Effects of Decapitation on Chlorophyll Metabolism, Endogenous Hormones, and Tillering Ability in Pinus yunnanensis Seedlings of Different Ages
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Decapitation Treatments
2.2. Observation of Sprouting Ability
2.3. Determination of Photosynthetic Pigments
2.4. Determination of Endogenous Hormone Content
2.5. Data Analysis
3. Results
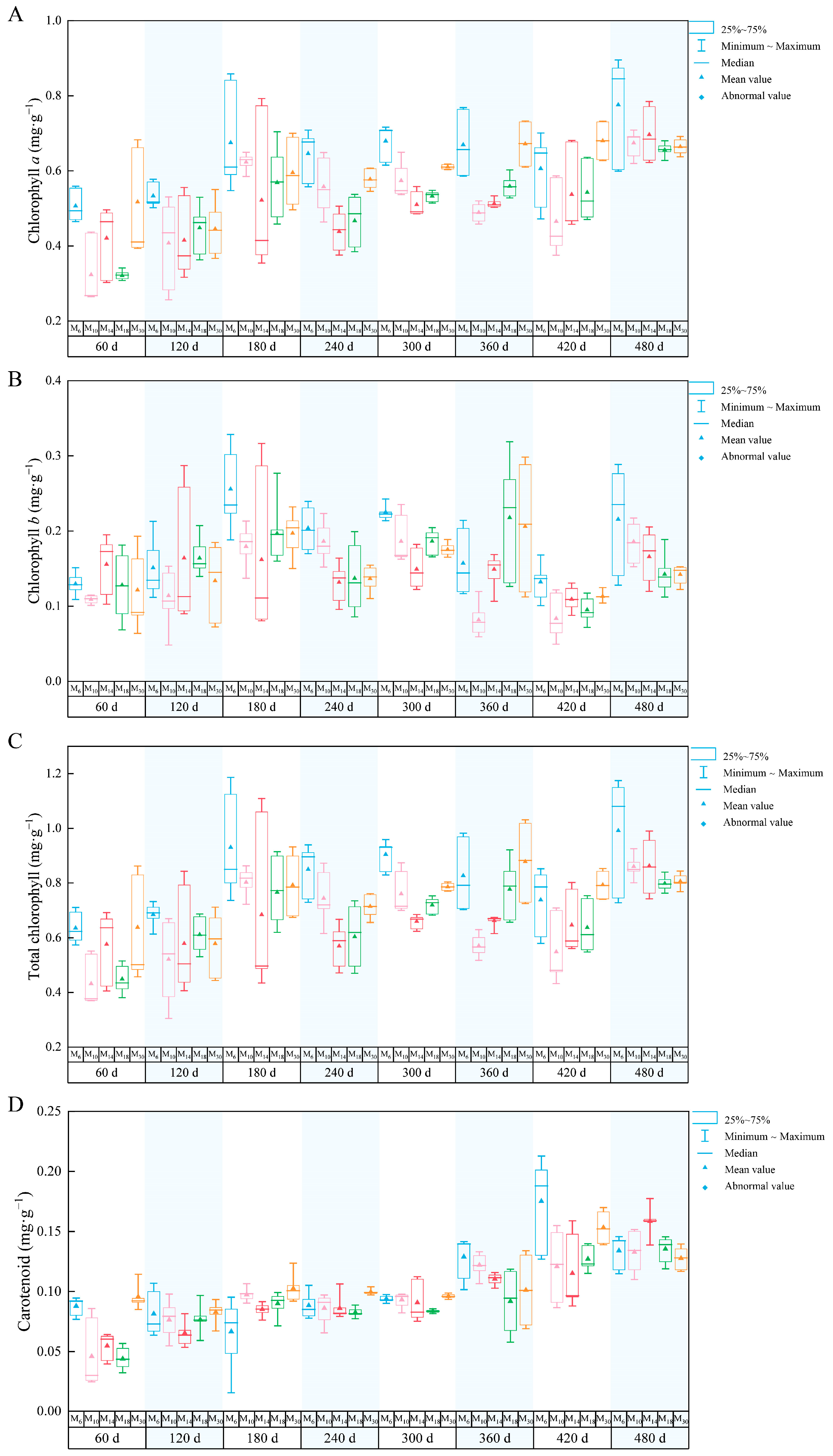
3.1. Photosynthetic Pigments in Seedlings with Different Seedling Ages
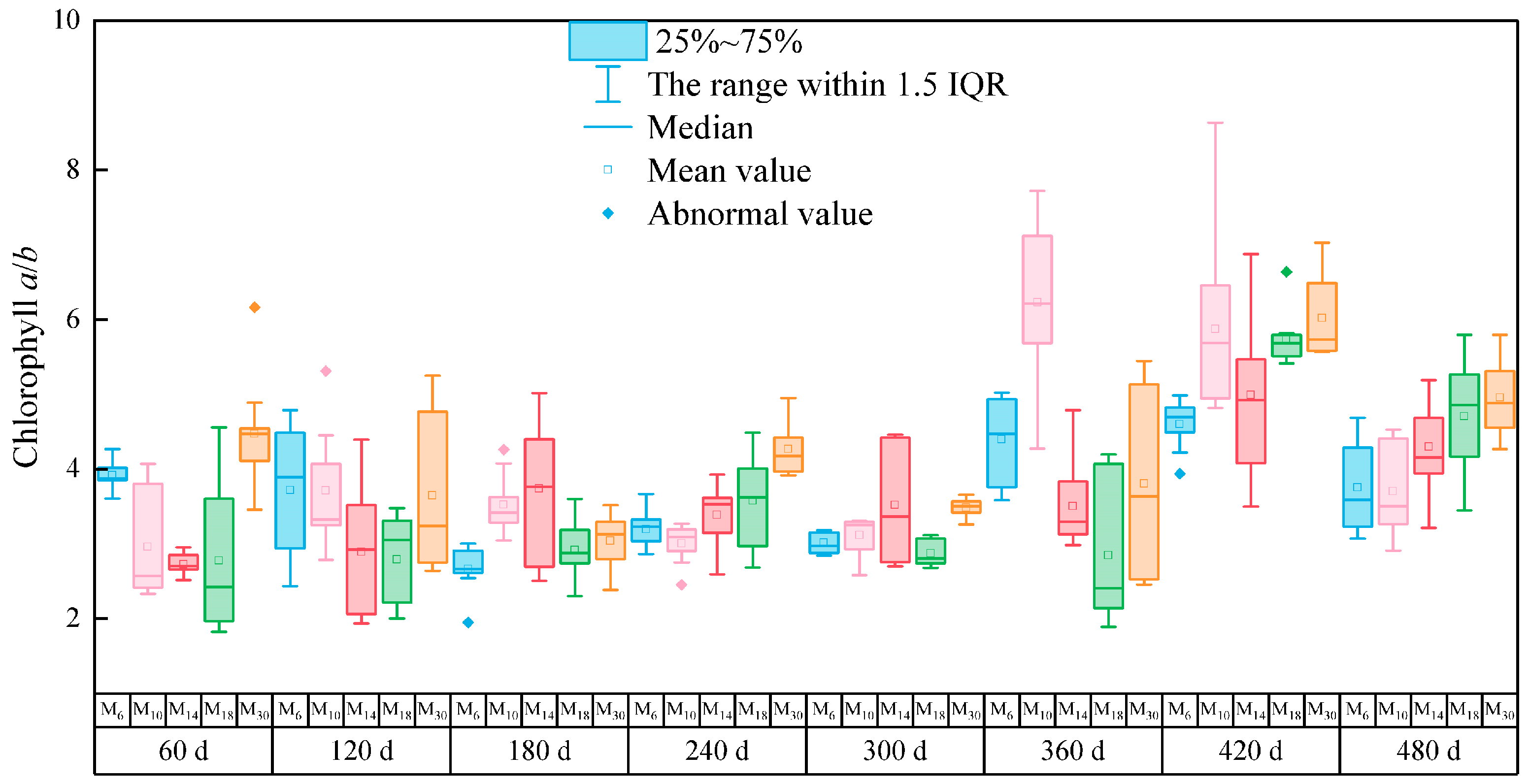
3.2. Effect of the Chlorophyll a/b Ratio on P. yunnanensis Seedlings Decapitated at Different Ages
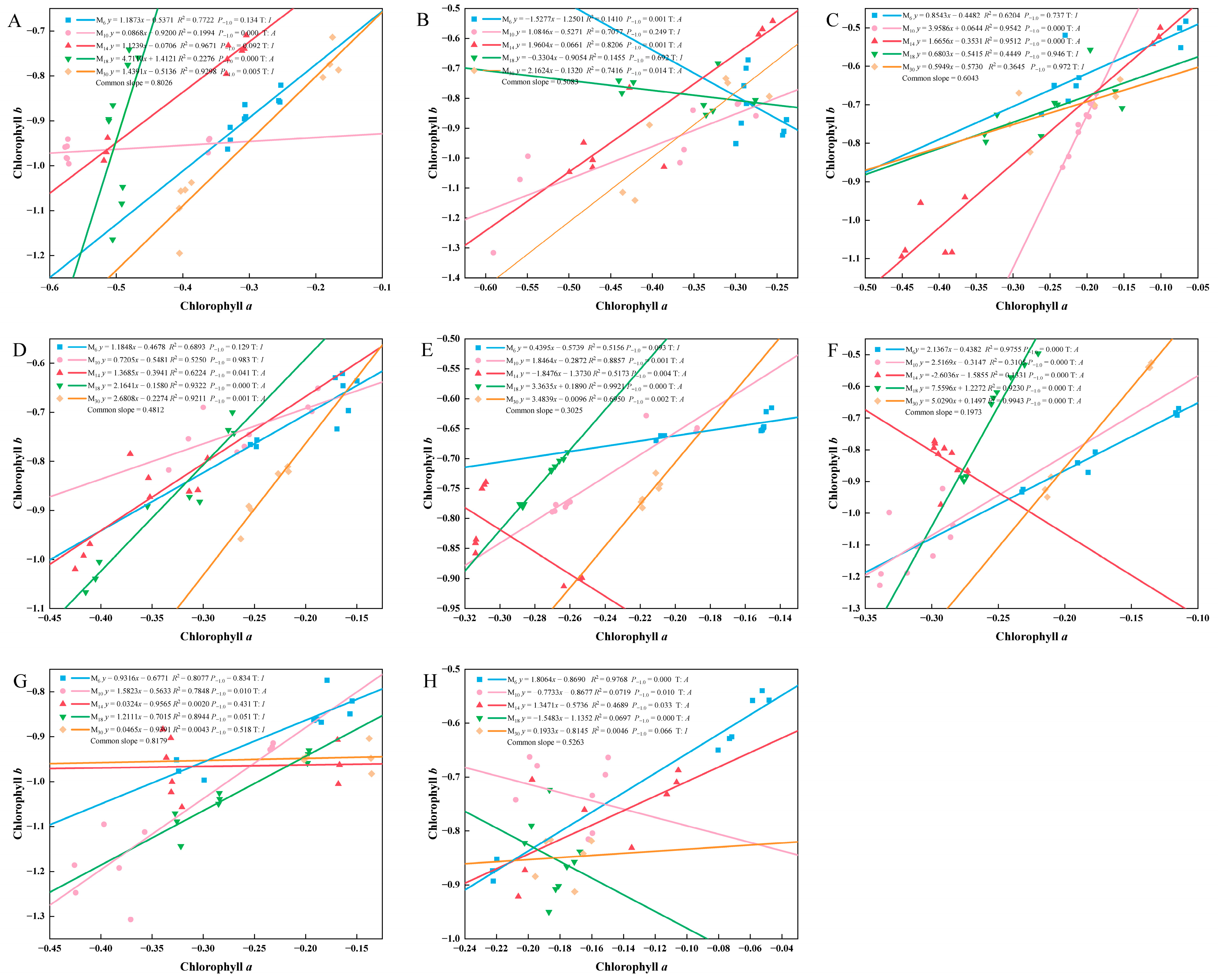
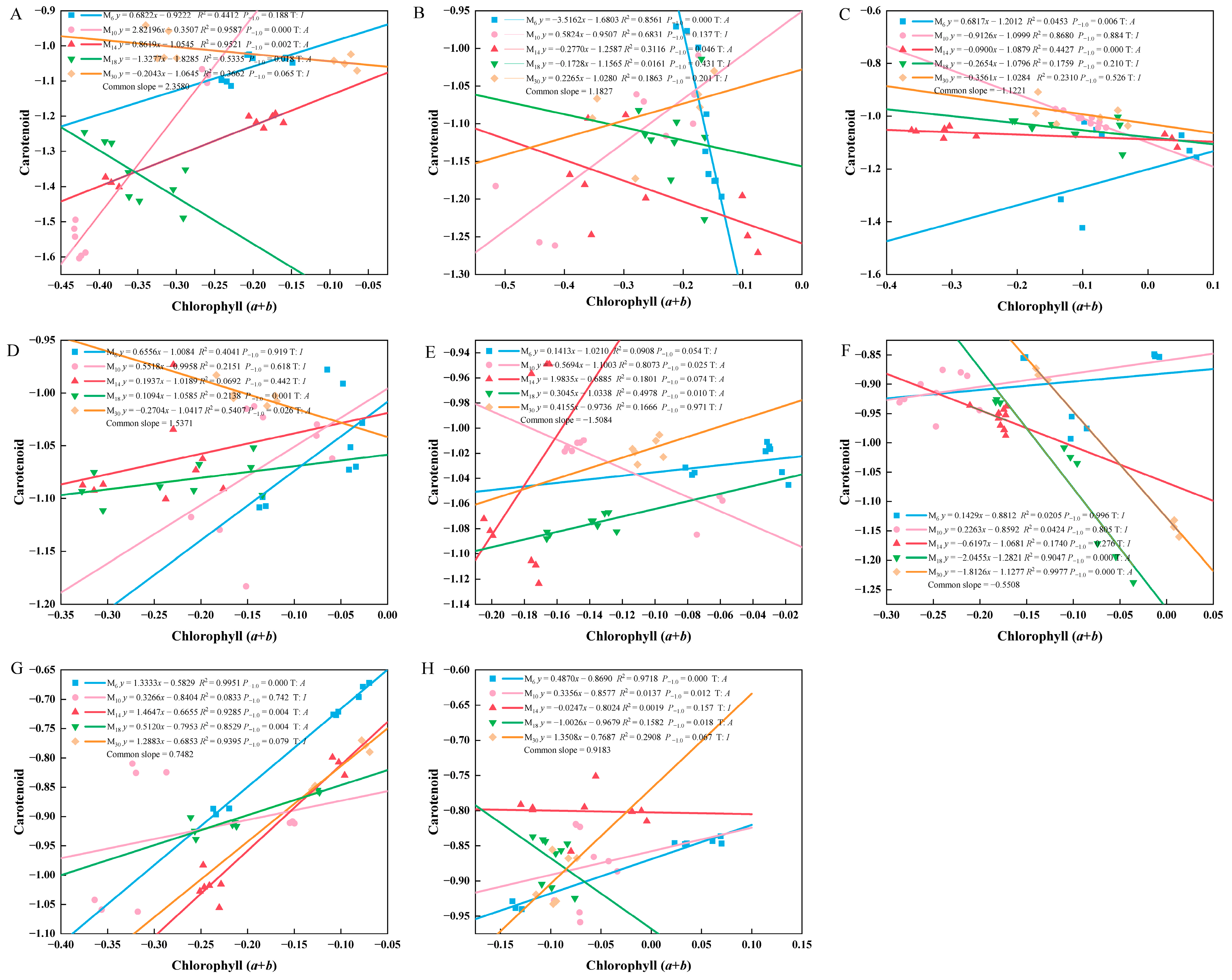
3.3. The Relationship Between the Relative Growth of the Photosynthetic Pigments of P. yunnanensis at Different Seedling Ages After Decapitation
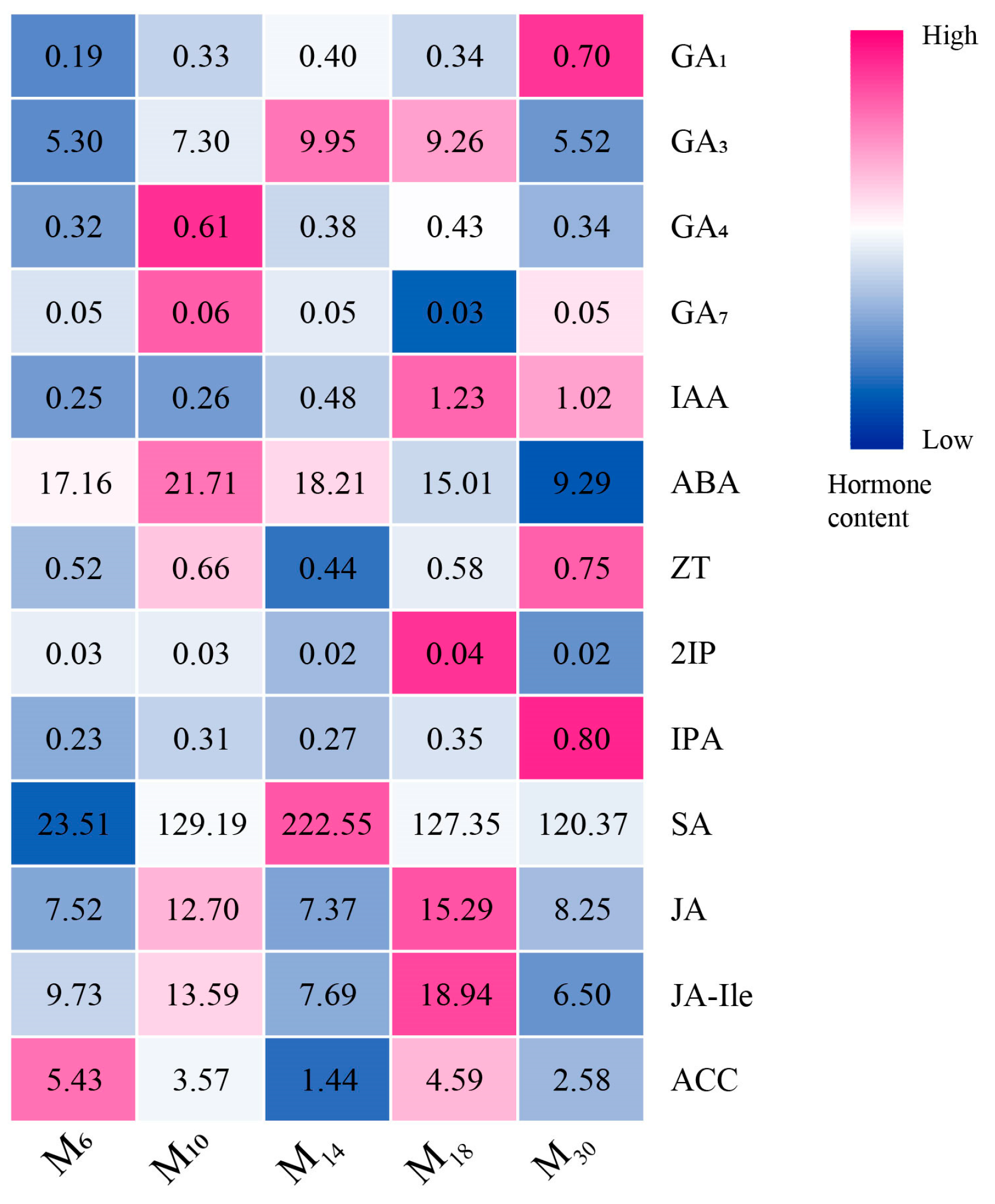
3.4. Response of Endogenous Hormone Content of P. yunnanensis at Different Seedling Ages After Decapitation
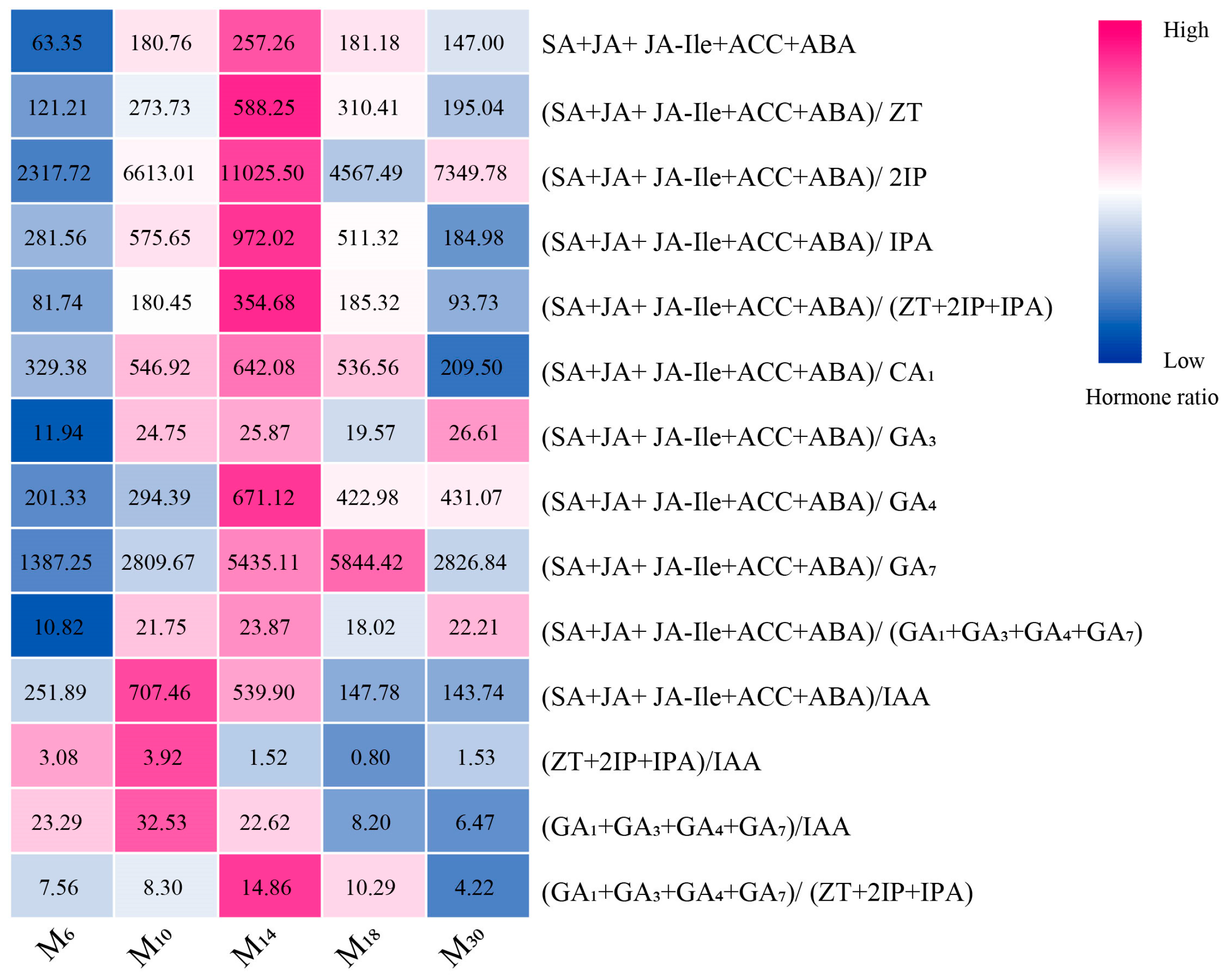
3.5. Response of the Endogenous Hormone Ratio of P. yunnanensis in Seedlings with Different Decapitation Ages
3.6. Effects of Endogenous Hormone Levels on Tillering Index and Tillering Capacity of Seedlings with Different Decapitation Ages
3.7. Principal Component Analysis of Seedling Age of Decapitation on Sprouting Index and Endogenous Hormone Ratios of P. yunnanensis
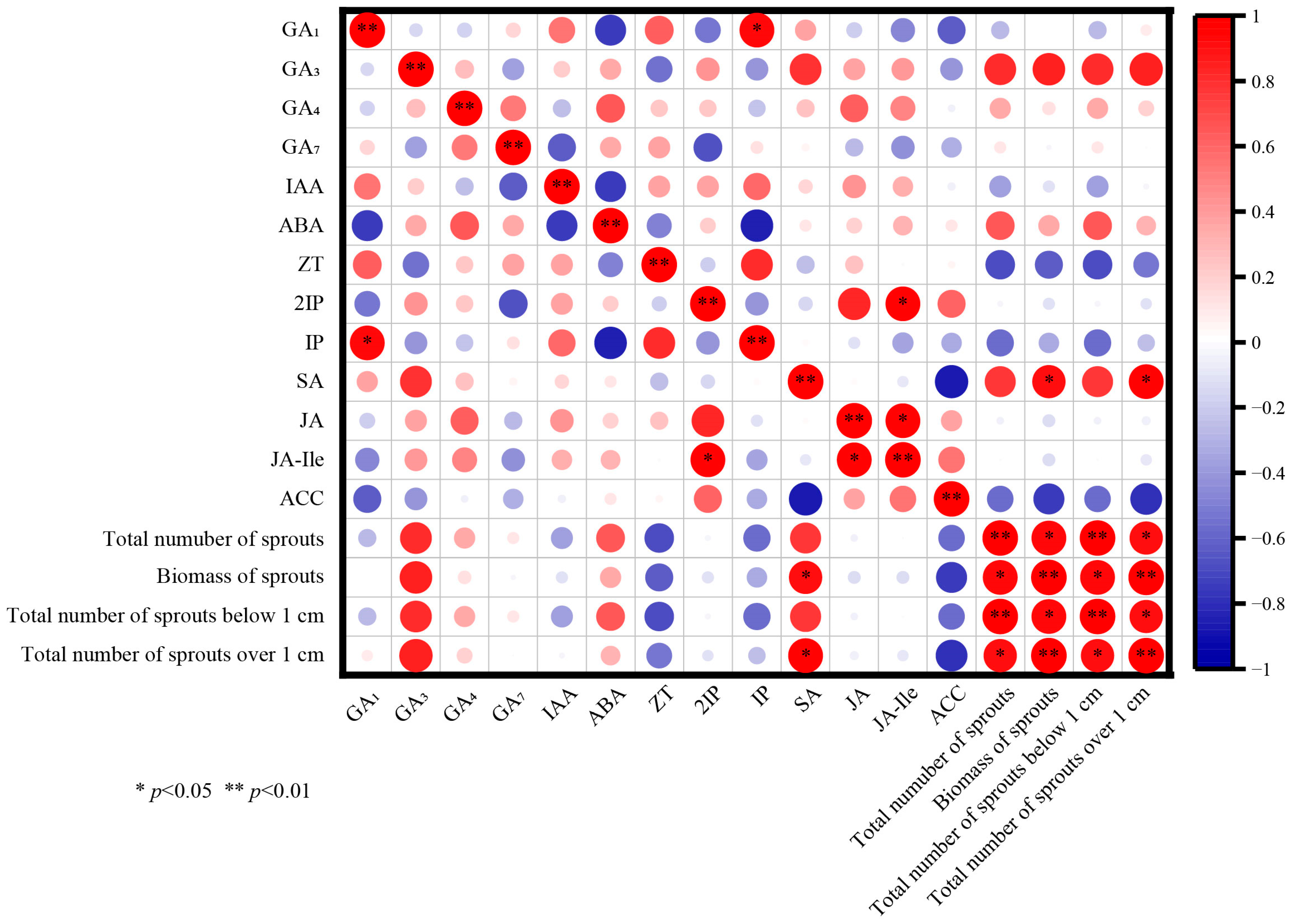
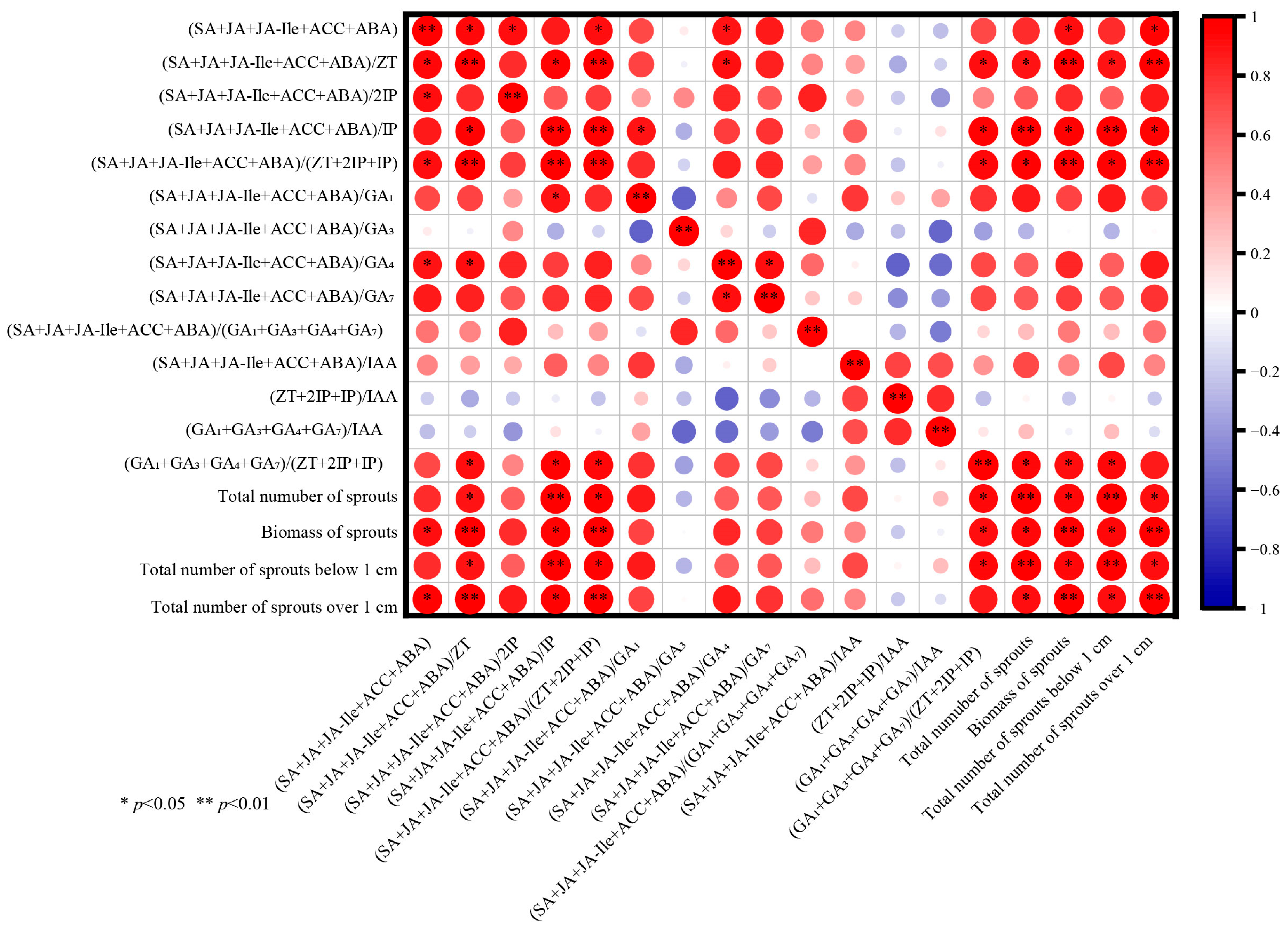
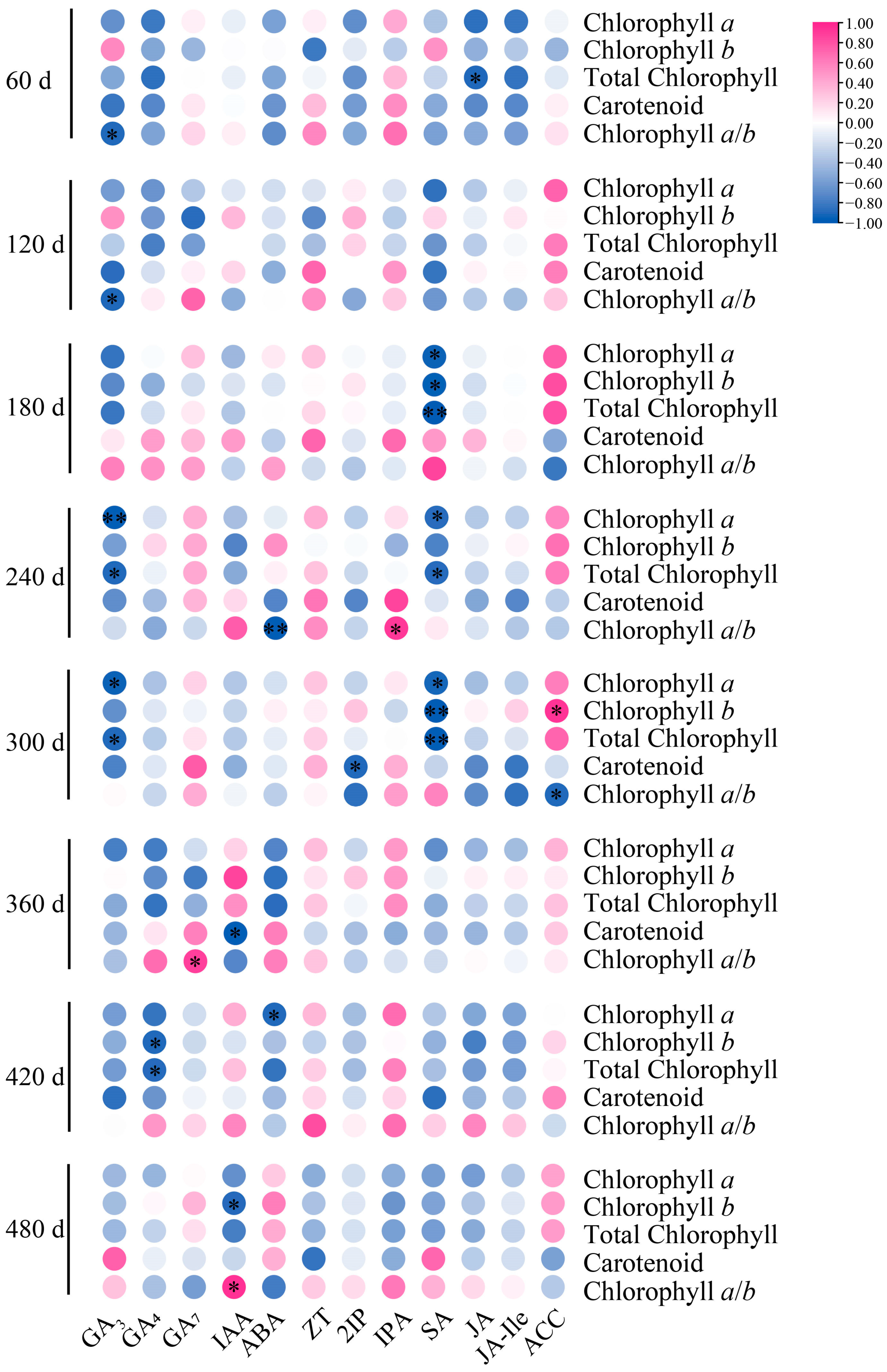
3.8. Correlation Analysis of Seedling Age on Photosynthetic Pigments and Endogenous Hormones of P. yunnanensis Seedlings
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhu, W.; Liu, Y.; Wu, J.; Li, C. Adaptations of Pinus yunnanensis Seedlings to Simulated Light Patches: Growth Dynamics and C:N:P Stoichiometry. Forests 2025, 16, 517. [Google Scholar] [CrossRef]
- Zhao, Y.; Duan, X.; Shu, S. The Influence of Typical Forest Types on Soil Erosion Resistance in the Water Source Areas of Central Yunnan. Asian Agric. Res. 2015, 7, 35–40. [Google Scholar]
- Miao, Y.; Gao, C.; Li, J.; Liu, Z.; Cui, K. Genetic diversity, population structure and a core collection establishment of Pinus yunnanensis using microsatellite markers. Eur. J. For. Res. 2023, 142, 1439–1451. [Google Scholar] [CrossRef]
- Li, Z.; Gao, C.; Li, J.; Wang, L.; Cui, K. Variation in growth traits and early evaluation of the selection of intra and interspecific hybrid progeny of Pinus yunnanensis. New For. 2024, 56, 14. [Google Scholar] [CrossRef]
- Hu, Z.; Cheng, S.; He, B.; Tang, G.; Chen, L.; Chen, S.; Tang, J.; Xu, Y.; Li, G.; Cai, N. Comparison of endogenous hormone content and balance in Pinus yunnanensis Franch. seedlings after decapitation. Front. Plant Sci. 2025, 16, 1531575. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Liu, X.; Wang, R.; Dong, X.; Guan, X.; Wang, Y.; Jiang, Y.; Shi, Z.; Qi, M.; Li, T. SlARF2a plays a negative role in mediating axillary shoot formation. Sci. Rep. 2016, 6, 33728. [Google Scholar] [CrossRef]
- Zhou, C.; Gu, X.; Li, J.; Su, X.; Chen, S.; Tang, J.; Chen, L.; Cai, N.; Xu, Y. Physiological Characteristics and Transcriptomic Responses of Pinus yunnanensis Lateral Branching to Different Shading Environments. Plants 2024, 13, 1588. [Google Scholar] [CrossRef]
- Zhou, C.; Kong, D.; Li, J.; Su, X.; Cai, N.; Xu, Y. The morphological and physiological responses of Pinus yunnanensis to different levels of shading after decapitation. Ind. Crop. Prod. 2025, 224, 120374. [Google Scholar] [CrossRef]
- Wang, Y.; Li, J.; Che, F.; Song, Y.; Wang, Q.; Yan, T.; Xu, Y.; Cai, N.; Chen, S. Effects of Stumping in Different Seasons on Germination Ability of Pinus yunnanensis. J. Southwest For. Univ. Nat. Sci. 2023, 43, 26–31. [Google Scholar]
- Cai, N.; Hu, Z.; He, B.; Cheng, S.; Chen, L.; Tang, J.; Chen, S.; Xu, Y.; Li, G. Response of sprouting ability of Pinus yunnanensis seedlings to stump-cut height. J. Northwest AF Univ. Nat. Sci. Ed. 2024, 52, 85–94. [Google Scholar] [CrossRef]
- Dong, L.; Wu, Y.; Zhang, J.; Deng, X.; Wang, T. Transcriptome Analysis Revealed Hormone Pathways and bZIP Genes Responsive to Decapitation in Sunflower. Genes. 2022, 13, 1737. [Google Scholar] [CrossRef] [PubMed]
- Fang, R.; Wu, Y.; Huang, X.; Hou, Z.; Zhang, J.; Wang, L.; Wang, Y.; Li, Y.; Chen, L.; Yang, H.; et al. Effects of decapitation on yield-related traits of total node number per plant in soybean. Field Crops Res. 2025, 321, 109664. [Google Scholar] [CrossRef]
- Anfang, M.; Shani, E. Transport mechanisms of plant hormones. Curr. Opin. Plant Biol. 2021, 63, 102055. [Google Scholar] [CrossRef]
- Bajguz, A.; Piotrowska-Niczyporuk, A. Biosynthetic Pathways of Hormones in Plants. Metabolites 2023, 13, 884. [Google Scholar] [CrossRef]
- Li, M.; Wei, Q.; Xiao, Y.; Peng, F. The effect of auxin and strigolactone on ATP/ADP isopentenyltransferase expression and the regulation of apical dominance in peach. Plant Cell Rep. 2018, 37, 1693–1705. [Google Scholar] [CrossRef]
- Yan, Y.; Zhao, N.; Tang, H.; Gong, B.; Shi, Q. Shoot branching regulation and signaling. Plant Growth Regul. 2020, 92, 131–140. [Google Scholar] [CrossRef]
- Zhu, J.; Li, X.; Huang, J.; Wang, L.; Zheng, Q.; Li, H.; Chen, Y.; Tang, J.; Hao, X.; Wang, X.; et al. Transcriptomics and plant hormone analysis reveal the mechanism of branching angle formation in tea plants (Camellia sinensis). Int. J. Mol. Sci. 2025, 26, 604. [Google Scholar] [CrossRef]
- Verma, K.; Kumari, K.; Rawat, M.; Devi, K.; Joshi, R. Crosstalk of Jasmonic acid and Salicylic acid with other Phytohormones Alleviates Abiotic and Biotic Stresses in Plants. J. Soil Sci. Plant Nutr. 2025, 25, 4997–5019. [Google Scholar] [CrossRef]
- Sun, D.; Zhang, L.; Yu, Q.; Zhang, J.; Li, P.; Zhang, Y.; Xing, X.; Ding, L.; Fang, W.; Chen, F.; et al. Integrated Signals of Jasmonates, Sugars, Cytokinins and Auxin Influence the Initial Growth of the Second Buds of Chrysanthemum after Decapitation. Biology 2021, 10, 440. [Google Scholar] [CrossRef] [PubMed]
- Everat-Bourbouloux, A.; Charnay, D. Endogenous abscisic acid levels in stems and axillary buds of intact or decapitated broad-bean plants (Vicia faba L.). Physiol. Plant. 1982, 54, 440–445. [Google Scholar] [CrossRef]
- Tanaka, A.; Ito, H. Chlorophyll degradation and its physiological function. Plant Cell Physiol. 2025, 66, 139–152. [Google Scholar] [CrossRef]
- Simkin, A.J.; Kapoor, L.; Doss, C.G.P.; Hofmann, T.A.; Lawson, T.; Ramamoorthy, S. The role of photosynthesis related pigments in light harvesting, photoprotection and enhancement of photosynthetic yield in planta. Photosynth. Res. 2022, 152, 23–42. [Google Scholar] [CrossRef]
- Li, X.; Zhang, W.; Niu, D.; Liu, X. Effects of abiotic stress on chlorophyll metabolism. Plant Sci. 2024, 342, 112030. [Google Scholar] [CrossRef]
- Sonobe, R.; Yamashita, H.; Mihara, H.; Morita, A.; Ikka, T. Estimation of Leaf Chlorophyll a, b and Carotenoid Contents and Their Ratios Using Hyperspectral Reflectance. Remote Sens. 2020, 12, 3265. [Google Scholar] [CrossRef]
- Zielewicz, W.; Wróbel, B.; Niedbała, G. Quantification of Chlorophyll and Carotene Pigments Content in Mountain Melick (Melica nutans L.) in Relation to Edaphic Variables. Forests 2020, 11, 1197. [Google Scholar] [CrossRef]
- Dhami, N.; Tissue, D.T.; Cazzonelli, C.I. Leaf-age dependent response of carotenoid accumulation to elevated CO2 in Arabidopsis. Arch. Biochem. Biophys. 2018, 647, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Yordanov, I.; Goltsev, V.; Stefanov, D.; Chernev, P.; Zaharieva, I.; Kirova, M.; Gecheva, V.; Strasser, R.J. Preservation of photosynthetic electron transport from senescence-induced inactivation in primary leaves after decapitation and defoliation of bean plants. J. Plant Physiol. 2008, 165, 1954–1963. [Google Scholar] [CrossRef]
- Walmsley, J.; Adamson, H. Chlorophyll accumulation and breakdown in light-grown barley transferred to darkness: Effect of seedling age. Physiol. Plant. 1989, 77, 312–319. [Google Scholar] [CrossRef]
- Liu, C.; Liang, S.; Wu, J.; Gu, J.; Duan, H. Response of growth and physiological-biochemical characteristics in Pinus yunnanensis seedlings to drought and re-watering. J. Northwest AF Univ. Nat. Sci. Ed. 2026, 54, 99–108. [Google Scholar] [CrossRef]
- Luo, Q.; Gu, L.; Li, S. Soil and Plant Nutrient Content in the Asexual Propagation Seed Orchard of Pinus yunnanensis. J. W. China Forest. Sci. 2025, 54, 112–119. [Google Scholar] [CrossRef]
- Cheng, S.; Wang, D.; He, B.; Hu, Z.; Chen, L.; Tang, J.; Chen, S.; Xu, Y.; Cai, N. Analysis of root morphological characteristics of Pinus yunnanensis seedlings at different stump-ages. J. Zhejiang AF Univ. 2024, 41, 322–332. [Google Scholar]
- Chao, E.; Wu, M.; Yue, D.; Yuan, Y.; Qiu, N.; Zhou, F. Promoting effect of low concentration strontium on photosynthetic performance of Chinese cabbage seedlings: Combined leaf characteristics, photosynthetic carbon assimilation and chlorophyll fluorescence. Ecotoxicol. Environ. Saf. 2024, 274, 116200. [Google Scholar] [CrossRef]
- Tang, G.; Wang, Y.; Lu, Z.; Cheng, S.; Hu, Z.; Chen, S.; Chen, L.; Tang, J.; Xu, Y.; Cai, N. Effects of Combined Nitrogen-Phosphorus on Biomass Accumulation, Allocation, and Allometric Growth Relationships in Pinus yunnanensis Seedlings after Top Pruning. Plants 2024, 13, 2450. [Google Scholar] [CrossRef]
- Wright, I.J.; Reich, P.B.; Westoby, M.; Ackerly, D.D.; Baruch, Z.; Bongers, F.; Cavender-Bares, J.; Chapin, T.; Cornelissen, J.H.C.; Diemer, M.; et al. The worldwide leaf economics spectrum. Nature 2004, 428, 821–827. [Google Scholar] [CrossRef] [PubMed]
- Cudjoe, E.; Bravo, F.; Pretzsch, H.; Bettinger, P.; Ruiz-Peinado, R. Competition in mixed Scots pine and Pyrenean oak stands modifies allometry and partially affects biomass allocation during early stand development. Ecol. Indic. 2025, 176, 113713. [Google Scholar] [CrossRef]
- Li, J.; Yan, X.; Zhang, P.; Zhuo, Z.; Wang, X.; Huang, K.; Wang, P.; Zhou, X.; Ma, M.; Zhao, Y.; et al. Multiple global change factors alter the scaling of nitrogen to phosphorus in alpine plants. Funct. Ecol. 2025, 39, 2044–2055. [Google Scholar] [CrossRef]
- Vanneste, S.; Pei, Y.; Friml, J. Mechanisms of auxin action in plant growth and development. Nat. Rev. Mol. Cell Biol. 2025, 1–19. [Google Scholar] [CrossRef]
- Leyser, O. Regulation of shoot branching by auxin. Trends Plant Sci. 2003, 8, 541–545. [Google Scholar] [CrossRef] [PubMed]
- Galinha, C.; Bilsborough, G.; Tsiantis, M. Hormonal input in plant meristems: A balancing act. Semin. Cell Dev. Biol. 2009, 20, 1149–1156. [Google Scholar] [CrossRef] [PubMed]
- Barbier, F.F.; Dun, E.A.; Beveridge, C.A. Apical dominance. Curr. Biol. 2017, 27, R864–R865. [Google Scholar] [CrossRef]
- Shen, J.; Zhang, Y.; Ge, D.; Wang, Z.; Song, W.; Gu, R.; Che, G.; Cheng, Z.; Liu, R.; Zhang, X. CsBRC1 inhibits axillary bud outgrowth by directly repressing the auxin efflux carrier CsPIN3 in cucumber. Proc. Natl. Acad. Sci. USA 2019, 116, 17105–17114. [Google Scholar] [CrossRef]
- Chen, Y.; Ling, Q.; Li, X.; Ma, Q.; Tang, S.; Yuanzhi, P.; Liu, Q.L.; Jia, Y.; Yong, X.; Jiang, B. Transcriptome analysis during axillary bud growth in chrysanthemum (chrysanthemum×morifolium). PeerJ 2023, 11, e16436. [Google Scholar] [CrossRef] [PubMed]
- Wolbang, C.M.; Chandler, P.M.; Smith, J.J.; Ross, J.J. Auxin from the Developing Inflorescence Is Required for the Biosynthesis of Active Gibberellins in Barley Stems. Plant Physiol. 2004, 134, 769–776. [Google Scholar] [CrossRef]
- Okada, K.; Wada, M.; Takebayashi, Y.; Kojima, M.; Sakakibara, H.; Nakayasu, M.; Mizutani, M.; Nakajima, M.; Moriya, S.; Shimizu, T.; et al. Columnar growth phenotype in apple results from gibberellin deficiency by ectopic expression of a dioxygenase gene. Tree Physiol. 2020, 40, 1205–1216. [Google Scholar] [CrossRef]
- Qiu, Y.; Guan, S.C.; Wen, C.; Li, P.; Gao, Z.; Chen, X. Auxin and cytokinin coordinate the dormancy and outgrowth of axillary bud in strawberry runner. BMC Plant Biol. 2019, 19, 528. [Google Scholar] [CrossRef]
- Shimizu-Sato, S.; Tanaka, M.; Mori, H. Auxin-cytokinin interactions in the control of shoot branching. Plant Mol. Biol. 2009, 69, 429–435. [Google Scholar] [CrossRef]
- Wang, D.; Su, P.; Yang, Z.; Chen, J.; Liao, R.; Gao, Y.; Kan, W.; Hou, J.; Wu, L. Combined analysis of metabolome and transcriptome in response to exogenous tZ treatment on axillary bud development in Eucommia ulmoides Oliver. Ind. Crop. Prod. 2025, 230, 121089. [Google Scholar] [CrossRef]
- Gomi, K. Jasmonic Acid: An Essential Plant Hormone. Int. J. Mol. Sci. 2020, 21, 1261. [Google Scholar] [CrossRef] [PubMed]
- Spoel, S.H.; Dong, X. Salicylic acid in plant immunity and beyond. Plant Cell 2024, 36, 1451–1464. [Google Scholar] [CrossRef] [PubMed]
- Brookbank, B.P.; Patel, J.; Gazzarrini, S.; Nambara, E. Role of Basal ABA in Plant Growth and Development. Genes 2021, 12, 1936. [Google Scholar] [CrossRef]
- Hartung, W.; Steigerwald, F. Abscisic acid and apical dominance in Phaseolus coccineus L. Planta 1977, 134, 295–299. [Google Scholar] [CrossRef] [PubMed]
- Gocal, G.F.; Pharis, R.P.; Yeung, E.C.; Pearce, D. Changes after decapitation in concentrations of Indole-3-Acetic acid and abscisic acid in the larger axillary bud of Phaseolus vulgaris L. cv Tender Green. Plant Physiol. 1991, 95, 344–350. [Google Scholar] [CrossRef]
- Binenbaum, J.; Weinstain, R.; Shani, E. Gibberellin Localization and Transport in Plants. Trends Plant Sci. 2018, 23, 410–421. [Google Scholar] [CrossRef] [PubMed]
- Van Staden, J.; Carmi, A. The effects of decapitation on the distribution of cytokinins and growth of Phaseolus vulgaris plants. Physiol. Plant. 1982, 55, 39–44. [Google Scholar] [CrossRef]
- Nowicka, B.; Ciura, J.; Szymańska, R.; Kruk, J. Improving photosynthesis, plant productivity and abiotic stress tolerance—Current trends and future perspectives. J. Plant Physiol. 2018, 231, 415–433. [Google Scholar] [CrossRef]
- Ye, J.; Liu, H.; Zhao, Z.; Xu, L.; Li, K.; Du, D. Fine mapping of the QTL cqSPDA2 for chlorophyll content in Brassica napus L. BMC Plant Biol. 2020, 20, 511. [Google Scholar] [CrossRef] [PubMed]
- Xiong, B.; Qiu, X.; Huang, S.; Wang, X.; Zhang, X.; Dong, T.; Wang, T.; Li, S.; Sun, G.; Zhu, J.; et al. Physiological and transcriptome analyses of photosynthesis and chlorophyll metabolism in variegated Citrus (Shiranuhi and Huangguogan) seedlings. Sci. Rep. 2019, 9, 15670. [Google Scholar] [CrossRef]
- Tschaplinski, T.J.; Blake, T.J. Photosynthetic reinvigoration of leaves following shoot decapitation and accelerated growth of coppice shoots. Physiol. Plant. 1989, 75, 157–165. [Google Scholar] [CrossRef]
- Blake, T.J.; Tschaplinski, T.J. Role of water relations and photosynthesis in the release of buds from apical dominance and the early reinvigoration of decapitated poplars. Physiol. Plant. 1986, 68, 287–293. [Google Scholar] [CrossRef]
- Satoh, M.; Kriedemann, P.E.; Loveys, B.R. Changes in Photosynthetic Activity and Related Processes Following Decapitation in Mulberry Trees. Physiol. Plant. 1977, 41, 203–210. [Google Scholar] [CrossRef]
- Müller, M.; Munné-Bosch, S. Hormonal impact on photosynthesis and photoprotection in plants. Plant Physiol. 2021, 185, 1500–1522. [Google Scholar] [CrossRef]
- Liu, X.; Li, Y.; Zhong, S. Interplay between Light and Plant Hormones in the Control of Arabidopsis Seedling Chlorophyll Biosynthesis. Front. Plant Sci. 2017, 8, 1433. [Google Scholar] [CrossRef]
- Yan, H.; Fu, K.; Li, J.; Li, M.; Li, S.; Dai, Z.; Jin, X. Photosynthesis, Chlorophyll Fluorescence, and Hormone Regulation in Tomato Exposed to Mechanical Wounding. Plants 2024, 13, 2594. [Google Scholar] [CrossRef]
- Chaiareekitwat, S.; Latif, S.; Mahayothee, B.; Khuwijitjaru, P.; Nagle, M.; Amawan, S.; Müller, J. Protein composition, chlorophyll, carotenoids, and cyanide content of cassava leaves (Manihot esculenta Crantz) as influenced by cultivar, plant age, and leaf position. Food Chem. 2022, 372, 131173. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Wang, F.; Wang, Y.; Zhong, X.; Zhu, S.; Zhang, X.; Li, S.; Lei, X.; Zang, Z.; Tan, G.; et al. Low-Temperature Regulates the Cell Structure and Chlorophyll in Addition to Cellulose Metabolism of Postharvest Red Toona sinensis Buds across Different Seasons. Int. J. Mol. Sci. 2024, 25, 7719. [Google Scholar] [CrossRef] [PubMed]
- Jinwen, L.; Jingping, Y.; Pinpin, F.; Junlan, S.; Dongsheng, L.; Changshui, G.; Wenyue, C. Responses of rice leaf thickness, SPAD readings and chlorophyll a/b ratios to different nitrogen supply rates in paddy field. Field Crops Res. 2009, 114, 426–432. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, W.; Su, X.; Cheng, S.; Wang, D.; Xu, Y.; Cai, N. Effects of Decapitation on Chlorophyll Metabolism, Endogenous Hormones, and Tillering Ability in Pinus yunnanensis Seedlings of Different Ages. Biology 2025, 14, 1070. https://doi.org/10.3390/biology14081070
Li W, Su X, Cheng S, Wang D, Xu Y, Cai N. Effects of Decapitation on Chlorophyll Metabolism, Endogenous Hormones, and Tillering Ability in Pinus yunnanensis Seedlings of Different Ages. Biology. 2025; 14(8):1070. https://doi.org/10.3390/biology14081070
Chicago/Turabian StyleLi, Wei, Xin Su, Sili Cheng, Dan Wang, Yulan Xu, and Nianhui Cai. 2025. "Effects of Decapitation on Chlorophyll Metabolism, Endogenous Hormones, and Tillering Ability in Pinus yunnanensis Seedlings of Different Ages" Biology 14, no. 8: 1070. https://doi.org/10.3390/biology14081070
APA StyleLi, W., Su, X., Cheng, S., Wang, D., Xu, Y., & Cai, N. (2025). Effects of Decapitation on Chlorophyll Metabolism, Endogenous Hormones, and Tillering Ability in Pinus yunnanensis Seedlings of Different Ages. Biology, 14(8), 1070. https://doi.org/10.3390/biology14081070





