Ex Vivo Characterization of Peritoneal Macrophages from Novel ABCA1-LSL and ABCG1-LSL Mice for Macrophage-Specific ABC-Transporter Overexpression
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Generation of ABCA1-LSL and ABCG1-LSL Mice
2.2. PM Isolation
2.3. PM Characterization
2.4. Plasmid DNA Transfection
2.5. Adenoviral Infection and Lentiviral Transduction
2.6. Gesicle Treatment
2.7. Western Blotting
2.8. Cholesterol Efflux
2.9. Statistical Analyses
3. Results
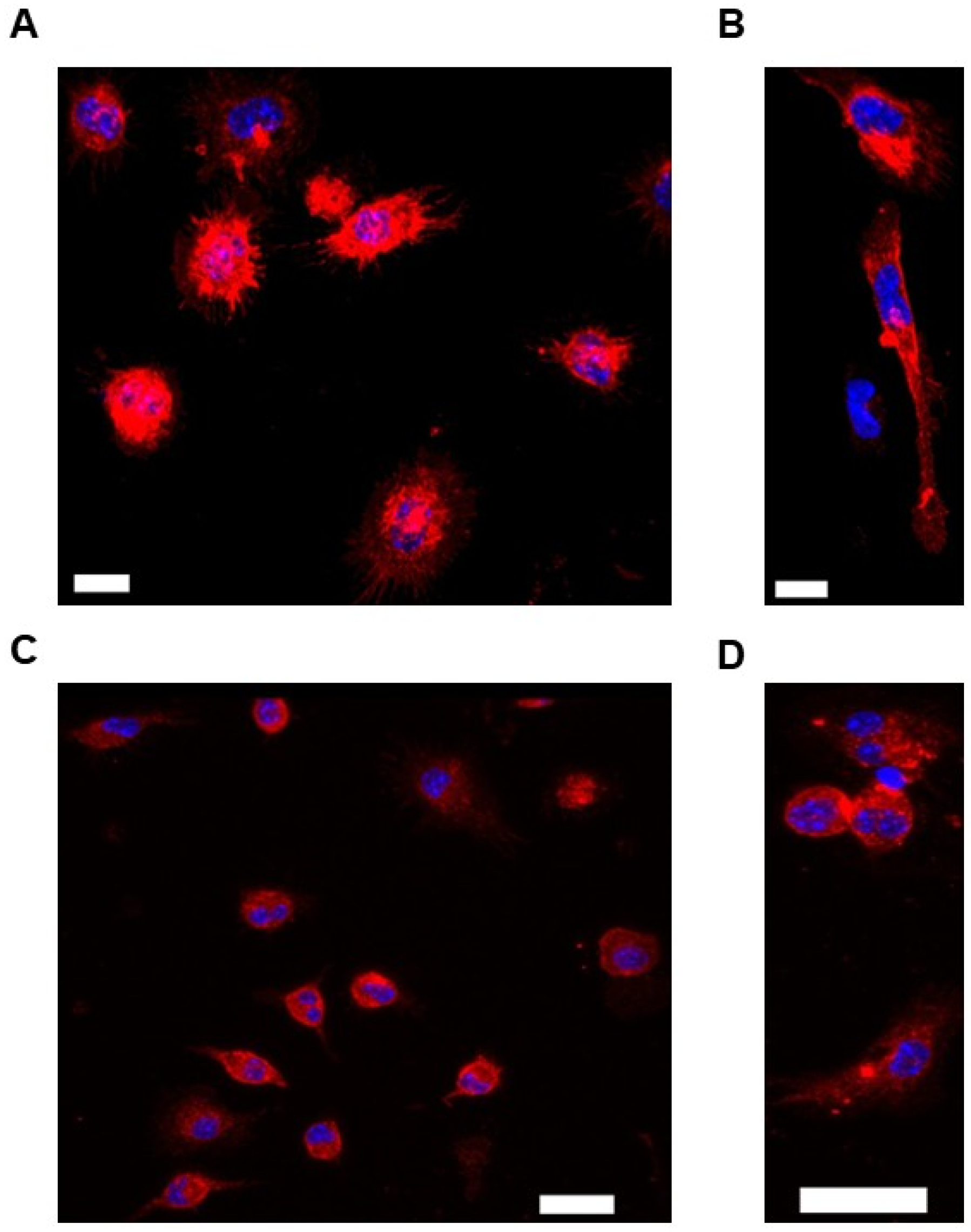
3.1. Use of Immunofluorescent Staining to Characterize Murine PMs Cultured Ex Vivo
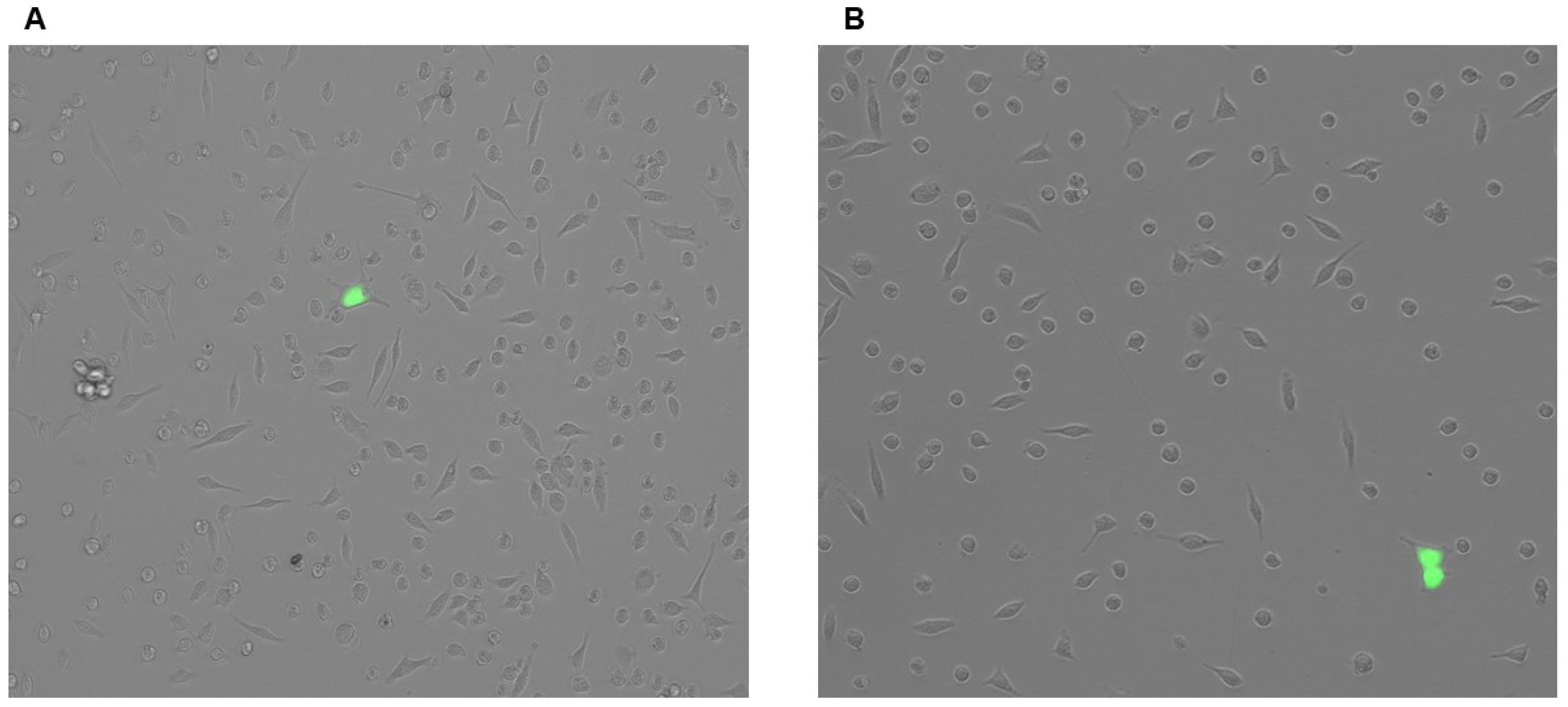
3.2. Plasmid Transfection Is Ineffective at Introducing Transgenes to PMs
3.3. Adenovirus and Lentiviral Vectors Fail at Robustly Introducing Transgenes to PMs
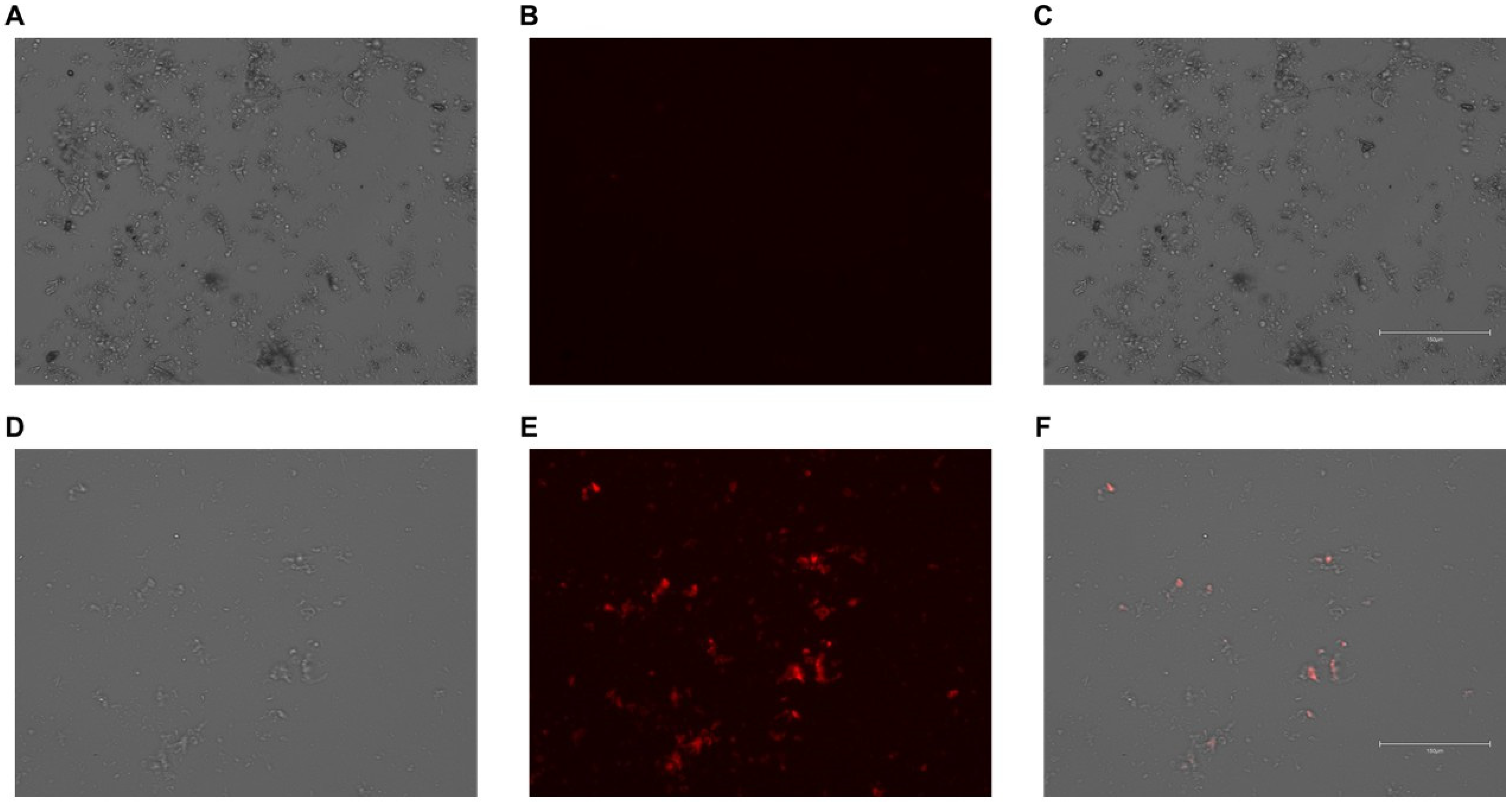
3.4. PMs Successfully Internalize Gesicles
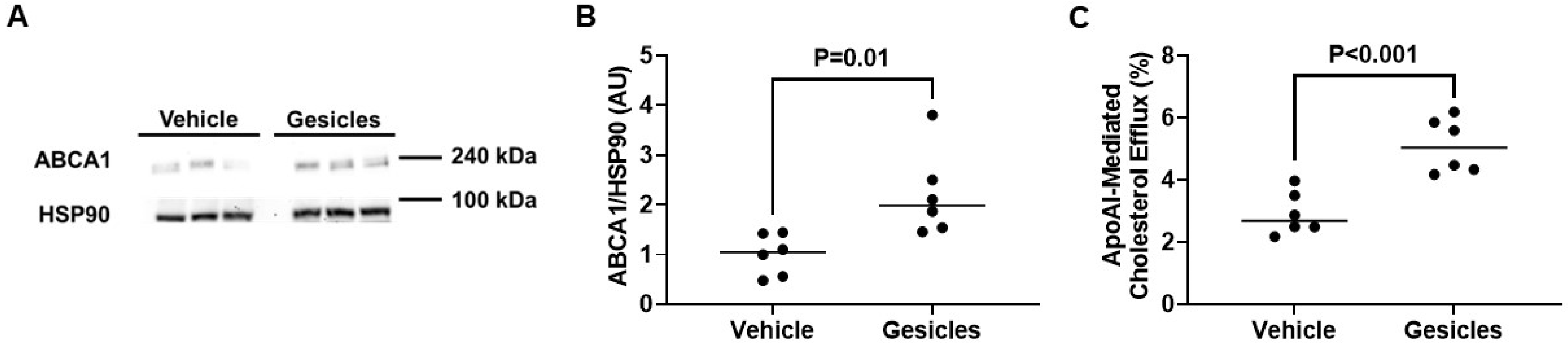
3.5. ABCA1/apoAI-Mediated Cholesterol Efflux Is Enhanced in ABCA1-LSL+/0 PMs Exposed to Gesicles Containing Cre Recombinase
3.6. Delivering Cre Recombinase Gesicles to ABCG1-LSL+/0 PMs Increases ABCG1/HDL-Mediated Cholesterol Efflux
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guyton, J.R. The arterial wall and the atherosclerotic lesion. Curr. Opin. Lipidol. 1994, 5, 376–381. [Google Scholar] [CrossRef] [PubMed]
- Mlynarska, E.; Czarnik, W.; Fularski, P.; Hajdys, J.; Majchrowicz, G.; Stabrawa, M.; Rysz, J.; Franczyk, B. From Atherosclerotic Plaque to Myocardial Infarction-The Leading Cause of Coronary Artery Occlusion. Int. J. Mol. Sci. 2024, 25, 7295. [Google Scholar] [CrossRef] [PubMed]
- Pahwa, R.; Jialal, I. Atherosclerosis. In StatPearls (Atherosclerosis); StatPearls: Treasure Island, FL, USA, 2020. [Google Scholar]
- Makover, M.E.; Shapiro, M.D.; Toth, P.P. There is urgent need to treat atherosclerotic cardiovascular disease risk earlier, more intensively, and with greater precision: A review of current practice and recommendations for improved effectiveness. Am. J. Prev. Cardiol. 2022, 12, 100371. [Google Scholar] [CrossRef]
- Stroes, E. Statins and LDL-cholesterol lowering: An overview. Curr. Med. Res. Opin. 2005, 21 (Suppl. 6), S9–S16. [Google Scholar] [CrossRef]
- Sucato, V.; Ortello, A.; Comparato, F.; Novo, G.; Galassi, A.R. Cholesterol-Lowering Strategies for Cardiovascular Disease Prevention: The Importance of Intensive Treatment and the Simplification of Medical Therapy. J. Clin. Med. 2024, 13, 1882. [Google Scholar] [CrossRef]
- Zhou, Q.; Liao, J.K. Statins and cardiovascular diseases: From cholesterol lowering to pleiotropy. Curr. Pharm. Des. 2009, 15, 467–478. [Google Scholar] [CrossRef]
- Kruth, H.S. Lipoprotein cholesterol and atherosclerosis. Curr. Mol. Med. 2001, 1, 633–653. [Google Scholar] [CrossRef]
- Cardiovascular disease: Risk assessment and reduction, including lipid modification: Evidence review C. In Evidence Review for Statins: Efficacy and Adverse Effects; National Institute for Health and Care Excellence (NICE): London, UK, 2023.
- Jebari-Benslaiman, S.; Galicia-Garcia, U.; Larrea-Sebal, A.; Olaetxea, J.R.; Alloza, I.; Vandenbroeck, K.; Benito-Vicente, A.; Martin, C. Pathophysiology of Atherosclerosis. Int. J. Mol. Sci. 2022, 23, 3346. [Google Scholar] [CrossRef]
- Yuan, Y.; Yuan, R.; Xin, Q.; Miao, Y.; Chen, Y.; Gao, R.; Cong, W. Tetramethylpyrazine and paeoniflorin combination (TMP-PF) inhibits angiogenesis in atherosclerosis via miR-126/VEGF/VEGFR2 signaling pathway. J. Future Foods 2024, 4, 280–287. [Google Scholar] [CrossRef]
- Li, J.; Xiong, T.; Wang, T.; Wang, M.; Wang, C.; Yang, F.; Wang, X.; Tan, Z.; Sun, W. Baicalein targets CD36 to prevent foam cell formation by suppressing the excessive uptake of oxLDL and accelerating ABCA1-mediated cholesterol efflux in oxLDL-induced THP-1 macrophages. J. Funct. Foods 2022, 97, 105253. [Google Scholar] [CrossRef]
- Lin, S.J. Risk factors, endothelial cell turnover and lipid transport in atherogenesis. Zhonghua Yi Xue Za Zhi (Taipei) 1996, 58, 309–316. [Google Scholar] [PubMed]
- Bielikova, Y.O.; Khranovskyi, A.M.; Motsak, T.M.; Kuzminets, A.A. The connection of systemic inflammation and atherosclerosis: What do we know nowadays? Wiad. Lek. 2024, 77, 2332–2339. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.K. Linking endothelial dysfunction with endothelial cell activation. J. Clin. Investig. 2013, 123, 540–541. [Google Scholar] [CrossRef] [PubMed]
- Poznyak, A.V.; Sadykhov, N.K.; Kartuesov, A.G.; Borisov, E.E.; Melnichenko, A.A.; Grechko, A.V.; Orekhov, A.N. Hypertension as a risk factor for atherosclerosis: Cardiovascular risk assessment. Front. Cardiovasc. Med. 2022, 9, 959285. [Google Scholar] [CrossRef]
- Batty, M.; Bennett, M.R.; Yu, E. The Role of Oxidative Stress in Atherosclerosis. Cells 2022, 11, 3843. [Google Scholar] [CrossRef]
- Prasad, K.; Mishra, M. Mechanism of Hypercholesterolemia-Induced Atherosclerosis. Rev. Cardiovasc. Med. 2022, 23, 212. [Google Scholar] [CrossRef]
- Camare, C.; Pucelle, M.; Negre-Salvayre, A.; Salvayre, R. Angiogenesis in the atherosclerotic plaque. Redox Biol. 2017, 12, 18–34. [Google Scholar] [CrossRef]
- Groenen, A.G.; Halmos, B.; Tall, A.R.; Westerterp, M. Cholesterol efflux pathways, inflammation, and atherosclerosis. Crit. Rev. Biochem. Mol. Biol. 2021, 56, 426–439. [Google Scholar] [CrossRef]
- Jesch, E.D.; Carr, T.P. Food Ingredients That Inhibit Cholesterol Absorption. Prev. Nutr. Food Sci. 2017, 22, 67–80. [Google Scholar] [CrossRef]
- Juhl, A.D.; Wustner, D. Pathways and Mechanisms of Cellular Cholesterol Efflux-Insight From Imaging. Front. Cell Dev. Biol. 2022, 10, 834408. [Google Scholar] [CrossRef]
- von Eckardstein, A. Cholesterol efflux from macrophages and other cells. Curr. Opin. Lipidol. 1996, 7, 308–319. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Westerterp, M. ABC Transporters, Cholesterol Efflux, and Implications for Cardiovascular Diseases. Adv. Exp. Med. Biol. 2020, 1276, 67–83. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Gong, K.; Xu, S.; Zhang, F.; Meng, X.; Han, J. Regulation of cholesterol homeostasis in health and diseases: From mechanisms to targeted therapeutics. Signal Transduct. Target. Ther. 2022, 7, 265. [Google Scholar] [CrossRef] [PubMed]
- Rosenson, R.S.; Brewer, H.B., Jr.; Davidson, W.S.; Fayad, Z.A.; Fuster, V.; Goldstein, J.; Hellerstein, M.; Jiang, X.C.; Phillips, M.C.; Rader, D.J.; et al. Cholesterol efflux and atheroprotection: Advancing the concept of reverse cholesterol transport. Circulation 2012, 125, 1905–1919. [Google Scholar] [CrossRef]
- Chistiakov, D.A.; Bobryshev, Y.V.; Orekhov, A.N. Macrophage-mediated cholesterol handling in atherosclerosis. J. Cell. Mol. Med. 2016, 20, 17–28. [Google Scholar] [CrossRef]
- Out, R.; Hoekstra, M.; Habets, K.; Meurs, I.; de Waard, V.; Hildebrand, R.B.; Wang, Y.; Chimini, G.; Kuiper, J.; Van Berkel, T.J.; et al. Combined deletion of macrophage ABCA1 and ABCG1 leads to massive lipid accumulation in tissue macrophages and distinct atherosclerosis at relatively low plasma cholesterol levels. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 258–264. [Google Scholar] [CrossRef]
- Choi, H.Y.; Ruel, I.; Choi, S.; Genest, J. New Strategies to Promote Macrophage Cholesterol Efflux. Front. Cardiovasc. Med. 2021, 8, 795868. [Google Scholar] [CrossRef]
- Ouimet, M.; Barrett, T.J.; Fisher, E.A. HDL and Reverse Cholesterol Transport. Circ. Res. 2019, 124, 1505–1518. [Google Scholar] [CrossRef]
- Tall, A.R. An overview of reverse cholesterol transport. Eur. Heart J. 1998, 19 (Suppl. A), A31–A35. [Google Scholar] [CrossRef]
- Fielding, C.J.; Fielding, P.E. Molecular physiology of reverse cholesterol transport. J. Lipid Res. 1995, 36, 211–228. [Google Scholar] [CrossRef]
- Kuzu, O.F.; Noory, M.A.; Robertson, G.P. The Role of Cholesterol in Cancer. Cancer Res. 2016, 76, 2063–2070. [Google Scholar] [CrossRef] [PubMed]
- Mordzinska-Rak, A.; Verdeil, G.; Hamon, Y.; Blaszczak, E.; Trombik, T. Dysregulation of cholesterol homeostasis in cancer pathogenesis. Cell. Mol. Life Sci. 2025, 82, 168. [Google Scholar] [CrossRef] [PubMed]
- Sharma, B.; Agnihotri, N. Role of cholesterol homeostasis and its efflux pathways in cancer progression. J. Steroid Biochem. Mol. Biol. 2019, 191, 105377. [Google Scholar] [CrossRef] [PubMed]
- Xiao, M.; Xu, J.; Wang, W.; Zhang, B.; Liu, J.; Li, J.; Xu, H.; Zhao, Y.; Yu, X.; Shi, S. Functional significance of cholesterol metabolism in cancer: From threat to treatment. Exp. Mol. Med. 2023, 55, 1982–1995. [Google Scholar] [CrossRef]
- He, Y.; Ronsein, G.E.; Tang, C.; Jarvik, G.P.; Davidson, W.S.; Kothari, V.; Song, H.D.; Segrest, J.P.; Bornfeldt, K.E.; Heinecke, J.W. Diabetes Impairs Cellular Cholesterol Efflux From ABCA1 to Small HDL Particles. Circ. Res. 2020, 127, 1198–1210. [Google Scholar] [CrossRef]
- Posadas-Sanchez, R.; Posadas-Romero, C.; Mendoza-Perez, E.; Caracas-Portilla, N.A.; Cardoso-Saldana, G.; Medina-Urrutia, A.; Jorge-Galarza, E.; Juarez-Rojas, J.G. Cholesterol efflux and metabolic abnormalities associated with low high-density-lipoprotein-cholesterol and high triglycerides in statin-treated coronary men with low-density lipoprotein-cholesterol <70 mg/dl. Am. J. Cardiol. 2012, 109, 636–641. [Google Scholar] [CrossRef]
- Stadler, J.T.; Marsche, G. Obesity-Related Changes in High-Density Lipoprotein Metabolism and Function. Int. J. Mol. Sci. 2020, 21, 8985. [Google Scholar] [CrossRef]
- Yvan-Charvet, L.; Swirski, F.K. Is defective cholesterol efflux an integral inflammatory component in myelopoiesis-driven cardiovascular diseases? Eur. Heart J. 2018, 39, 2168–2171. [Google Scholar] [CrossRef]
- Kruit, J.K.; Wijesekara, N.; Westwell-Roper, C.; Vanmierlo, T.; de Haan, W.; Bhattacharjee, A.; Tang, R.; Wellington, C.L.; LutJohann, D.; Johnson, J.D.; et al. Loss of both ABCA1 and ABCG1 results in increased disturbances in islet sterol homeostasis, inflammation, and impaired beta-cell function. Diabetes 2012, 61, 659–664. [Google Scholar] [CrossRef]
- Denimal, D.; Benanaya, S.; Monier, S.; Simoneau, I.; Pais de Barros, J.P.; Le Goff, W.; Bouillet, B.; Verges, B.; Duvillard, L. Normal HDL Cholesterol Efflux and Anti-Inflammatory Capacities in Type 2 Diabetes Despite Lipidomic Abnormalities. J. Clin. Endocrinol. Metab. 2022, 107, e3816–e3823. [Google Scholar] [CrossRef]
- Orozco Morales, J.A.; Medina Urrutia, A.X.; Tamayo, M.T.; Reyes Barrera, J.; Galarza, E.J.; Juarez Rojas, J.G.; Dies Suarez, P.; Mendez Sanchez, N.; Diaz Orozco, L.E.; Velazquez-Lopez, L.; et al. Impact of metabolic-associated fatty liver disease on the cholesterol efflux capacity of high-density lipoproteins in adolescents with type 2 diabetes. Front. Pediatr. 2024, 12, 1462406. [Google Scholar] [CrossRef] [PubMed]
- Yvan-Charvet, L.; Kling, J.; Pagler, T.; Li, H.; Hubbard, B.; Fisher, T.; Sparrow, C.P.; Taggart, A.K.; Tall, A.R. Cholesterol efflux potential and antiinflammatory properties of high-density lipoprotein after treatment with niacin or anacetrapib. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 1430–1438. [Google Scholar] [CrossRef] [PubMed]
- Phillips, M.C. Molecular mechanisms of cellular cholesterol efflux. J. Biol. Chem. 2014, 289, 24020–24029. [Google Scholar] [CrossRef]
- Frambach, S.; de Haas, R.; Smeitink, J.A.M.; Rongen, G.A.; Russel, F.G.M.; Schirris, T.J.J. Brothers in Arms: ABCA1- and ABCG1-Mediated Cholesterol Efflux as Promising Targets in Cardiovascular Disease Treatment. Pharmacol. Rev. 2020, 72, 152–190. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.H.; Tang, C.K. ABCA1, ABCG1, and Cholesterol Homeostasis. Adv. Exp. Med. Biol. 2022, 1377, 95–107. [Google Scholar] [CrossRef]
- Heinecke, J.W. Small HDL promotes cholesterol efflux by the ABCA1 pathway in macrophages: Implications for therapies targeted to HDL. Circ. Res. 2015, 116, 1101–1103. [Google Scholar] [CrossRef]
- Esobi, I.; Olanrewaju, O.; Echesabal-Chen, J.; Stamatikos, A. Utilizing the LoxP-Stop-LoxP System to Control Transgenic ABC-Transporter Expression In Vitro. Biomolecules 2022, 12, 679. [Google Scholar] [CrossRef]
- Du, X.M.; Kim, M.J.; Hou, L.; Le Goff, W.; Chapman, M.J.; Van Eck, M.; Curtiss, L.K.; Burnett, J.R.; Cartland, S.P.; Quinn, C.M.; et al. HDL particle size is a critical determinant of ABCA1-mediated macrophage cellular cholesterol export. Circ. Res. 2015, 116, 1133–1142. [Google Scholar] [CrossRef]
- Wang, X.; Collins, H.L.; Ranalletta, M.; Fuki, I.V.; Billheimer, J.T.; Rothblat, G.H.; Tall, A.R.; Rader, D.J. Macrophage ABCA1 and ABCG1, but not SR-BI, promote macrophage reverse cholesterol transport in vivo. J. Clin. Investig. 2007, 117, 2216–2224. [Google Scholar] [CrossRef]
- Matsuo, M. ABCA1 and ABCG1 as potential therapeutic targets for the prevention of atherosclerosis. J. Pharmacol. Sci. 2022, 148, 197–203. [Google Scholar] [CrossRef]
- Van Eck, M.; Singaraja, R.R.; Ye, D.; Hildebrand, R.B.; James, E.R.; Hayden, M.R.; Van Berkel, T.J. Macrophage ATP-binding cassette transporter A1 overexpression inhibits atherosclerotic lesion progression in low-density lipoprotein receptor knockout mice. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 929–934. [Google Scholar] [CrossRef]
- Santoro, S.W.; Schultz, P.G. Directed evolution of the site specificity of Cre recombinase. Proc. Natl. Acad. Sci. USA 2002, 99, 4185–4190. [Google Scholar] [CrossRef] [PubMed]
- Abremski, K.; Wierzbicki, A.; Frommer, B.; Hoess, R.H. Bacteriophage P1 Cre-loxP site-specific recombination. Site-specific DNA topoisomerase activity of the Cre recombination protein. J. Biol. Chem. 1986, 261, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Hoess, R.H.; Abremski, K. Interaction of the bacteriophage P1 recombinase Cre with the recombining site loxP. Proc. Natl. Acad. Sci. USA 1984, 81, 1026–1029. [Google Scholar] [CrossRef] [PubMed]
- Sauer, B.; Henderson, N. Site-specific DNA recombination in mammalian cells by the Cre recombinase of bacteriophage P1. Proc. Natl. Acad. Sci. USA 1988, 85, 5166–5170. [Google Scholar] [CrossRef]
- Guo, F.; Gopaul, D.N.; Van Duyne, G.D. Asymmetric DNA bending in the Cre-loxP site-specific recombination synapse. Proc. Natl. Acad. Sci. USA 1999, 96, 7143–7148. [Google Scholar] [CrossRef]
- Van Duyne, G.D. A structural view of cre-loxp site-specific recombination. Annu. Rev. Biophys. Biomol. Struct. 2001, 30, 87–104. [Google Scholar] [CrossRef]
- Kim, H.; Kim, M.; Im, S.K.; Fang, S. Mouse Cre-LoxP system: General principles to determine tissue-specific roles of target genes. Lab. Anim. Res. 2018, 34, 147–159. [Google Scholar] [CrossRef]
- Goodrich, M.M.; Talhouk, R.; Zhang, X.; Goodrich, D.W. An approach for controlling the timing and order of engineered mutations in mice. Genesis 2018, 56, e23243. [Google Scholar] [CrossRef]
- Safran, M.; Kim, W.Y.; Kung, A.L.; Horner, J.W.; DePinho, R.A.; Kaelin, W.G., Jr. Mouse reporter strain for noninvasive bioluminescent imaging of cells that have undergone Cre-mediated recombination. Mol. Imaging 2003, 2, 297–302. [Google Scholar] [CrossRef]
- Bapst, A.M.; Dahl, S.L.; Knopfel, T.; Wenger, R.H. Cre-mediated, loxP independent sequential recombination of a tripartite transcriptional stop cassette allows for partial read-through transcription. Biochim. Biophys. Acta Gene Regul. Mech. 2020, 1863, 194568. [Google Scholar] [CrossRef]
- Shi, J.; Hua, L.; Harmer, D.; Li, P.; Ren, G. Cre Driver Mice Targeting Macrophages. Methods Mol. Biol. 2018, 1784, 263–275. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, J.; Jiang, W.J.; Shan, X.W.; Yang, X.M.; Gao, J.G. Conditional gene manipulation: Cre-ating a new biological era. J. Zhejiang Univ. Sci. B 2012, 13, 511–524. [Google Scholar] [CrossRef] [PubMed]
- Demayo, J.L.; Wang, J.; Liang, D.; Zhang, R.; Demayo, F.J. Genetically Engineered Mice by Pronuclear DNA microinjection. Curr. Protoc. Mouse Biol. 2012, 2, 245–262. [Google Scholar] [CrossRef] [PubMed]
- Deaton, R.A.; Bulut, G.; Serbulea, V.; Salamon, A.; Shankman, L.S.; Nguyen, A.T.; Owens, G.K. A New Autosomal Myh11-CreER(T2) Smooth Muscle Cell Lineage Tracing and Gene Knockout Mouse Model-Brief Report. Arterioscler. Thromb. Vasc. Biol. 2023, 43, 203–211. [Google Scholar] [CrossRef]
- De Jesus, A.; Pusec, C.M.; Nguyen, T.; Keyhani-Nejad, F.; Gao, P.; Weinberg, S.E.; Ardehali, H. Optimized protocol to isolate primary mouse peritoneal macrophage metabolites. STAR Protoc. 2022, 3, 101668. [Google Scholar] [CrossRef]
- Huang, K.; Pokhrel, A.; Echesabal-Chen, J.; Scott, J.; Bruce, T.; Jo, H.; Stamatikos, A. Inhibiting MiR-33a-3p Expression Fails to Enhance ApoAI-Mediated Cholesterol Efflux in Pro-Inflammatory Endothelial Cells. Medicina 2025, 61, 329. [Google Scholar] [CrossRef]
- Chong, Z.X.; Yeap, S.K.; Ho, W.Y. Transfection types, methods and strategies: A technical review. PeerJ 2021, 9, e11165. [Google Scholar] [CrossRef]
- Leopold Wager, C.M.; Bonifacio, J.R.; Simper, J.; Naoun, A.A.; Arnett, E.; Schlesinger, L.S. Activation of transcription factor CREB in human macrophages by Mycobacterium tuberculosis promotes bacterial survival, reduces NF-kB nuclear transit and limits phagolysosome fusion by reduced necroptotic signaling. PLoS Pathog. 2023, 19, e1011297. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, S.; Liu, Y.; Gowda, Y.K.M.; Miao, J. Generation of Adenovirus for In Vitro and In Vivo Studies of Hepatocytes. Methods Mol. Biol. 2022, 2455, 343–358. [Google Scholar] [CrossRef]
- Oladosu, O.; Chin, E.; Barksdale, C.; Powell, R.R.; Bruce, T.; Stamatikos, A. Inhibition of miR-33a-5p in Macrophage-like Cells In Vitro Promotes apoAI-Mediated Cholesterol Efflux. Pathophysiology 2024, 31, 117–126. [Google Scholar] [CrossRef]
- Oladosu, O.; Esobi, I.C.; Powell, R.R.; Bruce, T.; Stamatikos, A. Dissecting the Impact of Vascular Smooth Muscle Cell ABCA1 versus ABCG1 Expression on Cholesterol Efflux and Macrophage-like Cell Transdifferentiation: The Role of SR-BI. J. Cardiovasc. Dev. Dis. 2023, 10, 416. [Google Scholar] [CrossRef] [PubMed]
- Campbell, L.A.; Coke, L.M.; Richie, C.T.; Fortuno, L.V.; Park, A.Y.; Harvey, B.K. Gesicle-Mediated Delivery of CRISPR/Cas9 Ribonucleoprotein Complex for Inactivating the HIV Provirus. Mol. Ther. 2019, 27, 151–163. [Google Scholar] [CrossRef] [PubMed]
- Campbell, L.A.; Richie, C.T.; Maggirwar, N.S.; Harvey, B.K. Cas9 Ribonucleoprotein Complex Delivery: Methods and Applications for Neuroinflammation. J. Neuroimmune Pharmacol. 2019, 14, 565–577. [Google Scholar] [CrossRef] [PubMed]
- Echesabal-Chen, J.; Huang, K.; Vojtech, L.; Oladosu, O.; Esobi, I.; Sachdeva, R.; Vyavahare, N.; Jo, H.; Stamatikos, A. Constructing Lipoparticles Capable of Endothelial Cell-Derived Exosome-Mediated Delivery of Anti-miR-33a-5p to Cultured Macrophages. Curr. Issues Mol. Biol. 2023, 45, 5631–5644. [Google Scholar] [CrossRef]
- Esobi, I.C.; Oladosu, O.; Echesabal-Chen, J.; Powell, R.R.; Bruce, T.; Stamatikos, A. miR-33a Expression Attenuates ABCA1-Dependent Cholesterol Efflux and Promotes Macrophage-Like Cell Transdifferentiation in Cultured Vascular Smooth Muscle Cells. J. Lipids 2023, 2023, 8241899. [Google Scholar] [CrossRef]
- Huang, K.; Pitman, M.; Oladosu, O.; Echesabal-Chen, J.; Vojtech, L.; Esobi, I.; Larsen, J.; Jo, H.; Stamatikos, A. The Impact of MiR-33a-5p Inhibition in Pro-Inflammatory Endothelial Cells. Diseases 2023, 11, 88. [Google Scholar] [CrossRef]
- Huang, K.; Garimella, S.; Clay-Gilmour, A.; Vojtech, L.; Armstrong, B.; Bessonny, M.; Stamatikos, A. Comparison of Human Urinary Exosomes Isolated via Ultracentrifugation Alone versus Ultracentrifugation Followed by SEC Column-Purification. J. Pers. Med. 2022, 12, 340. [Google Scholar] [CrossRef]
- Esobi, I.C.; Barksdale, C.; Heard-Tate, C.; Reigers Powell, R.; Bruce, T.F.; Stamatikos, A. MOVAS Cells: A Versatile Cell Line for Studying Vascular Smooth Muscle Cell Cholesterol Metabolism. Lipids 2021, 56, 413–422. [Google Scholar] [CrossRef]
- Huang, K.; Jo, H.; Echesabal-Chen, J.; Stamatikos, A. Combined LXR and RXR Agonist Therapy Increases ABCA1 Protein Expression and Enhances ApoAI-Mediated Cholesterol Efflux in Cultured Endothelial Cells. Metabolites 2021, 11, 640. [Google Scholar] [CrossRef]
- Christensen, J.E.; Andreasen, S.O.; Christensen, J.P.; Thomsen, A.R. CD11b expression as a marker to distinguish between recently activated effector CD8(+) T cells and memory cells. Int. Immunol. 2001, 13, 593–600. [Google Scholar] [CrossRef] [PubMed]
- Dos Anjos Cassado, A. F4/80 as a Major Macrophage Marker: The Case of the Peritoneum and Spleen. Results Probl. Cell Differ. 2017, 62, 161–179. [Google Scholar] [CrossRef] [PubMed]
- Khazen, W.; M’Bika, J.P.; Tomkiewicz, C.; Benelli, C.; Chany, C.; Achour, A.; Forest, C. Expression of macrophage-selective markers in human and rodent adipocytes. FEBS Lett. 2005, 579, 5631–5634. [Google Scholar] [CrossRef] [PubMed]
- Douglas, K.L. Toward development of artificial viruses for gene therapy: A comparative evaluation of viral and non-viral transfection. Biotechnol. Prog. 2008, 24, 871–883. [Google Scholar] [CrossRef]
- Ivanova, A.; Badertscher, L.; O’Driscoll, G.; Bergman, J.; Gordon, E.; Gunnarsson, A.; Johansson, C.; Munson, M.J.; Spinelli, C.; Torstensson, S.; et al. Creating Designer Engineered Extracellular Vesicles for Diverse Ligand Display, Target Recognition, and Controlled Protein Loading and Delivery. Adv. Sci. 2023, 10, e2304389. [Google Scholar] [CrossRef]
- Luciani, M.F.; Denizot, F.; Savary, S.; Mattei, M.G.; Chimini, G. Cloning of two novel ABC transporters mapping on human chromosome 9. Genomics 1994, 21, 150–159. [Google Scholar] [CrossRef]
- Puntoni, M.; Sbrana, F.; Bigazzi, F.; Sampietro, T. Tangier disease: Epidemiology, pathophysiology, and management. Am. J. Cardiovasc. Drugs 2012, 12, 303–311. [Google Scholar] [CrossRef]
- Oram, J.F. Tangier disease and ABCA1. Biochim. Biophys. Acta 2000, 1529, 321–330. [Google Scholar] [CrossRef]
- Rust, S.; Walter, M.; Funke, H.; von Eckardstein, A.; Cullen, P.; Kroes, H.Y.; Hordijk, R.; Geisel, J.; Kastelein, J.; Molhuizen, H.O.; et al. Assignment of Tangier disease to chromosome 9q31 by a graphical linkage exclusion strategy. Nat. Genet. 1998, 20, 96–98. [Google Scholar] [CrossRef]
- Bodzioch, M.; Orso, E.; Klucken, J.; Langmann, T.; Bottcher, A.; Diederich, W.; Drobnik, W.; Barlage, S.; Buchler, C.; Porsch-Ozcurumez, M.; et al. The gene encoding ATP-binding cassette transporter 1 is mutated in Tangier disease. Nat. Genet. 1999, 22, 347–351. [Google Scholar] [CrossRef]
- Brooks-Wilson, A.; Marcil, M.; Clee, S.M.; Zhang, L.H.; Roomp, K.; van Dam, M.; Yu, L.; Brewer, C.; Collins, J.A.; Molhuizen, H.O.; et al. Mutations in ABC1 in Tangier disease and familial high-density lipoprotein deficiency. Nat. Genet. 1999, 22, 336–345. [Google Scholar] [CrossRef] [PubMed]
- Rust, S.; Rosier, M.; Funke, H.; Real, J.; Amoura, Z.; Piette, J.C.; Deleuze, J.F.; Brewer, H.B.; Duverger, N.; Denefle, P.; et al. Tangier disease is caused by mutations in the gene encoding ATP-binding cassette transporter 1. Nat. Genet. 1999, 22, 352–355. [Google Scholar] [CrossRef] [PubMed]
- Oram, J.F.; Lawn, R.M. ABCA1. The gatekeeper for eliminating excess tissue cholesterol. J. Lipid Res. 2001, 42, 1173–1179. [Google Scholar] [CrossRef] [PubMed]
- Yin, K.; Liao, D.F.; Tang, C.K. ATP-binding membrane cassette transporter A1 (ABCA1): A possible link between inflammation and reverse cholesterol transport. Mol. Med. 2010, 16, 438–449. [Google Scholar] [CrossRef]
- Attie, A.D. ABCA1: At the nexus of cholesterol, HDL and atherosclerosis. Trends Biochem. Sci. 2007, 32, 172–179. [Google Scholar] [CrossRef]
- Soumian, S.; Albrecht, C.; Davies, A.H.; Gibbs, R.G. ABCA1 and atherosclerosis. Vasc. Med. 2005, 10, 109–119. [Google Scholar] [CrossRef]
- Mukhamedova, N.; Escher, G.; D’Souza, W.; Tchoua, U.; Grant, A.; Krozowski, Z.; Bukrinsky, M.; Sviridov, D. Enhancing apolipoprotein A-I-dependent cholesterol efflux elevates cholesterol export from macrophages in vivo. J. Lipid Res. 2008, 49, 2312–2322. [Google Scholar] [CrossRef]
- Wang, M.D.; Franklin, V.; Marcel, Y.L. In vivo reverse cholesterol transport from macrophages lacking ABCA1 expression is impaired. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 1837–1842. [Google Scholar] [CrossRef]
- Van Eck, M.; Van Berkel, T.J. ATP-binding cassette transporter A1 in lipoprotein metabolism and atherosclerosis: A new piece of the complex puzzle. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 2281–2283. [Google Scholar] [CrossRef]
- Chen, H.; Rossier, C.; Lalioti, M.D.; Lynn, A.; Chakravarti, A.; Perrin, G.; Antonarakis, S.E. Cloning of the cDNA for a human homologue of the Drosophila white gene and mapping to chromosome 21q22.3. Am. J. Hum. Genet. 1996, 59, 66–75. [Google Scholar]
- Savary, S.; Denizot, F.; Luciani, M.; Mattei, M.; Chimini, G. Molecular cloning of a mammalian ABC transporter homologous to Drosophila white gene. Mamm. Genome 1996, 7, 673–676. [Google Scholar] [CrossRef]
- Kotlyarov, S.; Kotlyarova, A. Biological Functions and Clinical Significance of the ABCG1 Transporter. Biology 2024, 14, 8. [Google Scholar] [CrossRef]
- Tarr, P.T.; Tarling, E.J.; Bojanic, D.D.; Edwards, P.A.; Baldan, A. Emerging new paradigms for ABCG transporters. Biochim. Biophys. Acta 2009, 1791, 584–593. [Google Scholar] [CrossRef]
- Woodward, O.M.; Kottgen, A.; Kottgen, M. ABCG transporters and disease. FEBS J. 2011, 278, 3215–3225. [Google Scholar] [CrossRef]
- Wu, Y.; Chen, L.; Xie, Z.; Wang, C.; Zhang, J.; Yan, X. Effects of ABCG1 knockout on proteomic composition of HDL in mice on a chow diet and a high-fat diet. Proteomics 2022, 22, e2100028. [Google Scholar] [CrossRef]
- Xu, D.; Li, Y.; Yang, F.; Sun, C.R.; Pan, J.; Wang, L.; Chen, Z.P.; Fang, S.C.; Yao, X.; Hou, W.T.; et al. Structure and transport mechanism of the human cholesterol transporter ABCG1. Cell Rep. 2022, 38, 110298. [Google Scholar] [CrossRef] [PubMed]
- Yvan-Charvet, L.; Wang, N.; Tall, A.R. Role of HDL, ABCA1, and ABCG1 transporters in cholesterol efflux and immune responses. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Tall, A.R.; Yvan-Charvet, L.; Terasaka, N.; Pagler, T.; Wang, N. HDL, ABC transporters, and cholesterol efflux: Implications for the treatment of atherosclerosis. Cell Metab. 2008, 7, 365–375. [Google Scholar] [CrossRef] [PubMed]
- Basso, F.; Amar, M.J.; Wagner, E.M.; Vaisman, B.; Paigen, B.; Santamarina-Fojo, S.; Remaley, A.T. Enhanced ABCG1 expression increases atherosclerosis in LDLr-KO mice on a western diet. Biochem. Biophys. Res. Commun. 2006, 351, 398–404. [Google Scholar] [CrossRef]
- Lammers, B.; Out, R.; Hildebrand, R.B.; Quinn, C.M.; Williamson, D.; Hoekstra, M.; Meurs, I.; Van Berkel, T.J.; Jessup, W.; Van Eck, M. Independent protective roles for macrophage Abcg1 and Apoe in the atherosclerotic lesion development. Atherosclerosis 2009, 205, 420–426. [Google Scholar] [CrossRef]
- Meurs, I.; Lammers, B.; Zhao, Y.; Out, R.; Hildebrand, R.B.; Hoekstra, M.; Van Berkel, T.J.; Van Eck, M. The effect of ABCG1 deficiency on atherosclerotic lesion development in LDL receptor knockout mice depends on the stage of atherogenesis. Atherosclerosis 2012, 221, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Ni, Z.L.; Zhao, S.P.; Wu, Z. ABCG1--a potential therapeutic target for atherosclerosis. Med. Hypotheses 2007, 69, 214–217. [Google Scholar] [CrossRef] [PubMed]
- Out, R.; Hoekstra, M.; Meurs, I.; de Vos, P.; Kuiper, J.; Van Eck, M.; Van Berkel, T.J. Total body ABCG1 expression protects against early atherosclerotic lesion development in mice. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 594–599. [Google Scholar] [CrossRef]
- Out, R.; Hoekstra, M.; Hildebrand, R.B.; Kruit, J.K.; Meurs, I.; Li, Z.; Kuipers, F.; Van Berkel, T.J.; Van Eck, M. Macrophage ABCG1 deletion disrupts lipid homeostasis in alveolar macrophages and moderately influences atherosclerotic lesion development in LDL receptor-deficient mice. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 2295–2300. [Google Scholar] [CrossRef] [PubMed]
- Herb, M.; Farid, A.; Gluschko, A.; Kronke, M.; Schramm, M. Highly Efficient Transfection of Primary Macrophages with In Vitro Transcribed mRNA. J. Vis. Exp. 2019, e60143. [Google Scholar] [CrossRef]
- Thompson, C.D.; Frazier-Jessen, M.R.; Rawat, R.; Nordan, R.P.; Brown, R.T. Evaluation of methods for transient transfection of a murine macrophage cell line, RAW 264.7. Biotechniques 1999, 27, 824–832. [Google Scholar] [CrossRef]
- Aluigi, M.; Fogli, M.; Curti, A.; Isidori, A.; Gruppioni, E.; Chiodoni, C.; Colombo, M.P.; Versura, P.; D’Errico-Grigioni, A.; Ferri, E.; et al. Nucleofection is an efficient nonviral transfection technique for human bone marrow-derived mesenchymal stem cells. Stem Cells 2006, 24, 454–461. [Google Scholar] [CrossRef]
- Iversen, N.; Birkenes, B.; Torsdalen, K.; Djurovic, S. Electroporation by nucleofector is the best nonviral transfection technique in human endothelial and smooth muscle cells. Genet. Vaccines Ther. 2005, 3, 2. [Google Scholar] [CrossRef]
- Luft, C.; Ketteler, R. Electroporation Knows No Boundaries: The Use of Electrostimulation for siRNA Delivery in Cells and Tissues. J. Biomol. Screen. 2015, 20, 932–942. [Google Scholar] [CrossRef]
- Potter, H. Transfection by electroporation. Curr. Protoc. Mol. Biol. 2003, 9, 9.3. [Google Scholar] [CrossRef]
- Zaragosi, L.E.; Billon, N.; Ailhaud, G.; Dani, C. Nucleofection is a valuable transfection method for transient and stable transgene expression in adipose tissue-derived stem cells. Stem Cells 2007, 25, 790–797. [Google Scholar] [CrossRef]
- Keller, A.A.; Maess, M.B.; Schnoor, M.; Scheiding, B.; Lorkowski, S. Transfecting Macrophages. Methods Mol. Biol. 2018, 1784, 187–195. [Google Scholar] [CrossRef]
- Maess, M.B.; Wittig, B.; Lorkowski, S. Highly efficient transfection of human THP-1 macrophages by nucleofection. J. Vis. Exp. 2014, e51960. [Google Scholar] [CrossRef]
- Kluth, D.C.; Ainslie, C.V.; Pearce, W.P.; Finlay, S.; Clarke, D.; Anegon, I.; Rees, A.J. Macrophages transfected with adenovirus to express IL-4 reduce inflammation in experimental glomerulonephritis. J. Immunol. 2001, 166, 4728–4736. [Google Scholar] [CrossRef]
- Stein, S.C.; Falck-Pedersen, E. Sensing adenovirus infection: Activation of interferon regulatory factor 3 in RAW 264.7 cells. J. Virol. 2012, 86, 4527–4537. [Google Scholar] [CrossRef]
- Tippimanchai, D.D.; Nolan, K.; Poczobutt, J.; Verzosa, G.; Li, H.; Scarborough, H.; Huang, J.; Young, C.; DeGregori, J.; Nemenoff, R.A.; et al. Adenoviral vectors transduce alveolar macrophages in lung cancer models. Oncoimmunology 2018, 7, e1438105. [Google Scholar] [CrossRef]
- Boehler, R.M.; Kuo, R.; Shin, S.; Goodman, A.G.; Pilecki, M.A.; Gower, R.M.; Leonard, J.N.; Shea, L.D. Lentivirus delivery of IL-10 to promote and sustain macrophage polarization towards an anti-inflammatory phenotype. Biotechnol. Bioeng. 2014, 111, 1210–1221. [Google Scholar] [CrossRef]
- Brempelis, K.J.; Cowan, C.M.; Kreuser, S.A.; Labadie, K.P.; Prieskorn, B.M.; Lieberman, N.A.P.; Ene, C.I.; Moyes, K.W.; Chinn, H.; DeGolier, K.R.; et al. Genetically engineered macrophages persist in solid tumors and locally deliver therapeutic proteins to activate immune responses. J. Immunother. Cancer 2020, 8, e001356. [Google Scholar] [CrossRef] [PubMed]
- Leyva, F.J.; Anzinger, J.J.; McCoy, J.P., Jr.; Kruth, H.S. Evaluation of transduction efficiency in macrophage colony-stimulating factor differentiated human macrophages using HIV-1 based lentiviral vectors. BMC Biotechnol. 2011, 11, 13. [Google Scholar] [CrossRef] [PubMed]
- Bulcha, J.T.; Wang, Y.; Ma, H.; Tai, P.W.L.; Gao, G. Viral vector platforms within the gene therapy landscape. Signal Transduct. Target. Ther. 2021, 6, 53. [Google Scholar] [CrossRef]
- Lundstrom, K. Viral Vectors in Gene Therapy. Diseases 2018, 6, 42. [Google Scholar] [CrossRef] [PubMed]
- Jarrosson-Wuilleme, L.; Goujon, C.; Bernaud, J.; Rigal, D.; Darlix, J.L.; Cimarelli, A. Transduction of nondividing human macrophages with gammaretrovirus-derived vectors. J. Virol. 2006, 80, 1152–1159. [Google Scholar] [CrossRef] [PubMed]
- Wotherspoon, S.; Dolnikov, A.; Symonds, G.; Nordon, R. Susceptibility of cell populations to transduction by retroviral vectors. J. Virol. 2004, 78, 5097–5102. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Edwards, J.P.; Mosser, D.M. The expression of exogenous genes in macrophages: Obstacles and opportunities. Methods Mol. Biol. 2009, 531, 123–143. [Google Scholar] [CrossRef] [PubMed]
- Cossarizza, A.; Chang, H.D.; Radbruch, A.; Abrignani, S.; Addo, R.; Akdis, M.; Andra, I.; Andreata, F.; Annunziato, F.; Arranz, E.; et al. Guidelines for the use of flow cytometry and cell sorting in immunological studies (third edition). Eur. J. Immunol. 2021, 51, 2708–3145. [Google Scholar] [CrossRef]
- McKinnon, K.M. Flow Cytometry: An Overview. Curr. Protoc. Immunol. 2018, 120, 5.1.1–5.1.11. [Google Scholar] [CrossRef]
- Vembadi, A.; Menachery, A.; Qasaimeh, M.A. Cell Cytometry: Review and Perspective on Biotechnological Advances. Front. Bioeng. Biotechnol. 2019, 7, 147. [Google Scholar] [CrossRef]
- Zhang, X.; Goncalves, R.; Mosser, D.M. The isolation and characterization of murine macrophages. Curr. Protoc. Immunol. 2008, 83, 14.1.1–14.1.14. [Google Scholar] [CrossRef]
- Fortier, A.H.; Falk, L.A. Isolation of murine macrophages. Curr. Protoc. Immunol. 2001, 14, 14.11.11–14.11.19. [Google Scholar] [CrossRef]
- Schmitz, G.; Drobnik, W. ATP-binding cassette transporters in macrophages: Promising drug targets for treatment of cardiovascular disease. Curr. Opin. Investig. Drugs 2002, 3, 853–858. [Google Scholar]
- Schmitz, G.; Kaminski, W.E.; Orso, E. ABC transporters in cellular lipid trafficking. Curr. Opin. Lipidol. 2000, 11, 493–501. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.; Lu, Y.; Wu, T. The impact of ATP-binding cassette transporters on metabolic diseases. Nutr. Metab. 2020, 17, 61. [Google Scholar] [CrossRef] [PubMed]
- Kotlyarov, S.; Kotlyarova, A. Clinical Significance of Lipid Transport Function of ABC Transporters in the Innate Immune System. Membranes 2022, 12, 83. [Google Scholar] [CrossRef]
- Choi, J.U.; Kim, Y.; Lee, D.Y.; Park, J.S.; Jeun, M.; Lee, H.K.; Park, C.H. Lentivirus-based production of human chimeric antigen receptor macrophages from peripheral blood. Biomark. Res. 2025, 13, 1. [Google Scholar] [CrossRef]
- Pay, S.L.; Qi, X.; Willard, J.F.; Godoy, J.; Sankhavaram, K.; Horton, R.; Mitter, S.K.; Quigley, J.L.; Chang, L.J.; Grant, M.B.; et al. Improving the Transduction of Bone Marrow-Derived Cells with an Integrase-Defective Lentiviral Vector. Hum. Gene Ther. Methods 2018, 29, 44–59. [Google Scholar] [CrossRef]
- Strack, A.; Deinzer, A.; Thirion, C.; Schrodel, S.; Dorrie, J.; Sauerer, T.; Steinkasserer, A.; Knippertz, I. Breaking Entry-and Species Barriers: LentiBOOST((R)) Plus Polybrene Enhances Transduction Efficacy of Dendritic Cells and Monocytes by Adenovirus 5. Viruses 2022, 14, 92. [Google Scholar] [CrossRef]
- Feil, S.; Valtcheva, N.; Feil, R. Inducible Cre mice. Methods Mol. Biol. 2009, 530, 343–363. [Google Scholar] [CrossRef]
- Hayashi, S.; McMahon, A.P. Efficient recombination in diverse tissues by a tamoxifen-inducible form of Cre: A tool for temporally regulated gene activation/inactivation in the mouse. Dev. Biol. 2002, 244, 305–318. [Google Scholar] [CrossRef]
- Canli, O.; Nicolas, A.M.; Gupta, J.; Finkelmeier, F.; Goncharova, O.; Pesic, M.; Neumann, T.; Horst, D.; Lower, M.; Sahin, U.; et al. Myeloid Cell-Derived Reactive Oxygen Species Induce Epithelial Mutagenesis. Cancer Cell 2017, 32, 869–883.e5. [Google Scholar] [CrossRef]
- Song, A.J.; Palmiter, R.D. Detecting and Avoiding Problems When Using the Cre-lox System. Trends Genet. 2018, 34, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Favari, E.; Chroni, A.; Tietge, U.J.; Zanotti, I.; Escola-Gil, J.C.; Bernini, F. Cholesterol efflux and reverse cholesterol transport. Handb. Exp. Pharmacol. 2015, 224, 181–206. [Google Scholar] [CrossRef]
- Ohashi, R.; Mu, H.; Wang, X.; Yao, Q.; Chen, C. Reverse cholesterol transport and cholesterol efflux in atherosclerosis. QJM 2005, 98, 845–856. [Google Scholar] [CrossRef]
- Cucuianu, M.; Coca, M.; Hancu, N. Reverse cholesterol transport and atherosclerosis. A mini review. Rom. J. Intern. Med. 2007, 45, 17–27. [Google Scholar]
- Goodwin, L.O.; Splinter, E.; Davis, T.L.; Urban, R.; He, H.; Braun, R.E.; Chesler, E.J.; Kumar, V.; van Min, M.; Ndukum, J.; et al. Large-scale discovery of mouse transgenic integration sites reveals frequent structural variation and insertional mutagenesis. Genome Res. 2019, 29, 494–505. [Google Scholar] [CrossRef]
- Haruyama, N.; Cho, A.; Kulkarni, A.B. Overview: Engineering transgenic constructs and mice. Curr. Protoc. Cell Biol. 2009, 42, 19.10.1–19.10.9. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Echesabal-Chen, J.; Fernando, L.; Brawner, A.; Pokhrel, A.; Huang, K.; Powell, R.R.; Bruce, T.; Guz, J.; Tang, F.-L.; Awgulewitsch, A.; et al. Ex Vivo Characterization of Peritoneal Macrophages from Novel ABCA1-LSL and ABCG1-LSL Mice for Macrophage-Specific ABC-Transporter Overexpression. Biology 2025, 14, 1073. https://doi.org/10.3390/biology14081073
Echesabal-Chen J, Fernando L, Brawner A, Pokhrel A, Huang K, Powell RR, Bruce T, Guz J, Tang F-L, Awgulewitsch A, et al. Ex Vivo Characterization of Peritoneal Macrophages from Novel ABCA1-LSL and ABCG1-LSL Mice for Macrophage-Specific ABC-Transporter Overexpression. Biology. 2025; 14(8):1073. https://doi.org/10.3390/biology14081073
Chicago/Turabian StyleEchesabal-Chen, Jing, Lawrence Fernando, Ally Brawner, Achala Pokhrel, Kun Huang, Rhonda Reigers Powell, Terri Bruce, Jan Guz, Fu-Lei Tang, Alexander Awgulewitsch, and et al. 2025. "Ex Vivo Characterization of Peritoneal Macrophages from Novel ABCA1-LSL and ABCG1-LSL Mice for Macrophage-Specific ABC-Transporter Overexpression" Biology 14, no. 8: 1073. https://doi.org/10.3390/biology14081073
APA StyleEchesabal-Chen, J., Fernando, L., Brawner, A., Pokhrel, A., Huang, K., Powell, R. R., Bruce, T., Guz, J., Tang, F.-L., Awgulewitsch, A., & Stamatikos, A. (2025). Ex Vivo Characterization of Peritoneal Macrophages from Novel ABCA1-LSL and ABCG1-LSL Mice for Macrophage-Specific ABC-Transporter Overexpression. Biology, 14(8), 1073. https://doi.org/10.3390/biology14081073





