Aquaculture Strategy and Genetic Diversity of Argopecten irradians concentricus in Beibu Gulf, China
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Sites and Scallops
2.2. Growth Experiment
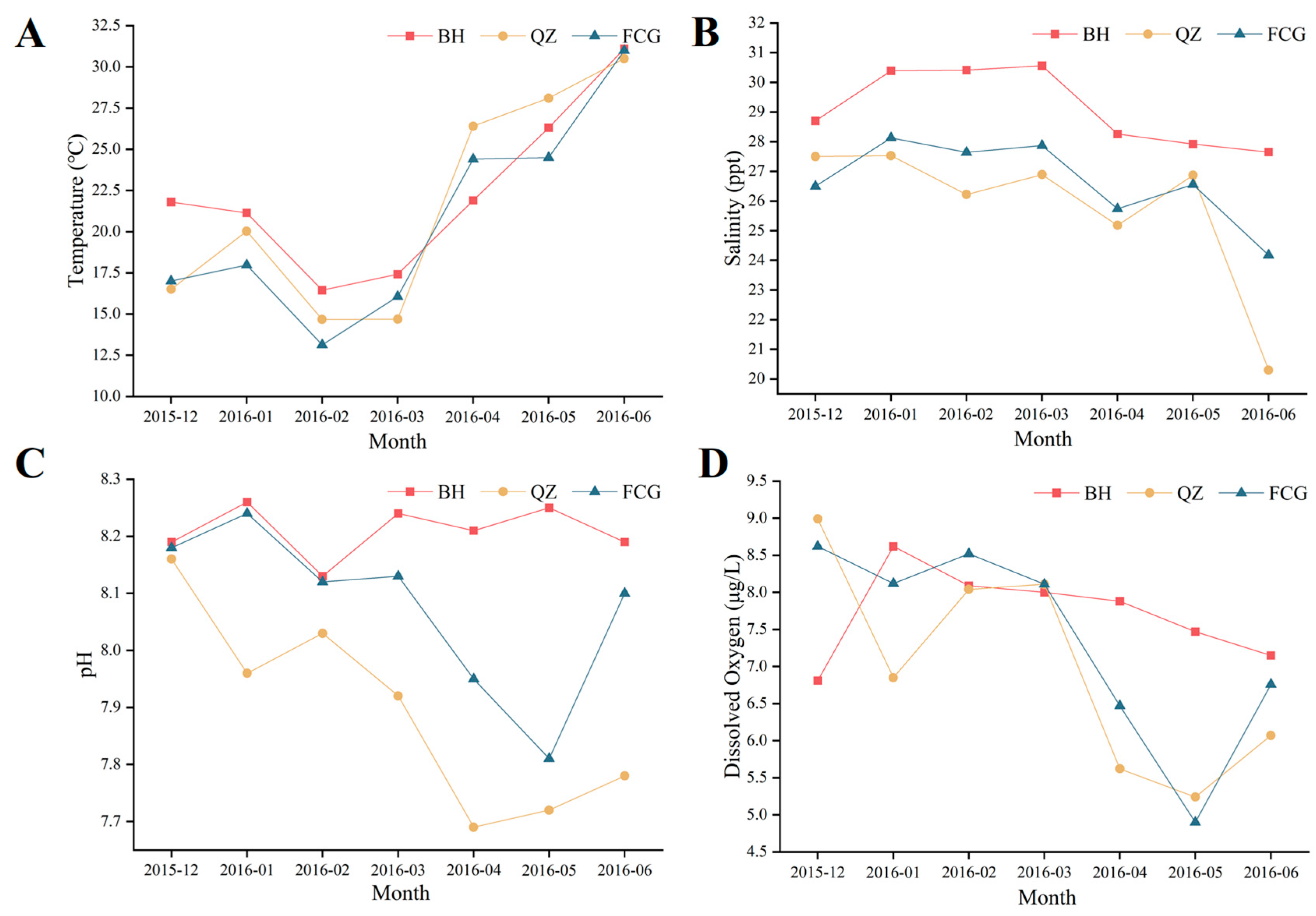
2.3. Environmental Factor and Phytoplankton Analysis
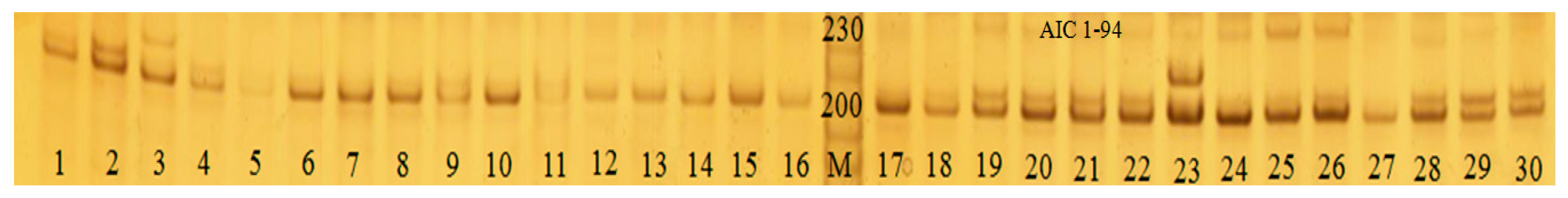
2.4. DNA Isolation and PCR Amplification
2.5. Statistical Analysis
3. Results
3.1. Effect of Stocking Density, Site, and Strain on the Growth of A. i. concentricus
3.2. Effect of Different Months on the Mortality of A. i. concentricus in Adult Culture Stage
3.3. Seawater Quality and Plankton at the Three Sites
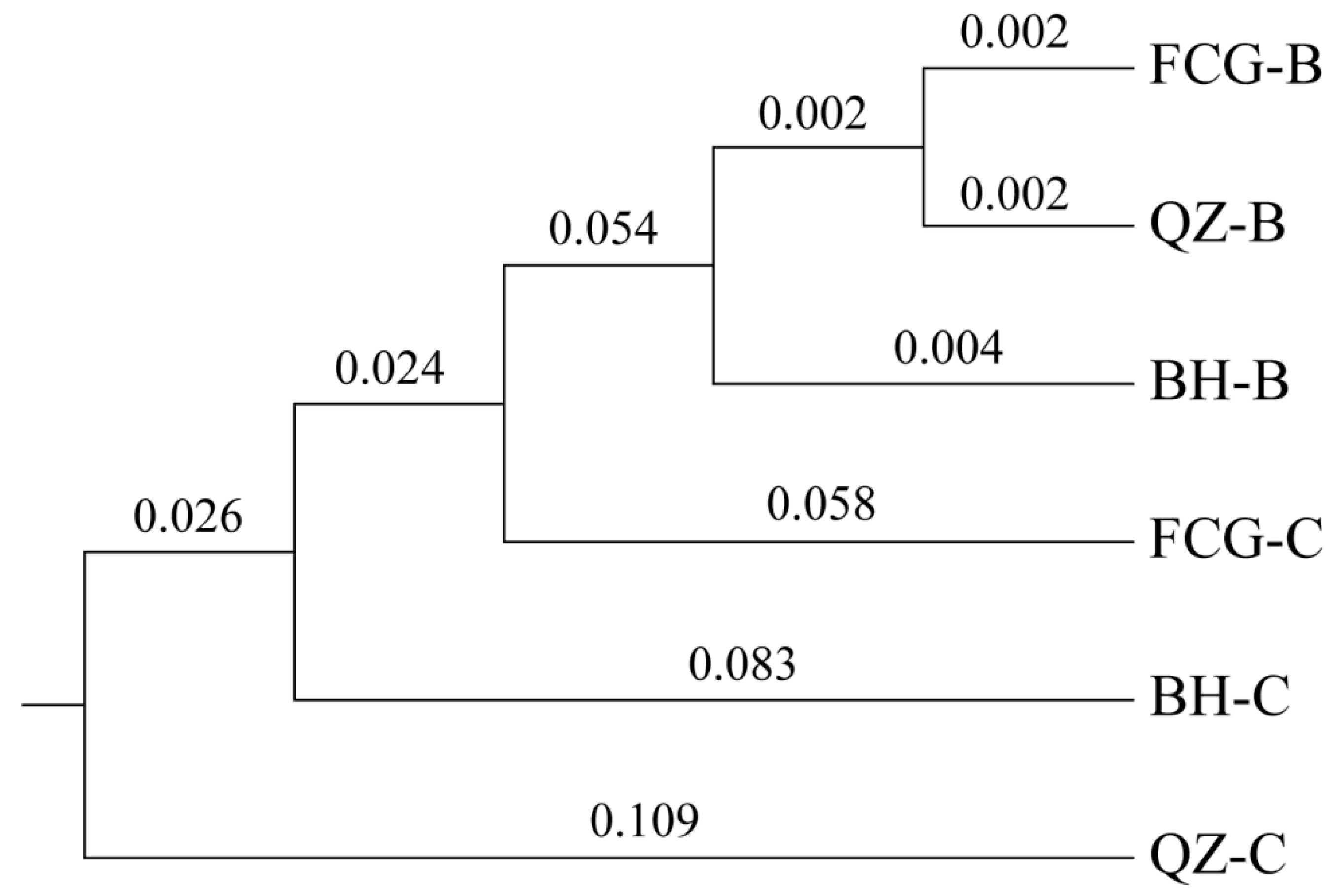
3.4. Genetic Variability of Six Scallop Cultured Populations
4. Discussion
4.1. Effects of Different Stocking Densities on Growth Performance
4.2. Effects of Different Sites on Growth Performance
4.3. Comparison of the Genetic Diversity of Populations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, H.; Liu, X.; Zhang, G.; Wang, C. Growth and survival of reciprocal crosses between two bay scallops, Argopecten irradians concentricus Say and A. irradians irradians Lamarck. Aquaculture 2007, 272 (Suppl. 1), S88–S93. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, T.; Zhou, J. Historical occurrence of algal blooms in the northern Beibu gulf of China and implications for future trends. Front. Microbiol. 2019, 10, 451. [Google Scholar] [CrossRef]
- Ji, Y.; Yan, G.; Wang, G.; Liu, J.; Tang, Z.; Yan, Y.; Qiu, J.; Zhang, L.; Pan, W.; Fu, Y.; et al. Prevalence and distribution of domoic acid and cyclic imines in bivalve mollusks from beibu gulf, China. J. Hazard. Mater. 2022, 423, 127078. [Google Scholar] [CrossRef]
- Tan, J. Transcriptome Analysis of Argopecten irradians Concentricus and Study on the Molecular Markers Ofits Progeny Crossed with Argopecten bohaihong. Master’s Thesis, Guangdong Ocean University, Zhanjiang, China, 2018. (In Chinese). [Google Scholar]
- Liu, J.; Liu, Z.; Sun, X. The effects of inbreeding on production traits of the southern bay scallop Argopecten Irradians concentricus. J. Shellfish Res. 2011, 30, 109–113. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, H.; Zheng, Y. The effect of parental selection on inbred first filial generation of Argopecten irradians concentricus Say. J. Fish. China 2007, 31, 443–451. (In Chinese) [Google Scholar]
- Yang, C.; Wang, L.; Liu, C.; Zhou, Z.; Xin, Z.; Song, L. The polymorphisms in the promoter of HSP90 gene and their association with heat tolerance of bay scallop. Cell Stress Chaperon. 2015, 20, 297–308. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Li, C.; Chen, M.; Liu, B.; Yan, X.; Wang, C. Draft genomes of two Atlantic bay scallop subspecies Argopecten irradians irradians and A. i. concentricus. Sci. Data 2020, 7, 99. [Google Scholar] [CrossRef]
- Zheng, H.; Xu, F.; Zhang, G. Crosses between two subspecies of bay scallop Argopecten irradians and heterosis for yield traits at harvest. Aquac. Res. 2011, 42, 602–612. [Google Scholar] [CrossRef]
- Griffith, A.W.; Harke, M.J.; Depasquale, E.; Berry, D.L.; Gobler, C. The harmful algae, Cochlodinium polykrikoides and Aureococcus anophagefferens, elicit stronger transcriptomic and mortality response in larval bivalves (Argopecten irradians) than climate change stressors. Ecol. Evol. 2019, 9, 4931–4948. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Gao, X.; Zhao, J.; Liu, Y.; Xie, L.; Lv, X.; Xing, Q. Summer deoxygenation in a bay scallop (Argopecten irradians) farming area: The decisive role of water temperature, stratification and beyond. Mar. Pollut. Bull. 2021, 173, 113092. [Google Scholar] [CrossRef]
- Li, H.; Liu, X.; Zhang, G. A consensus microsatellite-based linkage map for the hermaphroditic bay scallop (Argopecten irradians) and its application in size-related QTL analysis. PLoS ONE 2012, 7, e46926. [Google Scholar] [CrossRef] [PubMed]
- Hu, P.; Liu, H.; Pan, Y.; Qu, Y.; Tian, X.; Wang, K.; Wei, Y.; Wu, X.; Xing, X.; Zhang, Z.; et al. Microsatellite records for volume 11, issue 3. Conserv. Genet. Resour. 2019, 11, 365–368. [Google Scholar] [CrossRef]
- Wei, Z.; Qin, Y.; Liu, H.; Xing, Q.; Yu, Z.; Zhang, Y.; Pan, Y. Aquaculture performance and genetic diversity of a new [(Crassostrea hongkongensis ♀ × C. gigas ♂) ♂ × C. hongkongensis ♀] variety of the oyster “south China No. 1” in Beibu Gulf, China. Biology 2024, 13, 297. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, J.; Mei, S. Effects of tide levelculture density and season on growth and survival of wrinkled clam, Meretrix lyrata, juveniles. Mar. Sci. 2011, 35, 34–41. [Google Scholar] [CrossRef]
- Gao, X.; Zhang, M.; Li, X.; Wu, F.; Song, C.; Liu, Y. Effects of stocking density on survival, growth, and food intake of Haliotis discus hannai Ino in recirculating aquaculture systems. Aquaculture 2018, 482, 221–230. [Google Scholar] [CrossRef]
- Miyoshi, K.; Chiba, S. Predation risk management of sea stars (Asterias amurensis and Distolasterias nipon) by adjusting the density and size of seeded scallops (Mizuhopecten yessoensis): An improvement to local mariculture. Aquacult. Int. 2022, 30, 429–443. [Google Scholar] [CrossRef]
- Xiao, J.; Ford, S.; Yang, H.; Zhang, G.; Zhang, F.; Guo, X. Studies on mass summer mortality of cultured zhikong scallops (Chlamys farreri Jones et Preston) in China. Aquaculture 2005, 250, 602–615. [Google Scholar] [CrossRef]
- He, M.; Zhang, H.; Yuan, T. The growth and survival of a selected line in pearl oyster Pinctada martensii cultured in different densities, water, layers and sites. J. Trop. Oceanogr. 2009, 28, 68–71. [Google Scholar] [CrossRef]
- Liu, S.; Cui, J.; Lin, Y. Effects of self-thinning in the sea of raft culture of Pationopecten yessoensis. J. Fish. China 2013, 37, 1513–1520. (In Chinese) [Google Scholar] [CrossRef]
- Li, Z.; Liu, Z.; Liu, F.; Zhong, Y. Cultured density of new Argopecten irradians concentricus line in nursery and adult culture stage. Oceanol. Limnol. Sin. 2013, 44, 1557–1565. (In Chinese) [Google Scholar]
- De Oliveira, I.B.; Lavander, H.D.; Lima, P.; Oliveira, C.Y.B.; De Dantas, D.M.; Olivera Gálvez, A. Effect of stocking density on the growth and survival of Anomalocardia brasiliana (Gmelin, 1791) (Bivalvia: Veneridae) post-larvae. Cienc. Rural 2019, 49, e20190420. [Google Scholar] [CrossRef]
- Zhang, J.; Ma, W.; Song, X.; Lin, Q.; Gui, J.; Mei, J. Characterization and development of EST-SSR markers derived from transcriptome of yellow catfish. Molecules 2014, 19, 16402–16415. [Google Scholar] [CrossRef]
- Botstein, D.; White, R.L.; Skolnick, M.; Davis, R.W. Construction of a genetic linkage map in man using restriction fragment length polymorphisms. Am. J. Hum. Genet. 1980, 32, 314–331. [Google Scholar]
- Jonasson, J.P.; Thorarinsdottir, G.G.; Eiriksson, H.; Marteinsdottir, G. Temperature tolerance of Iceland scallop, Chlamys islandica (O.F. Muller) under controlled experimental conditions. Aquac. Res. 2004, 35, 1405–1414. [Google Scholar] [CrossRef]
- Wang, T.; Li, Q.; Zhang, J.; Yu, R. Effects of salinity, stocking density, and algal density on growth and survival of Iwagaki oyster Crassostrea nippona larvae. Aquacult. Int. 2018, 26, 947–958. [Google Scholar] [CrossRef]
- Li, Y.; Sunila, I.; Wikfors, G.H. Bioactive effects of Prorocentrum minimum on juvenile bay scallops (Argopecten irradians irradians) are dependent upon algal physiological status. Bot. Mar. 2012, 55, 19–29. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, M.; Wang, Z.; Wang, B. Assessment of bivalve carrying capacities and seeding densities in aquaculture areas of Jiaozhou Bay, China, using ecological modeling and the food balance. J. World Aquacult. Soc. 2021, 52, 1178–1193. [Google Scholar] [CrossRef]
- Grant, J.; Emerson, C.W.; Mallet, A.; Carver, C. Growth advantages of ear hanging compared to cage culture for sea scallops, Placopecten magellanicus. Aquaculture 2003, 217, 301–323. [Google Scholar] [CrossRef]
- Façanha, F.N.; Oliveira-Neto, A.R.; Figueiredo-Silva, C.; Nunes, A.J.P. Effect of shrimp stocking density and graded levels of dietary methionine over the growth performance of Litopenaeus vannamei reared in a green-water system. Aquaculture 2016, 463, 16–21. [Google Scholar] [CrossRef]
- DiMaggio, M.A.; Ohs, C.L.; Broach, J.S.; Sink, T.D. Effects of Stocking Density on Growth, Survival, and Stress Physiology of Pigfish. N. Am. J. Aquacult. 2014, 76, 201–210. [Google Scholar] [CrossRef]
- Iguchi, K.I.; Ogawa, K.; Nagae, M.; Ito, F. The influence of rearing density on stress response and disease susceptibility of ayu (Plecoglossus altivelis). Aquaculture 2003, 220, 515–523. [Google Scholar] [CrossRef]
- Marshall, R.D.; Dunham, A. Effects of culture media and stocking density on biofouling, shell shape, growth, and survival of the Pacific oyster (Crassostrea gigas) and the Manila clam (Venerupis philippinarum) in suspended culture. Aquaculture 2013, 406–407, 68–78. [Google Scholar] [CrossRef]
- Calabrese, S.; Nilsen, T.O.; Kolarevic, J.; Ebbesson, L.O.E.; Pedrosa, C.; Fivelstad, S.; Hosfeld, C.; Stefansson, S.; Terjesen, B.; Takle, H.; et al. Stocking density limits for post-smolt Atlantic salmon (Salmo salar L.) with emphasis on production performance and welfare. Aquaculture 2017, 468, 363–370. [Google Scholar] [CrossRef]
- Liu, Z.G.; Liu, J.Y.; Wang, H.; Zheng, Y.L. Study on adaptability of juveniles of Argopecten irradians concentricus say to salinity. J. Zhanjiang Ocean Univ. 2006, 26, 12–16. (In Chinese) [Google Scholar]
- Li, H.M.; Zhang, Y.Y.; Liang, Y.T.; Chen, J.; Zhu, Y.C.; Zhao, Y.T.; Jiao, N. Impacts of maricultural activities on characteristics of dissolved organic carbon and nutrients in a typical raft-culture area of the Yellow Sea, North China. Mar. Pollut. Bull. 2018, 137, 456–464. [Google Scholar] [CrossRef] [PubMed]
- Lage, S.; Costa, P.R.; Moita, T.; Eriksson, J.; Rasmussen, U.; Rydberg, S.J. BMAA in shellfish from two Portuguese transitional water bodies suggests the marine dinoflagellate Gymnodinium catenatum as a potential BMAA source. Aquat. Toxicol. 2014, 152, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Yu, H.; Yu, R. Genetic variability assessed by microsatellites in cultured populations of the Pacific oyster (Crassostrea gigas) in China. Aquaculture 2006, 259, 95–102. [Google Scholar] [CrossRef]
- Nei, M.; Tajima, F.; Tateno, Y. Accuracy of estimated phylogenetic trees from molecular data. J. Mol. Evol. 1983, 19, 153–170. [Google Scholar] [CrossRef]
| Locus | Repeat Motif | Primer Sequences | Tm (°C) | Size (bp) |
|---|---|---|---|---|
| AIC1-50 | (GT)17 | F: AGGGTGGTGAAGCAGGGAC R: GGATTACGCCAGCTATTTAGGTG | 55 | 320–353 |
| AIC1-94 | (GT)16 | F: TTTATGAGAACAGGCACAGC R: TGGACATGACATGATTACGC | 51 | 151–182 |
| AIC2-9 | (GT)16 | F: CCTTGTTATCTGCCATTTCG R: GACATGATTACGCCCAGCTAT | 51 | 646–677 |
| AIC2-77 | (TG)18 | F: TACAATGAACAGGGAAAGTCGG R: CGTACGCCAGCTATTTAGGTGA | 55 | 190–225 |
| AIC3-45 | (CA)5 … (TG)5 … (TG)16 | F: ACGACAGGTTTCCCGACTT R: TTAACGCCAGGGTTTTCC | 53 | 277–391 |
| AIC3-47 | (GT)16 | F: CTCATCCAGAGGACCAACTT R: GGTGATTACGCCAGCTATTT | 51 | 430–461 |
| AIC4-74 | (CA)35 | F: TGACCATGATTACGCCAAGT R: GGCACTCGTCGTAGTAGTAACAT | 54 | 113–182 |
| AIC4-78 | (CA)17 | F: ATTACGCCAAGCTATTTCGG R: CGTCGGCAACAGTTTGAGTA | 53 | 114–147 |
| AIC5-25 | (TG)18 | F:CGATTCCCACCCTACCTAT R:AGACATGATTACGCCAGCTA | 53 | 140–175 |
| AIC5-39 | (TG)16 | F: TGGTGGTAAGAGGGTGAGTT R: TGCCAAGCTATTTAGGTGAC | 51 | 150–181 |
| Stage | Site | Strain | Density | Shell Height (mm) | Shell Length (mm) | Shell Width (mm) | Body Weight (g) |
|---|---|---|---|---|---|---|---|
| Nursery | BH | Breeding | 100 | 31.73 ± 3.40 Aa | 32.30 ± 3.68 Aa | 13.55 ± 1.59 Aa | 6.18 ± 1.96 Aa |
| 200 | 29.10 ± 2.17 Ab | 29.61 ± 2.25 Ab | 12.35 ± 1.10 Ab | 4.64 ± 1.00 Ab | |||
| 300 | 26.67 ± 2.95 Ac | 26.89 ± 3.10 Ac | 11.44 ± 1.38 Ac | 3.70 ± 1.09 Ac | |||
| Control | 100 | 33.02 ± 2.43 Ba | 33.51 ± 3.71 Ba | 14.38 ± 1.69 Ba | 7.28 ± 2.18 Ba | ||
| 200 | 28.93 ± 2.93 Ab | 29.16 ± 3.04 Ab | 12.58 ± 1.50 Ab | 4.88 ± 1.33 Ab | |||
| 300 | 26.60 ± 2.60 Ac | 26.44 ± 2.76 Ac | 11.34 ± 1.23 Ac | 3.75 ± 1.23 Ac | |||
| QZ | Breeding | 100 | 27.06 ± 2.46 Aa | 27.32 ± 2.73 Aa | 11.37 ± 1.29 Aa | 4.03 ± 1.10 Aa | |
| 200 | 24.73 ± 2.55 Ab | 24.55 ± 2.70 Ab | 11.30 ± 1.65 Aac | 2.99 ± 0.81 Ab | |||
| 300 | 23.08 ± 2.58 Ac | 22.86 ± 2.72 Ac | 9.58 ± 1.22 Ac | 2.47 ± 0.82 Ac | |||
| Control | 100 | 26.53 ± 3.32 Ba | 26.35 ± 3.58 Aa | 10.80 ± 1.81 Ba | 3.84 ± 1.84 Aa | ||
| 200 | 24.36 ± 3.44 Ab | 24.15 ± 3.21 Ab | 9.92 ± 1.54 Ab | 3.34 ± 1.35 Bb | |||
| 300 | 22.70 ± 2.74 Ac | 22.23 ± 2.86 Ac | 9.11 ± 1.27 Bc | 2.35 ± 0.84 Ac | |||
| FCG | Breeding | 100 | 31.29 ± 3.30 Aa | 32.32 ± 3.78 Aa | 13.51 ± 1.73 Aa | 6.65 ± 2.06 Aa | |
| 200 | 29.19 ± 3.60 Ab | 29.78 ± 3.73 Ab | 12.62 ± 1.76 Ab | 5.32 ± 1.77 Ab | |||
| 300 | 26.77 ± 2.90 Ac | 27.02 ± 3.21 Ac | 11.37 ± 1.47 Ac | 4.01 ± 1.09 Ac | |||
| Control | 100 | 30.89 ± 3.06 Aa | 31.48 ± 3.33 Aa | 13.15 ± 1.59 Aa | 6.26 ± 1.80 Aa | ||
| 200 | 27.96 ± 3.08 Bb | 28.07 ± 3.39 Ab | 11.52 ± 1.55 Bb | 4.48 ± 1.43 Bb | |||
| 300 | 26.64 ± 3.29 Ac | 26.74 ± 3.51 Ac | 11.03 ± 1.72 Ac | 3.95 ± 1.47 Ac | |||
| Adult | BH | Breeding | 30 | 54.49 ± 2.38 Aa | 56.95 ± 2.62 Aa | 27.32 ± 1.02 Aa | 35.00 ± 2.78 Aa |
| 45 | 51.99 ± 1.94 Ab | 54.10 ± 1.82 Ab | 26.71 ± 5.12 Ab | 29.61 ± 1.61 Ab | |||
| 60 | 49.08 ± 1.83 Ac | 50.92 ± 2.07 Ac | 24.41 ± 1.23 Ac | 25.76 ± 2.03 Ac | |||
| Control | 30 | 44.81 ± 1.71 Ba | 46.85 ± 2.22 Ba | 22.24 ± 1.21 Ba | 26.41 ± 5.09 Ba | ||
| 45 | 42.30 ± 2.07 Bb | 43.47 ± 2.01 Bb | 20.72 ± 1.16 Bb | 22.49 ± 4.32 Bb | |||
| 60 | 38.98 ± 2.02 Bc | 40.06 ± 2.36 Bc | 19.26 ± 1.05 Bc | 18.85 ± 4.09 Bc | |||
| QZ | Breeding | 30 | 53.43 ± 2.91 Aa | 55.45 ± 2.96 Aa | 26.84 ± 1.56 Aa | 33.56 ± 3.59 Aa | |
| 45 | 51.40 ± 2.40 Ab | 53.13 ± 2.74 Ab | 26.07 ± 1.10 Ab | 30.02 ± 2.90 Ab | |||
| 60 | 49.59 ± 2.33 Ac | 50.47 ± 2.48 Ac | 25.00 ± 1.11 Ac | 27.31 ± 3.98 Ac | |||
| Control | 30 | 41.65 ± 2.44 Ba | 43.66 ± 2.85 Ba | 20.83 ± 1.68 Ba | 24.52 ± 6.12 Ba | ||
| 45 | 40.06 ± 3.16 Bb | 41.67 ± 2.87 Bb | 20.11 ± 2.33 Bb | 21.20 ± 5.32 Bb | |||
| 60 | 37.96 ± 3.66 Bc | 39.18 ± 2.89 Bc | 18.57 ± 1.51 Bc | 19.98 ± 5.34 Bc | |||
| FCG | Breeding | 30 | 55.79 ± 2.13 Aa | 58.79 ± 2.18 Aa | 29.45 ± 1.74 Aa | 39.88 ± 3.59 Aa | |
| 45 | 54.39 ± 1.64 Ab | 56.62 ± 1.77 Ab | 27.86 ± 1.47 Ab | 35.48 ± 2.13 Ab | |||
| 60 | 52.30 ± 1.68 Ac | 53.40 ± 5.49 Ac | 27.15 ± 1.57 Ac | 33.29 ± 3.53 Ac | |||
| Control | 30 | 46.86 ± 1.92 Ba | 48.39 ± 1.85 Ba | 24.34 ± 1.82 Ba | 29.07 ± 5.22 Ba | ||
| 45 | 44.54 ± 2.62 Bb | 46.37 ± 2.97 Bb | 23.15 ± 2.32 Bb | 27.28 ± 5.16 Bb | |||
| 60 | 40.21 ± 2.31 Bc | 42.06 ± 2.32 Bc | 20.82 ± 1.36 Bc | 23.15 ± 5.91 Bc |
| Site | Strain | Density | Mortality (%) | |||
|---|---|---|---|---|---|---|
| 2016-03 | 2016-04 | 2016-05 | 2016-06 | |||
| BH | Breeding | 30 | 0.56 | 1.11 | 2.78 | 10.00 |
| 45 | 1.85 | 0.00 | 2.59 | 10.00 | ||
| 60 | 1.11 | 1.94 | 1.11 | 10.56 | ||
| Control | 30 | 1.11 | 0.56 | 1.67 | 5.56 | |
| 45 | 1.48 | 1.11 | 0.37 | 5.56 | ||
| 60 | 1.39 | 0.28 | 1.11 | 8.06 | ||
| QZ | Breeding | 30 | 2.22 | 0.00 | 2.78 | 27.22 |
| 45 | 0.74 | 1.11 | 1.85 | 22.22 | ||
| 60 | 1.94 | 0.56 | 0.83 | 16.94 | ||
| Control | 30 | 3.33 | 2.78 | 1.67 | 29.44 | |
| 45 | 1.85 | 1.85 | 1.85 | 17.78 | ||
| 60 | 5.00 | 2.50 | 1.39 | 18.61 | ||
| FCG | Breeding | 30 | 3.89 | 0.56 | 1.67 | 9.44 |
| 45 | 1.48 | 1.48 | 0.37 | 7.78 | ||
| 60 | 1.39 | 1.11 | 1.94 | 6.11 | ||
| Control | 30 | 2.78 | 0.00 | 0.56 | 6.67 | |
| 45 | 1.48 | 0.74 | 2.59 | 7.04 | ||
| 60 | 2.78 | 0.00 | 1.39 | 6.94 | ||
| Site | Genera | Average Dominance | Frequency of Occurrence (%) | Average Genera Abundance (cells∙L−1) |
|---|---|---|---|---|
| BH | Coscinodiscus | 0.06 | 85.71 | 877 |
| Thalassionema | 0.01 | 57.14 | 5607 | |
| Pleurosigma | 0.05 | 71.43 | 297 | |
| Skeletonema | 0.01 | 14.29 | 52,000 | |
| Melosira | 0.00 | 14.29 | 1 | |
| Ditylum | 0.00 | 14.29 | 144 | |
| Synedra | 0.00 | 71.43 | 262 | |
| Rhizosolenia | 0.01 | 71.43 | 1140 | |
| Pinnularia | 0.00 | 42.86 | 19 | |
| Odontella | 0.00 | 57.14 | 290 | |
| Cerataulina | 0.12 | 57.14 | 31,499 | |
| Chaetoceros | 0.28 | 100.00 | 60,613 | |
| Bacteriastraceae | 0.00 | 14.29 | 9 | |
| Hemiaulus | 0.00 | 14.29 | 29 | |
| Navicula | 0.03 | 71.43 | 124 | |
| Schroderella | 0.00 | 14.29 | 1 | |
| Nitzschia | 0.02 | 57.14 | 105 | |
| Corethron | 0.00 | 14.29 | 4 | |
| Licmophora | 0.01 | 71.43 | 61 | |
| Bacillaria | 0.01 | 28.57 | 267 | |
| Pseudo-nitzschia | 0.00 | 14.29 | 643 | |
| Leptocylindrus | 0.01 | 28.57 | 469 | |
| Planktoniella | 0.00 | 14.29 | 0 | |
| Triceratium | 0.00 | 14.29 | 7 | |
| Eucampia | 0.00 | 28.57 | 289 | |
| Asteroplanus | 0.00 | 14.29 | 436 | |
| Guinardia | 0.00 | 14.29 | 4 | |
| Ceratium furca | 0.08 | 85.71 | 173 | |
| QZ | Coscinodiscus | 0.04 | 100.00 | 33 |
| Fragilaria | 0.01 | 71.43 | 13 | |
| Thalassionema | 0.01 | 57.14 | 23 | |
| Pleurosigma | 0.25 | 100.00 | 210 | |
| Skeletonema | 0.03 | 71.43 | 46 | |
| Melosira | 0.04 | 71.43 | 57 | |
| Ditylum | 0.00 | 28.57 | 9 | |
| Synedra | 0.03 | 85.71 | 34 | |
| Rhizosolenia | 0.01 | 42.86 | 8 | |
| Pinnularia | 0.01 | 57.14 | 6 | |
| Cerataulina | 0.01 | 28.57 | 42 | |
| Chaetoceros | 0.01 | 28.57 | 38 | |
| Hemiaulus | 0.00 | 14.29 | 4 | |
| Navicula | 0.14 | 100.00 | 148 | |
| Schroderella | 0.00 | 14.29 | 2 | |
| Nitzschia | 0.05 | 100.00 | 43 | |
| Corethron | 0.00 | 14.29 | 1 | |
| Licmophora | 0.04 | 71.43 | 56 | |
| Gyrosigma | 0.01 | 71.43 | 9 | |
| Bacillaria | 0.06 | 85.71 | 60 | |
| Pseudo-nitzschia | 0.00 | 28.57 | 12 | |
| Leptocylindrus | 0.01 | 28.57 | 22 | |
| Fragilariopsis | 0.00 | 14.29 | 0 | |
| Gossleriella | 0.00 | 14.29 | 2 | |
| Thalassiosira | 0.00 | 14.29 | 2 | |
| Stephanopyxis | 0.00 | 14.29 | 45 | |
| Guinardia | 0.00 | 14.29 | 4 | |
| Ceratium furca | 0.00 | 57.14 | 2 | |
| Phormidiaceae | 0.00 | 14.29 | 0 | |
| FCG | Coscinodiscus | 0.04 | 100.00 | 163 |
| Fragilaria | 0.01 | 57.14 | 49 | |
| Thalassionema | 0.15 | 100.00 | 3784 | |
| Pleurosigma | 0.03 | 100.00 | 248 | |
| Skeletonema | 0.04 | 57.14 | 480 | |
| Melosira | 0.00 | 14.29 | 17 | |
| Ditylum | 0.00 | 57.14 | 80 | |
| Synedra | 0.03 | 85.71 | 888 | |
| Rhizosolenia | 0.05 | 57.14 | 413 | |
| Pinnularia | 0.01 | 100.00 | 81 | |
| Odontella | 0.00 | 71.43 | 19 | |
| Cerataulina | 0.06 | 57.14 | 1008 | |
| Chaetoceros | 0.29 | 85.71 | 38,666 | |
| Bacteriastraceae | 0.00 | 14.29 | 0 | |
| Hemiaulus | 0.00 | 42.86 | 79 | |
| Navicula | 0.01 | 85.71 | 115 | |
| Nitzschia | 0.00 | 71.43 | 176 | |
| Gyrosigma | 0.00 | 14.29 | 6 | |
| Bacillaria | 0.00 | 14.29 | 157 | |
| Pseudo-nitzschia | 0.00 | 28.57 | 67 | |
| Leptocylindrus | 0.00 | 28.57 | 414 | |
| Fragilariopsis | 0.00 | 42.86 | 153 | |
| Detonula | 0.00 | 28.57 | 183 | |
| Thalassiosira | 0.00 | 14.29 | 23 | |
| Eucampia | 0.00 | 14.29 | 204 | |
| Guinardia | 0.01 | 28.57 | 416 | |
| Ceratium furca | 0.01 | 100.00 | 68 |
| Populations | Average NA | Aveage NE | Average HO | Average HE | Average PIC |
|---|---|---|---|---|---|
| BH-B | 2.80 | 2.13 | 0.37 | 0.49 | 0.41 |
| QZ-B | 2.80 | 1.82 | 0.42 | 0.44 | 0.37 |
| FCG-B | 3.70 | 2.07 | 0.42 | 0.53 | 0.46 |
| BH-C | 4.40 | 3.04 | 0.38 | 0.62 | 0.55 |
| QZ-C | 3.70 | 1.98 | 0.46 | 0.48 | 0.42 |
| FCG-C | 4.20 | 2.61 | 0.40 | 0.62 | 0.55 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Q.; Feng, J.; Qin, Y.; Pan, Y. Aquaculture Strategy and Genetic Diversity of Argopecten irradians concentricus in Beibu Gulf, China. Biology 2025, 14, 1103. https://doi.org/10.3390/biology14081103
Wang Q, Feng J, Qin Y, Pan Y. Aquaculture Strategy and Genetic Diversity of Argopecten irradians concentricus in Beibu Gulf, China. Biology. 2025; 14(8):1103. https://doi.org/10.3390/biology14081103
Chicago/Turabian StyleWang, Qishuai, Jie Feng, Yanping Qin, and Ying Pan. 2025. "Aquaculture Strategy and Genetic Diversity of Argopecten irradians concentricus in Beibu Gulf, China" Biology 14, no. 8: 1103. https://doi.org/10.3390/biology14081103
APA StyleWang, Q., Feng, J., Qin, Y., & Pan, Y. (2025). Aquaculture Strategy and Genetic Diversity of Argopecten irradians concentricus in Beibu Gulf, China. Biology, 14(8), 1103. https://doi.org/10.3390/biology14081103






