier, A.L.; Liu, S.; Zhang, Z.; Zhou, L. Betaine increases mitochondrial content and improves hepatic lipid metabolism. Food Funct. 2019, 10, 216–223. [Google Scholar] [CrossRef] [PubMed]
- Lone, S.H.; Bhat, K.A.; Khuroo, M.A. Arglabin: From isolation to antitumor evaluation. Chem.-Biol. Interact. 2015, 240, 180–198. [Google Scholar] [CrossRef] [PubMed]
- Li, C.J.; Guo, S.F.; Shi, T.M. Culture supernatants of breast cancer cell line MDA-MB-231 treated with parthenolide inhibit the proliferation, migration, and lumen formation capacity of human umbilical vein endothelial cells. Chin. Med. J. 2012, 125, 2195–2199. [Google Scholar] [PubMed]
- Li, Z.; Qin, B.; Qi, X.; Mao, J.; Wu, D. Isoalantolactone induces apoptosis in human breast cancer cells via ROS-mediated mitochondrial pathway and downregulation of SIRT1. Arch. Pharmacal Res. 2016, 39, 1441–1453. [Google Scholar] [CrossRef] [PubMed]
- Yen, M.C.; Shih, Y.C.; Hsu, Y.L.; Lin, E.S.; Lin, Y.S.; Tsai, E.M.; Ho, Y.W.; Hou, M.F.; Kuo, P.L. Isolinderalactone enhances the inhibition of SOCS3 on STAT3 activity by decreasing miR-30c in breast cancer. Oncol. Rep. 2016, 35, 1356–1364. [Google Scholar] [CrossRef] [PubMed]
- Kotammagari, T.K.; Paul, S.; Barik, G.K.; Santra, M.K.; Bhattacharya, A.K. Synthesis of artemisinic acid derived glycoconjugates and their anticancer studies. Org. Biomol. Chem. 2020, 18, 2252–2263. [Google Scholar] [CrossRef] [PubMed]
- Harbeck, N.; Gnant, M. Breast cancer. Lancet 2017, 389, 1134–1150. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, H.; Merkher, Y.; Chen, L.; Liu, N.; Leonov, S.; Chen, Y. Recent advances in therapeutic strategies for triple-negative breast cancer. J. Hematol. Oncol. 2022, 15, 121. [Google Scholar] [CrossRef] [PubMed]
- Di Nardo, P.; Lisanti, C.; Garutti, M.; Buriolla, S.; Alberti, M.; Mazzeo, R.; Puglisi, F. Chemotherapy in patients with early breast cancer: Clinical overview and management of long-term side effects. Expert Opin. Drug Saf. 2022, 21, 1341–1355. [Google Scholar] [CrossRef] [PubMed]
- Kang, L.; Miao, M.S.; Song, Y.G.; Fang, X.Y.; Zhang, J.; Zhang, Y.N.; Miao, J.X. Total flavonoids of Taraxacum mongolicum inhibit non-small cell lung cancer by regulating immune function. J. Ethnopharmacol. 2021, 281, 114514. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.J.; Liu, S.T.; Yang, R.C.; Wang, L.H.; Tsai, C.C.; Chen, T.W.; Huang, S.M. Anticancer Effects of Taraxacum via Cell Cycle Arrest, Necrosis, Apoptosis, and Endoplasmic Reticulum Stress. Am. J. Chin. Med. 2022, 50, 569–587. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Cui, L.; Feng, L.; Zhang, Z.; Song, J.; Liu, D.; Jia, X. Isoalantolactone inhibits the migration and invasion of human breast cancer MDA-MB-231 cells via suppression of the p38 MAPK/NF-κB signaling pathway. Oncol. Rep. 2016, 36, 1269–1276. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Meng, X.; Li, Y.; Yang, C.; Liu, Y. Growth inhibition effects of isoalantolactone on K562/A02 cells: Caspase-dependent apoptotic pathways, S phase arrest, and downregulation of Bcr/Abl. Phytother. Res. PTR 2014, 28, 1679–1686. [Google Scholar] [CrossRef] [PubMed]
- Lang, S.J.; Schmiech, M.; Hafner, S.; Paetz, C.; Werner, K.; El Gaafary, M.; Schmidt, C.Q.; Syrovets, T.; Simmet, T. Chrysosplenol d, a Flavonol from Artemisia annua, Induces ERK1/2-Mediated Apoptosis in Triple Negative Human Breast Cancer Cells. Int. J. Mol. Sci. 2020, 21, 4090. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Kang, Y.H.; Hwang, S.C.; Oh, S.H.; Byun, J.H. Parthenolide Has Negative Effects on In Vitro Enhanced Osteogenic Phenotypes by Inflammatory Cytokine TNF-α via Inhibiting JNK Signaling. Int. J. Mol. Sci. 2020, 21, 5433. [Google Scholar] [CrossRef] [PubMed]
- Denda, Y.; Matsuo, Y.; Sugita, S.; Eguchi, Y.; Nonoyama, K.; Murase, H.; Kato, T.; Imafuji, H.; Saito, K.; Morimoto, M.; et al. The Natural Product Parthenolide Inhibits Both Angiogenesis and Invasiveness and Improves Gemcitabine Resistance by Suppressing Nuclear Factor κB Activation in Pancreatic Cancer Cell Lines. Nutrients 2024, 16, 705. [Google Scholar] [CrossRef] [PubMed]
- Asghar, U.; Witkiewicz, A.K.; Turner, N.C.; Knudsen, E.S. The history and future of targeting cyclin-dependent kinases in cancer therapy. Nat. Rev. Drug Discov. 2015, 14, 130–146. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.J.; Kim, G.H. Daidzein causes cell cycle arrest at the G1 and G2/M phases in human breast cancer MCF-7 and MDA-MB-453 cells. Phytomed. Int. J. Phytother. Phytopharm. 2008, 15, 683–690. [Google Scholar] [CrossRef] [PubMed]
- Maggio, B.; Raimondi, M.V.; Raffa, D.; Plescia, F.; Scherrmann, M.C.; Prosa, N.; Lauricella, M.; D’Anneo, A.; Daidone, G. Synthesis and antiproliferative activity of a natural like glycoconjugate polycyclic compound. Eur. J. Med. Chem. 2016, 122, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Yu, H.; Dong, G.; Cai, L.; Bai, Y. Chamaejasmine arrests cell cycle, induces apoptosis and inhibits nuclear NF-κB translocation in the human breast cancer cell line MDA-MB-231. Molecules 2013, 18, 845–858. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Xiao, Y.; Liu, S.; Luo, F.; Tang, D.; Yu, Y.; Xie, Y. Isorhamnetin induces cell cycle arrest and apoptosis by triggering DNA damage and regulating the AMPK/mTOR/p70S6K signaling pathway in doxorubicin-resistant breast cancer. Phytomed. Int. J. Phytother. Phytopharm. 2023, 114, 154780. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Cong, X.; Zhai, L.; Hu, H.; Xu, J.Y.; Zhao, W.; Zhu, M.; Tan, M.; Ye, B.C. Comparative evaluation of label-free quantification strategies. J. Proteom. 2020, 215, 103669. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Jiang, H.D.; Kan, H.Y.; Zhang, L.; Rao, Y.X.; Jiang, X.B.; Li, M.H.; Wang, Q. RIT1 Promotes the Proliferation of Gliomas Through the Regulation of the PI3K/AKT/c-Myc Signalling Pathway. J. Cell. Mol. Med. 2025, 29, e70362. [Google Scholar] [CrossRef] [PubMed]
- Qian, X.; Bi, Q.Y.; Wang, Z.N.; Han, F.; Liu, L.M.; Song, L.B.; Li, C.Y.; Zhang, A.Q.; Ji, X.M. Qingyihuaji Formula promotes apoptosis and autophagy through inhibition of MAPK/ERK and PI3K/Akt/mTOR signaling pathway on pancreatic cancer in vivo and in vitro. J. Ethnopharmacol. 2023, 307, 116198. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhou, J.; Cheng, A.; Liu, Y.; Guo, J.; Li, X.; Chen, M.; Hu, D.; Wu, J. Artesunate-binding FABP5 promotes apoptosis in lung cancer cells via the PPARγ-SCD pathway. Int. Immunopharmacol. 2024, 143, 113381. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.B.; Hou, P.P.; Liu, F.Y.; Hong, W.B.; Chen, H.Z.; Sun, X.Y.; Li, P.; Zhang, Y.; Ju, C.Y.; Luo, L.J.; et al. Blocking PPARγ interaction facilitates Nur77 interdiction of fatty acid uptake and suppresses breast cancer progression. Proc. Natl. Acad. Sci. USA 2020, 117, 27412–27422. [Google Scholar] [CrossRef] [PubMed]
| No. | Compound | Rt (min) | Molecular Formula | Quantifier Ion | mzVault Best Match | |
|---|---|---|---|---|---|---|
| Type | m/z | |||||
| 1 | Betaine | 0.587 | C5H11NO2 | [M + H]+ | 118.0865 | 84.6 |
| 2 | (R)-Mandelic acid | 3.027 | C8H8O3 | [M − H]− | 151.0399 | 77.9 |
| 3 | Azelaic acid | 5.057 | C9H16O4 | [M − H]− | 187.0974 | 85.3 |
| 4 | Arglabin | 5.806 | C15H18O3 | [M + H]+ | 247.1329 | 76.7 |
| 5 | Dehydrocostus lactone | 5.258 | C15H18O2 | [M + H]+ | 231.1380 | 93.5 |
| 6 | Arteannuin | 6.071 | C15H20O3 | [M + H]+ | 249.1479 | 83.5 |
| 7 | Nicotinic acid | 0.819 | C6H5NO2 | [M + H]+ | 124.0395 | 80.6 |
| 8 | Atractylenolide II | 4.757 | C15H20O2 | [M + H]+ | 233.1532 | 83.7 |
| 9 | Parthenolide | 6.068 | C15H20O3 | [M + H]+ | 249.1099 | 90.3 |
| 10 | Linderalactone | 4.699 | C15H16O3 | [M + H]+ | 245.1172 | 89.0 |
| 11 | Artemisinic acid | 5.261 | C15H22O2 | [M + H]+ | 235.1693 | 84.6 |
| 12 | Isoalantolactone | 4.762 | C15H20O2 | [M − H]− | 233.1535 | 86.2 |
| Category | Members | Main Biological Activities |
|---|---|---|
| Sesquiterpene lactones | Arglabin, Dehydrocostus lactone, Atractylenolide II, Parthenolide, Isoalantolactone, Linderalactone | Anti-inflammatory [15,16], antitumor [17,18,19], apoptosis induction [20,21] |
| Sesquiterpenes (atypical) | Arteannuin, Artemisinic acid | Antimalarial [22], anti-inflammatory [23,24], anticancer [25] |
| Organic acids | (R)-Mandelic acid, Azelaic acid, Nicotinic acid | Antibacterial [26], anti-inflammatory [27,28] |
| Amino acid derivatives | Betaine | Cytoprotective [29], antioxidant [30], metabolic regulation [31] |
| Time (hours) | Cell Viability | IC50 (mg/mL) |
|---|---|---|
| 12 | 0.72 ± 0.11 | 1.577 ± 0.23 |
| 24 | 0.53 ± 0.12 * | 1.124 ± 0.18 * |
| 36 | 0.51 ± 0.10 * | 1.032 ± 0.12 * |
| 48 | 0.32 ± 0.11 ** | 0.821 ± 0.20 * |
| Active Compounds | Binding Energy/ (kcal·mol−1) | ||||
|---|---|---|---|---|---|
| PI3K | PAK1 | JAK1 | STAT3 | FABP4 | |
| Betaine | −4.2 | −3.2 | −3.6 | −3.5 | −4.5 |
| (R)-Mandelic acid | −6.6 | −5.3 | −5.9 | −5.3 | −4.6 |
| Azelaic acid | −5.9 | −4.8 | −3.5 | −5.4 | −4.2 |
| Arglabin | −7.9 | −6.1 | −8.7 | −6.7 | −8.6 |
| Dehydrocostus lactone | −7.8 | −6.9 | −8.5 | −6.7 | −7.0 |
| Arteannuin | −8.4 | −7.3 | −9.3 | −7.5 | −8.9 |
| Nicotinic acid | −5.6 | −3.8 | −4.8 | −4.6 | −4.9 |
| Atractylenolide II | −8.2 | −6.8 | −7.8 | −6.8 | −6.5 |
| Parthenolide | −7.9 | −4.2 | −7.5 | −6.7 | −7.4 |
| Linderalactone | −8.1 | −6.6 | −7.8 | −6.6 | −8.1 |
| Artemisinic acid | −7.5 | −6.1 | −7.2 | −6.6 | −5.5 |
| Isoalantolactone | −8.3 | −6.8 | −8.6 | −6.4 | −6.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mou, W.; Zhang, P.; Cui, Y.; Yang, D.; Zhao, G.; Xu, H.; Zhang, D.; Liang, Y. Mechanistic Study on the Inhibitory Effect of Dandelion Extract on Breast Cancer Cell Proliferation and Its Induction of Apoptosis. Biology 2025, 14, 910. https://doi.org/10.3390/biology14080910
Mou W, Zhang P, Cui Y, Yang D, Zhao G, Xu H, Zhang D, Liang Y. Mechanistic Study on the Inhibitory Effect of Dandelion Extract on Breast Cancer Cell Proliferation and Its Induction of Apoptosis. Biology. 2025; 14(8):910. https://doi.org/10.3390/biology14080910
Chicago/Turabian StyleMou, Weifeng, Ping Zhang, Yu Cui, Doudou Yang, Guanjie Zhao, Haijun Xu, Dandan Zhang, and Yinku Liang. 2025. "Mechanistic Study on the Inhibitory Effect of Dandelion Extract on Breast Cancer Cell Proliferation and Its Induction of Apoptosis" Biology 14, no. 8: 910. https://doi.org/10.3390/biology14080910
APA StyleMou, W., Zhang, P., Cui, Y., Yang, D., Zhao, G., Xu, H., Zhang, D., & Liang, Y. (2025). Mechanistic Study on the Inhibitory Effect of Dandelion Extract on Breast Cancer Cell Proliferation and Its Induction of Apoptosis. Biology, 14(8), 910. https://doi.org/10.3390/biology14080910







