Paternal Cocaine Exposure and Its Testicular Legacy: Epigenetic, Physiological, and Intergenerational Consequences
Simple Summary
Abstract
1. Introduction
2. Cocaine as a Global Health Issue: Epidemiology and Neurobiological Mechanisms
3. Cocaine and Male Reproductive Physiology: From Hormonal Disruption to Cellular Effects
3.1. Neuroendocrine Disruption and Hormonal Imbalance
3.2. Wired for Damage: Local Mechanisms of Cocaine Toxicity in the Testis
3.3. Somatic and Germ Cell Vulnerability
4. Germline Epigenetic Alterations and Intergenerational Outcomes of Paternal Cocaine Exposure
4.1. Epigenetic Remodeling of Germ Cells and Sperm
4.2. From Sperm to Offspring: Intergenerational Effects of Paternal Cocaine Use
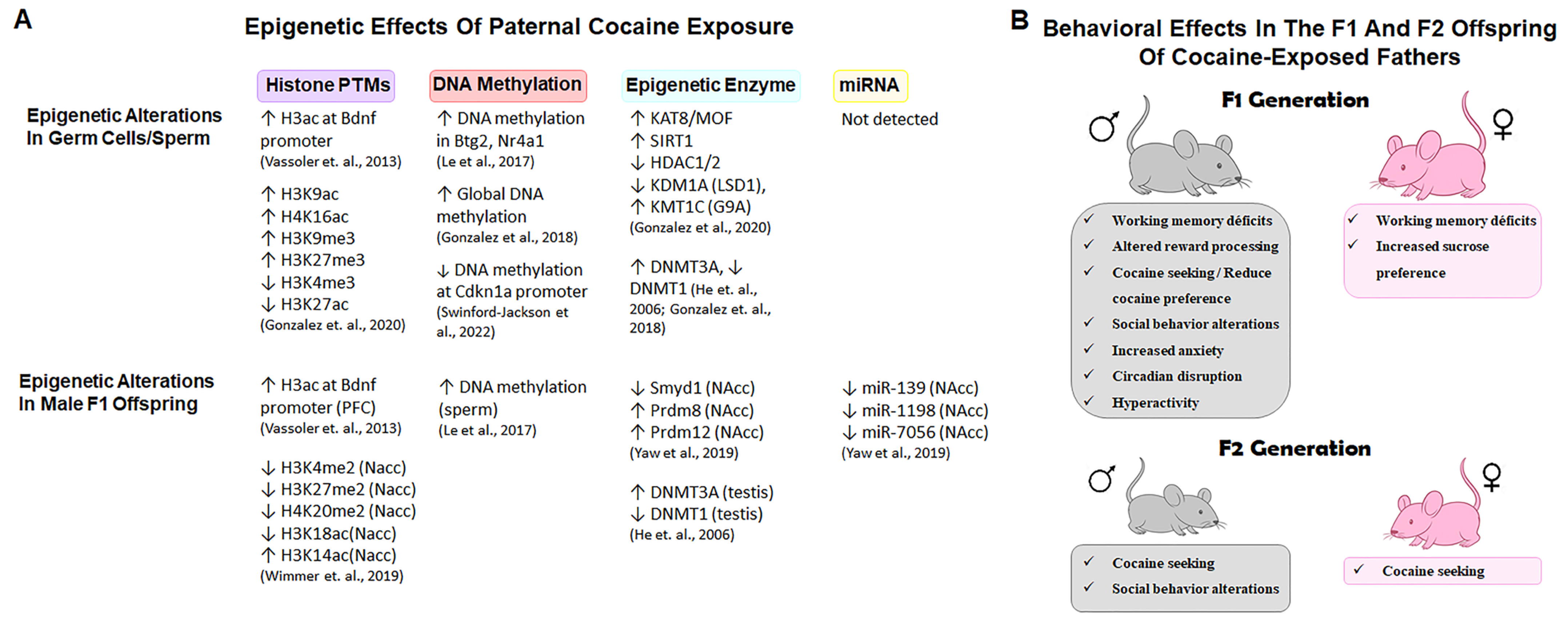
5. Conclusions and Future Perspectives
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- González, C.R.; González, B. Exploring the Stress Impact in the Paternal Germ Cells Epigenome: Can Catecholamines Induce Epigenetic Reprogramming? Front. Endocrinol. 2021, 11, 630948. [Google Scholar] [CrossRef]
- Naro, C.; Sette, C. Splicing regulation in brain and testis: Common themes for highly specialized organs. Cell Cycle 2021, 20, 480–489. [Google Scholar] [CrossRef]
- Soumillon, M.; Necsulea, A.; Weier, M.; Brawand, D.; Zhang, X.; Gu, H.; Barthès, P.; Kokkinaki, M.; Nef, S.; Gnirke, A.; et al. Cellular source and mechanisms of high transcriptome complexity in the mammalian testis. Cell Rep. 2013, 3, 2179–2190. [Google Scholar] [CrossRef] [PubMed]
- Bohacek, J.; Mansuy, I.M. Molecular insights into transgenerational non-genetic inheritance of acquired behaviours. Nat. Rev. Genet. 2015, 16, 641–652. [Google Scholar] [CrossRef] [PubMed]
- United Nations Office on Drugs and Crime (UNODC). Global Report on Cocaine 2023; UNODC: Vienna, Austria, 2023. [Google Scholar]
- Sora, I.; Hall, F.S.; Andrews, A.M.; Itokawa, M.; Li, X.F.; Wei, H.B.; Wichems, C.; Lesch, K.P.; Murphy, D.L.; Uhl, G.R. Molecular Mechanisms of Cocaine Reward: Combined Dopamine and Serotonin Transporter Knockouts Eliminate Cocaine Place Preference. Proc. Natl. Acad. Sci. USA 2001, 98, 5300–5305. [Google Scholar] [CrossRef]
- Cadet, J.L.; Jayanthi, S.; McCoy, M.T.; Beauvais, G.; Cai, N.S. Dopamine D1 Receptors, Regulation of Gene Expression in the Brain, and Neurodegeneration. CNS Neurol. Disord. Drug Targets 2010, 9, 526–538. [Google Scholar] [CrossRef]
- Chen, N.; Reith, M.E.A. Structure and Function of the Dopamine Transporter. Eur. J. Pharmacol. 2000, 405, 329–339. [Google Scholar] [CrossRef]
- Reith, M.E.A.; Xu, C.; Chen, N.H. Pharmacology and Regulation of the Neuronal Dopamine Transporter. Eur. J. Pharmacol. 1997, 324, 1–10. [Google Scholar] [CrossRef]
- Berke, J.D.; Hyman, S.E. Addiction, Dopamine, and the Molecular Mechanisms of Memory. Neuron 2000, 25, 515–532. [Google Scholar] [CrossRef]
- Volkow, N.D.; Morales, M. The Brain on Drugs: From Reward to Addiction. Cell 2015, 162, 712–725. [Google Scholar] [CrossRef]
- Torres, G.E.; Gainetdinov, R.R.; Caron, M.G. Plasma Membrane Monoamine Transporters: Structure, Regulation and Function. Nat. Rev. Neurosci. 2003, 4, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, A.S.; Andersen, J.; Jørgensen, T.N.; Sørensen, L.; Eriksen, J.; Loland, C.J.; Strømgaard, K.; Gether, U. SLC6 Neurotransmitter Transporters: Structure, Function, and Regulation. Pharmacol. Rev. 2011, 63, 585–640. [Google Scholar] [CrossRef] [PubMed]
- Sofuoglu, M.; Sewell, R.A. Norepinephrine and Stimulant Addiction. Addict. Biol. 2009, 14, 119–129. [Google Scholar] [CrossRef]
- Frungieri, M.B.; Mayerhofer, A. Biogenic Amines in the Testis: Sources, Receptors and Actions. Front. Endocrinol. 2024, 15, 1392917. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, M.; Fukushima, S.; Shen, H.W.; Hall, F.S.; Uhl, G.R.; Numachi, Y.; Kobayashi, H.; Sora, I. Norepinephrine Transporter Blockade Can Normalize the Prepulse Inhibition Deficits Found in Dopamine Transporter Knockout Mice. Neuropsychopharmacology 2006, 31, 2132–2139. [Google Scholar] [CrossRef]
- Eshleman, A.J.; Carmolli, M.; Cumbay, M.; Martens, C.R.; Neve, K.A.; Janowsky, A. Characteristics of Drug Interactions with Recombinant Biogenic Amine Transporters Expressed in the Same Cell Type. J. Pharmacol. Exp. Ther. 1999, 289, 877–885. [Google Scholar] [CrossRef]
- Carboni, E.; Tanda, G.L.; Frau, R.; Di Chiara, G. Blockade of the Noradrenaline Carrier Increases Extracellular Dopamine Concentrations in the Prefrontal Cortex: Evidence That Dopamine Is Taken Up In Vivo by Noradrenergic Terminals. J. Neurochem. 1990, 55, 1067–1070. [Google Scholar] [CrossRef]
- Wee, S.; Ordway, G.A.; Woolverton, W.L. Reinforcing Effect of Pseudoephedrine Isomers and the Mechanism of Action. Eur. J. Pharmacol. 2004, 493, 117–125. [Google Scholar] [CrossRef]
- Gillis, R.A.; Hernandez, Y.M.; Erzouki, H.K.; Raczkowski, V.F.; Mandal, A.K.; Kuhn, F.E.; Dretchen, K.L. Sympathetic Nervous System Mediated Cardiovascular Effects of Cocaine Are Primarily Due to a Peripheral Site of Action of the Drug. Drug Alcohol Depend. 1995, 37, 217–230. [Google Scholar] [CrossRef]
- Ziu, E.; Hadden, C.; Li, Y.; Lowery, C.L., III; Singh, P.; Ucer, S.S.; Mercado, C.P.; Gu, H.H.; Kilic, F. Effect of Serotonin on Platelet Function in Cocaine Exposed Blood. Sci. Rep. 2014, 4, 5945. [Google Scholar] [CrossRef]
- Simmler, L.D.; Blakely, R.D. The SERT Met172 Mouse: An Engineered Model to Elucidate the Contributions of Serotonin Signaling to Cocaine Action. ACS Chem. Neurosci. 2019, 10, 3053–3060. [Google Scholar] [CrossRef]
- Taracha, E. The Role of Serotoninergic System in Psychostimulant Effects. Postepy Psychiatr. Neurol. 2021, 30, 258–269. [Google Scholar] [CrossRef]
- Knowles, L.G.; Armanious, A.J.; Peng, Y.; Welsh, W.J.; James, M.H. Recent Advances in Drug Discovery Efforts Targeting the Sigma 1 Receptor System: Implications for Novel Medications Designed to Reduce Excessive Drug and Food Seeking. Addict. Neurosci. 2023, 8, 100126. [Google Scholar] [CrossRef]
- Hayashi, T.; Su, T.P. Sigma-1 Receptor Chaperones at the ER-Mitochondrion Interface Regulate Ca(2+) Signaling and Cell Survival. Cell 2007, 131, 596–610. [Google Scholar] [CrossRef]
- Goguadze, N.; Zhuravliova, E.; Morin, D.; Mikeladze, D.; Maurice, T. Sigma-1 Receptor Agonists Induce Oxidative Stress in Mitochondria and Enhance Complex I Activity in Physiological Condition but Protect Against Pathological Oxidative Stress. Neurotox. Res. 2019, 35, 1–18. [Google Scholar] [CrossRef]
- Jang, E.Y.; Ryu, Y.H.; Lee, B.H.; Chang, S.C.; Yeo, M.J.; Kim, S.H.; Folsom, R.J.; Schilaty, N.D.; Kim, K.J.; Yang, C.H.; et al. Involvement of Reactive Oxygen Species in Cocaine-Taking Behaviors in Rats. Addict. Biol. 2015, 20, 663–675. [Google Scholar] [CrossRef] [PubMed]
- Thangaraj, A.; Periyasamy, P.; Guo, M.L.; Chivero, E.T.; Callen, S.; Buch, S. Mitigation of Cocaine-Mediated Mitochondrial Damage, Defective Mitophagy and Microglial Activation by Superoxide Dismutase Mimetics. Autophagy 2020, 16, 289–312. [Google Scholar] [CrossRef] [PubMed]
- Drake, L.R.; Scott, P.J.H. DARK Classics in Chemical Neuroscience: Cocaine. ACS Chem. Neurosci 2018, 9, 2358–2372. [Google Scholar] [CrossRef] [PubMed]
- O’Leary, M.E.; Hancox, J.C. Role of voltage-gated sodium, potassium and calcium channels in the development of cocaine-associated cardiac arrhythmias. Br. J. Clin. Pharmacol. 2010, 69, 427–442. [Google Scholar] [CrossRef]
- Kiyatkin, E.A.; Brown, P.L. The Role of Peripheral and Central Sodium Channels in Mediating Brain Temperature Fluctuations Induced by Intravenous Cocaine. Brain Res. 2006, 1117, 38–53. [Google Scholar] [CrossRef]
- Leslie, S.W.; Soon-Sutton, T.L.; Khan, M.A.B. StatPearls. In Male Infertility; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Rodprasert, W.; Toppari, J.; Virtanen, H.E. Environmental toxicants and male fertility. Best Pract. Res. Clin. Obstet. Gynaecol. 2023, 86, 102298. [Google Scholar] [CrossRef] [PubMed]
- Chianese, T.; Trinchese, G.; Leandri, R.; De Falco, M.; Mollica, M.P.; Scudiero, R.; Rosati, L. Glyphosate Exposure Induces Cytotoxicity, Mitochondrial Dysfunction and Activation of ERα and ERβ Estrogen Receptors in Human Prostate PNT1A Cells. Int. J. Mol. Sci. 2024, 25, 7039. [Google Scholar] [CrossRef] [PubMed]
- Meston, C.M.; Bradford, A.; Higgins, J.; Heiman, J.; Schonfeld, K. Psychoactive Drugs and Human Sexual Behavior: The Role of Serotonergic Activity. J. Psychoact. Drugs 1992, 24, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Bracken, M.B.; Eskenazi, B.; Sachse, K.; McSharry, J.E.; Hellenbrand, K.; Leo-Summers, L. Association of Cocaine Use with Sperm Concentration, Motility, and Morphology. Fertil. Steril. 1990, 53, 315–322. [Google Scholar] [CrossRef]
- Abel, E.L.; Moore, C.; Waselewsky, D.; Zajac, C.; Russell, L.D. Effects of Cocaine Hydrochloride on Reproductive Function and Sexual Behavior of Male Rats and on the Behavior of Their Offspring. J. Androl. 1989, 10, 17–27. [Google Scholar] [CrossRef]
- Yelian, F.D.; Sacco, A.G.; Ginsburg, K.A.; Doerr, P.A.; Armant, D.R. The effects of in vitro cocaine exposure on human sperm motility, intracellular calcium, and oocyte penetration. Fertil. Steril. 1994, 61, 915–921. [Google Scholar] [CrossRef]
- Yazigi, R.A.; Odem, R.R.; Polakoski, K.L. Demonstration of Specific Binding of Cocaine to Human Spermatozoa. J. Am. Med. Assoc. 1991, 266, 1956–1959. [Google Scholar] [CrossRef]
- Misra, A.L.; Giri, V.V.; Patel, M.N.; Alluri, V.R.; Mulé, S.J. Disposition and Metabolism of [3H] Cocaine in Acutely and Chronically Treated Monkeys. Drug Alcohol Depend. 1977, 2, 261–272. [Google Scholar] [CrossRef]
- Li, H.; George, V.K.; Crossland, W.J.; Anderson, G.F.; Dhabuwala, C.B. Characterization of Cocaine Binding Sites in the Rat Testes. J. Urol. 1997, 158, 962–965. [Google Scholar] [CrossRef]
- Mendelson, J.H.; Sholar, M.B.; Mutschler, N.H.; Jaszyna-Gasior, M.; Goletiani, N.V.; Siegel, A.J.; Mello, N.K. Effects of Intravenous Cocaine and Cigarette Smoking on Luteinizing Hormone, Testosterone, and Prolactin in Men. J. Pharmacol. Exp. Ther. 2003, 307, 339–348. [Google Scholar] [CrossRef]
- Chin, J.; Sternin, O.; Wu, H.B.; Burrell, S.; Lu, D.; Jenab, S.; Perrotti, L.I.; Quiñones-Jenab, V. Endogenous Gonadal Hormones Modulate Behavioral and Neurochemical Responses to Acute and Chronic Cocaine Administration. Brain Res. 2002, 945, 123–130. [Google Scholar] [CrossRef]
- Ersche, K.D.; Stochl, J.; Brühl, A.B.; Gurnell, M. Evidence of Hypothalamic–Pituitary–Adrenal and –Gonadal Dysfunction in Cocaine-Addicted Men. Eur. Addict. Res. 2024, 30, 114–120. [Google Scholar] [CrossRef]
- Berul, C.I.; Harclerode, J.E. Effects of Cocaine Hydrochloride on the Male Reproductive System. Life Sci. 1989, 45, 91–95. [Google Scholar] [CrossRef]
- Sarnyai, Z.; Dhabhar, F.S.; McEwen, B.S.; Kreek, M.J. Neuroendocrine-Related Effects of Long-Term, ‘Binge’ Cocaine Administration: Diminished Individual Differences in Stress-Induced Corticosterone Response. Neuroendocrinology 1998, 68, 334–344. [Google Scholar] [CrossRef] [PubMed]
- Festa, E.D.; Jenab, S.; Chin, J.; Gazi, F.M.; Wu, H.B.; Russo, S.J.; Quinones-Jenab, V. Frequency of Cocaine Administration Affects Behavioral and Endocrine Responses in Male and Female Fischer Rats. Cell. Mol. Biol. 2003, 49, 1275–1280. [Google Scholar]
- Ben-Jonathan, N.; Hnasko, R. Dopamine as a Prolactin (PRL) Inhibitor. Endocr. Rev. 2001, 22, 724–763. [Google Scholar] [CrossRef] [PubMed]
- Rosati, L.; Chianese, T.; Mileo, A.; De Falco, M.; Capaldo, A. Cocaine Effects on Reproductive Behavior and Fertility: An Overview. Vet. Sci. 2023, 10, 484. [Google Scholar] [CrossRef] [PubMed]
- Heesch, C.M.; Negus, B.H.; Keffer, J.H.; Snyder, R.W., II; Risser, R.C.; Eichhorn, E.J. Effects of Cocaine on Cortisol Secretion in Humans. Am. J. Med. Sci. 1995, 310, 61–64. [Google Scholar] [CrossRef]
- Sinha, R.; Garcia, M.; Paliwal, P.; Kreek, M.J.; Rounsaville, B.J. Stress-Induced Cocaine Craving and Hypothalamic–Pituitary–Adrenal Responses Are Predictive of Cocaine Relapse Outcomes. Arch. Gen. Psychiatry 2006, 63, 324–331. [Google Scholar] [CrossRef]
- Sarnyai, Z.; Mello, N.K.; Mendelson, J.H.; Erös-Sarnyai, M.; Mercer, G. Effects of Cocaine on Pulsatile Activity of Hypothalamic–Pituitary–Adrenal Axis in Male Rhesus Monkeys: Neuroendocrine and Behavioral Correlates. J. Pharmacol. Exp. Ther. 1996, 277, 225–234. [Google Scholar] [CrossRef]
- Erb, S.; Shaham, Y.; Stewart, J. The Role of Corticotropin-Releasing Factor and Corticosterone in Stress- and Cocaine-Induced Relapse to Cocaine Seeking in Rats. J. Neurosci. 1998, 18, 5529–5536. [Google Scholar] [CrossRef]
- Sakaue, M.; Hoffman, B.B. Glucocorticoids Induce Transcription and Expression of the Alpha 1B Adrenergic Receptor Gene in DTT1 MF-2 Smooth Muscle Cells. J. Clin. Investig. 1991, 88, 385–389. [Google Scholar] [CrossRef] [PubMed]
- Kiely, J.; Hadcock, J.R.; Bahouth, S.W.; Malbon, C.C. Glucocorticoids Down-Regulate Beta 1-Adrenergic-Receptor Expression by Suppressing Transcription of the Receptor Gene. Biochem. J. 1994, 302, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Davidoff, M.S.; Ungefroren, H.; Middendorff, R.; Koeva, Y.; Bakalska, M.; Atanassova, N.; Holstein, A.F.; Jezek, D.; Pusch, W.; Müller, D. Catecholamine-synthesizing enzymes in the adult and prenatal human testis. Histochem. Cell Biol. 2005, 124, 313–323. [Google Scholar] [CrossRef] [PubMed]
- González, C.R.; González, B.; Matzkin, M.E.; Muñiz, J.A.; Cadet, J.L.; Garcia-Rill, E.; Urbano, F.J.; Vitullo, A.D.; Bisagno, V. Psychostimulant-Induced Testicular Toxicity in Mice: Evidence of Cocaine and Caffeine Effects on the Local Dopaminergic System. PLoS ONE 2015, 10, e0142713. [Google Scholar] [CrossRef]
- Uhlén, M.; Fagerberg, L.; Hallström, B.M.; Lindskog, C.; Oksvold, P.; Mardinoglu, A.; Sivertsson, Å.; Kampf, C.; Sjöstedt, E.; Asplund, A.; et al. Proteomics. Tissue-based Map of the Human Proteome. Science 2015, 347, 1260419. [Google Scholar] [CrossRef]
- Flierl, M.A.; Rittirsch, D.; Huber-Lang, M.; Sarma, J.V.; Ward, P.A. Catecholamines—Crafty Weapons in the Inflammatory Arsenal of Immune/Inflammatory Cells or Opening Pandora’s Box? Mol. Med. 2008, 14, 195–204. [Google Scholar] [CrossRef]
- Sorriento, D.; Santulli, G.; Del Giudice, C.; Anastasio, A.; Trimarco, B.; Iaccarino, G. Endothelial Cells Are Able to Synthesize and Release Catecholamines Both In Vitro and In Vivo. Hypertension 2012, 60, 129–136. [Google Scholar] [CrossRef]
- Mayerhofer, A.; Danilchik, M.; Pau, K.Y.; Lara, H.E.; Russell, L.D.; Ojeda, S.R. Testis of Prepubertal Rhesus Monkeys Receives a Dual Catecholaminergic Input Provided by the Extrinsic Innervation and an Intragondal Source of Catecholamines. Biol. Reprod. 1996, 55, 509–518. [Google Scholar] [CrossRef]
- Mayerhofer, A.; Frungieri, M.B.; Fritz, S.; Bulling, A.; Jessberger, B.; Vogt, H.J. Evidence for Catecholaminergic, Neuronlike Cells in the Adult Human Testis: Changes Associated with Testicular Pathologies. J. Androl. 1999, 20, 341–347. [Google Scholar] [CrossRef]
- Zieher, L.M.; Debeljuk, L.; Iturriza, F.; Mancini, R.E. Biogenic Amine Concentration in Testes of Rats at Different Ages. Endocrinology 1971, 88, 351–354. [Google Scholar] [CrossRef]
- González, B.; Gancedo, S.N.; Garazatua, S.A.J.; Roldán, E.; Vitullo, A.D.; González, C.R. Dopamine Receptor D1 Contributes to Cocaine Epigenetic Reprogramming of Histone Modifications in Male Germ Cells. Front. Cell Dev. Biol. 2020, 8, 216. [Google Scholar] [CrossRef]
- Wolfe, S.A., Jr.; Culp, S.G.; De Souza, E.B. Sigma-Receptors in Endocrine Organs: Identification, Characterization, and Autoradiographic Localization in Rat Pituitary, Adrenal, Testis, and Ovary. Endocrinology 1989, 124, 1160–1172. [Google Scholar] [CrossRef] [PubMed]
- Cunha-Oliveira, T.; Rego, A.C.; Oliveira, C.R. Cellular and Molecular Mechanisms Involved in the Neurotoxicity of Opioid and Psychostimulant Drugs. Brain Res. Rev. 2008, 58, 192–208. [Google Scholar] [CrossRef]
- Rasool, A.; Manzoor, R.; Ullah, K.; Afzal, R.; Ul-Haq, A.; Imran, H.; Kaleem, I.; Akhtar, T.; Farrukh, A.; Hameed, S.; et al. Oxidative Stress and Dopaminergic Metabolism: A Major PD Pathogenic Mechanism and Basis of Potential Antioxidant Therapies. CNS Neurol. Disord. Drug Targets 2024, 23, 852–864. [Google Scholar] [CrossRef] [PubMed]
- Pires, A.; Pieri, P.; Hage, M.; Santos, A.B.; Medeiros, M.C.; Garcia, R.C.; Yonamine, M.; Hallak, J.; Saldiva, P.H.; Zorzetto, J.C.; et al. Repeated Inhalation of Crack-Cocaine Affects Spermatogenesis in Young and Adult Mice. Inhal. Toxicol. 2012, 24, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Csaba, Z.; Csernus, V.; Gerendai, I. Intratesticular Serotonin Affects Steroidogenesis in the Rat Testis. J. Neuroendocr. 1998, 10, 371–376. [Google Scholar] [CrossRef]
- James, P.; Rivier, C.; Lee, S. Presence of Corticotrophin-Releasing Factor and/or Tyrosine Hydroxylase in Cells of a Neural Brain–Testicular Pathway That Are Labelled by a Transganglionic Tracer. J. Neuroendocr. 2008, 20, 173–181. [Google Scholar] [CrossRef]
- Zhang, H.; Loughlin, K.R. The Effect of Cocaine and Its Metabolites on Sertoli Cell Function. J. Urol. 1996, 155, 163–166. [Google Scholar] [CrossRef]
- Rodriguez, M.C.; Sanchez-Yague, J.; Paniagua, R. Effects of Cocaine on Testicular Structure in the Rat. Reprod. Toxicol. 1992, 6, 51–55. [Google Scholar] [CrossRef]
- George, V.K.; Li, H.; Teloken, C.; Grignon, D.J.; Lawrence, W.D.; Dhabuwala, C.B. Effects of Long-Term Cocaine Exposure on Spermatogenesis and Fertility in Peripubertal Male Rats. J. Urol. 1996, 155, 327–331. [Google Scholar] [CrossRef] [PubMed]
- Nazmara, Z.; Ebrahimi, B.; Makhdoumi, P.; Noori, L.; Mahdavi, S.A.; Hassanzadeh, G. Effects of Illicit Drugs on Structural and Functional Impairment of Testis, Endocrinal Disorders, and Molecular Alterations of the Semen. Iran. J. Basic. Med. Sci. 2021, 24, 856–867. [Google Scholar] [PubMed]
- Jaenisch, R.; Bird, A. Epigenetic Regulation of Gene Expression: How the Genome Integrates Intrinsic and Environmental Signals. Nat. Genet. 2003, 33 (Suppl. S3), 245–254. [Google Scholar] [CrossRef] [PubMed]
- Allis, C.D.; Jenuwein, T. The Molecular Hallmarks of Epigenetic Control. Nat. Rev. Genet. 2016, 17, 487–500. [Google Scholar] [CrossRef]
- Hackett, J.A.; Surani, M.A. Beyond DNA: Programming and Inheritance of Parental Methylomes. Cell 2013, 153, 737–739. [Google Scholar] [CrossRef]
- Wimmer, M.E.; Briand, L.A.; Fant, B.; Guercio, L.A.; Arreola, A.C.; Schmidt, H.D.; Sidoli, S.; Han, Y.; Garcia, B.A.; Pierce, R.C. Paternal Cocaine Taking Elicits Epigenetic Remodeling and Memory Deficits in Male Progeny. Mol. Psychiatry 2017, 22, 1653. [Google Scholar] [CrossRef]
- Pierce, R.C.; Fant, B.; Swinford-Jackson, S.E.; Heller, E.A.; Berrettini, W.H.; Wimmer, M.E. Environmental, Genetic and Epigenetic Contributions to Cocaine Addiction. Neuropsychopharmacology 2018, 43, 1471–1480. [Google Scholar] [CrossRef]
- Urb, M.; Niinep, K.; Matsalu, T.; Kipper, K.; Herodes, K.; Zharkovsky, A.; Timmusk, T.; Anier, K.; Kalda, A. The Role of DNA Methyltransferase Activity in Cocaine Treatment and Withdrawal in the Nucleus Accumbens of Mice. Addict. Biol. 2020, 25, e12720. [Google Scholar] [CrossRef]
- Nestler, E.J. Epigenetic Mechanisms of Drug Addiction. Neuropharmacology 2014, 76, 259–268. [Google Scholar] [CrossRef]
- Walker, D.M.; Cates, H.M.; Heller, E.A.; Nestler, E.J. Regulation of Chromatin States by Drugs of Abuse. Curr. Opin. Neurobiol. 2015, 30, 112–121. [Google Scholar] [CrossRef]
- Hammoud, S.S.; Low, D.H.; Yi, C.; Carrell, D.T.; Guccione, E.; Cairns, B.R. Chromatin and Transcription Transitions of Mammalian Adult Germline Stem Cells and Spermatogenesis. Cell Stem Cell 2014, 15, 239–253. [Google Scholar] [CrossRef]
- Rodgers, A.B.; Bale, T.L. Germ Cell Origins of Posttraumatic Stress Disorder Risk: The Transgenerational Impact of Parental Stress Experience. Biol. Psychiatry 2015, 78, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Gapp, K.; Bohacek, J. Epigenetic Germline Inheritance in Mammals: Looking to the Past to Understand the Future. Genes Brain Behav. 2018, 17, e12407. [Google Scholar] [CrossRef] [PubMed]
- Duffy, K.A.; Bale, T.L.; Epperson, C.N. Germ Cell Drivers: Transmission of Preconception Stress Across Generations. Front. Hum. Neurosci. 2021, 15, 642762. [Google Scholar] [CrossRef] [PubMed]
- Fischer, V.; Kretschmer, M.; Germain, P.L.; Kaur, J.; Mompart-Barrenechea, S.; Pelczar, P.; Schürmann, D.; Schär, P.; Gapp, K. Sperm Chromatin Accessibility’s Involvement in the Intergenerational Effects of Stress Hormone Receptor Activation. Transl. Psychiatry 2023, 13, 378. [Google Scholar] [CrossRef]
- Kretschmer, M.; Fischer, V.; Gapp, K. When Dad’s Stress Gets under Kid’s Skin—Impacts of Stress on Germline Cargo and Embryonic Development. Biomolecules 2023, 13, 1750. [Google Scholar] [CrossRef]
- Vassoler, F.M.; White, S.L.; Schmidt, H.D.; Sadri-Vakili, G.; Pierce, R.C. Epigenetic Inheritance of a Cocaine-Resistance Phenotype. Nat. Neurosci. 2013, 16, 42–47. [Google Scholar] [CrossRef]
- Lepack, A.E.; Werner, C.T.; Stewart, A.F.; Fulton, S.L.; Zhong, P.; Farrelly, L.A.; Smith, A.C.W.; Ramakrishnan, A.; Lyu, Y.; Bastle, R.M.; et al. Dopaminylation of Histone H3 in Ventral Tegmental Area Regulates Cocaine Seeking. Science 2020, 368, 197–201. [Google Scholar] [CrossRef]
- He, F.; Lidow, I.A.; Lidow, M.S. Consequences of Paternal Cocaine Exposure in Mice. Neurotoxicol. Teratol. 2006, 28, 198–209. [Google Scholar] [CrossRef]
- González, B.; Pantoja, C.R.G.; Sosa, M.H.; Vitullo, A.D.; Bisagno, V.; González, C.R. Cocaine Alters the Mouse Testicular Epigenome with Direct Impact on Histone Acetylation and DNA Methylation Marks. Reprod. Biomed. Online 2018, 37, 269–278. [Google Scholar] [CrossRef]
- Le, Q.; Yan, B.; Yu, X.; Li, Y.; Song, H.; Zhu, H.; Hou, W.; Ma, D.; Wu, F.; Zhou, Y.; et al. Drug-Seeking Motivation Level in Male Rats Determines Offspring Susceptibility or Resistance to Cocaine-Seeking Behaviour. Nat. Commun. 2017, 8, 15527. [Google Scholar] [CrossRef]
- Swinford-Jackson, S.E.; Fant, B.; Wimmer, M.E.; Chan, D.; Knouse, M.C.; Sarmiento, M.; Thomas, A.S.; Huffman, P.J.; Mankame, S.; Worobey, S.J.; et al. Cocaine-Induced Changes in Sperm Cdkn1a Methylation Are Associated with Cocaine Resistance in Male Offspring. J. Neurosci. 2022, 42, 2905–2916. [Google Scholar] [CrossRef]
- Naveed, M.; Shen, Z.; Bao, J. Sperm-Borne Small Non-Coding RNAs: Potential Functions and Mechanisms as Epigenetic Carriers. Cell Biosci. 2025, 15, 5. [Google Scholar] [CrossRef]
- Rodgers, A.B.; Morgan, C.P.; Bronson, S.L.; Revello, S.; Bale, T.L. Paternal Stress Exposure Alters Sperm microRNA Content and Reprograms Offspring HPA Stress Axis Regulation. J. Neurosci. 2013, 33, 9003–9012. [Google Scholar] [CrossRef]
- González, B.; González, C.R. Sperm-Borne mRNAs: Potential Roles in Zygote Genome Activation and Epigenetic Inheritance. Open Biol. 2025, 15, 240321. [Google Scholar] [CrossRef]
- Jirtle, R.L.; Skinner, M.K. Environmental Epigenomics and Disease Susceptibility. Nat. Rev. Genet. 2007, 8, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Rice, R.C.; Gil, D.V.; Baratta, A.M.; Frawley, R.R.; Hill, S.Y.; Farris, S.P.; Homanics, G.E. Inter- and Transgenerational Heritability of Preconception Chronic Stress or Alcohol Exposure: Translational Outcomes in Brain and Behavior. Neurobiol. Stress 2023, 29, 100603. [Google Scholar] [CrossRef] [PubMed]
- Lo, J.O.; D’Mello, R.J.; Watch, L.; Schust, D.J.; Murphy, S.K. An Epigenetic Synopsis of Parental Substance Use. Epigenomics 2023, 15, 453–473. [Google Scholar] [CrossRef]
- Zheng, X.; Li, Z.; Wang, G.; Wang, H.; Zhou, Y.; Zhao, X.; Cheng, C.Y.; Qiao, Y.; Sun, F. Sperm Epigenetic Alterations Contribute to Inter- and Transgenerational Effects of Paternal Exposure to Long-Term Psychological Stress via Evading Offspring Embryonic Reprogramming. Cell Discov. 2021, 7, 101. [Google Scholar] [CrossRef]
- Anway, M.D.; Memon, M.A.; Uzumcu, M.; Skinner, M.K. Transgenerational Effect of the Endocrine Disruptor Vinclozolin on Male Spermatogenesis. J. Androl. 2006, 27, 868–879. [Google Scholar] [CrossRef]
- Wimmer, M.E.; Vassoler, F.M.; White, S.L.; Schmidt, H.D.; Sidoli, S.; Han, Y.; Garcia, B.A.; Pierce, R.C. Impaired Cocaine-Induced Behavioral Plasticity in the Male Offspring of Cocaine-Experienced Sires. Eur. J. Neurosci. 2019, 49, 1115–1126. [Google Scholar] [CrossRef] [PubMed]
- Huang, N.; Cui, J.; Fan, G.; Pan, T.; Han, K.; Xu, K.; Jiang, C.; Liu, X.; Wang, F.; Ma, L.; et al. Transcriptomic Effects of Paternal Cocaine-Seeking on the Reward Circuitry of Male Offspring. Transl. Psychiatry 2024, 14, 120. [Google Scholar] [CrossRef] [PubMed]
- Killinger, C.E.; Robinson, S.; Stanwood, G.D. Subtle Biobehavioral Effects Produced by Paternal Cocaine Exposure. Synapse 2012, 66, 902–908. [Google Scholar] [CrossRef] [PubMed]
- Fischer, D.K.; Rice, R.C.; Martinez Rivera, A.; Donohoe, M.; Rajadhyaksha, A.M. Altered Reward Sensitivity in Female Offspring of Cocaine-Exposed Fathers. Behav. Brain Res. 2017, 332, 23–31. [Google Scholar] [CrossRef]
- White, S.L.; Vassoler, F.M.; Schmidt, H.D.; Pierce, R.C.; Wimmer, M.E. Enhanced Anxiety in the Male Offspring of Sires That Self-Administered Cocaine. Addict. Biol. 2016, 21, 802–810. [Google Scholar] [CrossRef]
- Yaw, A.M.; Prosser, R.A.; Jones, P.C.; Garcia, B.J.; Jacobson, D.A.; Glass, J.D. Epigenetic Effects of Paternal Cocaine on Reward Stimulus Behavior and Accumbens Gene Expression in Mice. Behav. Brain Res. 2019, 367, 68–81. [Google Scholar] [CrossRef]
- Yaw, A.M.; Woodruff, R.W.; Prosser, R.A.; Glass, J.D. Paternal Cocaine Disrupts Offspring Circadian Clock Function in a Sex-Dependent Manner in Mice. Neuroscience 2018, 379, 257–268. [Google Scholar] [CrossRef]
- Yaw, A.M.; Glass, J.D.; Prosser, R.A.; Caldwell, H.K. Paternal Cocaine in Mice Alters Social Behavior and Brain Oxytocin Receptor Density in First Generation Offspring. Neuroscience 2022, 485, 65–77. [Google Scholar] [CrossRef]
- Verstegen, R.H.J.; Wang, G.; Langenberg-Ververgaert, K.P.S.; Ren, L.Y.; Nulman, I. Paternal exposure to recreational drugs before conception and its effect on live-born offspring: A scoping review. Birth Defects Res. 2020, 112, 970–988. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González, C.R.; González, B. Paternal Cocaine Exposure and Its Testicular Legacy: Epigenetic, Physiological, and Intergenerational Consequences. Biology 2025, 14, 1072. https://doi.org/10.3390/biology14081072
González CR, González B. Paternal Cocaine Exposure and Its Testicular Legacy: Epigenetic, Physiological, and Intergenerational Consequences. Biology. 2025; 14(8):1072. https://doi.org/10.3390/biology14081072
Chicago/Turabian StyleGonzález, Candela R., and Betina González. 2025. "Paternal Cocaine Exposure and Its Testicular Legacy: Epigenetic, Physiological, and Intergenerational Consequences" Biology 14, no. 8: 1072. https://doi.org/10.3390/biology14081072
APA StyleGonzález, C. R., & González, B. (2025). Paternal Cocaine Exposure and Its Testicular Legacy: Epigenetic, Physiological, and Intergenerational Consequences. Biology, 14(8), 1072. https://doi.org/10.3390/biology14081072






