Parasitic Fauna of Lepus europaeus and Lepus timidus in Kazakhstan: Parasitological Profile and Molecular Identification
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection and Analysis
2.2. Parasitological Methods
2.3. Coprological Investigations
2.4. DNA Extraction Method
2.5. PCR and Sequencing
2.6. Phylogenetic Analysis
2.7. Statistical Analysis
3. Results
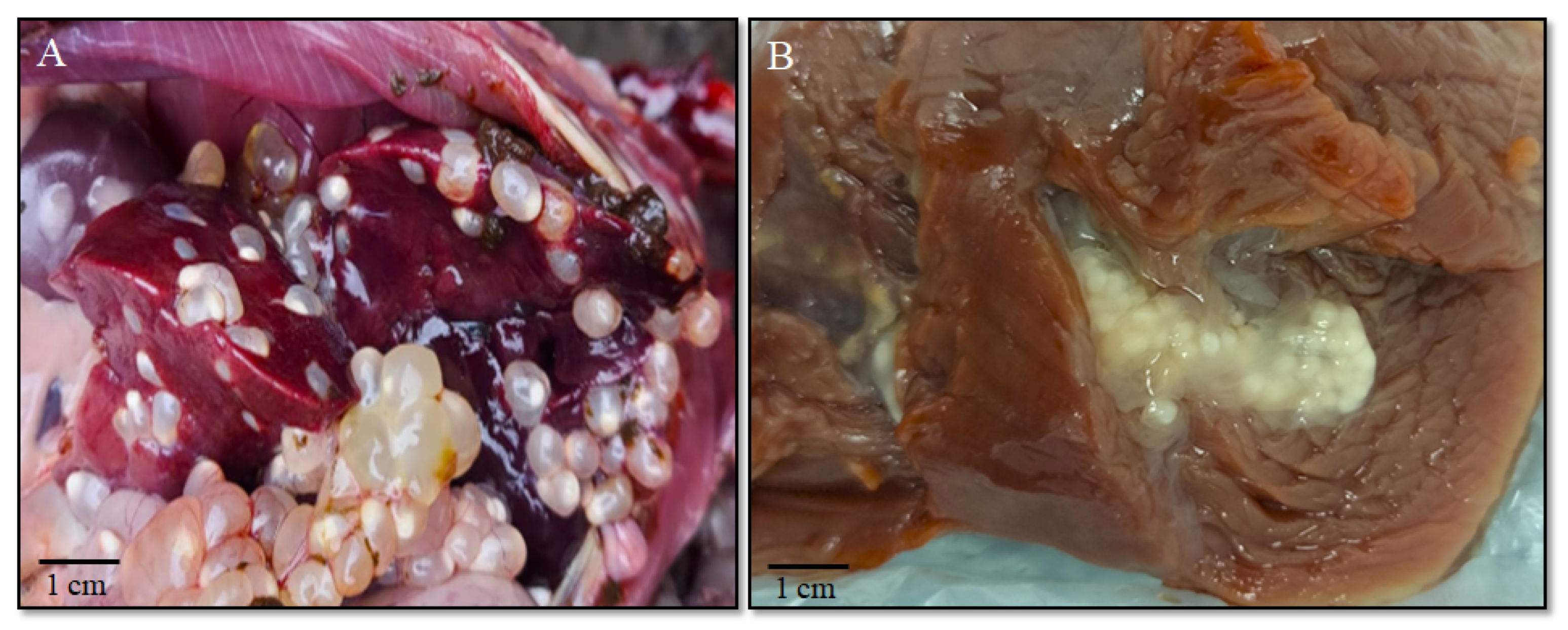
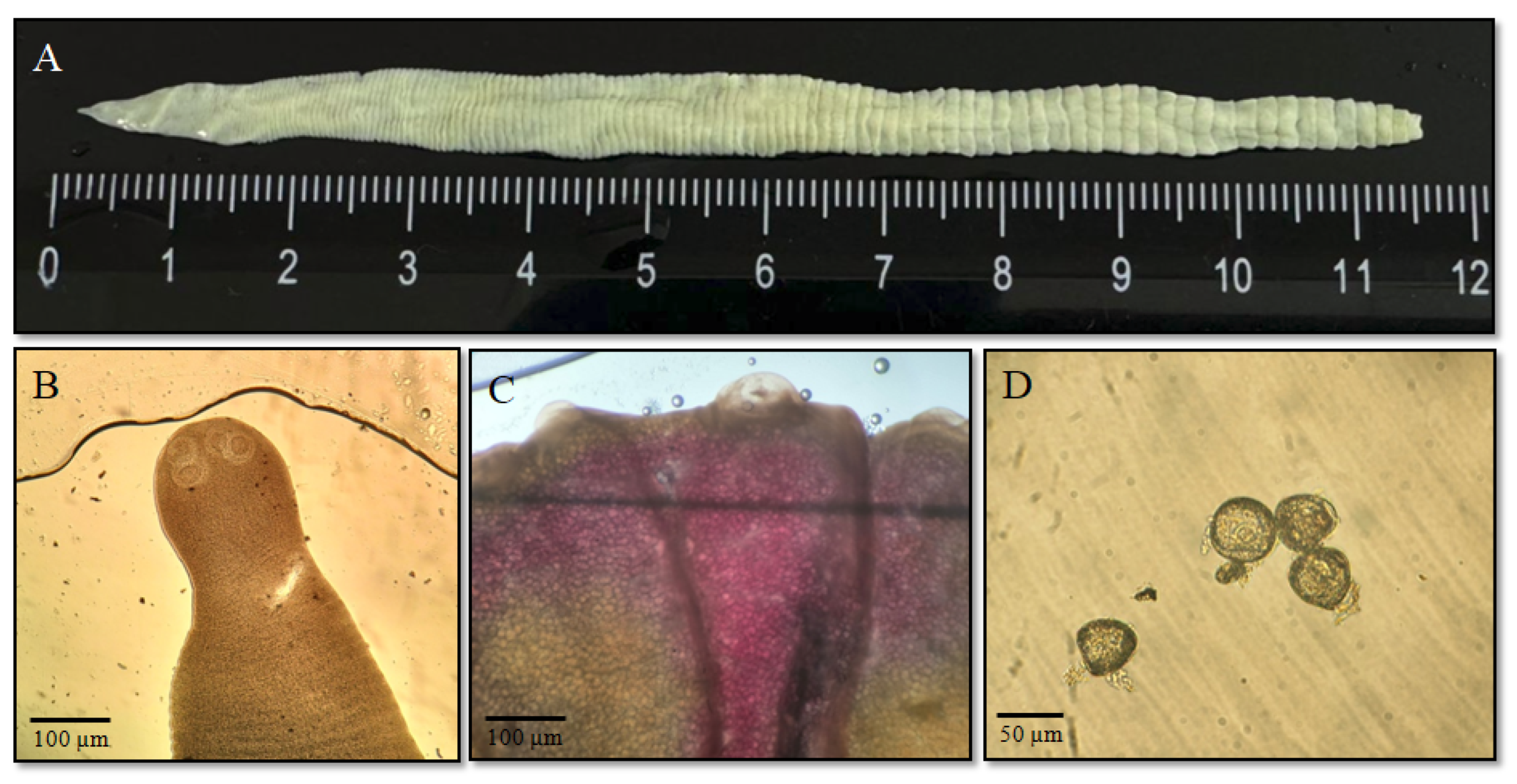
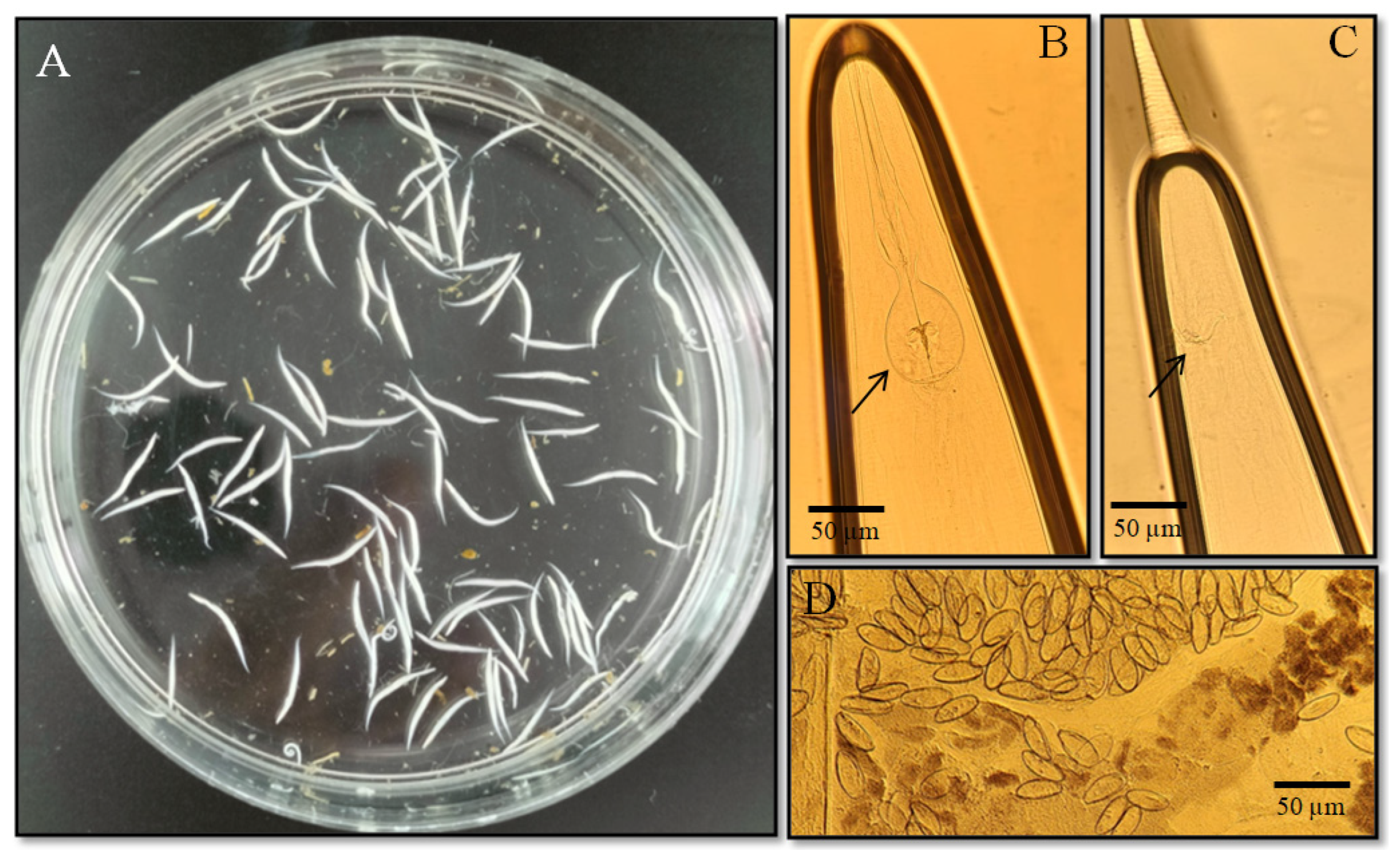
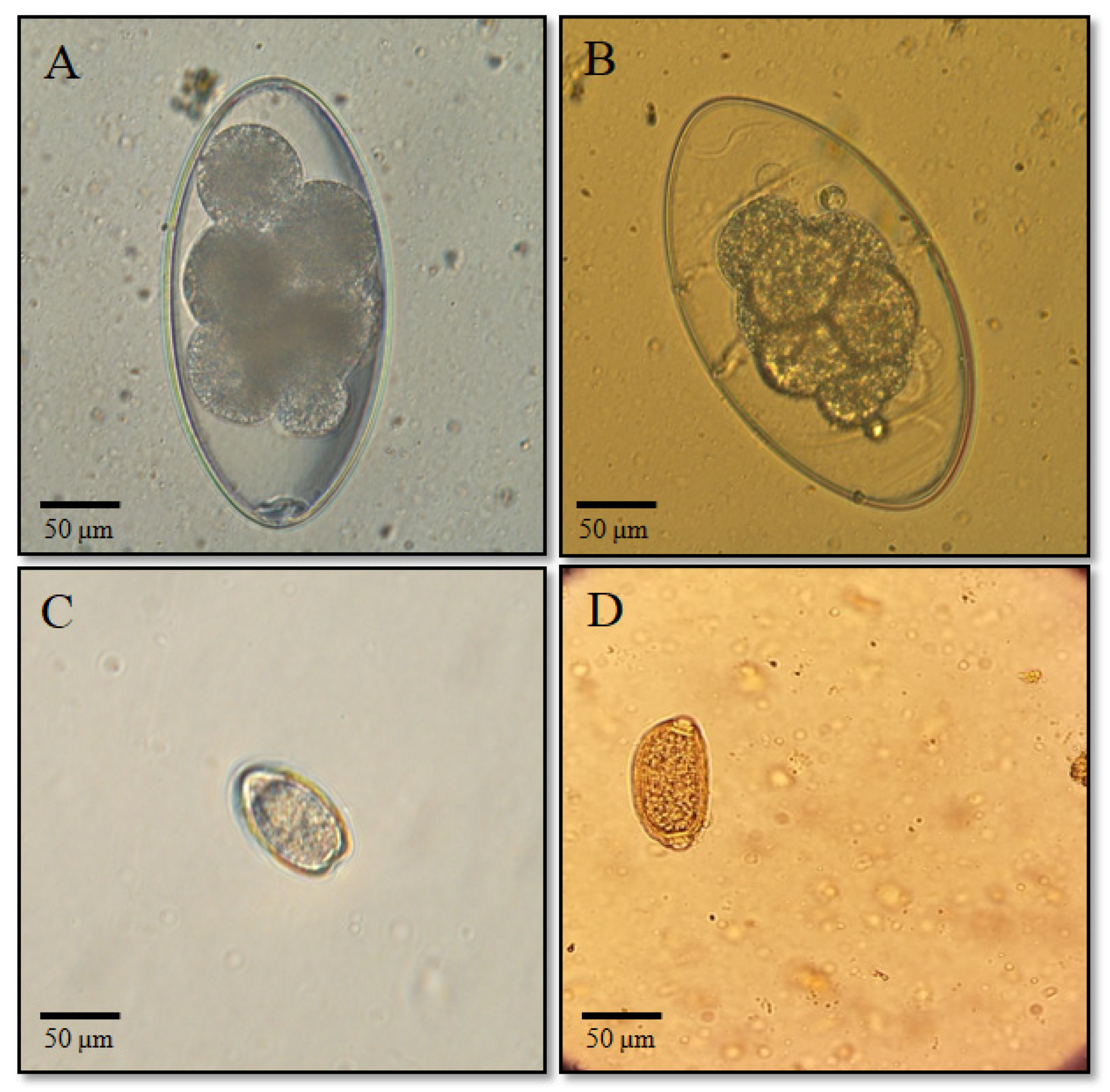
3.1. Parasitological Research
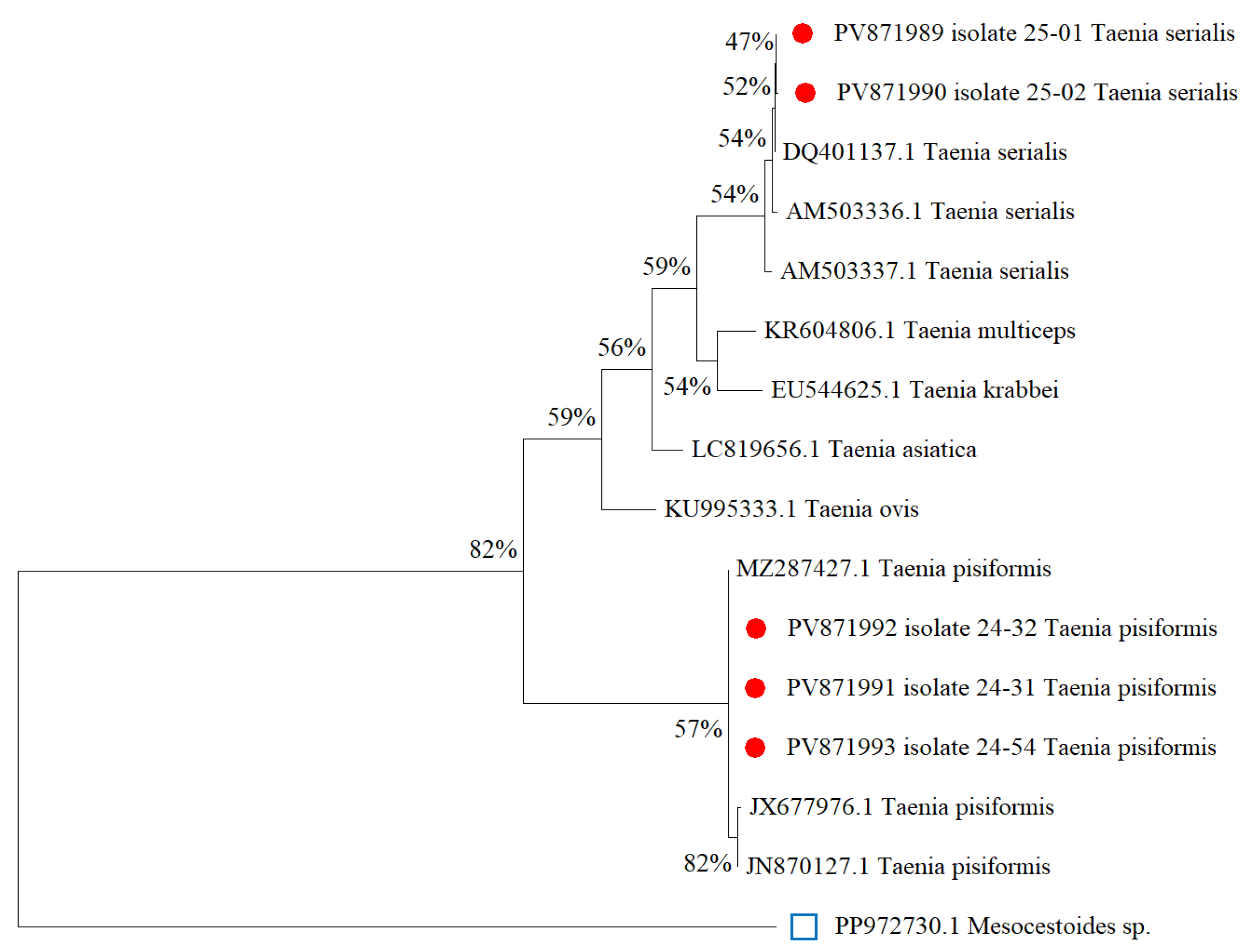
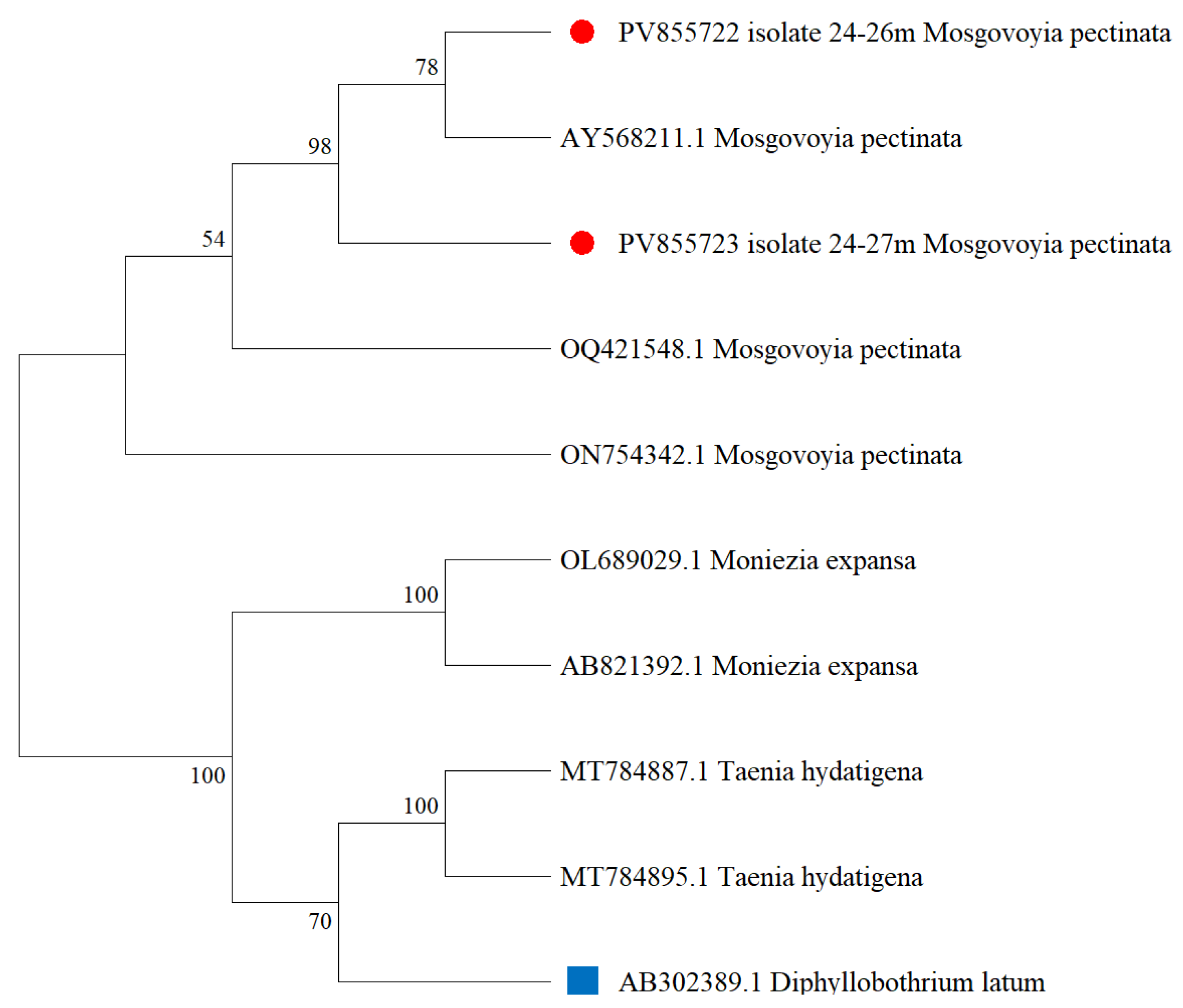
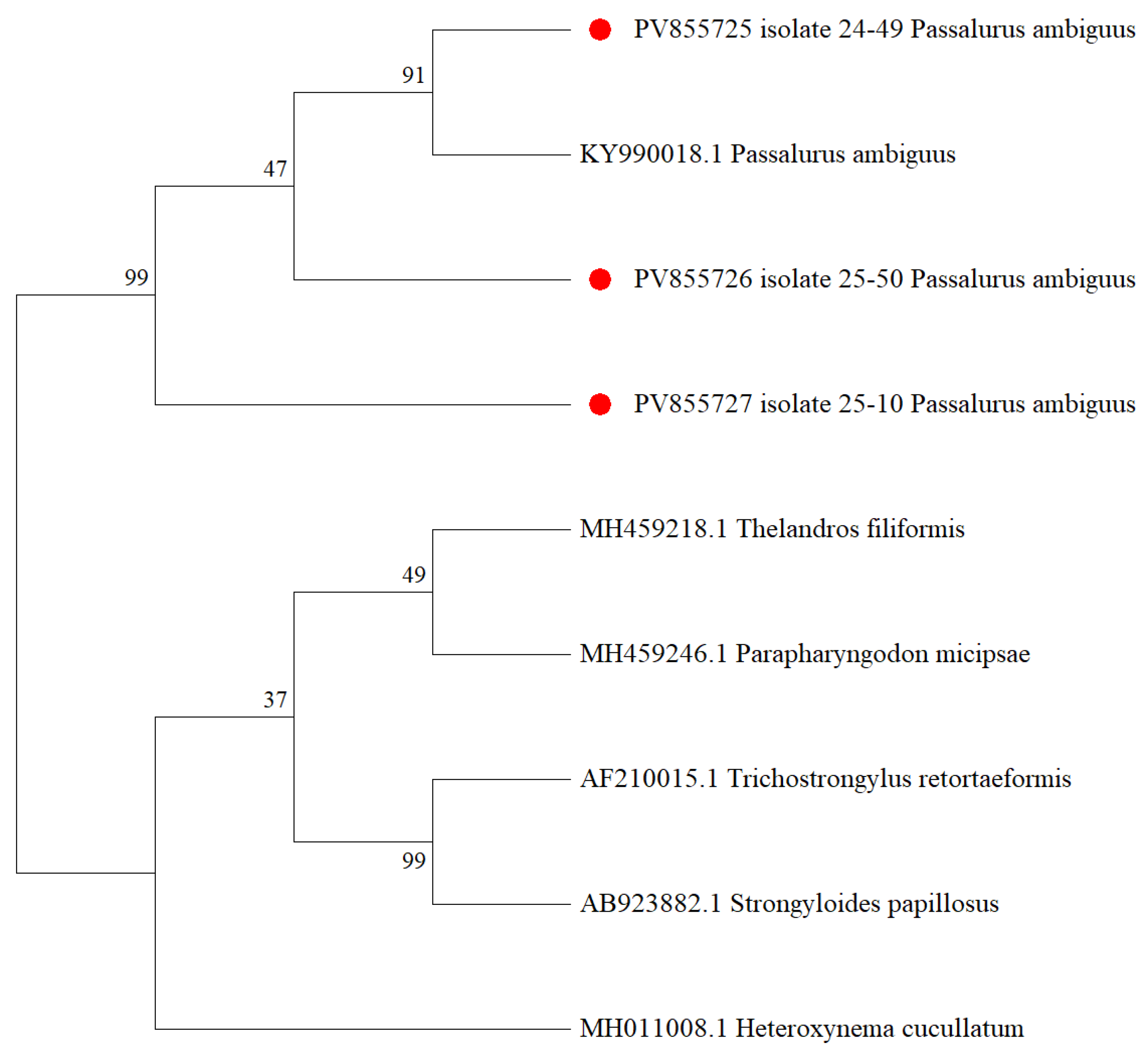
3.2. Molecular Genetics Identification
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| N | number |
| bp | base pairs |
| CA | California |
| CI | confidence interval |
| cox1 | cytochrome c oxidase subunit 1 |
| DNA | deoxyribonucleic acid |
| F | Forward |
| MD | Maryland |
| ML | Maximum Likelihood |
| nad1 | NADH dehydrogenase subunit 1 |
| OR | Odds Ratio |
| R | Revers |
| SD | standard deviation |
| TBE | Tris-borate-EDTA buffer |
| USA | United States of America |
| UV | ultraviolet |
References
- Smith, R.K.; Vaughan Jennings, N.; Harris, S. A quantitative analysis of the abundance and demography of European hares Lepus europaeus in relation to habitat type, intensity of agriculture and climate. Mammal Rev. 2005, 35, 1–24. [Google Scholar] [CrossRef]
- Vilkov, V.S.; Pashkov, S.V. The state of the populations of white hare (Lepus timidus) and hare (L. europaeus) in the forest-steppe of Kazakhstan. Ecosystems 2019, 20, 149–162. (In Russian) [Google Scholar]
- Dubinský, P.; Vasilková, Z.; Hurníková, Z.; Miterpáková, M.; Slamečka, J.; Jurčík, R. Parasitic infections of the European brown hare (Lepus europaeus Pallas, 1778) in south-western Slovakia. Helminthologia 2010, 47, 219–225. [Google Scholar] [CrossRef]
- Kornaś, S.; Wierzbowska, I.A.; Wajdzik, M.; Kowal, J.; Basiaga, M.; Paweł Nosal, P. Endoparasites of European Brown Hare (Lepus Europaeus) from Southern Poland Based on Necropsy. Ann. Anim. Sci. 2014, 14, 297–306. [Google Scholar] [CrossRef]
- Sergi, V.; Romeo, G.; Serafini, M.; Torretta, E.; Macchioni, F. Endoparasites of the European Hare (Lepus Europaeus) (Pallas, 1778) in Central Italy. Helminthologia 2018, 55, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Samorek-Pieróg, M.; Karamon, J.; Brzana, A.; Bilska-Zając, E.; Zdybel, J.; Cencek, T. Molecular Confirmation of Massive Taenia pisiformis Cysticercosis in One Rabbit in Poland. Pathogens 2021, 10, 1029. [Google Scholar] [CrossRef] [PubMed]
- Brustenga, L.; Franciosini, M.P.; Diaferia, M.; Rigamonti, G.; Musa, L.; Russomanno, B.L.; Veronesi, F. Parasitological Survey in European Brown Hare (Lepus europaeus Pallas, 1778) Breeding Facilities in Southern Italy. Pathogens 2023, 12, 208. [Google Scholar] [CrossRef]
- Magnussen, E.; Stensvold, C.R.; Berg, R.; Jokelainen, P.; Haukisalmi, V. Identification of the tapeworm Mosgovoyia pectinata (Anoplocephalidae) in Faroese mountain hares (Lepus timidus). Int. J. Parasitol. Parasites Wildl. 2023, 21, 17–21. [Google Scholar] [CrossRef]
- Wells, K.; Gibson, D.I.; Clark, N.J.; Ribas, A.; Morand, S.; McCallum, H.I. Global spread of helminth parasites at the human-domestic animal-wildlife interface. Glob. Change Biol. 2018, 24, 3254–3265. [Google Scholar] [CrossRef]
- Macchioni, F.; Romeo, G.; Trocchi, V.; Usai, F.; Cecchi, F.; Monni, G.; Stancampiano, L. Intestinal helminths of the endemic Italian hare, Lepus corsicanus (De Winton, 1898), in Sicily (Italy). Hystrix Ital. J. Mammal. 2022, 33, 98–100. [Google Scholar] [CrossRef]
- Treml, F.; Pikula, J.; Bandouchova, H.; Horakova, J. European brown hare as a potential source of zoonotic agents. Vet. Med. 2007, 52, 451–456. [Google Scholar] [CrossRef]
- Ebani, V.V.; Poli, A.; Rocchigiani, G.; Bertelloni, F.; Nardoni, S.; Papini, R.A.; Mancianti, F. Serological survey on some pathogens in wild brown hares (Lepus europaeus) in Central Italy. Asian Pac. J. Trop. Med. 2016, 9, 465–469. [Google Scholar] [CrossRef]
- Molina, R.; Jiménez, M.I.; Cruz, I.; Iriso, A.; Martín-Martín, I.; Sevillano, O.; Melero, S.; Bernal, J. The hare (Lepus granatensis) as potential sylvatic reservoir of Leishmania infantum in Spain. Vet. Parasitol. 2012, 190, 268–271. [Google Scholar] [CrossRef]
- Yan, X.; He, S.; Liu, Y.; Han, B.; Zhang, N.; Deng, H.; Wang, Y.; Liu, M. Molecular identification and phylogenetic analysis of gastrointestinal nematodes in different populations of Kazakh sheep. Exp. Parasitol. 2023, 254, 108625. [Google Scholar] [CrossRef] [PubMed]
- Newey, S.; Shaw, D.J.; Kirby, A.; Montieth, P.; Hudson, P.J.; Thirgood, S.J. Prevalence, intensity and aggregation of intestinal parasites in mountain hares and their potential impact on population dynamics. Int. J. Parasitol. 2005, 35, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Hussein, N.M.; Rabie, S.A.H.; Abuelwafa, W.A.; El Din, M.M.M. Morphometry, molecular identification and histopathology of Passalurus ambiguus Rudolphi, 1819 in domestic rabbits (Oryctolagus cuniculus) in Qena, Upper Egypt. J. Parasit. Dis. 2022, 46, 511–525. [Google Scholar] [CrossRef] [PubMed]
- Faehndrich, M.; Woelfing, B.; Klink, J.C.; Roller, M.; Baumgärtner, W.; Wohlsein, P.; Raue, K.; Strube, C.; Ewers, C.; Prenger-Berninghoff, E.; et al. Pathomorphological Findings and Infectious Diseases in Selecte European Brown Hare (Lepus europaeus Pallas, 1778) Populations from Schleswig-Holstein, Germany. Pathogens 2023, 12, 1317. [Google Scholar] [CrossRef]
- Hughes, K. Endoparasites of rabbits and hares. J. Vet. Diagn. Investig. 2024, 36, 599–616. [Google Scholar] [CrossRef]
- Eberhardt, A.T.; Robles, M.D.R.; Monje, L.D.; Beldomenico, P.M.; Callejón, R. A new Trichuris species (Nematoda: Trichuridae) from capybaras: Morphological-molecular characterization and phylogenetic relationships. Acta Trop. 2019, 190, 244–252. [Google Scholar] [CrossRef]
- Ryzhikov, K.M.; Gubanov, N.M.; Fedorov, K.P. Biology of Mosgovoyia pectinata, hare cestodes. Sci. Notes V. I. Lenin Mosc. State Pedagog. Inst. 1956, 96, 147–150. (In Russian) [Google Scholar]
- Fadeyev, V.A. European hare—Lepus europaeus Pall., 1778. In Mammalia of Kazakhstan; Sludskiy, A.A., Strautman, E.A., Eds.; Science of the Kazakh SSR: Alma-Ata, Kazakhstan, 1980; Volume 2, pp. 79–112. (In Russian) [Google Scholar]
- Hulbert, I.A.; Boag, B. The potential role of habitat on intestinal helminths of mountain hares, Lepus timidus. J. Helminthol. 2001, 75, 345–349. [Google Scholar] [CrossRef]
- Boag, B.; Iason, G. The occurrence and abundance of helminth parasites of the mountain hare Lepus timidus (L.) and the wild rabbit Oryctolagus cuniculus (L.) in Aberdeenshire, Scotland. J. Helminthol. 1986, 60, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Kapitonov, V.I.; Utinov, S.R. Mountain hare—Lepus timidus Linnaeus, 1758. In Mammalia of Kazakhstan; Sludskiy, A.A., Strautman, E.A., Eds.; Science of the Kazakh SSR: Alma-Ata, Kazakhstan, 1980; Volume 2, pp. 9–51. (In Russian) [Google Scholar]
- Bobrinsky, N.A.; Kuznetsov, B.A.; Kuzyakin, A.P. Identifier of Mammals of the USSR; Prosveshchenie: Moscow, Russia, 1965; pp. 244–245. (In Russian) [Google Scholar]
- Skrjabin, K.I. Method of Complete Helminthological Dissection of Vertebrates, Including Humans; Publishing House of 1st Moscow State University: Moscow, Russia, 1928; pp. 40–48. (In Russian) [Google Scholar]
- Deplazes, P.; Eckert, J.; Mathis, A.; Von Samson-Himmelstjerna, G.; Zahner, H. Parasitology in Veterinary Medicine; Wageningen Academic Publishers: Wageningen, The Netherlands, 2016; pp. 224–239. [Google Scholar]
- De Bellocq, J.G.; Ferté, H.; Depaquit, J.; Justine, J.L.; Tillier, A.; Durette-Desset, M.C. Phylogeny of the Trichostrongylina (Nematoda) inferred from 28S rDNA sequences. Mol. Phylogenet. Evol. 2001, 19, 430–442. [Google Scholar] [CrossRef] [PubMed]
- Dryden, M.W.; Payne, P.A.; Smith, V. Comparison of common fecal flotation techniques for the recovery of parasite eggs and oocysts. Vet. Ther. 2005, 6, 15–18. [Google Scholar] [PubMed]
- Zajac, A.M.; Conboy, G.A.; Little, S.E.; Reichard, M.V. Veterinary Clinical Parasitology, 9th ed.; Wiley & Sons: Hoboken, NJ, USA, 2021; pp. 4–8. [Google Scholar]
- Cunningham, S.W.; Tessler, M.; Johnson-Rosemond, J.; Whittaker, I.S.; Brugler, M.R. Environmental DNA Isolation, Validation, and Preservation Methods. Methods Mol. Biol. 2024, 2744, 171–180. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Baran, A.I.; Alvandi, G.; Firouzamandi, M.; Norouzi, R.; Katiraee, F. Study of morphology and phylogeny of Mosgovoyia pectinata (Cestoda; Anoplocephalidae) from European wild hares (Lepus europaeus) in Northwestern Iran. J. Anim. Environ. 2021, 13, 13–20. [Google Scholar] [CrossRef]
- Kalmykov, A.P.; Vlasenkov, S.A.; Tulendeev, R.N. The first registration of the cestode Mosgovoyia pectinata (Goeze, 1782) Spassky, 1951 at the European hare (Lepus Europaeus Pall. 1821) in the Volga delta. In Biodiversity, Rational Use of Biological Resources and Biotechnology. Proceedings of the International Scientific and Practical Online Conference, Astrakhan State University, Astrakhan, Russia; Publishing house “Astrakhan University”: Astrakhan, Russia, 2021; pp. 12–15. (In Russian) [Google Scholar]
- Tamura, K.; Nei, M. Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Mol. Biol. Evol. 1993, 10, 512–526. [Google Scholar] [CrossRef]
- Felsenstein, J. Confidence limits on phylogenies: An approach using the bootstrap. Evolution 1985, 39, 783–791. [Google Scholar] [CrossRef]
- Khalil, L.F.; Jones, A.; Bray, R.A. Order Cyclophyllidea (diagnosis and key to families). In Keys to the Cestode Parasites of Vertebrates; Khalil, L.F., Jones, A., Bray, R.A., Eds.; CAB International: Wallingford, UK, 1994; pp. 305–307. [Google Scholar]
- Stunkard, H.W. Studies on the life history of the Anoplocephaline Cestodes of Hares and Rabbits. J. Parasit. Urbana. 1941, XXVII, 299–325. [Google Scholar] [CrossRef]
- Ball, S.; Kelly, T.; Butler, F. Endoparasites of the endemic Irish hare Lepus timidus hibernicus. J. Wildl. Dis. 2020, 19, 337–341. [Google Scholar] [CrossRef]
- Lin, Y.; Lingxian, H. Studies on the life cycle of Ctenotaenia citelli (Kirshenblat) and Mosgovoyia pectinata (Goeze). Acta Zool. Sin. 1986, 32, 144–151. [Google Scholar]
- Berg, C. Endoparasitism in Wild Norwegian Hares (Lepus timidus). A National Survey and a More Detailed Study of Two Isolated Subpopulations. Bachelor’s Thesis, Veterinary College of Norway, Oslo, Norway, 1980; p. 71. [Google Scholar]
- Soveri, T.; Valtonen, M. Endoparasites of hares (Lepus timidus L. and L. europaeus Pallas) in Finland. J. Wildl. Dis. 1983, 19, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Yushkov, V.F. Fauna of the European North-East of Russia; Nauka: St. Petersburg, Russia, 1995; pp. 80–85. (In Russian) [Google Scholar]
- Maslennikova, O.V.; Limendova, S.A. Monitoring of helminths of some mammal species in the Kirov region. In Ecology of the native land: Problems and solutions. Proceedings of the XVII All-Russian Scientific and Practical Conference with International Participation, Vyatka State University, Kirov, Russia, 26–27 April 2022; Vyatka State University Publishing House: Kirov, Russia, 2022; pp. 317–320. (In Russian) [Google Scholar]
- Sedalishchev, V.T.; Anufriev, A.I. Ecological and physiological characteristics of the mountain hare Lepus timidus (Leporidae) in Central Yakutia. Bull. North-East. Sci. Cent. Far East. Branch Russ. Acad. Sci. 2013, 1, 69–74. (In Russian) [Google Scholar]
- Siben, A.N.; Nikonova, A.A. Helminthofauna of the white hare (Lepus timidus) in Russia. Literature review. Agro-Ind. Complex Innov. Technol. 2022, 4, 31–38. (In Russian) [Google Scholar] [CrossRef]
- Vysotskaya, S.O. Helminths of small mammals of the Transcarpathian region (Eastern Carpathians, Ukraine). Parasitology 1997, 31, 346–355. (In Russian) [Google Scholar]
- Cadenatsii, A.N. Helminth fauna of mammals of Crimea and experience of recovery of domestic animals from main helminthiases. Work. GELAN 1957, 1, 109. (In Russian) [Google Scholar]
- Stryukov, A.A.; Leonov, S.V.; Sorgin, D.A. Endoparasites of the brown hare (Lepus europaeus) in the Crimea: An annotated checklist and invasion indicators. Ecosystems 2022, 29, 58–69. (In Russian) [Google Scholar]
- Khidaibergenov, A.D.j. Current status of trematodes and cestodes wild mammals of the Kyrgyzstan. Sci. New Technol. 2015, 2, 113–115. (In Russian) [Google Scholar]
- Fataliev, G.G. Helminth fauna of the brown hare of Azerbaijan and the ways of its formation. Bull. Zaporizhzhskiy Natl. Univ. Collect. Sci. Work. Biol. Sci. 2013, 3, 53–59. [Google Scholar]
- Movsesyan, S.O.; Chubaryan, F.A.; Nikogosyan, M.A. Cestodes of the Fauna of the Southern Lesser Caucasus; Nauka, Institute of Parasitology RAS: Moscow, Russia, 2006; 331p. (In Russian) [Google Scholar]
- Kontrimavicius, V.L. Helminth fauna of hares of the USSR and the experience of its ecological-geographical analysis. Work. GELAN 1959, 9, 133–144. [Google Scholar]
- Jia, W.Z.; Yan, H.B.; Guo, A.J.; Zhu, X.Q.; Wang, Y.C.; Shi, W.G.; Chen, H.T.; Zhan, F.; Zhang, S.H.; Fu, B.Q.; et al. Complete mitochondrial genomes of Taenia multiceps, T. hydatigena and T. pisiformis: Additional molecular markers for a tapeworm genus of human and animal health significance. BMC Genom. 2010, 11, 447. [Google Scholar] [CrossRef]
- Loos-Frank, B. An up-date of Verster’s (1969) “Taxonomic revision of the genus Taenia Linnaeus” (Cestoda) in table format. Syst. Parasitol. 2000, 45, 155–183. [Google Scholar] [CrossRef]
- Stancampiano, L.; Ravagnan, S.; Capelli, G.; Militerno, G. Cysticercosis by Taenia pisiformis in Brown Hare (Lepus europaeus) in Northern Italy: Epidemiologic and pathologic features. Int. J. Parasitol. Parasites Wild 2019, 9, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Keith, L.B.; Cary, J.R.; Yuill, T.M.; Keith, I.M. Prevalence of helminths in a cyclic snowshoe hare population. J. Wild Dis. 1985, 21, 233–254. [Google Scholar] [CrossRef] [PubMed]
- Dominguez-Roldan, R.; Perez-Martinez, M.; Rosetti, M.F.; Arias-Hernandez, D.; Bernal-Fernandez, G.; Flores-Perez, F.I.; Hallal Calleros, C. High frequency of Taenia pisiformis metacestodes and high sex-associated susceptibility to cysticercosis in naturally infected wild rabbits. Parasitol. Res. 2018, 117, 2201–2206. [Google Scholar] [CrossRef] [PubMed]
- Dubina, I.N.; Subbotin, A.M. Epizootology of Taenia pisiformis and its larval stage Cysticercus pisiformis. Vet. Sci. Abstr. J. 2000, 1, 71–74. (In Russian) [Google Scholar]
- Mykhailiutenko, S.; Kruchynenko, O.; Kuzmenko, L.; Klymenko, O.; Dmytrenko, N. Morpho-anatomical traits of Cysticercus pisiformis in European rabbits (Oryctolagus cuniculus). Bulg. J. Vet. Med. 2024, 27, 593–601. [Google Scholar] [CrossRef]
- Krivko, M.S.; Tambiev, T.S.; Krivko, A.S.; Kazhanova, M.D.; Lipkovich, A.D.; Tazayan, A.N.; Gak, Y.M.; Ivanchenko, Y.V. The incidence of Cysticercosis in brown hares in Rostov oblast. J. Agric. Environ. 2024, 5, 45. [Google Scholar] [CrossRef]
- Garedaghi, Y.; Firouzivand, Y.; Luca, I. Prevalence of Endoparasites and their Zoonotic Significance in Wild Rabbits of Ahar City, Iran. Am. J. Anim. Vet. Sci. 2022, 17, 31–34. [Google Scholar] [CrossRef]
- Zhaxylykov, A.; Uakhit, R.; Kiyan, V. Study of prevalence and morphological features of cestodes in foxes (Vulpes vulpes) in the territory of Kazakhstan. J. Biol. Res. 2024, 1, 31–38. [Google Scholar] [CrossRef]
- Uakhit, R.; Smagulova, A.; Lider, L.; Leontyev, S.; Kiyan, V. Epizootiological monitoring of wolf helminths in Northern and Central Kazakhstan. Vet. World 2024, 17, 1648–1654. [Google Scholar] [CrossRef] [PubMed]
- Akhmetbekov, N.A. Zonal distribution of helminthiasis in Akmola and Karaganda regions. Sci. Bull. S. Seifullin Kazakh Agrotech. Univ. 2001, 4, 55–61. [Google Scholar]
- Zhaksylykova, A.A.; Abdybekova, F.M.; Sayakova, Z.Z.; Kenesary, S.A.; Kydyrkhanova, E.A. Epizootic situation of echinococcosis of animals (dogs) in the territory of the Republic of Kazakhstan. Endless Light Sci. 2024, 30, 5–10. [Google Scholar]
- Hoberg, E.P. Taenia tapeworms: Their biology, evolution and socioeconomic significance. Microbes Infect. 2002, 4, 859–866. [Google Scholar] [CrossRef]
- Eckert, J. Interactions between cestodes and their vertebrate hosts. In Parasite-Host Associations: Coexistence or Conflict? Toft, C.A., Aeschlimann, A., Bolis, L., Eds.; Oxford University Press: Oxford, UK, 1991; pp. 201–227. [Google Scholar] [CrossRef]
- Morandi, B.; Bazzucchi, A.; Gambini, S.; Crotti, S.; Cruciani, D.; Morandi, F.; Napoleoni, M.; Piseddu, T.; Di Donato, A.; Gavaudan, S. A novel intermediate host for Taenia serialis (Gervais, 1847): The European roe deer (Capreolus capreolus L. 1758) from the Monti Sibillini National Park (MSNP), Italy. Int. J. Parasitol. Parasites Wildl. 2021, 17, 110–113. [Google Scholar] [CrossRef]
- Hough, I. Subcutaneous larval Taenia serialis in a ring-tailed possum (Pseudocheirus peregrinus). Aust. Vet. J. 2000, 78, 468. [Google Scholar] [CrossRef]
- Segovia, J.M.; Torres, J.; Miquel, J.; Llaneza, L.; Feliu, C. Helminths in the wolf, Canis lupus, from north-western Spain. J. Helminthol. 2001, 75, 183–192. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Jian, Y.N.; Ma, L.Q.; Li, X.P.; Karanis, P. A Case of Coenurosis in a Wild Rabbit (Lepus sinensis) Caused by Taenia serialis Metacestode in Qinghai Tibetan Plateau Area, China. Korean J. Parasitol. 2018, 56, 195–198. [Google Scholar] [CrossRef]
- Schneider-Crease, I.; Griffin, R.H.; Gomery, M.A.; Dorny, P.; Noh, J.C.; Handali, S.; Chastain, H.M.; Wilkins, P.P.; Nunn, C.L.; Snyder-Mackler, N.; et al. Identifying wildlife reservoirs of neglected taeniid tapeworms: Non-invasive diagnosis of endemic Taenia serialis infection in a wild primate population. PLoS Neglected Trop. Dis. 2017, 11, e0005709. [Google Scholar] [CrossRef]
- Tappe, D.; Berkholz, J.; Mahlke, U.; Lobeck, H.; Nagel, T.; Haeupler, A.; Muntau, B.; Racz, P.; Poppert, S. Molecular Identification of Zoonotic Tissue-Invasive Tapeworm Larvae Other than Taenia solium in Suspected Human Cysticercosis Cases. J. Clin. Microbiol. 2016, 54, 172–174. [Google Scholar] [CrossRef]
- Rinaldi, L.; Russo, T.; Schioppi, M.; Pennacchio, S.; Cringoli, G. Passalurus ambiguus: New insights into copromicroscopic diagnosis and circadian rhythm of egg excretion. Parasitol. Res. 2007, 101, 557–561. [Google Scholar] [CrossRef]
- Mäkitaipale, J.; Karvinen, I.; Virtala, A.K.; Näreaho, A. Prevalence of intestinal parasites and risk factor analysis for Eimeria infections in Finnish pet rabbits. Vet. Parasitol. Reg. Stud. Rep. 2017, 9, 34–40. [Google Scholar] [CrossRef]
- Raue, K.; Heuer, L.; Böhm, C.; Wolken, S.; Epe, C.; Strube, C. 10-year parasitological examination results (2003 to 2012) of faecal samples from horses, ruminants, pigs, dogs, cats, rabbits and hedgehogs. Parasitol. Res. 2017, 116, 3315–3330. [Google Scholar] [CrossRef] [PubMed]
- Grigoryan, G.A. On the study of the fauna of parasitic worms of hares (Lepus europaeus cyrensis Sat., 1905) of Armenia. Zool. Collect. 1950, 7, 111–120. (In Russian) [Google Scholar]
- Allan, J.C.; Craig, P.S.; Sherington, J.; Rogan, M.T.; Storey, D.M.; Heath, S.; Iball, K. Helminth parasites of the wild rabbit Oryctolagus cuniculus near Malham Tarn, Yorkshire, UK. J. Helminthol. 1999, 73, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Grishina, E.A. Study of markers of blood lymphocytes apoptosis in animals at helminthiases. Russ. J. Parasitol. 2017, 40, 162–167. (In Russian) [Google Scholar]
- Kabilov, T.K.; Saidullaev, S.S.; Dadaev, S.; Kabilova, S.h.T. On the study of helminths of rare and endangered species of wild mammals of the fauna of Uzbekistan. In Proceedings of the Scientific Conference Problems of Protection and Sustainable Use of Biodiversity of the Animal World of Kazakhstan, Almaty, Kazakhstan, 6–8 April 1999; p. 67. [Google Scholar]
- Abdel-Gaber, R.; Ataya, F.; Fouad, D.; Daoud, M.; Alzuhairy, S. Prevalence, Morphological and Molecular Phylogenetic Analyses of the Rabbit Pinworm, Passalurus ambiguus Rudolphi 1819, in the Domestic Rabbits Oryctolagus cuniculus. Acta Parasitol. 2019, 64, 316–330. [Google Scholar] [CrossRef]
- Harcourt-Brown, F.M. Digestive system disease. In BSAVA Manual of Rabbit Medicine; Meredith, A., Lord, B., Eds.; British Small Animal Veterinary Association: Gloucester, UK, 2014; pp. 168–190. [Google Scholar]
| No. | Target | Primer | Sequence | PCR Parameters | Reference |
|---|---|---|---|---|---|
| 1 | nad1 | JB11F JB12R | 5′-ACCACTAACTAATTCACTTTC-3′ 5′-AGATTCGTAAGGGGCCTAATA-3′ | initial denaturation 95 °C 3 min | [3] |
| 35 cycles denaturation at 95 °C for 60 s annealing at 50 °C for 60 s extension at 72 °C for 60 s | |||||
| final extension at 72 °C 5 min | |||||
| 2 | cox1 | COX-F COX-R | 5′-GATGTTTTCTTTACATTTATCTGGTG-3′ 5′-GCCACCACAAATCAAGTATC-3′ | initial denaturation 95 °C 3 min | [8] |
| 35 cycles denaturation at 95 °C for 30 s annealing at 53 °C for 60 s extension at 72 °C for 60 s | |||||
| final extension at 72 °C 7 min | |||||
| 3 | 28S | C1F D2R | 5′-ACCCGCTGAATTTAAGCAT-3′ 5′-TCCGTGTTTCAAGACGG-3′ | initial denaturation 95 °C 2 min | [16,28] |
| 35 cycles denaturation at 95 °C for 15 s annealing at 50 °C for 20 s extension at 72 °C for 30 s | |||||
| final extension at 72 °C 1 min |
| Host | Species Identified | N Infected/N Examined | % Prevalence (95% CI) | N Helminth Found | Range of Intensity | Mean (SD) Intensity | Odds | p-Value |
|---|---|---|---|---|---|---|---|---|
| >0.05 | ||||||||
| Mountain hare (Lepus timidus) | Mosgovoyia pectinata | 7/61 | 11.5 (4.7–22.2) | 55 | 3–15 | 7.9 (4.5) | 0.1296 | 0.5100 |
| Taenia serialis | 2/61 | 3.3 (0.4–11.3) | 70 | 30–40 | 35.0 (7.1) | 0.0339 | 0.5052 | |
| Taenia pisiformis | 2/61 | 3.3 (0.4–11.3) | 148 | 34–114 | 74.0 (56.57) | 0.0339 | 1.0000 | |
| Passalurus ambiguus | 3/61 | 4.9 (10.3–13.7) | 103 | 1–87 | 34.3 (49.7) | 0.0517 | 1.0000 | |
| European hare (Lepus europaeus) | Mosgovoyia pectinata | 3/46 | 6.5 (1.4–18.0) | 34 | 2–28 | 11.3 (13.1) | 0.0698 | 0.5100 |
| Taenia pisiformis | 2/46 | 4.3 (0.5–14.9) | 107 | 46–61 | 53.5 (10.6) | 0.0455 | 1.0000 | |
| Passalurus ambiguus | 3/46 | 6.5 (1.4–18.0) | 141 | 5–95 | 47.0 (45.6) | 0.0698 | 1.0000 |
| Host | Species Identified | N Infected/N Examined | % Prevalence (95% CI) | Odds Ratio (95% CI) | p-Value |
|---|---|---|---|---|---|
| >0.05 | |||||
| Mountain hare (Lepus timidus) | Nematodirus leporis | 7/52 | 13.4 (5.6–25.8) | 0.1556 (6.68–25.27) | 0.0000 |
| Eimeria spp. | 2/52 | 3.8 (0.4–13.2) | 0.40 (0.03–4.85) | 1.0000 | |
| Trichuris leporis | 1/52 | 1.9 (0.5–10.3) | - | - | |
| European hare (Lepus europaeus) | Nematodirus leporis | 4/11 | 36.6 (10.1–69.2) | 0.5714 (15.17–64.62) | 0.0000 |
| Eimeria spp. | 1/11 | 9.1 (0.2–41.3) | 2.50 (0.21–30.29) | 1.0000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kiyan, V.; Smagulova, A.; Manapov, N.; Jazina, K.; Uakhit, R.; Bulashev, A.; Lider, L.; Leontyev, S. Parasitic Fauna of Lepus europaeus and Lepus timidus in Kazakhstan: Parasitological Profile and Molecular Identification. Biology 2025, 14, 1083. https://doi.org/10.3390/biology14081083
Kiyan V, Smagulova A, Manapov N, Jazina K, Uakhit R, Bulashev A, Lider L, Leontyev S. Parasitic Fauna of Lepus europaeus and Lepus timidus in Kazakhstan: Parasitological Profile and Molecular Identification. Biology. 2025; 14(8):1083. https://doi.org/10.3390/biology14081083
Chicago/Turabian StyleKiyan, Vladimir, Ainura Smagulova, Nurassyl Manapov, Karina Jazina, Rabiga Uakhit, Aitbay Bulashev, Lyudmila Lider, and Sergey Leontyev. 2025. "Parasitic Fauna of Lepus europaeus and Lepus timidus in Kazakhstan: Parasitological Profile and Molecular Identification" Biology 14, no. 8: 1083. https://doi.org/10.3390/biology14081083
APA StyleKiyan, V., Smagulova, A., Manapov, N., Jazina, K., Uakhit, R., Bulashev, A., Lider, L., & Leontyev, S. (2025). Parasitic Fauna of Lepus europaeus and Lepus timidus in Kazakhstan: Parasitological Profile and Molecular Identification. Biology, 14(8), 1083. https://doi.org/10.3390/biology14081083







