Mimicking Gastric Cancer Collagen Reorganization with Decellularized ECM-Based Scaffolds
Simple Summary
Abstract
1. Introduction
2. Gastric Cancer Overview
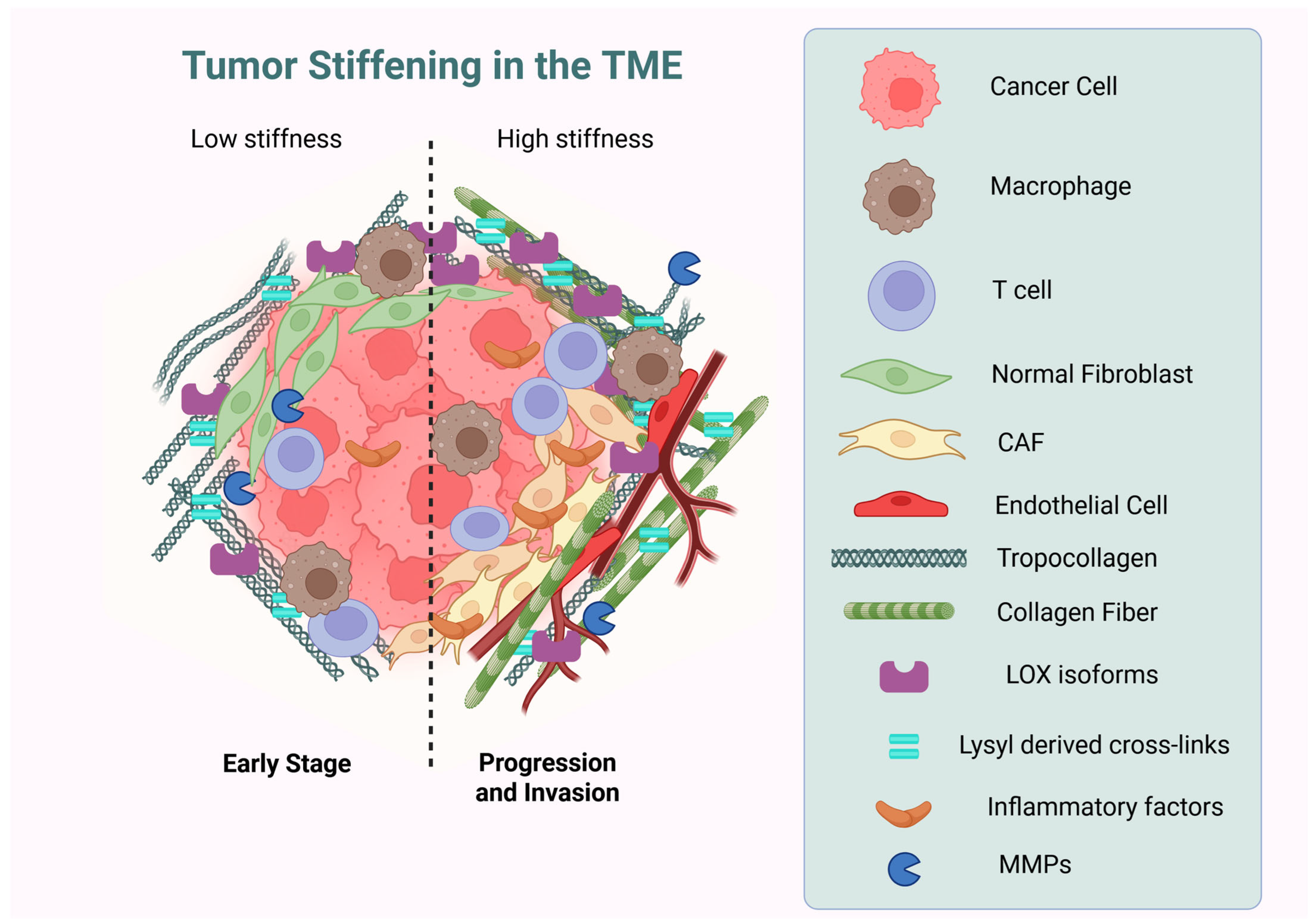
3. Collagens in Gastric Cancer: Expression, Deposition, Assembly, and Reorganization
3.1. Upregulation of Collagens Expression and Deposition in Gastric Cancer
| Type | Genes Involved in GC | Normal Gastric Tissue | GC Tissue | Biological Role in GC | References |
|---|---|---|---|---|---|
| Collagen I | COL1A1 COL1A2 | Moderate (structural support) | High in stromal fibrosis, linked to invasion and metastasis. Potential role as a negative prognostic indicator | Promotes EMT, TME stiffness | [29,41,63,64,65,66,67] |
| Collagen III | COL3A1 | Moderate (maintains mucosal integrity) | Moderate (co-localizes with Collagen I in desmoplasia). Co-overexpression with COL5A2 is proposed as a predictor of poor survival in late-stage GC | Stromal remodeling | [67,68,69,70] |
| Collagen IV | COL4A1 COL4A2 | Moderate (basement membrane) | High. Proposed as a biomarker for poor survival prognosis | Promote EMT, migration, tumor invasiveness, and metastasis | [71,72,73,74] |
| Collagen V | COL5A1 COL5A2 | Moderate | High. Co-overexpression with COL3A1 is proposed as a predictor of poor survival in late-stage GC | Promotes EMT, cell migration | [49,50,69,70,75,76] |
| Collagen VI | COL6A3 | Moderate | Moderate, High. Proposed as a therapeutic target to slow GC progression, blocking specific integrins that bond with Collagen VI | Cell differentiation, migration, and adhesion | [77] |
| Collagen VII | COL7A1 | Moderate | High. Proposed as a biomarker for poor survival prognosis | Tumor invasiveness and metastasis | [78] |
| Collagen X | COL10A1 | Moderate (basement membrane) | High. Proposed as a biomarker for poor survival prognosis and poor immune response. Because of its restricted high expression (only in cartilage and tumor), elevated plasma levels of Collagen X fragments have been found in GC patients | Promotes EMT, tumor vasculature | [48,79,80,81] |
| Collagen XI | COL11A1 | Moderate | High. Proposed as a biomarker for poor survival prognosis and chemotherapy resistance. It has been proposed as a target for CAFs focused therapies in GC | Tumor progression and metastasis, promotes EMT | [62,64,82] |
| Collagen XII | COL12A1 | Non-detected | High. Potential role as a poor survival prognosis. As a therapeutic target, blocking or decreasing Collagen XII can potentially mitigate metastatic | Tumor invasiveness and metastasis | [47] |
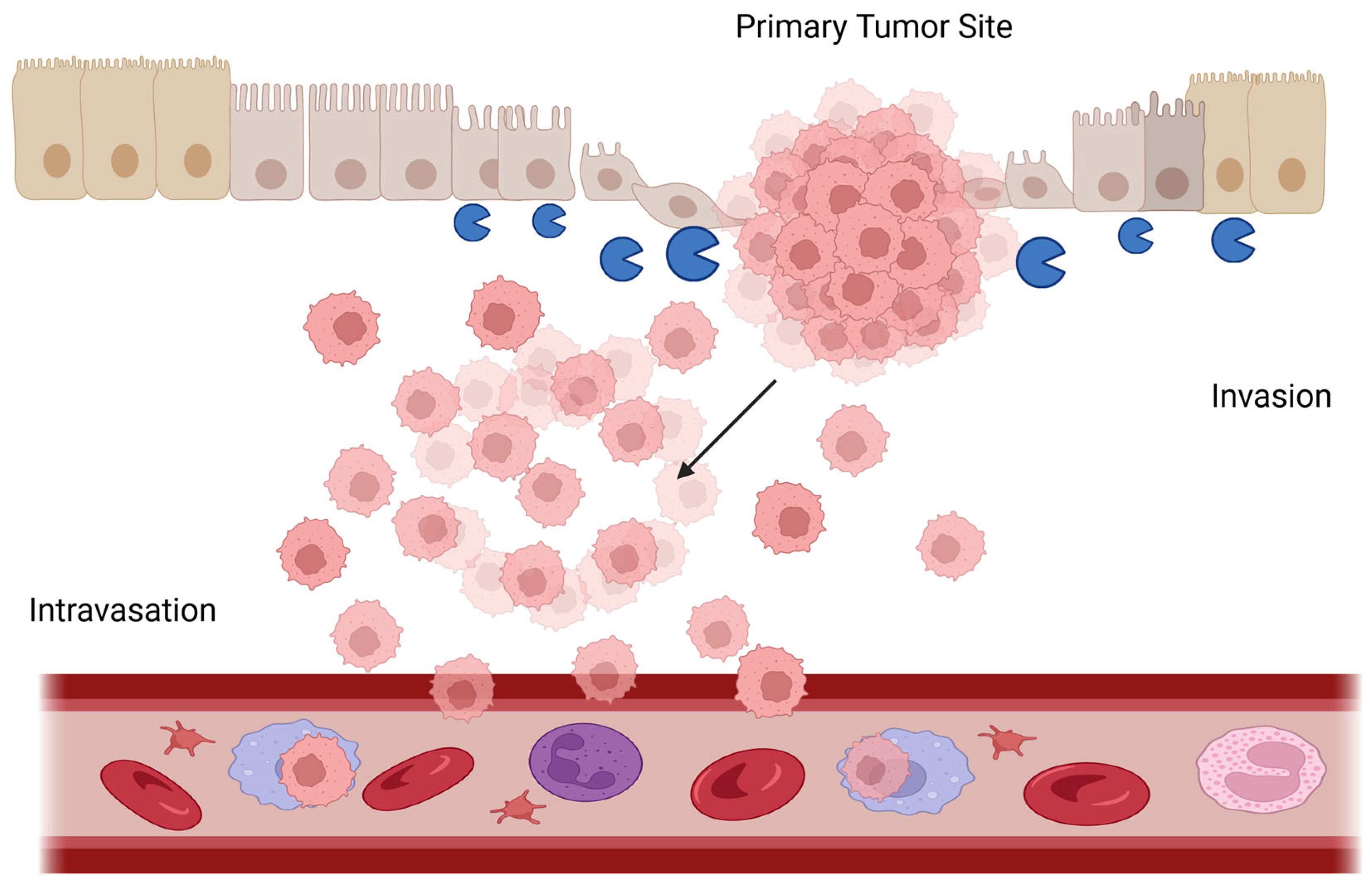
3.2. Collagen Reorganization and Assembly in Gastric Cancer
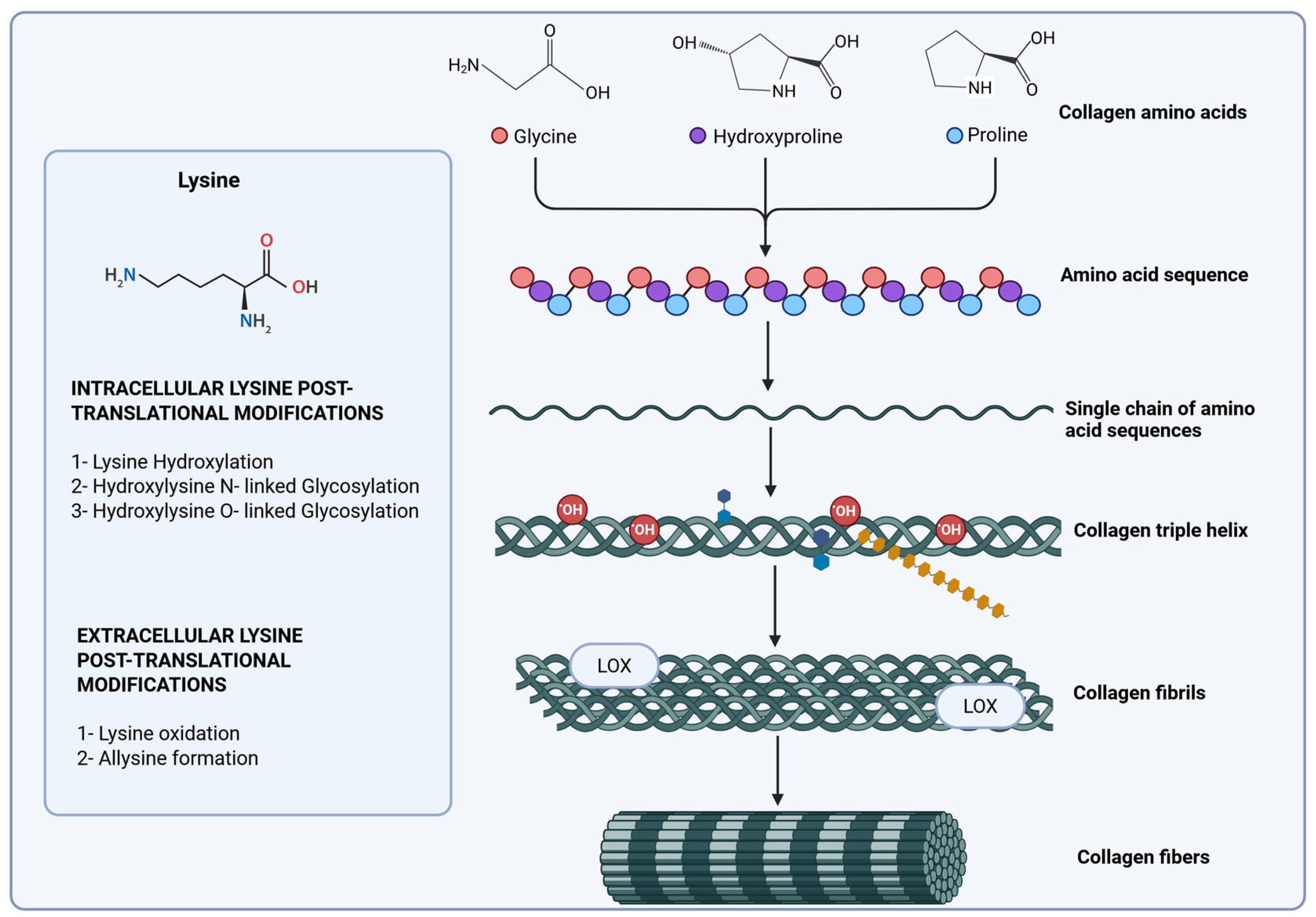
4. Collagen Biosynthesis and Degradation in Gastric Cancer
4.1. Collagen Hydroxylases
4.2. Lysyl Oxidases
4.3. Matrix Metalloproteinases (MMPs)
4.4. Collagen Cross-Linking by Advanced Glycation Products
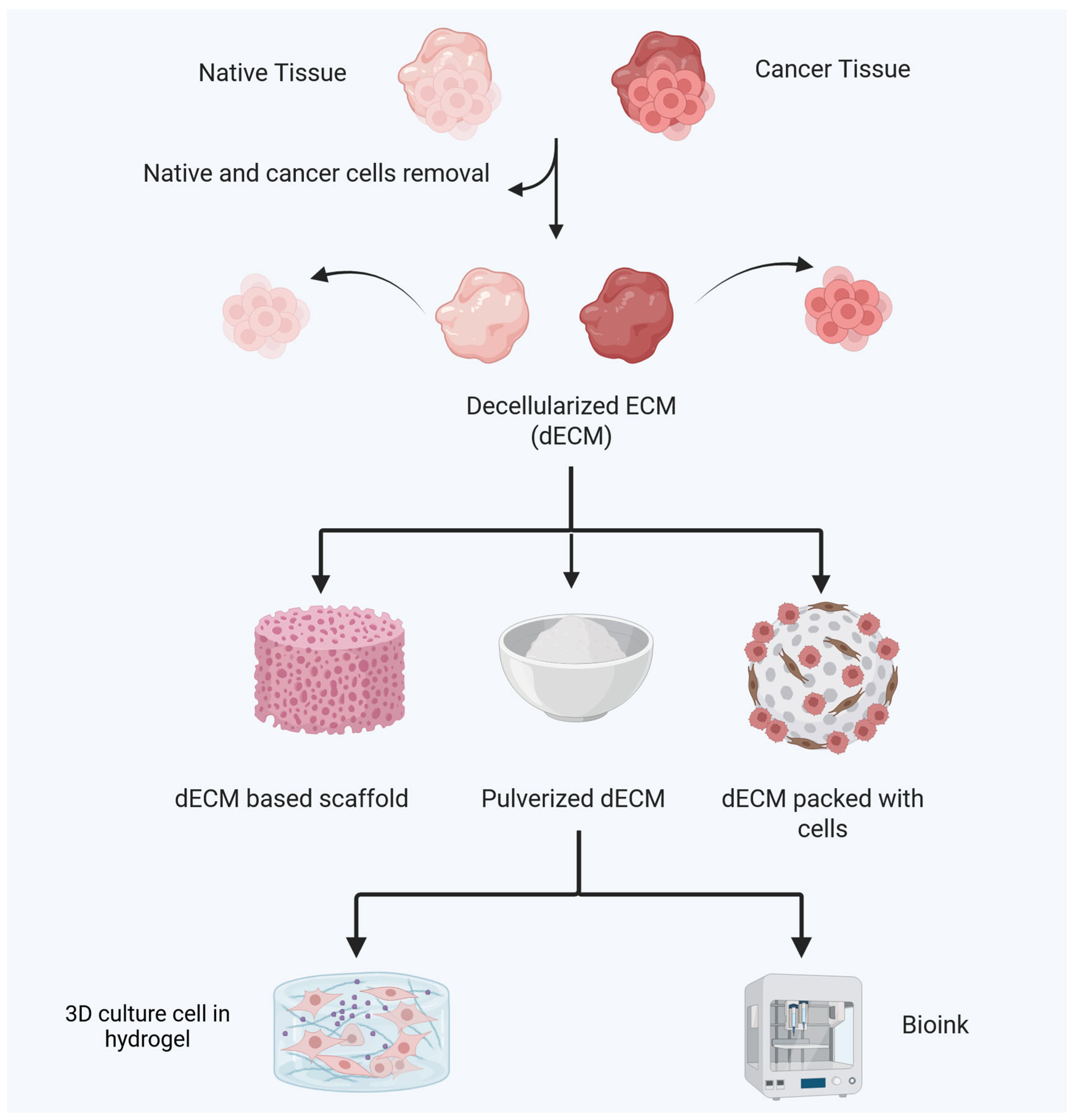
5. Decellularized Tissues as Scaffolds for Biomimetic 3D In Vitro Models in Gastric Cancer
5.1. Comparison of Features of dECM Sources
5.2. Techniques for Decellularization for Obtaining dECM Materials
5.3. Studies in Cancer Initiation and Progression Using dECM
5.4. dECM Scaffolds to Study Collagen Assembly in the Gastric Tumor Microenvironment
6. Conclusions and Future Research Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Li, W.; Ng, J.M.-K.; Wong, C.C.; Ng, E.K.W.; Yu, J. Molecular alterations of cancer cell and tumour microenvironment in metastatic gastric cancer. Oncogene 2018, 37, 4903–4920. [Google Scholar] [CrossRef] [PubMed]
- Shah, D.; Bentrem, D. Environmental and genetic risk factors for gastric cancer. J. Surg. Oncol. 2022, 125, 1096–1103. [Google Scholar] [CrossRef] [PubMed]
- Smyth, E.C.; Nilsson, M.; Grabsch, H.I.; van Grieken, N.C.; Lordick, F. Gastric cancer. Lancet 2020, 396, 635–648. [Google Scholar] [CrossRef]
- Van Cutsem, E.; Sagaert, X.; Topal, B.; Haustermans, K.; Prenen, H. Gastric cancer. Lancet 2016, 388, 2654–2664. [Google Scholar] [CrossRef]
- Page-McCaw, A.; Ewald, A.J.; Werb, Z. Matrix metalloproteinases and the regulation of tissue remodelling. Nat. Rev. Mol. Cell Biol. 2007, 8, 221–233. [Google Scholar] [CrossRef]
- Turley, S.J.; Cremasco, V.; Astarita, J.L. Immunological hallmarks of stromal cells in the tumour microenvironment. Nat. Rev. Immunol. 2015, 15, 669–682. [Google Scholar] [CrossRef] [PubMed]
- Moreira, A.M.; Pereira, J.; Melo, S.; Fernandes, M.S.; Carneiro, P.; Seruca, R.; Figueiredo, J. The Extracellular Matrix: An Accomplice in Gastric Cancer Development and Progression. Cells 2020, 9, 394. [Google Scholar] [CrossRef] [PubMed]
- Najafi, M.; Farhood, B.; Mortezaee, K. Extracellular matrix (ECM) stiffness and degradation as cancer drivers. J. Cell Biochem. 2019, 120, 2782–2790. [Google Scholar] [CrossRef]
- Mai, Z.; Lin, Y.; Lin, P.; Zhao, X.; Cui, L. Modulating extracellular matrix stiffness: A strategic approach to boost cancer immunotherapy. Cell Death Dis. 2024, 15, 307. [Google Scholar] [CrossRef]
- Caballero, D.; Kundu, B.; Abreu, C.M.; Amorim, S.; Fernandes, D.C.; Pires, R.A.; Oliveira, J.M.; Correlo, V.M.; Reis, R.L.; Kundu, S.C. Forecast cancer: The importance of biomimetic 3D in vitro models in cancer drug testing/discovery and therapy. Vitr. Model. 2022, 1, 119–123. [Google Scholar] [CrossRef] [PubMed]
- Cristescu, R.; Lee, J.; Nebozhyn, M.; Kim, K.M.; Ting, J.C.; Wong, S.S.; Liu, J.; Yue, Y.G.; Wang, J.; Yu, K.; et al. Molecular analysis of gastric cancer identifies subtypes associated with distinct clinical outcomes. Nat. Med. 2015, 21, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Shao, X.; Zhang, Y.; Zhu, M.; Wang, F.X.C.; Mu, J.; Li, J.; Yao, H.; Chen, K. Role of tumor microenvironment in cancer progression and therapeutic strategy. Cancer Med. 2023, 12, 11149–11165. [Google Scholar] [CrossRef]
- Tamayo-Angorrilla, M.; López de Andrés, J.; Jiménez, G.; Marchal, J.A. The biomimetic extracellular matrix: A therapeutic tool for breast cancer research. Transl. Res. 2022, 247, 117–136. [Google Scholar] [CrossRef]
- Röcken, C. Molecular classification of gastric cancer. Expert. Rev. Mol. Diagn. 2017, 17, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Ajani, J.A.; Lee, J.; Sano, T.; Janjigian, Y.Y.; Fan, D.; Song, S. Gastric adenocarcinoma. Nat. Rev. Dis. Primers 2017, 3, 17036. [Google Scholar] [CrossRef]
- Seeneevassen, L.; Bessède, E.; Mégraud, F.; Lehours, P.; Dubus, P.; Varon, C. Gastric Cancer: Advances in Carcinogenesis Research and New Therapeutic Strategies. Int. J. Mol. Sci. 2021, 22, 3418. [Google Scholar] [CrossRef]
- Johnston, F.M.; Beckman, M. Updates on Management of Gastric Cancer. Curr. Oncol. Rep. 2019, 21, 67. [Google Scholar] [CrossRef]
- Lauren, P. THE TWO HISTOLOGICAL MAIN TYPES OF GASTRIC CARCINOMA: DIFFUSE AND SO-CALLED INTESTINAL-TYPE CARCINOMA. AN ATTEMPT AT A HISTO-CLINICAL CLASSIFICATION. Acta Pathol. Microbiol. Scand. 1965, 64, 31–49. [Google Scholar] [CrossRef]
- Cisło, M.; Filip, A.A.; Arnold Offerhaus, G.J.; Ciseł, B.; Rawicz-Pruszyński, K.; Skierucha, M.; Polkowski, W.P. Distinct molecular subtypes of gastric cancer: From Laurén to molecular pathology. Oncotarget 2018, 9, 19427–19442. [Google Scholar] [CrossRef] [PubMed]
- Zaafouri, H.; Jouini, R.; Khedhiri, N.; Khanchel, F.; Cherif, M.; Mesbahi, M.; Daghmouri, A.; Mahmoudi, W.; Akremi, S.; Sabbah, M.; et al. Comparison between signet-ring cell carcinoma and non-signet-ring cell carcinoma of the stomach: Clinicopathological parameters, epidemiological data, outcome, and prognosis-a cohort study of 123 patients from a non-endemic country. World J. Surg. Oncol. 2022, 20, 238. [Google Scholar] [CrossRef] [PubMed]
- Machlowska, J.; Pucułek, M.; Sitarz, M.; Terlecki, P.; Maciejewski, R.; Sitarz, R. State of the art for gastric signet ring cell carcinoma: From classification, prognosis, and genomic characteristics to specified treatments. Cancer Manag. Res. 2019, 11, 2151–2161. [Google Scholar] [CrossRef]
- Correa, P.; Piazuelo, M.B. The gastric precancerous cascade. J. Dig. Dis. 2012, 13, 2–9. [Google Scholar] [CrossRef]
- Carcas, L.P. Gastric cancer review. J. Carcinog. 2014, 13, 14. [Google Scholar] [CrossRef]
- Yamaguchi, H.; Yoshida, N.; Takanashi, M.; Ito, Y.; Fukami, K.; Yanagihara, K.; Yashiro, M.; Sakai, R. Stromal fibroblasts mediate extracellular matrix remodeling and invasion of scirrhous gastric carcinoma cells. PLoS ONE 2014, 9, e85485. [Google Scholar] [CrossRef]
- Cosma, L.-S.; Schlosser, S.; Tews, H.C.; Müller, M.; Kandulski, A. Hereditary Diffuse Gastric Cancer: Molecular Genetics, Biological Mechanisms and Current Therapeutic Approaches. Int. J. Mol. Sci. 2022, 23, 7821. [Google Scholar] [CrossRef]
- Zheng, H.C.; Li, X.H.; Hara, T.; Masuda, S.; Yang, X.H.; Guan, Y.F.; Takano, Y. Mixed-type gastric carcinomas exhibit more aggressive features and indicate the histogenesis of carcinomas. Virchows Arch. 2008, 452, 525–534. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.H.; Ji, C.D.; Xiao, H.L.; Zhao, H.B.; Cui, Y.H.; Bian, X.W. Reorganized Collagen in the Tumor Microenvironment of Gastric Cancer and Its Association with Prognosis. J. Cancer 2017, 8, 1466–1476. [Google Scholar] [CrossRef]
- Duarte, I.; Llanos, O. Patterns of metastases in intestinal and diffuse types of carcinoma of the stomach. Hum. Pathol. 1981, 12, 237–242. [Google Scholar] [CrossRef]
- Schauer, M.; Peiper, M.; Theisen, J.; Knoefel, W. Prognostic factors in patients with diffuse type gastric cancer (linitis plastica) after operative treatment. Eur. J. Med. Res. 2011, 16, 29–33. [Google Scholar] [CrossRef]
- Ming, S.C. Gastric carcinoma. A pathobiological classification. Cancer 1977, 39, 2475–2485. [Google Scholar] [CrossRef]
- Ricard-Blum, S. The collagen family. Cold Spring Harb. Perspect. Biol. 2011, 3, a004978. [Google Scholar] [CrossRef] [PubMed]
- Kular, J.K.; Basu, S.; Sharma, R.I. The extracellular matrix: Structure, composition, age-related differences, tools for analysis and applications for tissue engineering. J. Tissue Eng. 2014, 5, 2041731414557112. [Google Scholar] [CrossRef]
- Zhou, N.; Liu, Y.D.; Zhang, Y.; Gu, T.W.; Peng, L.H. Pharmacological Functions, Synthesis, and Delivery Progress for Collagen as Biodrug and Biomaterial. Pharmaceutics 2023, 15, 1443. [Google Scholar] [CrossRef]
- Sato, H.; Naito, I.; Momota, R.; Naomoto, Y.; Yamatsuji, T.; Sado, Y.; Ninomiya, Y.; Ohtsuka, A. The differential distribution of type IV collagen alpha chains in the subepithelial basement membrane of the human alimentary canal. Arch. Histol. Cytol. 2007, 70, 313–323. [Google Scholar] [CrossRef]
- Cox, T.R. The matrix in cancer. Nat. Rev. Cancer 2021, 21, 217–238. [Google Scholar] [CrossRef]
- Zhou, Y.; Jiang, Z.; Cao, L.; Yang, J. The role of various collagen types in tumor biology: A review. Front. Oncol. 2025, 15, 1549797. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Li, J.; Yang, X.; Li, X.; Kong, J.; Qi, D.; Zhang, F.; Sun, B.; Liu, Y.; Liu, T. Carcinoma-associated fibroblast-derived lysyl oxidase-rich extracellular vesicles mediate collagen crosslinking and promote epithelial-mesenchymal transition via p-FAK/p-paxillin/YAP signaling. Int. J. Oral. Sci. 2023, 15, 32. [Google Scholar] [CrossRef] [PubMed]
- Guszczyn, T.; Sobolewski, K. Deregulation of collagen metabolism in human stomach cancer. Pathobiology 2004, 71, 308–313. [Google Scholar] [CrossRef]
- Sun, H.; Wang, Y.; Wang, S.; Xie, Y.; Sun, K.; Li, S.; Cui, W.; Wang, K. The involvement of collagen family genes in tumor enlargement of gastric cancer. Sci. Rep. 2023, 13, 100. [Google Scholar] [CrossRef]
- Mari, V.; Angerilli, V.; Munari, G.; Scarpa, M.; Bao, Q.R.; Pucciarelli, S.; Fassan, M.; Spolverato, G. Molecular Determinants of Peritoneal Dissemination in Gastric Adenocarcinoma. Dig. Dis. 2023, 41, 49–65. [Google Scholar] [CrossRef]
- Han, W.; Chen, S.; Yuan, W.; Fan, Q.; Tian, J.; Wang, X.; Chen, L.; Zhang, X.; Wei, W.; Liu, R.; et al. Oriented collagen fibers direct tumor cell intravasation. Proc. Natl. Acad. Sci. USA 2016, 113, 11208–11213. [Google Scholar] [CrossRef]
- Chen, D.; Liu, Z.; Liu, W.; Fu, M.; Jiang, W.; Xu, S.; Wang, G.; Chen, F.; Lu, J.; Chen, H.; et al. Predicting postoperative peritoneal metastasis in gastric cancer with serosal invasion using a collagen nomogram. Nat. Commun. 2021, 12, 179. [Google Scholar] [CrossRef] [PubMed]
- Provenzano, P.P.; Inman, D.R.; Eliceiri, K.W.; Knittel, J.G.; Yan, L.; Rueden, C.T.; White, J.G.; Keely, P.J. Collagen density promotes mammary tumor initiation and progression. BMC Med. 2008, 6, 11. [Google Scholar] [CrossRef] [PubMed]
- Conklin, M.W.; Eickhoff, J.C.; Riching, K.M.; Pehlke, C.A.; Eliceiri, K.W.; Provenzano, P.P.; Friedl, A.; Keely, P.J. Aligned collagen is a prognostic signature for survival in human breast carcinoma. Am. J. Pathol. 2011, 178, 1221–1232. [Google Scholar] [CrossRef]
- Jiang, X.; Wu, M.; Xu, X.; Zhang, L.; Huang, Y.; Xu, Z.; He, K.; Wang, H.; Wang, H.; Teng, L. COL12A1, a novel potential prognostic factor and therapeutic target in gastric cancer. Mol. Med. Rep. 2019, 20, 3103–3112. [Google Scholar] [CrossRef]
- Necula, L.; Matei, L.; Dragu, D.; Pitica, I.; Neagu, A.I.; Bleotu, C.; Dima, S.; Popescu, I.; Diaconu, C.C.; Chivu-Economescu, M. High plasma levels of COL10A1 are associated with advanced tumor stage in gastric cancer patients. World J. Gastroenterol. 2020, 26, 3024–3033. [Google Scholar] [CrossRef]
- Ding, Y.L.; Sun, S.F.; Zhao, G.L. COL5A2 as a potential clinical biomarker for gastric cancer and renal metastasis. Medicine 2021, 100, e24561. [Google Scholar] [CrossRef]
- Jin, Y.; Song, X.; Sun, X.; Ding, Y. Up-regulation of collagen type V alpha 2 (COL5A2) promotes malignant phenotypes in gastric cancer cell via inducing epithelial-mesenchymal transition (EMT). Open Med. 2023, 18, 20220593. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Li, X.; He, Y.; Wang, Y.; Shen, J.; Wang, S.; You, Q.; Zhai, J.; Shen, L. Cancer-associated fibroblasts-derived HAPLN1 promotes tumour invasion through extracellular matrix remodeling in gastric cancer. Gastric Cancer 2022, 25, 346–359. [Google Scholar] [CrossRef]
- Ozmen, E.; Demir, T.D.; Ozcan, G. Cancer-associated fibroblasts: Protagonists of the tumor microenvironment in gastric cancer. Front. Mol. Biosci. 2024, 11, 1340124. [Google Scholar] [CrossRef]
- Nissen, N.I.; Karsdal, M.; Willumsen, N. Collagens and Cancer associated fibroblasts in the reactive stroma and its relation to Cancer biology. J. Exp. Clin. Cancer Res. 2019, 38, 115. [Google Scholar] [CrossRef]
- Zheng, H.; Tan, J.; Qin, F.; Zheng, Y.; Yang, X.; Qin, X.; Liao, H. Analysis of cancer-associated fibroblasts related genes identifies COL11A1 associated with lung adenocarcinoma prognosis. BMC Med. Genom. 2024, 17, 97. [Google Scholar] [CrossRef]
- Nallanthighal, S.; Heiserman, J.P.; Cheon, D.-J. Collagen Type XI Alpha 1 (COL11A1): A Novel Biomarker and a Key Player in Cancer. Cancers 2021, 13, 935. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Li, J.; Lin, J.; Zhuo, W.; Si, J. COL11A1 is overexpressed in gastric cancer tissues and regulates proliferation, migration and invasion of HGC-27 gastric cancer cells in vitro. Oncol. Rep. 2017, 37, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Fuyuhiro, Y.; Yashiro, M.; Noda, S.; Kashiwagi, S.; Matsuoka, J.; Doi, Y.; Kato, Y.; Muguruma, K.; Sawada, T.; Hirakawa, K. Myofibroblasts are associated with the progression of scirrhous gastric carcinoma. Exp. Ther. Med. 2010, 1, 547–551. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Sun, H.; Yu, H.; Wang, L.; Gao, C.; Mei, H.; Jiang, X.; Ji, M. Tumor-associated-fibrosis and active collagen-CD44 axis characterize a poor-prognosis subtype of gastric cancer and contribute to tumor immunosuppression. J. Transl. Med. 2025, 23, 123. [Google Scholar] [CrossRef]
- Xu, G.; Zhang, B.; Ye, J.; Cao, S.; Shi, J.; Zhao, Y.; Wang, Y.; Sang, J.; Yao, Y.; Guan, W.; et al. Exosomal miRNA-139 in cancer-associated fibroblasts inhibits gastric cancer progression by repressing MMP11 expression. Int. J. Biol. Sci. 2019, 15, 2320–2329. [Google Scholar] [CrossRef]
- Sun, C.; Fukui, H.; Hara, K.; Zhang, X.; Kitayama, Y.; Eda, H.; Tomita, T.; Oshima, T.; Kikuchi, S.; Watari, J.; et al. FGF9 from cancer-associated fibroblasts is a possible mediator of invasion and anti-apoptosis of gastric cancer cells. BMC Cancer 2015, 15, 333. [Google Scholar] [CrossRef]
- Zhang, Q.; Peng, C. Cancer-associated fibroblasts regulate the biological behavior of cancer cells and stroma in gastric cancer. Oncol. Lett. 2018, 15, 691–698. [Google Scholar] [CrossRef]
- Zhang, J.; Lu, S.; Lu, T.; Han, D.; Zhang, K.; Gan, L.; Wu, X.; Li, Y.; Zhao, X.; Li, Z. Single-cell analysis reveals the COL11A1+ fibroblasts are cancer-specific fibroblasts that promote tumor progression. Front. Pharmacol. 2023, 14, 1121586. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.N.; Zhu, H.L.; Xia, M.T.; Liao, J.; Huang, X.T.; Xiao, J.W.; Yuan, C. A panel of collagen genes are associated with prognosis of patients with gastric cancer and regulated by microRNA-29c-3p: An integrated bioinformatics analysis and experimental validation. Cancer Manag. Res. 2019, 11, 4757–4772. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhou, T.; Li, A.; Yao, H.; He, F.; Wang, L.; Si, J. A potential role of collagens expression in distinguishing between premalignant and malignant lesions in stomach. Anat. Rec. 2009, 292, 692–700. [Google Scholar] [CrossRef] [PubMed]
- Jang, M.; Koh, I.; Lee, J.E.; Lim, J.Y.; Cheong, J.H.; Kim, P. Increased extracellular matrix density disrupts E-cadherin/β-catenin complex in gastric cancer cells. Biomater. Sci. 2018, 6, 2704–2713. [Google Scholar] [CrossRef]
- Hu, Y.; Li, J.; Luo, H.; Song, W.; Yang, J. Differential Expression of COL1A1, COL1A2, COL6A3, and SULF1 as Prognostic Biomarkers in Gastric Cancer. Int. J. Gen. Med. 2021, 14, 5835–5843. [Google Scholar] [CrossRef]
- Wang, Y.; Zheng, K.; Chen, X.; Chen, R.; Zou, Y. Bioinformatics analysis identifies COL1A1, THBS2 and SPP1 as potential predictors of patient prognosis and immunotherapy response in gastric cancer. Biosci. Rep. 2021, 41, BSR20202564. [Google Scholar] [CrossRef] [PubMed]
- Naito, Y.; Sakamoto, N.; Oue, N.; Yashiro, M.; Sentani, K.; Yanagihara, K.; Hirakawa, K.; Yasui, W. MicroRNA-143 regulates collagen type III expression in stromal fibroblasts of scirrhous type gastric cancer. Cancer Sci. 2014, 105, 228–235. [Google Scholar] [CrossRef]
- Chen, M.; Zhu, X.; Zhang, L.; Zhao, D. COL5A2 is a prognostic-related biomarker and correlated with immune infiltrates in gastric cancer based on transcriptomics and single-cell RNA sequencing. BMC Med. Genom. 2023, 16, 220. [Google Scholar] [CrossRef]
- Han, S.; Wang, Z.; Liu, J.; Wang, H.D.; Yuan, Q. miR-29a-3p-dependent COL3A1 and COL5A1 expression reduction assists sulforaphane to inhibit gastric cancer progression. Biochem. Pharmacol. 2021, 188, 114539. [Google Scholar] [CrossRef]
- Wang, Z.N.; Xu, H.M. Relationship between collagen IV expression and biological behavior of gastric cancer. World J. Gastroenterol. 2000, 6, 438–439. [Google Scholar] [CrossRef]
- Cui, X.; Shan, T.; Qiao, L. Collagen type IV alpha 1 (COL4A1) silence hampers the invasion, migration and epithelial–mesenchymal transition (EMT) of gastric cancer cells through blocking Hedgehog signaling pathway. Bioengineered 2022, 13, 8972–8981. [Google Scholar] [CrossRef]
- Xu, Y.; Jin, H.; Chen, Y.; Yang, Z.; Xu, D.; Zhang, X.; Yang, J.; Wang, Y. Comprehensive analysis of the expression, prognostic, and immune infiltration for COL4s in stomach adenocarcinoma. BMC Med. Genom. 2024, 17, 168. [Google Scholar] [CrossRef]
- Qian, X.; Jia, W.; Li, Y.; Chen, J.; Zhang, J.; Sun, Y. COL4A1 Promotes Gastric Cancer Progression by Regulating Tumor Invasion, Tumor Microenvironment and Drug Sensitivity. Curr. Med. Chem. 2025, 32, 1–19. [Google Scholar] [CrossRef]
- Tan, Y.; Chen, Q.; Xing, Y.; Zhang, C.; Pan, S.; An, W.; Xu, H. High expression of COL5A2, a member of COL5 family, indicates the poor survival and facilitates cell migration in gastric cancer. Biosci. Rep. 2021, 41. [Google Scholar] [CrossRef]
- Shen, H.; Wang, L.; Chen, Q.; Xu, J.; Zhang, J.; Fang, L.; Wang, J.; Fan, W. The prognostic value of COL3A1/FBN1/COL5A2/SPARC-mir-29a-3p-H19 associated ceRNA network in Gastric Cancer through bioinformatic exploration. J. Cancer 2020, 11, 4933–4946. [Google Scholar] [CrossRef]
- Xie, X.; Liu, X.; Zhang, Q.; Yu, J. Overexpression of collagen VI α3 in gastric cancer. Oncol. Lett. 2014, 7, 1537–1543. [Google Scholar] [CrossRef]
- Oh, S.E.; Oh, M.Y.; An, J.Y.; Lee, J.H.; Sohn, T.S.; Bae, J.M.; Choi, M.G.; Kim, K.M. Prognostic Value of Highly Expressed Type VII Collagen (COL7A1) in Patients with Gastric Cancer. Pathol. Oncol. Res. 2021, 27, 1609860. [Google Scholar] [CrossRef]
- Shen, N.; Zhu, S.; Zhang, Z.; Yong, X. High Expression of COL10A1 Is an Independent Predictive Poor Prognostic Biomarker and Associated with Immune Infiltration in Advanced Gastric Cancer Microenvironment. J. Oncol. 2022, 2022, 1463316. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Huang, H.; Shi, G.; Zhao, L.; Li, T.; Zhang, Z.; Liu, R.; Hu, Y.; Liu, H.; Yu, J.; et al. TGF-β1-SOX9 axis-inducible COL10A1 promotes invasion and metastasis in gastric cancer via epithelial-to-mesenchymal transition. Cell Death Dis. 2018, 9, 849. [Google Scholar] [CrossRef]
- Chen, S.; Wei, Y.; Liu, H.; Gong, Y.; Zhou, Y.; Yang, H.; Tang, L. Analysis of Collagen type X alpha 1 (COL10A1) expression and prognostic significance in gastric cancer based on bioinformatics. Bioengineered 2021, 12, 127–137. [Google Scholar] [CrossRef]
- Salimian, N.; Peymani, M.; Ghaedi, K.; Hashemi, M.; Rahimi, E. Collagen 1A1 (COL1A1) and Collagen11A1(COL11A1) as diagnostic biomarkers in Breast, colorectal and gastric cancers. Gene 2024, 892, 147867. [Google Scholar] [CrossRef] [PubMed]
- Sangaletti, S.; Chiodoni, C.; Tripodo, C.; Colombo, M.P. The good and bad of targeting cancer-associated extracellular matrix. Curr. Opin. Pharmacol. 2017, 35, 75–82. [Google Scholar] [CrossRef]
- Li, J.; Wang, X.; Zheng, K.; Liu, Y.; Li, J.; Wang, S.; Liu, K.; Song, X.; Li, N.; Xie, S.; et al. The clinical significance of collagen family gene expression in esophageal squamous cell carcinoma. PeerJ 2019, 7, e7705. [Google Scholar] [CrossRef]
- Revell, C.K.; Jensen, O.E.; Shearer, T.; Lu, Y.; Holmes, D.F.; Kadler, K.E. Collagen fibril assembly: New approaches to unanswered questions. Matrix Biol. Plus 2021, 12, 100079. [Google Scholar] [CrossRef]
- Uzawa, K.; Kasamatsu, A.; Yamauchi, M. Collagen cross-linking in oral cancer. Oral. Sci. Int. 2024, 21, 3–14. [Google Scholar] [CrossRef]
- Reiser, K.M.; Amigable, M.A.; Last, J.A. Nonenzymatic glycation of type I collagen. The effects of aging on preferential glycation sites. J. Biol. Chem. 1992, 267, 24207–24216. [Google Scholar] [CrossRef] [PubMed]
- Gautieri, A.; Redaelli, A.; Buehler, M.J.; Vesentini, S. Age-and diabetes-related nonenzymatic crosslinks in collagen fibrils: Candidate amino acids involved in advanced glycation end-products. Matrix Biol. 2014, 34, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Gaar, J.; Naffa, R.; Brimble, M. Enzymatic and non-enzymatic crosslinks found in collagen and elastin and their chemical synthesis. Org. Chem. Front. 2020, 7, 2789–2814. [Google Scholar] [CrossRef]
- Huerta-López, C.; Clemente-Manteca, A.; Velázquez-Carreras, D.; Espinosa, F.M.; Sanchez, J.G.; Martínez-Del-Pozo, Á.; García-García, M.; Martín-Colomo, S.; Rodríguez-Blanco, A.; Esteban-González, R.; et al. Cell response to extracellular matrix viscous energy dissipation outweighs high-rigidity sensing. Sci. Adv. 2024, 10, eadf9758. [Google Scholar] [CrossRef]
- Xu, Y.J.; Gong, H.L.; Hu, B.; Hu, B. Role of "Stiff Rim" sign obtained by shear wave elastography in diagnosis and guiding therapy of breast cancer. Int. J. Med. Sci. 2021, 18, 3615–3623. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Todd, L.; Huang, L.; Noguera-Ortega, E.; Lu, Z.; Huang, L.; Kopp, M.; Li, Y.; Pattada, N.; Zhong, W.; et al. Desmoplastic stroma restricts T cell extravasation and mediates immune exclusion and immunosuppression in solid tumors. Nat. Commun. 2023, 14, 5110. [Google Scholar] [CrossRef] [PubMed]
- Jang, M.; An, J.; Oh, S.W.; Lim, J.Y.; Kim, J.; Choi, J.K.; Cheong, J.H.; Kim, P. Matrix stiffness epigenetically regulates the oncogenic activation of the Yes-associated protein in gastric cancer. Nat. Biomed. Eng. 2021, 5, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Deng, B.; Zhao, Z.; Kong, W.; Han, C.; Shen, X.; Zhou, C. Biological role of matrix stiffness in tumor growth and treatment. J. Transl. Med. 2022, 20, 540. [Google Scholar] [CrossRef]
- Wang, Z.; Fan, G.; Zhu, H.; Yu, L.; She, D.; Wei, Y.; Huang, J.; Li, T.; Zhan, S.; Zhou, S.; et al. PLOD2 high expression associates with immune infiltration and facilitates cancer progression in osteosarcoma. Front. Oncol. 2022, 12, 980390. [Google Scholar] [CrossRef]
- Liu, D.; Shi, K.; Fu, M.; Chen, F. Melatonin indirectly decreases gastric cancer cell proliferation and invasion via effects on cancer-associated fibroblasts. Life Sci. 2021, 277, 119497. [Google Scholar] [CrossRef]
- Bonnans, C.; Chou, J.; Werb, Z. Remodelling the extracellular matrix in development and disease. Nat. Rev. Mol. Cell Biol. 2014, 15, 786–801. [Google Scholar] [CrossRef]
- Xiao, Z.; Puré, E. The fibroinflammatory response in cancer. Nat. Rev. Cancer 2025, 25, 399–425. [Google Scholar] [CrossRef]
- Xi, G.; Qiu, L.; Xu, S.; Guo, W.; Fu, F.; Kang, D.; Zheng, L.; He, J.; Zhang, Q.; Li, L.; et al. Computer-assisted quantification of tumor-associated collagen signatures to improve the prognosis prediction of breast cancer. BMC Med. 2021, 19, 273. [Google Scholar] [CrossRef]
- Sutherland, T.E.; Dyer, D.P.; Allen, J.E. The extracellular matrix and the immune system: A mutually dependent relationship. Science 2023, 379, eabp8964. [Google Scholar] [CrossRef]
- Tvaroška, I. Glycosylation Modulates the Structure and Functions of Collagen: A Review. Molecules 2024, 29, 1417. [Google Scholar] [CrossRef]
- Borst, R.; Meyaard, L.; Pascoal Ramos, M.I. Understanding the matrix: Collagen modifications in tumors and their implications for immunotherapy. J. Transl. Med. 2024, 22, 382. [Google Scholar] [CrossRef]
- Inoue, T.; Yashiro, M.; Nishimura, S.; Maeda, K.; Sawada, T.; Ogawa, Y.; Sowa, M.; Chung, K.H. Matrix metalloproteinase-1 expression is a prognostic factor for patients with advanced gastric cancer. Int. J. Mol. Med. 1999, 4, 73–77. [Google Scholar] [CrossRef]
- Añazco, C.; Delgado-López, F.; Araya, P.; González, I.; Morales, E.; Pérez-Castro, R.; Romero, J.; Rojas, A. Lysyl Oxidase Isoforms in Gastric Cancer. Biomark. Med. 2016, 10, 987–998. [Google Scholar] [CrossRef]
- Tong, Y.; Qi, Y.; Xiong, G.; Li, J.; Scott, T.L.; Chen, J.; He, D.; Li, L.; Wang, C.; Lane, A.N.; et al. The PLOD2/succinate axis regulates the epithelial–mesenchymal plasticity and cancer cell stemness. Proc. Natl. Acad. Sci. USA 2023, 120, e2214942120. [Google Scholar] [CrossRef]
- Du, H.; Chen, Y.; Hou, X.; Huang, Y.; Wei, X.; Yu, X.; Feng, S.; Wu, Y.; Zhan, M.; Shi, X.; et al. PLOD2 regulated by transcription factor FOXA1 promotes metastasis in NSCLC. Cell Death Dis. 2017, 8, e3143. [Google Scholar] [CrossRef]
- Kang, H.; Strong, A.L.; Sun, Y.; Guo, L.; Juan, C.; Bancroft, A.C.; Choi, J.H.; Pagani, C.A.; Fernandes, A.A.; Woodard, M.; et al. The HIF-1α/PLOD2 axis integrates extracellular matrix organization and cell metabolism leading to aberrant musculoskeletal repair. Bone Res. 2024, 12, 17. [Google Scholar] [CrossRef] [PubMed]
- Jafari, E.; Safinejad, S.; Dabiri, S.; Naghibzadeh-Tahami, A. Study of the Relationship between MMP-2 and MMP-9 and Her2/neu Overexpression in Gastric Cancer: Clinico-Pathological Correlations. Asian Pac. J. Cancer Prev. 2021, 22, 811–817. [Google Scholar] [CrossRef] [PubMed]
- Prathipaa, R.; Priyathersini, N.; Thanka, J. Expression of Matrix Metalloproteinase-9 in Gastric Cancer. Cureus 2021, 13, e18195. [Google Scholar] [CrossRef]
- Kubben, F.J.G.M.; Sier, C.F.M.; van Duijn, W.; Griffioen, G.; Hanemaaijer, R.; van de Velde, C.J.H.; van Krieken, J.H.J.M.; Lamers, C.B.H.W.; Verspaget, H.W. Matrix metalloproteinase-2 is a consistent prognostic factor in gastric cancer. Br. J. Cancer 2006, 94, 1035–1040. [Google Scholar] [CrossRef] [PubMed]
- Kasashima, H.; Yashiro, M.; Kinoshita, H.; Fukuoka, T.; Morisaki, T.; Masuda, G.; Sakurai, K.; Kubo, N.; Ohira, M.; Hirakawa, K. Lysyl oxidase is associated with the epithelial–mesenchymal transition of gastric cancer cells in hypoxia. Gastric Cancer 2016, 19, 431–442. [Google Scholar] [CrossRef]
- Zhao, L.; Niu, H.; Liu, Y.; Wang, L.; Zhang, N.; Zhang, G.; Liu, R.; Han, M. LOX inhibition downregulates MMP-2 and MMP-9 in gastric cancer tissues and cells. J. Cancer 2019, 10, 6481–6490. [Google Scholar] [CrossRef]
- Gonzalez-Avila, G.; Sommer, B.; Mendoza-Posada, D.A.; Ramos, C.; Garcia-Hernandez, A.A.; Falfan-Valencia, R. Matrix metalloproteinases participation in the metastatic process and their diagnostic and therapeutic applications in cancer. Crit. Rev. Oncol./Hematol. 2019, 137, 57–83. [Google Scholar] [CrossRef] [PubMed]
- Cetin, M.; Saatci, O.; Rezaeian, A.-H.; Rao, C.N.; Beneker, C.; Sreenivas, K.; Taylor, H.; Pederson, B.; Chatzistamou, I.; Buckley, B.; et al. A highly potent bi-thiazole inhibitor of LOX rewires collagen architecture and enhances chemoresponse in triple-negative breast cancer. Cell Chem. Biol. 2024, 31, 1926–1941.e1911. [Google Scholar] [CrossRef]
- Lan, J.; Zhang, S.; Zheng, L.; Long, X.; Chen, J.; Liu, X.; Zhou, M.; Zhou, J. PLOD2 promotes colorectal cancer progression by stabilizing USP15 to activate the AKT/mTOR signaling pathway. Cancer Sci. 2023, 114, 3190–3202. [Google Scholar] [CrossRef]
- Yue, W.; Zhang, H.; Gao, Y.; Ding, J.; Xue, R.; Dong, C.; Liu, F.; Yang, L.; Yang, L.; Li, L. Procollagen-lysine 2-oxoglutarate 5-dioxygenase 2 promotes collagen cross-linking and ECM stiffening to induce liver fibrosis. Biochim. Biophys. Acta Mol. Basis Dis. 2024, 1870, 167205. [Google Scholar] [CrossRef]
- Di, W.Y.; Kang, X.H.; Zhang, J.H.; Wang, Y.; Kou, W.Z.; Su, W. Expression of PLOD2 in esophageal squamous cell carcinoma and its correlation with invasion and metastasis. Zhonghua Bing. Li Xue Za Zhi 2019, 48, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Pang, M.; Hou, X.; Yuan, S.; Sun, L. PLOD2 in cancer research. Biomed. Pharmacother. 2017, 90, 670–676. [Google Scholar] [CrossRef]
- Xu, F.; Zhang, J.; Hu, G.; Liu, L.; Liang, W. Hypoxia and TGF-β1 induced PLOD2 expression improve the migration and invasion of cervical cancer cells by promoting epithelial-to-mesenchymal transition (EMT) and focal adhesion formation. Cancer Cell Int. 2017, 17, 54. [Google Scholar] [CrossRef] [PubMed]
- Li, S.S.; Lian, Y.F.; Huang, Y.L.; Huang, Y.H.; Xiao, J. Overexpressing PLOD family genes predict poor prognosis in gastric cancer. J. Cancer 2020, 11, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Kiyozumi, Y.; Iwatsuki, M.; Kurashige, J.; Ogata, Y.; Yamashita, K.; Koga, Y.; Toihata, T.; Hiyoshi, Y.; Ishimoto, T.; Baba, Y.; et al. PLOD2 as a potential regulator of peritoneal dissemination in gastric cancer. Int. J. Cancer 2018, 143, 1202–1211. [Google Scholar] [CrossRef]
- Yamauchi, M.; Sricholpech, M. Lysine post-translational modifications of collagen. Essays Biochem. 2012, 52, 113–133. [Google Scholar] [CrossRef]
- Chen, W.; Yang, A.; Jia, J.; Popov, Y.V.; Schuppan, D.; You, H. Lysyl Oxidase (LOX) Family Members: Rationale and Their Potential as Therapeutic Targets for Liver Fibrosis. Hepatology 2020, 72, 729–741. [Google Scholar] [CrossRef]
- Kasashima, H.; Yashiro, M.; Okuno, T.; Miki, Y.; Kitayama, K.; Masuda, G.; Kinoshita, H.; Morisaki, T.; Fukuoka, T.; Hasegawa, T.; et al. Significance of the Lysyl Oxidase Members Lysyl Oxidase Like 1, 3, and 4 in Gastric Cancer. Digestion 2018, 98, 238–248. [Google Scholar] [CrossRef]
- Wu, L.; Zhu, Y. The function and mechanisms of action of LOXL2 in cancer (Review). Int. J. Mol. Med. 2015, 36, 1200–1204. [Google Scholar] [CrossRef]
- Zhu, J.; Luo, C.; Zhao, J.; Zhu, X.; Lin, K.; Bu, F.; Yu, Z.; Zou, F.; Zhu, Z. Expression of LOX Suggests Poor Prognosis in Gastric Cancer. Front Med 2021, 8, 718986. [Google Scholar] [CrossRef]
- Ma, L.J.; Li, Y.G.; Huang, L.; Han, M.; Ma, B.J.; Sun, B.J.; Lin, J.J.; Song, T.G. Expression of LOX and MMP-2 in gastric cancer tissue and the effects of LOX and MMP-2 on tumor invasion and metastasis. Zhonghua Zhong Liu Za Zhi 2011, 33, 37–41. [Google Scholar] [PubMed]
- Hu, Q.; Masuda, T.; Kuramitsu, S.; Tobo, T.; Sato, K.; Kidogami, S.; Nambara, S.; Ueda, M.; Tsuruda, Y.; Kuroda, Y.; et al. Potential association of LOXL1 with peritoneal dissemination in gastric cancer possibly via promotion of EMT. PLoS ONE 2020, 15, e0241140. [Google Scholar] [CrossRef] [PubMed]
- Cano, A.; Santamaría, P.G.; Moreno-Bueno, G. LOXL2 in epithelial cell plasticity and tumor progression. Future Oncol. 2012, 8, 1095–1108. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wu, X.; Sun, Y.; Chu, Y.; Liu, F.; Chen, C. TRIM44 regulates tumor immunity in gastric cancer through LOXL2-dependent extracellular matrix remodeling. Cell Oncol. 2023, 46, 423–435. [Google Scholar] [CrossRef]
- Nishioka, T.; Eustace, A.; West, C. Lysyl oxidase: From basic science to future cancer treatment. Cell Struct. Funct. 2012, 37, 75–80. [Google Scholar] [CrossRef]
- Mayorca-Guiliani, A.; Erler, J.T. The potential for targeting extracellular LOX proteins in human malignancy. Onco Targets Ther. 2013, 6, 1729–1735. [Google Scholar] [CrossRef]
- Görögh, T.; Weise, J.B.; Holtmeier, C.; Rudolph, P.; Hedderich, J.; Gottschlich, S.; Hoffmann, M.; Ambrosch, P.; Csiszar, K. Selective upregulation and amplification of the lysyl oxidase like-4 (LOXL4) gene in head and neck squamous cell carcinoma. J. Pathol. 2007, 212, 74–82. [Google Scholar] [CrossRef]
- Weise, J.B.; Rudolph, P.; Heiser, A.; Kruse, M.-L.; Hedderich, J.; Cordes, C.; Hoffmann, M.; Brant, O.; Ambrosch, P.; Csiszar, K.; et al. LOXL4 is a selectively expressed candidate diagnostic antigen in head and neck cancer. Eur. J. Cancer 2008, 44, 1323–1331. [Google Scholar] [CrossRef] [PubMed]
- Li, R.K.; Zhao, W.Y.; Fang, F.; Zhuang, C.; Zhang, X.X.; Yang, X.M.; Jiang, S.H.; Kong, F.Z.; Tu, L.; Zhang, W.M.; et al. Lysyl oxidase-like 4 (LOXL4) promotes proliferation and metastasis of gastric cancer via FAK/Src pathway. J. Cancer Res. Clin. Oncol. 2015, 141, 269–281. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chen, C.; Huang, J.; Xie, Z.; Chen, X.; Zheng, Z.; Li, E.; Zou, H. The possibilities of LOXL4 as a prognostic marker for carcinomas. Amino Acids 2023, 55, 1519–1529. [Google Scholar] [CrossRef]
- Shan, Y.Q.; Ying, R.C.; Zhou, C.H.; Zhu, A.K.; Ye, J.; Zhu, W.; Ju, T.F.; Jin, H.C. MMP-9 is increased in the pathogenesis of gastric cancer by the mediation of HER2. Cancer Gene Ther. 2015, 22, 101–107. [Google Scholar] [CrossRef]
- Murray, G.I.; Duncan, M.E.; Arbuckle, E.; Melvin, W.T.; Fothergill, J.E. Matrix metalloproteinases and their inhibitors in gastric cancer. Gut 1998, 43, 791–797. [Google Scholar] [CrossRef]
- Xu, J.; E, C.; Yao, Y.; Ren, S.; Wang, G.; Jin, H. Matrix metalloproteinase expression and molecular interaction network analysis in gastric cancer. Oncol. Lett. 2016, 12, 2403–2408. [Google Scholar] [CrossRef]
- Kessenbrock, K.; Plaks, V.; Werb, Z. Matrix metalloproteinases: Regulators of the tumor microenvironment. Cell 2010, 141, 52–67. [Google Scholar] [CrossRef] [PubMed]
- Dalberg, K.; Eriksson, E.; Enberg, U.; Kjellman, M.; Bäckdahl, M. Gelatinase A, membrane type 1 matrix metalloproteinase, and extracellular matrix metalloproteinase inducer mRNA expression: Correlation with invasive growth of breast cancer. World J. Surg. 2000, 24, 334–340. [Google Scholar] [CrossRef]
- Mu, X.; Fan, Y.; Xu, J.; Xie, R. Exploration of the optimal regimen of gastric mucosal cleansing medication for the H. pylori population before ME-NBI screening: Study protocol for a single-center, single-blind, randomized controlled trial. Front Med 2025, 12, 1516271. [Google Scholar] [CrossRef]
- Sokolova, O.; Naumann, M. Matrix Metalloproteinases in Helicobacter pylori–Associated Gastritis and Gastric Cancer. Int. J. Mol. Sci. 2022, 23, 1883. [Google Scholar] [CrossRef] [PubMed]
- Juric, V.; O’Sullivan, C.; Stefanutti, E.; Kovalenko, M.; Greenstein, A.; Barry-Hamilton, V.; Mikaelian, I.; Degenhardt, J.; Yue, P.; Smith, V. MMP-9 inhibition promotes anti-tumor immunity through disruption of biochemical and physical barriers to T-cell trafficking to tumors. PLoS ONE 2018, 13, e0207255. [Google Scholar] [CrossRef] [PubMed]
- Quintero-Fabián, S.; Arreola, R.; Becerril-Villanueva, E.; Torres-Romero, J.C.; Arana-Argáez, V.; Lara-Riegos, J.; Ramírez-Camacho, M.A.; Alvarez-Sánchez, M.E. Role of Matrix Metalloproteinases in Angiogenesis and Cancer. Front. Oncol. 2019, 9, 1370. [Google Scholar] [CrossRef]
- Twarda-Clapa, A.; Olczak, A.; Białkowska, A.M.; Koziołkiewicz, M. Advanced Glycation End-Products (AGEs): Formation, Chemistry, Classification, Receptors, and Diseases Related to AGEs. Cells 2022, 11, 1312. [Google Scholar] [CrossRef]
- Haque, E.; Kamil, M.; Hasan, A.; Irfan, S.; Sheikh, S.; Khatoon, A.; Nazir, A.; Mir, S.S. Advanced glycation end products (AGEs), protein aggregation and their cross talk: New insight in tumorigenesis. Glycobiology 2019, 30, 49–57. [Google Scholar] [CrossRef] [PubMed]
- van der Lugt, T.; Opperhuizen, A.; Bast, A.; Vrolijk, M.F. Dietary Advanced Glycation Endproducts and the Gastrointestinal Tract. Nutrients 2020, 12, 2814. [Google Scholar] [CrossRef]
- Wulf, H.C.; Sandby-Møller, J.; Kobayasi, T.; Gniadecki, R. Skin aging and natural photoprotection. Micron 2004, 35, 185–191. [Google Scholar] [CrossRef]
- Naomi, R.; Ridzuan, P.M.; Bahari, H. Current Insights into Collagen Type I. Polymers 2021, 13, 2642. [Google Scholar] [CrossRef]
- Said, G.; Guilbert, M.; Millerot-Serrurot, E.; Van Gulick, L.; Terryn, C.; Garnotel, R.; Jeannesson, P. Impact of carbamylation and glycation of collagen type I on migration of HT1080 human fibrosarcoma cells. Int. J. Oncol. 2012, 40, 1797–1804. [Google Scholar] [CrossRef][Green Version]
- Bartling, B.; Desole, M.; Rohrbach, S.; Silber, R.E.; Simm, A. Age-associated changes of extracellular matrix collagen impair lung cancer cell migration. Faseb J. 2009, 23, 1510–1520. [Google Scholar] [CrossRef] [PubMed]
- Morita, K.; Urabe, K.; Moroi, Y.; Koga, T.; Nagai, R.; Horiuchi, S.; Furue, M. Migration of keratinocytes is impaired on glycated collagen I. Wound Repair. Regen. 2005, 13, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Bansode, S.; Bashtanova, U.; Li, R.; Clark, J.; Müller, K.H.; Puszkarska, A.; Goldberga, I.; Chetwood, H.H.; Reid, D.G.; Colwell, L.J.; et al. Glycation changes molecular organization and charge distribution in type I collagen fibrils. Sci. Rep. 2020, 10, 3397. [Google Scholar] [CrossRef]
- Jang, M.; Oh, S.W.; Lee, Y.; Kim, J.Y.; Ji, E.S.; Kim, P. Targeting extracellular matrix glycation to attenuate fibroblast activation. Acta Biomater. 2022, 141, 255–263. [Google Scholar] [CrossRef]
- Sloseris, D.; Forde, N.R. AGEing of collagen: The effects of glycation on collagen’s stability, mechanics and assembly. Matrix Biol. 2025, 135, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Suh, Y.J.; Hall, M.S.; Huang, Y.L.; Moon, S.Y.; Song, W.; Ma, M.; Bonassar, L.J.; Segall, J.E.; Wu, M. Glycation of collagen matrices promotes breast tumor cell invasion. Integr Biol 2019, 11, 109–117. [Google Scholar] [CrossRef]
- Radziejewska, I.; Supruniuk, K.; Nazaruk, J.; Karna, E.; Popławska, B.; Bielawska, A.; Galicka, A. Rosmarinic acid influences collagen, MMPs, TIMPs, glycosylation and MUC1 in CRL-1739 gastric cancer cell line. Biomed. Pharmacother. 2018, 107, 397–407. [Google Scholar] [CrossRef]
- Kuniyasu, H.; Oue, N.; Wakikawa, A.; Shigeishi, H.; Matsutani, N.; Kuraoka, K.; Ito, R.; Yokozaki, H.; Yasui, W. Expression of receptors for advanced glycation end-products (RAGE) is closely associated with the invasive and metastatic activity of gastric cancer. J. Pathol. 2002, 196, 163–170. [Google Scholar] [CrossRef]
- Pinho, S.S.; Carvalho, S.; Marcos-Pinto, R.; Magalhães, A.; Oliveira, C.; Gu, J.; Dinis-Ribeiro, M.; Carneiro, F.; Seruca, R.; Reis, C.A. Gastric cancer: Adding glycosylation to the equation. Trends Mol. Med. 2013, 19, 664–676. [Google Scholar] [CrossRef]
- Yakubov, G.E.; Papagiannopoulos, A.; Rat, E.; Easton, R.L.; Waigh, T.A. Molecular structure and rheological properties of short-side-chain heavily glycosylated porcine stomach mucin. Biomacromolecules 2007, 8, 3467–3477. [Google Scholar] [CrossRef] [PubMed]
- Rojas, A.; Añazco, C.; González, I.; Araya, P. Extracellular matrix glycation and receptor for advanced glycation end-products activation: A missing piece in the puzzle of the association between diabetes and cancer. Carcinogenesis 2018, 39, 515–521. [Google Scholar] [CrossRef]
- Pennarossa, G.; Arcuri, S.; De Iorio, T.; Gandolfi, F.; Brevini, T.A.L. Current Advances in 3D Tissue and Organ Reconstruction. Int. J. Mol. Sci. 2021, 22, 830. [Google Scholar] [CrossRef]
- Neal, J.T.; Li, X.; Zhu, J.; Giangarra, V.; Grzeskowiak, C.L.; Ju, J.; Liu, I.H.; Chiou, S.-H.; Salahudeen, A.A.; Smith, A.R.; et al. Organoid Modeling of the Tumor Immune Microenvironment. Cell 2018, 175, 1972–1988.e1916. [Google Scholar] [CrossRef]
- Varinelli, L.; Guaglio, M.; Brich, S.; Zanutto, S.; Belfiore, A.; Zanardi, F.; Iannelli, F.; Oldani, A.; Costa, E.; Chighizola, M.; et al. Decellularized extracellular matrix as scaffold for cancer organoid cultures of colorectal peritoneal metastases. J. Mol. Cell Biol. 2023, 14, mjac064. [Google Scholar] [CrossRef] [PubMed]
- Gvaramia, D.; Kern, J.; Jakob, Y.; Tritschler, H.; Brenner, R.E.; Breiter, R.; Kzhyshkowska, J.; Rotter, N. Modulation of the inflammatory response to decellularized collagen matrix for cartilage regeneration. J. Biomed. Mater. Res. A 2022, 110, 1021–1035. [Google Scholar] [CrossRef] [PubMed]
- Deng, M.; Xie, P.; Xue, H.; Chen, Q.; Zhou, Y.; Ming, J.; Ma, Y.; Liu, J.; Huang, H. Decellularized tissue matrices hydrogels functionalized with extracellular vesicles promote macrophage reprogramming and neural stem cell differentiation for spinal cord injury repair. J. Nanobiotechnol. 2025, 23, 139. [Google Scholar] [CrossRef]
- Lee, J.J.; Ng, K.Y.; Bakhtiar, A. Extracellular matrix: Unlocking new avenues in cancer treatment. Biomark. Res. 2025, 13, 78. [Google Scholar] [CrossRef]
- Isaeva, E.V.; Beketov, E.E.; Arguchinskaya, N.V.; Ivanov, S.; Shegay, P.V.; Kaprin, A.D. Decellularized Extracellular Matrix for Tissue Engineering (Review). Sovrem. Tekhnologii Med. 2022, 14, 57–68. [Google Scholar] [CrossRef]
- Hoshiba, T. Decellularized Extracellular Matrix for Cancer Research. Materials 2019, 12, 1311. [Google Scholar] [CrossRef]
- Dehghani, S.; Aghaee, Z.; Soleymani, S.; Tafazoli, M.; Ghabool, Y.; Tavassoli, A. An overview of the production of tissue extracellular matrix and decellularization process. Cell Tissue Bank. 2024, 25, 369–387. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, J.; Kankala, R.K.; Jiang, M.; Long, L.; Li, W.; Zou, L.; Chen, A.; Liu, Y. Decellularized extracellular matrix-based disease models for drug screening. Mater. Today Bio 2024, 29, 101280. [Google Scholar] [CrossRef]
- Kim, B.S.; Das, S.; Jang, J.; Cho, D.-W. Decellularized Extracellular Matrix-based Bioinks for Engineering Tissue- and Organ-specific Microenvironments. Chem. Rev. 2020, 120, 10608–10661. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, T.W.; Wognum, S.; Joyce, E.M.; Freytes, D.O.; Sacks, M.S.; Badylak, S.F. Collagen fiber alignment and biaxial mechanical behavior of porcine urinary bladder derived extracellular matrix. Biomaterials 2008, 29, 4775–4782. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Xu, Y.; Luo, S.; Dang, H.; Cao, M. Effect of cryoprotectants on rat kidney decellularization by freeze-thaw process. Cryobiology 2022, 105, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-H.; Hsia, K.; Su, C.-K.; Chen, C.-C.; Yeh, C.-C.; Ma, H.; Lu, J.-H. Sonication-Assisted Method for Decellularization of Human Umbilical Artery for Small-Caliber Vascular Tissue Engineering. Polymers 2021, 13, 1699. [Google Scholar] [CrossRef]
- Seo, Y.; Jung, Y.; Kim, S.H. Decellularized heart ECM hydrogel using supercritical carbon dioxide for improved angiogenesis. Acta Biomater. 2018, 67, 270–281. [Google Scholar] [CrossRef]
- Freytes, D.O.; Martin, J.; Velankar, S.S.; Lee, A.S.; Badylak, S.F. Preparation and rheological characterization of a gel form of the porcine urinary bladder matrix. Biomaterials 2008, 29, 1630–1637. [Google Scholar] [CrossRef]
- Liu, G.; Wang, B.; Li, S.; Jin, Q.; Dai, Y. Human breast cancer decellularized scaffolds promote epithelial-to-mesenchymal transitions and stemness of breast cancer cells in vitro. J. Cell. Physiol. 2019, 234, 9447–9456. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Wang, H.; Li, G.; Zhao, B. Three-dimensional decellularized tumor extracellular matrices with different stiffness as bioengineered tumor scaffolds. Bioact. Mater. 2021, 6, 2767–2782. [Google Scholar] [CrossRef]
- Leiva, M.C.; Garre, E.; Gustafsson, A.; Svanström, A.; Bogestål, Y.; Håkansson, J.; Ståhlberg, A.; Landberg, G. Breast cancer patient-derived scaffolds as a tool to monitor chemotherapy responses in human tumor microenvironments. J. Cell Physiol. 2021, 236, 4709–4724. [Google Scholar] [CrossRef] [PubMed]
- Sensi, F.; D’Angelo, E.; Biccari, A.; Marangio, A.; Battisti, G.; Crotti, S.; Fassan, M.; Laterza, C.; Giomo, M.; Elvassore, N.; et al. Establishment of a human 3D pancreatic adenocarcinoma model based on a patient-derived extracellular matrix scaffold. Transl. Res. 2023, 253, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Seifi, Z.; Khazaei, M.; Cheraghali, D.; Rezakhani, L. Decellularized tissues as platforms for digestive system cancer models. Heliyon 2024, 10, e31589. [Google Scholar] [CrossRef]
- Chaitin, H.; Lu, M.L.; Wallace, M.B.; Kang, Y. Development of a Decellularized Porcine Esophageal Matrix for Potential Applications in Cancer Modeling. Cells 2021, 10, 1055. [Google Scholar] [CrossRef]
- Heydari, Z.; Devasahayam Arokia Balaya, R.; Sarkar, G.; Boardman, L. The Role of Organoids in Advancing Colorectal Cancer Research: Insights and Future Directions. Cancers 2025, 17, 2129. [Google Scholar] [CrossRef]
- Kim, J.; Jang, J.; Cho, D.-W. Controlling cancer cell behavior by improving the stiffness of gastric tissue-decellularized ECM bioink with cellulose nanoparticles. Front. Bioeng. Biotechnol. 2021, 9, 605819. [Google Scholar] [CrossRef] [PubMed]
- Bonnesœur, S.; Morin-Grognet, S.; Thoumire, O.; Le Cerf, D.; Boyer, O.; Vannier, J.P.; Labat, B. Hyaluronan-based hydrogels as versatile tumor-like models: Tunable ECM and stiffness with genipin-crosslinking. J. Biomed. Mater. Res. A 2020, 108, 1256–1268. [Google Scholar] [CrossRef]
- Shigeta, Y.; Saleh, T.; Benedetti, G.; Caciolli, L.; Chang, J.; Zambaiti, E.; Wu, L.; Khalaf, S.; Song, W.; Pellegata, A.F.; et al. Stomach engineering: Region-specific characterization of the decellularized porcine stomach. Pediatr. Surg. Int. 2023, 40, 13. [Google Scholar] [CrossRef]
- Theocharis, A.D.; Manou, D.; Karamanos, N.K. The extracellular matrix as a multitasking player in disease. FEBS J. 2019, 286, 2830–2869. [Google Scholar] [CrossRef]
- Hinshaw, D.C.; Shevde, L.A. The Tumor Microenvironment Innately Modulates Cancer Progression. Cancer Res. 2019, 79, 4557–4566. [Google Scholar] [CrossRef]
- He, Y.; Liu, T.; Dai, S.; Xu, Z.; Wang, L.; Luo, F. Tumor-Associated Extracellular Matrix: How to Be a Potential Aide to Anti-tumor Immunotherapy? Front. Cell Dev. Biol. 2021, 9, 739161. [Google Scholar] [CrossRef] [PubMed]
- Daliri, K.; Hescheler, J.; Newby, G.A.; Clement, K.; Liu, D.R.; Pfannkuche, K. Modulating Collagen I Expression in Fibroblasts by CRISPR-Cas9 Base Editing of the Collagen 1A1 Promoter. Int. J. Mol. Sci. 2025, 26, 3041. [Google Scholar] [CrossRef] [PubMed]

| Feature | dECM | Matrigel |
|---|---|---|
| Source | Derived from decellularized native tissues (e.g., lung, heart, liver) | Basement membrane extract from Engelbreth–Holm–Swarm (EHS) mouse sarcoma |
| Composition | Contains native ECM proteins (collagens, proteoglycans, etc.) specific to the tissue of origin | Primarily composed of laminin, collagen IV, heparan sulfate proteoglycans, and entactin |
| 3D Structure | Preserves the native 3D ultrastructure of the tissue, providing a more physiological environment | Forms a dense, non-fibrous gel with a reticular structure |
| Cellular interactions | Supports cell adhesion, migration, proliferation, and differentiation in a tissue-specific manner | Supports cell adhesion, spreading, and migration, primarily through interactions with integrins |
| Growth Factors | Contains growth factors present in the original tissue, which can be tissue-specific | Contains growth factors, but their concentrations and types can vary and may not be tissue-specific |
| Variability | Can exhibit batch-to-batch variability due to differences in donor tissues and decellularization protocols | Known for batch-to-batch variability due to its source |
| Cost | It can be more expensive than Matrigel, depending on the tissue source and decellularization method | Relatively inexpensive and readily available |
| Applications | Tissue engineering, regenerative medicine, organoid culture, drug screening, and disease modeling | Cell culture, 3D cell culture, angiogenesis assays, and in vitro studies |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Corro, N.; Alarcón, S.; Astroza, Á.; González-Stegmaier, R.; Añazco, C. Mimicking Gastric Cancer Collagen Reorganization with Decellularized ECM-Based Scaffolds. Biology 2025, 14, 1067. https://doi.org/10.3390/biology14081067
Corro N, Alarcón S, Astroza Á, González-Stegmaier R, Añazco C. Mimicking Gastric Cancer Collagen Reorganization with Decellularized ECM-Based Scaffolds. Biology. 2025; 14(8):1067. https://doi.org/10.3390/biology14081067
Chicago/Turabian StyleCorro, Néstor, Sebastián Alarcón, Ángel Astroza, Roxana González-Stegmaier, and Carolina Añazco. 2025. "Mimicking Gastric Cancer Collagen Reorganization with Decellularized ECM-Based Scaffolds" Biology 14, no. 8: 1067. https://doi.org/10.3390/biology14081067
APA StyleCorro, N., Alarcón, S., Astroza, Á., González-Stegmaier, R., & Añazco, C. (2025). Mimicking Gastric Cancer Collagen Reorganization with Decellularized ECM-Based Scaffolds. Biology, 14(8), 1067. https://doi.org/10.3390/biology14081067







