Targeting Ferroptosis: Emerging Insights into Osteoporosis Mechanisms
Abstract
Simple Summary
Abstract
1. Introduction
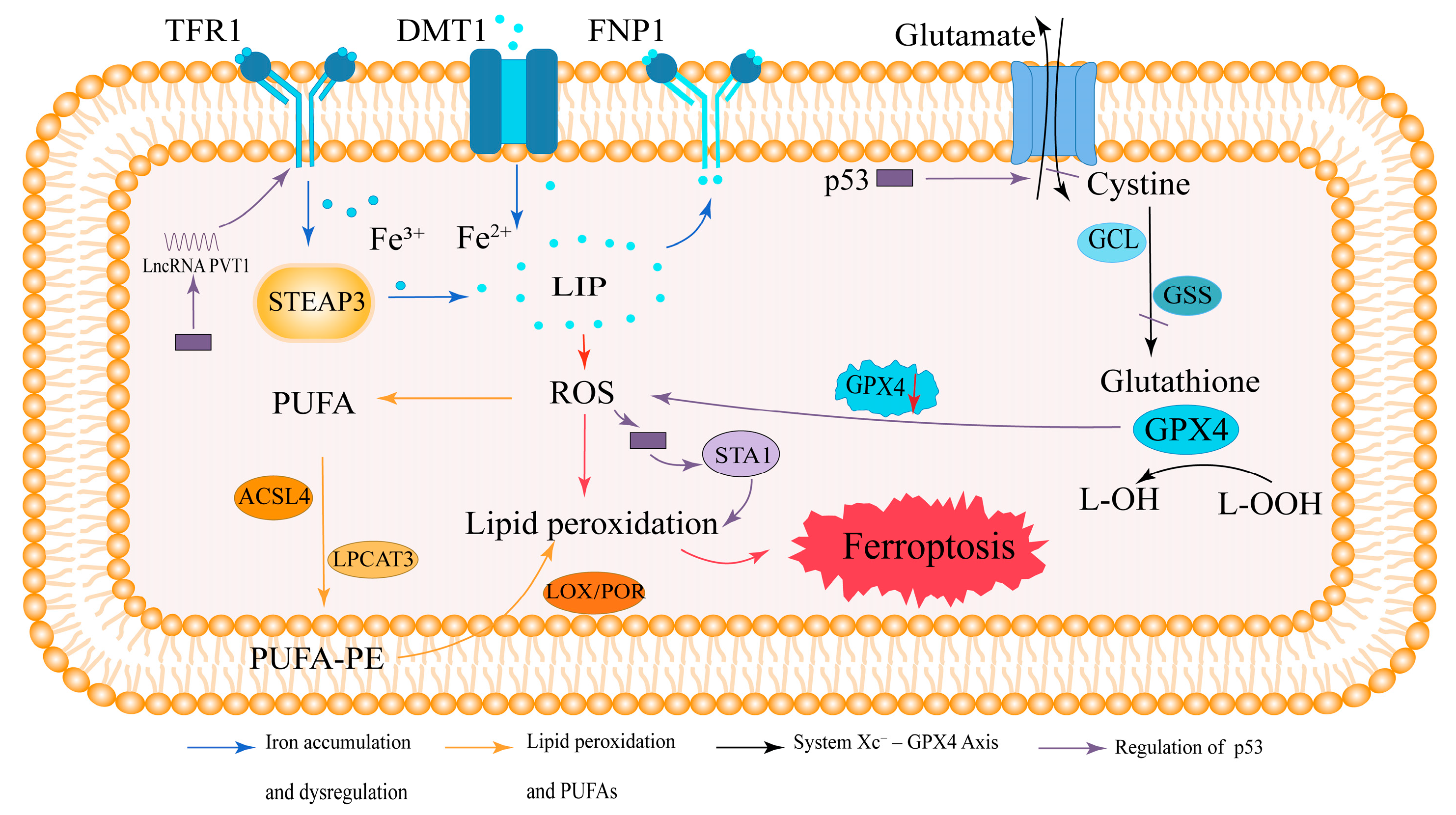
2. Overview of Ferroptosis
2.1. Iron Accumulation and Dysregulation in Ferroptosis
2.2. Lipid Peroxidation and Polyunsaturated Fatty Acids (PUFAs)
2.3. The System Xc−–GPX4 Axis in Antioxidant Defense
2.4. Regulation of Ferroptosis by p53
2.5. Summary of Ferroptosis Mechanisms
3. Pathogenesis of Osteoporosis
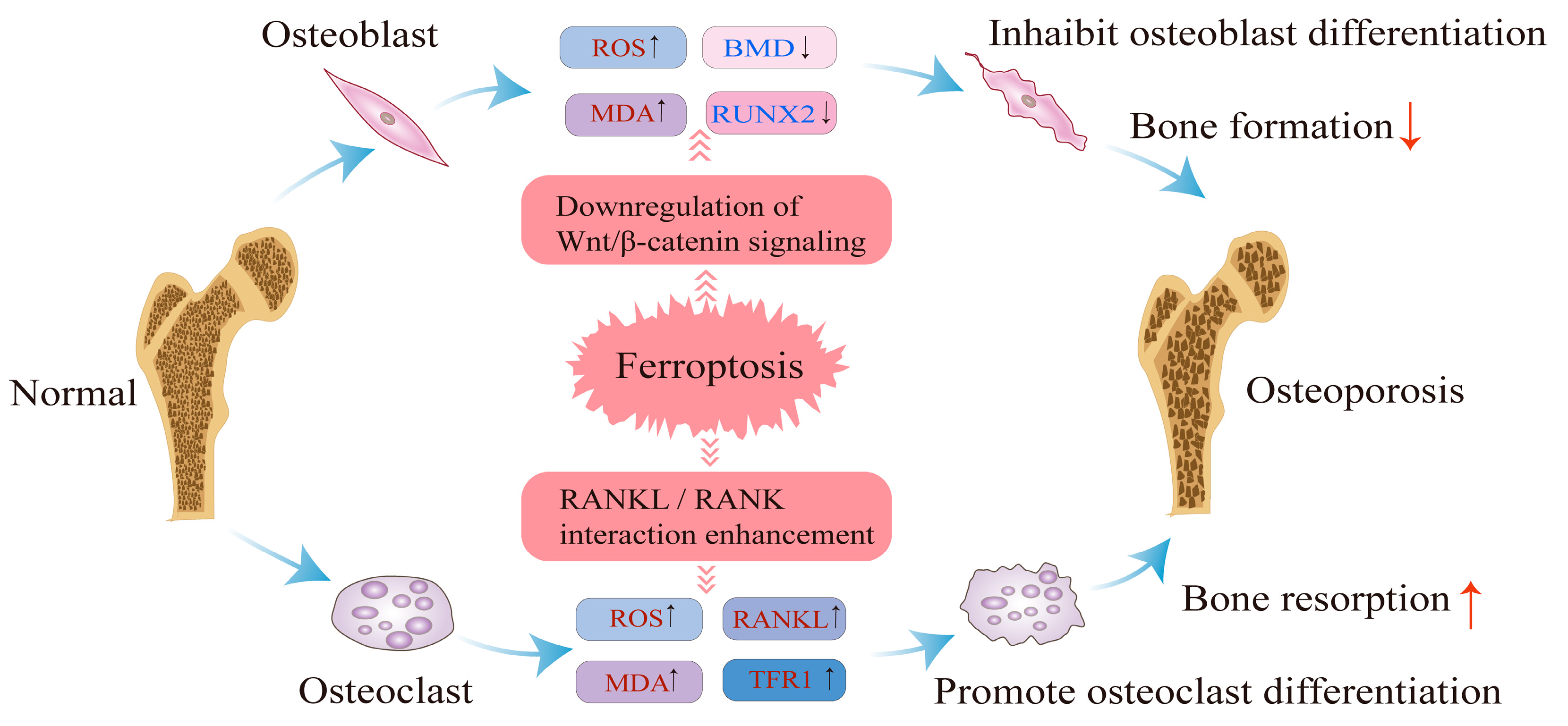
4. Regulatory Mechanisms of Ferroptosis in Osteoporosis
4.1. Impact of Ferroptosis on Osteoblast Differentiation and Bone Formation
4.2. Impact of Ferroptosis on Osteoclast Function and Bone Resorption
4.3. Summary of Regulatory Mechanisms
5. Factors Affecting the Regulation of Ferroptosis in Osteoporosis
5.1. Factors Associated with Ferroptosis
5.2. Genetic Factors and Ferroptosis in Osteoporosis
6. The Therapeutic Potential of Ferroptosis in Osteoporosis
6.1. Iron Chelators
6.2. Natural Compounds
6.3. Mitochondria-Related Targets and Therapeutic Strategies
| Therapeutic Strategy | Mechanism | Target Cell Type | References |
|---|---|---|---|
| Deferoxamine (DFO) | Chelates Fe2+; modulates GPX4, HMOX1, and SLC7A11 to suppress ferroptosis | Osteoblasts | [84,98] |
| Asparagine | Activates NRF2/HO-1 pathway; upregulates Runx2 to inhibit ferroptosis | Osteoblasts | [129] |
| Neferine | Suppresses RANKL-induced NF-κB signaling to reduce osteoclastogenesis | Osteoclasts | [130,131] |
| Melatonin | Chelates iron and scavenges ROS; activates MT2 and NF-κB pathways to promote osteogenesis | Osteoblasts, BM-MSCs | [128,132,133] |
| Overexpression of FtMt | Reducing excess ferrous ions inhibits the occurrence of ferroptosis | Osteoblasts | [135] |
7. Challenges and Future Prospects
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BMD | Bone mineral density |
| GPX4 | Glutathione peroxidase 4 |
| TFR1 | Transferrin receptor 1 |
| STEAP3 | Six-transmembrane epithelial antigen of prostate 3 |
| LIP | Labile iron pool |
| DMT1 | Divalent metal transporter 1 |
| FPN1 | Ferroportin 1 |
| FTH1 | Ferritin heavy chain 1 |
| FTL1 | Ferritin light chain 1 |
| NCOA4 | Nuclear receptor coactivator 4 |
| ROS | Reactive oxygen species |
| PUFA | Polyunsaturated fatty acid |
| AA | Arachidonic acid |
| AdA | Adrenic acid |
| ACSL4 | Acyl-CoA synthetase long-chain family member 4 |
| LPCAT3 | Lysophosphatidylcholine acyltransferase 3 |
| LOX | Lipoxygenase |
| POR | Cytochrome P450 oxidoreductase |
| GSH | Glutathione |
| GCL | Glutamate–cysteine ligase |
| GSS | Glutathione synthetase |
| SLC7A11 | Solute carrier family 7 member 11 |
| SAT1 | Spermidine/spermine N1-acetyltransferase 1 |
| MDA | Malondialdehyde |
| ARE | Antioxidant response element |
| MAPK | Mitogen-activated protein kinase |
| JNK | c-Jun N-terminal kinase |
| Nrf2 | Nuclear factor erythroid 2-related factor 2 |
| KEAP1 | Kelch-like ECH-associated protein 1 |
| TGF-β | Transforming growth factor beta |
| BMP | Bone morphogenetic protein |
| RUNX2 | Runt-related transcription factor 2 |
| BM-MSCs | Bone marrow mesenchymal stem cells |
| ALP | Alkaline phosphatase |
| TNF-α | Tumor necrosis factor alpha |
| NF-κB | Nuclear factor kappa B |
| RANKL | Receptor activator of nuclear factor κB ligand |
| OPG | Osteoprotegerin |
| HIFs | Hypoxia-inducible factors |
| DFO | Deferoxamine |
| DFP | Deferiprone |
| DFX | Deferasirox |
| OCN | Osteocalcin |
| MT2 | Melatonin receptor 2 |
| FtMt | Mitochondrial ferritin |
| MitoQ | Mitoquinone |
References
- Yao, X.; Sun, K.; Yu, S.; Luo, J.; Guo, J.; Lin, J.; Wang, G.; Guo, Z.; Ye, Y.; Guo, F. Chondrocyte Ferroptosis Contribute to the Progression of Osteoarthritis. J. Orthop. Translat. 2021, 27, 33–43. [Google Scholar] [CrossRef]
- Ru, Q.; Li, Y.; Xie, W.; Ding, Y.; Chen, L.; Xu, G.; Wu, Y.; Wang, F. Fighting Age-Related Orthopedic Diseases: Focusing on Ferroptosis. Bone Res. 2023, 11, 12. [Google Scholar] [CrossRef]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An Iron-Dependent Form of Nonapoptotic Cell Death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef]
- Dolma, S.; Lessnick, S.L.; Hahn, W.C.; Stockwell, B.R. Identification of Genotype-Selective Antitumor Agents Using Synthetic Lethal Chemical Screening in Engineered Human Tumor Cells. Cancer Cell 2003, 3, 285–296. [Google Scholar] [CrossRef]
- Le, Y.; Zhang, Z.; Wang, C.; Lu, D. Ferroptotic Cell Death: New Regulatory Mechanisms for Metabolic Diseases. Endocr. Metab. Immune Disord. Drug Targets 2021, 21, 785–800. [Google Scholar] [CrossRef]
- Mao, C.; Liu, X.; Zhang, Y.; Lei, G.; Yan, Y.; Lee, H.; Koppula, P.; Wu, S.; Zhuang, L.; Fang, B.; et al. Dhodh-Mediated Ferroptosis Defence Is a Targetable Vulnerability in Cancer. Nature 2021, 593, 586–590. [Google Scholar] [CrossRef]
- Wen, S.; Aki, T.; Unuma, K.; Uemura, K. Chemically Induced Models of Parkinson’s Disease: History and Perspectives for the Involvement of Ferroptosis. Front. Cell. Neurosci. 2020, 14, 581191. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Liao, Y.; Xia, Q.; Zhou, S.; Li, X. Ferroptosis Regulatory Signaling Pathway and Its Research Progress in Related Diseases. Chin. J. Clin. Pharmacol. Ther. 2022, 27, 227–234. [Google Scholar] [CrossRef]
- Rachner, T.D.; Khosla, S.; Hofbauer, L.C. Osteoporosis: Now and the Future. Lancet 2011, 377, 1276–1287. [Google Scholar] [CrossRef] [PubMed]
- Subarajan, P.; Arceo-Mendoza, R.M.; Camacho, P.M. Postmenopausal Osteoporosis: A Review of Latest Guidelines. Endocrinol. Metab. Clin. N. Am. 2024, 53, 497–512. [Google Scholar] [CrossRef]
- Salari, N.; Ghasemi, H.; Mohammadi, L.; Behzadi, M.H.; Rabieenia, E.; Shohaimi, S.; Mohammadi, M. The Global Prevalence of Osteoporosis in the World: A Comprehensive Systematic Review and Meta-Analysis. J. Orthop. Surg. Res. 2021, 16, 609. [Google Scholar] [CrossRef]
- Feng, X.; McDonald, J.M. Disorders of Bone Remodeling. Annu. Rev. Pathol. 2011, 6, 121–145. [Google Scholar] [CrossRef] [PubMed]
- Ralston, S.H. Bone Structure and Metabolism. Medicine 2013, 41, 581–585. [Google Scholar] [CrossRef]
- Siddiqui, J.A.; Partridge, N.C. Physiological Bone Remodeling: Systemic Regulation and Growth Factor Involvement. Physiology 2016, 31, 233–245. [Google Scholar] [CrossRef]
- Brown, C. Osteoporosis: Staying Strong. Nature 2017, 550, S15–S17. [Google Scholar] [CrossRef] [PubMed]
- Park-Min, K.H. Mechanisms Involved in Normal and Pathological Osteoclastogenesis. Cell. Mol. Life Sci. 2018, 75, 2519–2528. [Google Scholar] [CrossRef]
- Zhang, J.; Qiu, X.; Xi, K.; Hu, W.; Pei, H.; Nie, J.; Wang, Z.; Ding, J.; Shang, P.; Li, B.; et al. Therapeutic Ionizing Radiation Induced Bone Loss: A Review of in Vivo and in Vitro Findings. Connect. Tissue Res 2018, 59, 509–522. [Google Scholar] [CrossRef]
- Ni, S.; Yuan, Y.; Kuang, Y.; Li, X. Iron Metabolism and Immune Regulation. Front. Immunol. 2022, 13, 816282. [Google Scholar] [CrossRef]
- Liu, P.; Wang, W.; Li, Z.; Li, Y.; Yu, X.; Tu, J.; Zhang, Z. Ferroptosis: A New Regulatory Mechanism in Osteoporosis. Oxid. Med. Cell. Longev. 2022, 2022, 2634431. [Google Scholar] [CrossRef]
- Jang, N.; Kim, I.K.; Jung, D.; Chung, Y.; Kang, Y.P. Regulation of Ferroptosis in Cancer and Immune Cells. Immune Netw. 2025, 25, e6. [Google Scholar] [CrossRef]
- Dixon, S.J.; Olzmann, J.A. The Cell Biology of Ferroptosis. Nat. Rev. Mol. Cell Biol. 2024, 25, 424–442. [Google Scholar] [CrossRef]
- Moujalled, D.; Strasser, A.; Liddell, J.R. Molecular Mechanisms of Cell Death in Neurological Diseases. Cell Death Differ. 2021, 28, 2029–2044. [Google Scholar] [CrossRef]
- Elmore, S. Apoptosis: A Review of Programmed Cell Death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef] [PubMed]
- Green, D.R.; Reed, J.C. Mitochondria and Apoptosis. Science 1998, 281, 1309–1312. [Google Scholar] [CrossRef] [PubMed]
- Kerr, J.F.; Wyllie, A.H.; Currie, A.R. Apoptosis: A Basic Biological Phenomenon with Wide-Ranging Implications in Tissue Kinetics. Br. J. Cancer 1972, 26, 239–257. [Google Scholar] [CrossRef] [PubMed]
- Taylor, R.C.; Cullen, S.P.; Martin, S.J. Apoptosis: Controlled Demolition at the Cellular Level. Nat. Rev. Mol. Cell Biol. 2008, 9, 231–241. [Google Scholar] [CrossRef]
- Galluzzi, L.; Keep, O.; Krautwald, S.; Kroemer, G.; Linkermann, A. Molecular Mechanisms of Regulated Necrosis. Semin. Cell Dev. Biol. 2014, 35, 24–32. [Google Scholar] [CrossRef]
- Jorgensen, I.; Rayamajhi, M.; Miao, E.A. Programmed Cell Death as a Defence against Infection. Nat. Rev. Immunol. 2017, 17, 151–164. [Google Scholar] [CrossRef]
- Mizushima, N.; Komatsu, M. Autophagy: Renovation of Cells and Tissues. Cell 2011, 147, 728–741. [Google Scholar] [CrossRef]
- Stockwell, B.R.; Angeli, J.P.F.; Bayir, H.; Bush, A.I.; Conrad, M.; Dixon, S.J.; Fulda, S.; Gascón, S.; Hatzios, S.K.; Kagan, V.E.; et al. Ferroptosis: A Regulated Cell Death Nexus Linking Metabolism, Redox Biology, and Disease. Cell 2017, 171, 273–285. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, X.; Gueydan, C.; Han, J. Plasma Membrane Changes During Programmed Cell Deaths. Cell Res. 2018, 28, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Hou, W.; Song, X.; Yu, Y.; Huang, J.; Sun, X.; Kang, R.; Tang, D. Ferroptosis: Process and Function. Cell Death Differ. 2016, 23, 369–379. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Guo, P.; Xie, X.; Wang, Y.; Chen, G. Ferroptosis, a New Form of Cell Death, and Its Relationships with Tumourous Diseases. J. Cell. Mol. Med. 2017, 21, 648–657. [Google Scholar] [CrossRef]
- Dai, E.; Chen, X.; Linkermann, A.; Jiang, X.; Kang, R.; Kagan, V.E.; Bayir, H.; Yang, W.S.; Garcia-Saez, A.J.; Ioannou, M.S.; et al. A Guideline on the Molecular Ecosystem Regulating Ferroptosis. Nat. Cell Biol. 2024, 26, 1447–1457. [Google Scholar] [CrossRef] [PubMed]
- Dodson, M.; Castro-Portuguez, R.; Zhang, D.D. Nrf2 Plays a Critical Role in Mitigating Lipid Peroxidation and Ferroptosis. Redox Biol. 2019, 23, 101107. [Google Scholar] [CrossRef]
- Fan, Z.; Wirth, A.K.; Chen, D.; Wruck, C.J.; Rauh, M.; Buchfelder, M.; Savaskan, N. Nrf2-Keap1 Pathway Promotes Cell Proliferation and Diminishes Ferroptosis. Oncogenesis 2017, 6, e371. [Google Scholar] [CrossRef]
- Kagan, V.E.; Mao, G.; Qu, F.; Angeli, J.P.; Doll, S.; Croix, C.S.; Dar, H.H.; Liu, B.; Tyurin, V.A.; Ritov, V.B.; et al. Oxidized Arachidonic and Adrenic Pes Navigate Cells to Ferroptosis. Nat. Chem. Biol. 2017, 13, 81–90. [Google Scholar] [CrossRef]
- Koppula, P.; Zhuang, L.; Gan, B. Cystine Transporter Slc7a11/Xct in Cancer: Ferroptosis, Nutrient Dependency, and Cancer Therapy. Protein Cell 2021, 12, 599–620. [Google Scholar] [CrossRef]
- Lei, G.; Zhang, Y.; Hong, T.; Zhang, X.; Liu, X.; Mao, C.; Yan, Y.; Koppula, P.; Cheng, W.; Sood, A.K.; et al. Ferroptosis as a Mechanism to Mediate P53 Function in Tumor Radiosensitivity. Oncogene 2021, 40, 3533–3547. [Google Scholar] [CrossRef]
- Lei, G.; Zhang, Y.; Koppula, P.; Liu, X.; Zhang, J.; Lin, S.H.; Ajani, J.A.; Xiao, Q.; Liao, Z.; Wang, H.; et al. The Role of Ferroptosis in Ionizing Radiation-Induced Cell Death and Tumor Suppression. Cell Res. 2020, 30, 146–162. [Google Scholar] [CrossRef]
- Zhou, R.P.; Chen, Y.; Wei, X.; Yu, B.; Xiong, Z.G.; Lu, C.; Hu, W. Novel Insights into Ferroptosis: Implications for Age-Related Diseases. Theranostics 2020, 10, 11976–11997. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Palte, M.J.; Deik, A.A.; Li, H.; Eaton, J.K.; Wang, W.; Tseng, Y.Y.; Deasy, R.; Kost-Alimova, M.; Dančík, V.; et al. A Gpx4-Dependent Cancer Cell State Underlies the Clear-Cell Morphology and Confers Sensitivity to Ferroptosis. Nat. Commun. 2019, 10, 1617. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Naguro, I.; Ichijo, H. Iron Homeostasis and Iron-Regulated Ros in Cell Death, Senescence and Human Diseases. Biochim. Biophys. Acta Gen. Subj. 2019, 1863, 1398–1409. [Google Scholar] [CrossRef] [PubMed]
- Donovan, A.; Lima, C.A.; Pinkus, J.L.; Pinkus, G.S.; Zon, L.I.; Robine, S.; Andrews, N.C. The Iron Exporter Ferroportin/Slc40a1 Is Essential for Iron Homeostasis. Cell Metab. 2005, 1, 191–200. [Google Scholar] [CrossRef]
- Dowdle, W.E.; Nyfeler, B.; Nagel, J.; Elling, R.A.; Liu, S.; Triantafellow, E.; Menon, S.; Wang, Z.; Honda, A.; Pardee, G.; et al. Selective Vps34 Inhibitor Blocks Autophagy and Uncovers a Role for Ncoa4 in Ferritin Degradation and Iron Homeostasis in Vivo. Nat. Cell Biol. 2014, 16, 1069–1079. [Google Scholar] [CrossRef]
- Fang, Y.; Chen, X.; Tan, Q.; Zhou, H.; Xu, J.; Gu, Q. Inhibiting Ferroptosis through Disrupting the Ncoa4–Fth1 Interaction: A New Mechanism of Action. ACS Cent. Sci. 2021, 7, 980–989. [Google Scholar] [CrossRef]
- Mikulska-Ruminska, K.; Anthonymuthu, T.S.; Levkina, A.; Shrivastava, I.H.; Kapralov, A.A.; Bayır, H.; Kagan, V.E.; Bahar, I. No(●) Represses the Oxygenation of Arachidonoyl Pe by 15lox/Pebp1: Mechanism and Role in Ferroptosis. Int. J. Mol. Sci. 2021, 22, 5253. [Google Scholar] [CrossRef]
- Doll, S.; Proneth, B.; Tyurina, Y.Y.; Panzilius, E.; Kobayashi, S.; Ingold, I.; Irmler, M.; Beckers, J.; Aichler, M.; Walch, A.; et al. Acsl4 Dictates Ferroptosis Sensitivity by Shaping Cellular Lipid Composition. Nat. Chem. Biol. 2017, 13, 91–98. [Google Scholar] [CrossRef]
- Poltorack, C.D.; Dixon, S.J. Understanding the Role of Cysteine in Ferroptosis: Progress & Paradoxes. FEBS J. 2022, 289, 374–385. [Google Scholar] [CrossRef]
- Proneth, B.; Conrad, M. Ferroptosis and Necroinflammation, a yet Poorly Explored Link. Cell Death Differ. 2019, 26, 14–24. [Google Scholar] [CrossRef]
- Tu, H.; Tang, L.J.; Luo, X.J.; Ai, K.L.; Peng, J. Insights into the Novel Function of System Xc- in Regulated Cell Death. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 1650–1662. [Google Scholar] [CrossRef]
- Kanapathipillai, M. Treating P53 Mutant Aggregation-Associated Cancer. Cancers 2018, 10, 154. [Google Scholar] [CrossRef]
- Ong, A.L.C.; Ramasamy, T.S. Role of Sirtuin1-P53 Regulatory Axis in Aging, Cancer and Cellular Reprogramming. Ageing Res. Rev. 2018, 43, 64–80. [Google Scholar] [CrossRef] [PubMed]
- Ou, Y.; Wang, S.J.; Li, D.; Chu, B.; Gu, W. Activation of Sat1 Engages Polyamine Metabolism with P53-Mediated Ferroptotic Responses. Proc. Natl. Acad. Sci. USA 2016, 113, E6806–E6812. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Xu, F.; Lu, H. Lncrna Pvt1 Regulates Ferroptosis through Mir-214-Mediated Tfr1 and P53. Life Sci. 2020, 260, 118305. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Wang, W.; Zhang, W. Ferroptosis and the Bidirectional Regulatory Factor P53. Cell Death Discov. 2023, 9, 197. [Google Scholar] [CrossRef]
- Wang, L.; You, X.; Zhang, L.; Zhang, C.; Zou, W. Mechanical Regulation of Bone Remodeling. Bone Res. 2022, 10, 16. [Google Scholar] [CrossRef]
- Holick, M.F. Vitamin D Deficiency. N. Engl. J. Med. 2007, 357, 266–281. [Google Scholar] [CrossRef]
- Raisz, L.G. Pathogenesis of Osteoporosis: Concepts, Conflicts, and Prospects. J. Clin. Investig. 2005, 115, 3318–3325. [Google Scholar] [CrossRef]
- Ralston, S.H.; Uitterlinden, A.G. Genetics of Osteoporosis. Endocr. Rev. 2010, 31, 629–662. [Google Scholar] [CrossRef]
- Riggs, B.L.; Wahner, H.W.; Seeman, E.; Offord, K.P.; Dunn, W.L.; Mazess, R.B.; Johnson, K.A.; Melton, L.J., 3rd. Changes in Bone Mineral Density of the Proximal Femur and Spine with Aging. Differences between the Postmenopausal and Senile Osteoporosis Syndromes. J. Clin. Investig. 1982, 70, 716–723. [Google Scholar] [CrossRef]
- Kim, B.J.; Ahn, S.H.; Bae, S.J.; Kim, E.H.; Lee, S.H.; Kim, H.K.; Choe, J.W.; Koh, J.M.; Kim, G.S. Iron Overload Accelerates Bone Loss in Healthy Postmenopausal Women and Middle-Aged Men: A 3-Year Retrospective Longitudinal Study. J. Bone Miner. Res. 2012, 27, 2279–2290. [Google Scholar] [CrossRef]
- Liu, G.; Men, P.; Kenner, G.H.; Miller, S.C. Age-Associated Iron Accumulation in Bone: Implications for Postmenopausal Osteoporosis and a New Target for Prevention and Treatment by Chelation. Biometals 2006, 19, 245–251. [Google Scholar] [CrossRef]
- Wang, X.; Chen, B.; Sun, J.; Jiang, Y.; Zhang, H.; Zhang, P.; Fei, B.; Xu, Y. Iron-Induced Oxidative Stress Stimulates Osteoclast Differentiation Via Nf-Κb Signaling Pathway in Mouse Model. Metabolism 2018, 83, 167–176. [Google Scholar] [CrossRef]
- Baschant, U.; Rauner, M.; Balaian, E.; Weidner, H.; Roetto, A.; Platzbecker, U.; Hofbauer, L.C. Wnt5a Is a Key Target for the Pro-Osteogenic Effects of Iron Chelation on Osteoblast Progenitors. Haematologica 2016, 101, 1499–1507. [Google Scholar] [CrossRef]
- Ishii, K.A.; Fumoto, T.; Iwai, K.; Takeshita, S.; Ito, M.; Shimohata, N.; Aburatani, H.; Taketani, S.; Lelliott, C.J.; Vidal-Puig, A.; et al. Coordination of Pgc-1beta and Iron Uptake in Mitochondrial Biogenesis and Osteoclast Activation. Nat. Med. 2009, 15, 259–266. [Google Scholar] [CrossRef]
- Soltanoff, C.S.; Yang, S.; Chen, W.; Li, Y.P. Signaling Networks That Control the Lineage Commitment and Differentiation of Bone Cells. Crit. Rev. Eukaryot. Gene Expr. 2009, 19, 1–46. [Google Scholar] [CrossRef]
- Tang, Y.; Wu, X.; Lei, W.; Pang, L.; Wan, C.; Shi, Z.; Zhao, L.; Nagy, T.R.; Peng, X.; Hu, J.; et al. Tgf-Beta1-Induced Migration of Bone Mesenchymal Stem Cells Couples Bone Resorption with Formation. Nat. Med. 2009, 15, 757–765. [Google Scholar] [CrossRef]
- Boyce, B.F.; Xing, L. Biology of Rank, Rankl, and Osteoprotegerin. Arthritis Res. Ther. 2007, 9 (Suppl. 1), S1. [Google Scholar] [CrossRef]
- Hofbauer, L.C.; Kühne, C.A.; Viereck, V. The Opg/Rankl/Rank System in Metabolic Bone Diseases. J. Musculoskelet. Neuronal Interact. 2004, 4, 268–275. [Google Scholar]
- Roodman, G.D. Osteoclasts Pump Iron. Cell Metab. 2009, 9, 405–406. [Google Scholar] [CrossRef]
- Tsay, J.; Yang, Z.; Ross, F.P.; Cunningham-Rundles, S.; Lin, H.; Coleman, R.; Mayer-Kuckuk, P.; Doty, S.B.; Grady, R.W.; Giardina, P.J.; et al. Bone Loss Caused by Iron Overload in a Murine Model: Importance of Oxidative Stress. Blood 2010, 116, 2582–2589. [Google Scholar] [CrossRef] [PubMed]
- Messer, J.G.; Kilbarger, A.K.; Erikson, K.M.; Kipp, D.E. Iron Overload Alters Iron-Regulatory Genes and Proteins, Down-Regulates Osteoblastic Phenotype, and Is Associated with Apoptosis in Fetal Rat Calvaria Cultures. Bone 2009, 45, 972–979. [Google Scholar] [CrossRef] [PubMed]
- Che, J.; Yang, J.; Zhao, B.; Zhang, G.; Wang, L.; Peng, S.; Shang, P. The Effect of Abnormal Iron Metabolism on Osteoporosis. Biol. Trace Elem. Res. 2020, 195, 353–365. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.J.; Shin, H.L.; Kim, B.S.; Kim, H.J.; Ryoo, H.M. Runx2-Modifying Enzymes: Therapeutic Targets for Bone Diseases. Exp. Mol. Med. 2020, 52, 1178–1184. [Google Scholar] [CrossRef]
- Chen, B.; Yan, Y.L.; Liu, C.; Bo, L.; Li, G.F.; Wang, H.; Xu, Y.J. Therapeutic Effect of Deferoxamine on Iron Overload-Induced Inhibition of Osteogenesis in a Zebrafish Model. Calcif. Tissue Int. 2014, 94, 353–360. [Google Scholar] [CrossRef]
- Balogh, E.; Tolnai, E.; Nagy, B., Jr.; Nagy, B.; Balla, G.; Balla, J.; Jeney, V. Iron Overload Inhibits Osteogenic Commitment and Differentiation of Mesenchymal Stem Cells Via the Induction of Ferritin. Biochim. Biophys. Acta 2016, 1862, 1640–1649. [Google Scholar] [CrossRef]
- Gammella, E.; Recalcati, S.; Cairo, G. Dual Role of Ros as Signal and Stress Agents: Iron Tips the Balance in Favor of Toxic Effects. Oxid. Med. Cell. Longev. 2016, 2016, 8629024. [Google Scholar] [CrossRef]
- Domazetovic, V.; Marcucci, G.; Iantomasi, T.; Brandi, M.L.; Vincenzini, M.T. Oxidative Stress in Bone Remodeling: Role of Antioxidants. Clin. Cases Miner. Bone Metab. 2017, 14, 209–216. [Google Scholar] [CrossRef]
- Bai, X.C.; Lu, D.; Bai, J.; Zheng, H.; Ke, Z.Y.; Li, X.M.; Luo, S.Q. Oxidative Stress Inhibits Osteoblastic Differentiation of Bone Cells by Erk and Nf-Kappab. Biochem. Biophys. Res. Commun. 2004, 314, 197–207. [Google Scholar] [CrossRef]
- Jin, W.; Zhu, X.; Yao, F.; Xu, X.; Chen, X.; Luo, Z.; Zhao, D.; Li, X.; Leng, X.; Sun, L. Cytoprotective Effect of Fufang Lurong Jiangu Capsule against Hydrogen Peroxide-Induced Oxidative Stress in Bone Marrow Stromal Cell-Derived Osteoblasts through the Nrf2/Ho-1 Signaling Pathway. Biomed. Pharmacother. 2020, 121, 109676. [Google Scholar] [CrossRef]
- Gong, W.; Liu, M.; Zhang, Q.; Zhang, Q.; Wang, Y.; Zhao, Q.; Xiang, L.; Zheng, C.; Zhang, Q.; Qin, L. Orcinol Glucoside Improves Senile Osteoporosis through Attenuating Oxidative Stress and Autophagy of Osteoclast Via Activating Nrf2/Keap1 and Mtor Signaling Pathway. Oxid. Med. Cell. Longev. 2022, 2022, 5410377. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Lin, S.; Li, H.; Si, S.; Wang, Z. Myeloperoxidase Controls Bone Turnover by Suppressing Osteoclast Differentiation through Modulating Reactive Oxygen Species Level. J. Bone Miner. Res. 2021, 36, 591–603. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Wang, H.; Qi, G.; Jiang, C.; Chen, K.; Yan, Z. Iron Overload-Induced Ferroptosis of Osteoblasts Inhibits Osteogenesis and Promotes Osteoporosis: An in Vitro and in Vivo Study. IUBMB Life 2022, 74, 1052–1069. [Google Scholar] [CrossRef] [PubMed]
- Maeda, K.; Kobayashi, Y.; Koide, M.; Uehara, S.; Okamoto, M.; Ishihara, A.; Kayama, T.; Saito, M.; Marumo, K. The Regulation of Bone Metabolism and Disorders by Wnt Signaling. Int. J. Mol. Sci. 2019, 20, 5525. [Google Scholar] [CrossRef]
- Gaur, T.; Lengner, C.J.; Hovhannisyan, H.; Bhat, R.A.; Bodine, P.V.; Komm, B.S.; Javed, A.; van Wijnen, A.J.; Stein, J.L.; Stein, G.S.; et al. Canonical Wnt Signaling Promotes Osteogenesis by Directly Stimulating Runx2 Gene Expression. J. Biol. Chem. 2005, 280, 33132–33140. [Google Scholar] [CrossRef]
- Deng, Q.; Ping, L.; Manju, C.; Jiajia, L.; Soma, B.; Gang, M.; Lin, H.; Zhanying, W.; Zhenlin, Z.; Yingzi, Y.; et al. Activation of Hedgehog Signaling in Mesenchymal Stem Cells Induces Cartilage and Bone Tumor Formation Via Wnt/Β-Catenin. Elife 2019, 8, e50208. [Google Scholar] [CrossRef]
- Zanotti, S.; Canalis, E. Notch Signaling and the Skeleton. Endocr. Rev. 2016, 37, 223–253. [Google Scholar] [CrossRef]
- Chen, G.; Deng, C.; Li, Y.P. Tgf-Β and Bmp Signaling in Osteoblast Differentiation and Bone Formation. Int. J. Biol. Sci. 2012, 8, 272–288. [Google Scholar] [CrossRef]
- Luo, C.; Xu, W.; Tang, X.; Liu, X.; Cheng, Y.; Wu, Y.; Xie, Z.; Wu, X.; He, X.; Wang, Q.; et al. Canonical Wnt Signaling Works Downstream of Iron Overload to Prevent Ferroptosis from Damaging Osteoblast Differentiation. Free Radic. Biol. Med. 2022, 188, 337–350. [Google Scholar] [CrossRef]
- Sun, Y.X.; Xu, A.H.; Yang, Y.; Li, J. Role of Nrf2 in Bone Metabolism. J. Biomed. Sci. 2015, 22, 101. [Google Scholar] [CrossRef] [PubMed]
- Motohashi, H.; Yamamoto, M. Nrf2-Keap1 Defines a Physiologically Important Stress Response Mechanism. Trends Mol. Med. 2004, 10, 549–557. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.; Zhang, J.; An, F.; Wang, J.; Shi, Y.; Yuan, L.; Lv, D.; Zhao, Y.; Wang, Y. Research Progress of Ferroptosis Regulatory Network and Bone Remodeling in Osteoporosis. Front. Public Health 2022, 10, 910675. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Lin, B.; Deng, X.; Huang, K.; Zhang, Y.; Wang, N. Vdr Activation Attenuates Osteoblastic Ferroptosis and Senescence by Stimulating the Nrf2/Gpx4 Pathway in Age-Related Osteoporosis. Free. Radic. Biol. Med. 2022, 193, 720–735. [Google Scholar] [CrossRef]
- Ma, H.; Wang, X.; Zhang, W.; Li, H.; Zhao, W.; Sun, J.; Yang, M. Melatonin Suppresses Ferroptosis Induced by High Glucose via Activation of the Nrf2/HO-1 Signaling Pathway in Type 2 Diabetic Osteoporosis. Oxid. Med. Cell. Longev. 2020, 2020, 9067610. [Google Scholar] [CrossRef]
- Kamata, T. Roles of Nox1 and Other Nox Isoforms in Cancer Development. Cancer Sci. 2009, 100, 1382–1388. [Google Scholar] [CrossRef]
- Vermot, A.; Petit-Härtlein, I.; Smith, S.M.E.; Fieschi, F. NADPH Oxidases (NOX): An Overview from Discovery, Molecular Mechanisms to Physiology and Pathology. Antioxidants 2021, 10, 890. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, A.; Li, G.; Zhai, Q.; Huang, Z.; Wang, X.; Cao, Z.; Liu, L.; Liu, G.; Chen, B.; et al. Osteoporotic Bone Loss from Excess Iron Accumulation Is Driven by Nox4-Triggered Ferroptosis in Osteoblasts. Free Radic. Biol. Med. 2023, 198, 123–136. [Google Scholar] [CrossRef]
- Ni, S.; Yuan, Y.; Qian, Z.; Zhong, Z.; Lv, T.; Kuang, Y.; Yu, B. Hypoxia Inhibits Rankl-Induced Ferritinophagy and Protects Osteoclasts from Ferroptosis. Free Radic. Biol. Med. 2021, 169, 271–282. [Google Scholar] [CrossRef]
- Wu, Y.; Yang, Y.; Wang, L.; Chen, Y.; Han, X.; Sun, L.; Chen, H.; Chen, Q. Effect of Bifidobacterium on Osteoclasts: Tnf-A/Nf-Κb Inflammatory Signal Pathway-Mediated Mechanism. Front. Endocrinol. 2023, 14, 1109296. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, L.; Yao, G.; Zhao, H.; Wu, S. Nrf2 Is Essential for Iron-Overload Stimulated Osteoclast Differentiation through Regulation of Redox and Iron Homeostasis. Cell Biol. Toxicol. 2023, 39, 3305–3321. [Google Scholar] [CrossRef]
- Ling, H.; Xiao, H.; Luo, T.; Lin, H.; Deng, J. Role of Ferroptosis in Regulating the Epithelial-Mesenchymal Transition in Pulmonary Fibrosis. Biomedicines 2023, 11, 163. [Google Scholar] [CrossRef]
- Juárez, P.; Fournier, P.G.J.; Mohammad, K.S.; McKenna, R.C.; Davis, H.W.; Peng, X.H.; Niewolna, M.; Mauviel, A.; Chirgwin, J.M.; Guise, T.A. Halofuginone Inhibits Tgf-Β/Bmp Signaling and in Combination with Zoledronic Acid Enhances Inhibition of Breast Cancer Bone Metastasis. Oncotarget 2017, 8, 86447–86462. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Yao, H.; Encarnacion, A.M.; Jeong, J.; Choi, Y.; Park, S.; Lee, S.; Lee, T. Novel Inhibitor of Keap1-Nrf2 Protein-Protein Interaction Attenuates Osteoclastogenesis In Vitro and Prevents OVX-Induced Bone Loss In Vivo. Antioxidants 2024, 13, 850. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Hu, W.; Qian, D.; Bai, X.; He, H.; Li, L.; Sun, S. The Mechanisms of Ferroptosis Under Hypoxia. Cell. Mol. Neurobiol. 2023, 43, 3329–3341. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Yang, K.; An, J.; Jiang, N.; Fu, S.; Tang, X. The Role of NRF2 in Bone Metabolism—Friend or Foe? Front. Endocrinol. 2022, 13, 813057. [Google Scholar] [CrossRef]
- Xue, C.; Luo, H.; Wang, L.; Deng, Q.; Kui, W.; Da, W.; Chen, L.; Liu, S.; Xue, Y.; Yang, J.; et al. Aconine Attenuates Osteoclast-Mediated Bone Resorption and Ferroptosis to Improve Osteoporosis Via Inhibiting Nf-Κb Signaling. Front. Endocrinol. 2023, 14, 1234563. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, X.; Qi, B.; Sun, C.; Sun, K.; Liu, N.; Zhu, L.; Wei, X. Ferroptosis and Musculoskeletal Diseases: "Iron Maiden" Cell Death May Be a Promising Therapeutic Target. Front. Immunol. 2022, 13, 972753. [Google Scholar] [CrossRef]
- Li, Y.; Huang, Z.; Pan, S.; Feng, Y.; He, H.; Cheng, S.; Wang, L.; Wang, L.; Pathak, J.L. Resveratrol Alleviates Diabetic Periodontitis-Induced Alveolar Osteocyte Ferroptosis Possibly via Regulation of SLC7A11/GPX4. Nutrients 2023, 15, 2115. [Google Scholar] [CrossRef]
- Hou, W.; Xie, Y.; Song, X.; Sun, X.; Lotze, M.T.; Zeh, H.J., 3rd; Kang, R.; Tang, D. Autophagy Promotes Ferroptosis by Degradation of Ferritin. Autophagy 2016, 12, 1425–1428. [Google Scholar] [CrossRef]
- Chen, X.; Li, J.; Kang, R.; Klionsky, D.J.; Tang, D. Ferroptosis: Machinery and Regulation. Autophagy 2021, 17, 2054–2081. [Google Scholar] [CrossRef]
- Ye, Z.; Liu, W.; Zhuo, Q.; Hu, Q.; Liu, M.; Sun, Q.; Zhang, Z.; Fan, G.; Xu, W.; Ji, S.; et al. Ferroptosis: Final Destination for Cancer? Cell Prolif. 2020, 53, e12761. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.S.; SriRamaratnam, R.; Welsch, M.E.; Shimada, K.; Skouta, R.; Viswanathan, V.S.; Cheah, J.H.; Clemons, P.A.; Shamji, A.F.; Clish, C.B.; et al. Regulation of Ferroptotic Cancer Cell Death by Gpx4. Cell 2014, 156, 317–331. [Google Scholar] [CrossRef] [PubMed]
- Friedmann Angeli, J.P.; Schneider, M.; Proneth, B.; Tyurina, Y.Y.; Tyurin, V.A.; Hammond, V.J.; Herbach, N.; Aichler, M.; Walch, A.; Eggenhofer, E.; et al. Inactivation of the Ferroptosis Regulator Gpx4 Triggers Acute Renal Failure in Mice. Nat. Cell Biol. 2014, 16, 1180–1191. [Google Scholar] [CrossRef] [PubMed]
- Skouta, R.; Dixon, S.J.; Wang, J.; Dunn, D.E.; Orman, M.; Shimada, K.; Rosenberg, P.A.; Lo, D.C.; Weinberg, J.M.; Linkermann, A.; et al. Ferrostatins Inhibit Oxidative Lipid Damage and Cell Death in Diverse Disease Models. J. Am. Chem. Soc. 2014, 136, 4551–4556. [Google Scholar] [CrossRef]
- Zheng, X.; Liang, Y.; Zhang, C. Ferroptosis Regulated by Hypoxia in Cells. Cells 2023, 12, 1050. [Google Scholar] [CrossRef]
- Henning, Y.; Blind, U.S.; Larafa, S.; Matschke, J.; Fandrey, J. Hypoxia Aggravates Ferroptosis in Rpe Cells by Promoting the Fenton Reaction. Cell Death Dis. 2022, 13, 662. [Google Scholar] [CrossRef]
- Singhal, R.; Mitta, S.R.; Das, N.K.; Kerk, S.A.; Sajjakulnukit, P.; Solanki, S.; Andren, A.; Kumar, R.; Olive, K.P.; Banerjee, R.; et al. HIF-2α Activation Potentiates Oxidative Cell Death in Colorectal Cancers by Increasing Cellular Iron. J. Clin. Investig. 2021, 131, e143691. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, J.; Zhang, M.; OuYang, H.; Li, M.; Jia, D.; Wang, R.; Zhou, W.; Liu, H.; Hu, Y.J.E.P. Cadmium Exposure During Puberty Damages Testicular Development and Spermatogenesis Via Ferroptosis Caused by Intracellular Iron Overload and Oxidative Stress in Mice. Environ. Pollut. 2023, 325, 121434. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, M. Pm2.5 Induces Ferroptosis in Human Endothelial Cells through Iron Overload and Redox Imbalance. Environ. Pollut. 2019, 254, 112937. [Google Scholar] [CrossRef]
- Banaszkiewicz, K.; Sikorska, K.; Panas, D.; Sworczak, K. The Role of the Trabecular Bone Score in the Assessment of Osteoarticular Disorders in Patients with Hfe-Hemochromatosis: A Single-Center Study from Poland. Genes 2021, 12, 1304. [Google Scholar] [CrossRef] [PubMed]
- Ward, D.M.; Kaplan, J. Ferroportin-Mediated Iron Transport: Expression and Regulation. Biochim. Biophys. Acta 2012, 1823, 1426–1433. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Fang, B.; Fujiwara, T.; Krager, K.; Gorantla, A.; Li, C.; Feng, J.Q.; Jennings, M.L.; Zhou, J.; Aykin-Burns, N.; et al. Deletion of Ferroportin in Murine Myeloid Cells Increases Iron Accumulation and Stimulates Osteoclastogenesis in Vitro and in Vivo. J. Biol. Chem. 2018, 293, 9248–9264. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Xue, Y.; Wang, J. Screening Diagnostic Markers of Osteoporosis Based on Ferroptosis of Osteoblast and Osteoclast. Aging 2023, 15, 9391–9407. [Google Scholar] [CrossRef]
- Du, Y.X.; Zhao, Y.T.; Sun, Y.X.; Xu, A.H. Acid Sphingomyelinase Mediates Ferroptosis Induced by High Glucose Via Autophagic Degradation of Gpx4 in Type 2 Diabetic Osteoporosis. Mol. Med. 2023, 29, 125. [Google Scholar] [CrossRef]
- Di Paola, A.; Tortora, C.; Argenziano, M.; Marrapodi, M.M.; Rossi, F. Emerging Roles of the Iron Chelators in Inflammation. Int. J. Mol. Sci. 2022, 23, 7977. [Google Scholar] [CrossRef]
- Mobarra, N.; Shanaki, M.; Ehteram, H.; Nasiri, H.; Sahmani, M.; Saeidi, M.; Goudarzi, M.; Pourkarim, H.; Azad, M. A Review on Iron Chelators in Treatment of Iron Overload Syndromes. Int. J. Hematol. -Oncol. Stem Cell Res. 2016, 10, 239–247. [Google Scholar]
- Yang, J.; Tang, Q.; Zeng, Y. Melatonin: Potential Avenue for Treating Iron Overload Disorders. Ageing Res. Rev. 2022, 81, 101717. [Google Scholar] [CrossRef]
- Liu, S.; Fang, T.; Yang, L.; Chen, Z.; Mu, S.; Fu, Q. Gastrodin Protects Mc3t3-E1 Osteoblasts from Dexamethasone-Induced Cellular Dysfunction and Promotes Bone Formation Via Induction of the Nrf2 Signaling Pathway. Int. J. Mol. Med. 2018, 41, 2059–2069. [Google Scholar] [CrossRef]
- Chen, S.; Chu, B.; Chen, Y.; Cheng, X.; Guo, D.; Chen, L.; Wang, J.; Li, Z.; Hong, Z.; Hong, D. Neferine Suppresses Osteoclast Differentiation through Suppressing Nf-Κb Signal Pathway but Not Mapks and Promote Osteogenesis. J. Cell. Physiol. 2019, 234, 22960–22971. [Google Scholar] [CrossRef]
- Li, H.; Gao, L.; Min, J.; Yang, Y.; Zhang, R. Neferine Suppresses Autophagy-Induced Inflammation, Oxidative Stress and Adipocyte Differentiation in Graves’ Orbitopathy. J. Cell. Mol. Med. 2021, 25, 1949–1957. [Google Scholar] [CrossRef]
- Li, T.; Jiang, S.; Lu, C.; Yang, W.; Yang, Z.; Hu, W.; Xin, Z.; Yang, Y. Melatonin: Another Avenue for Treating Osteoporosis? J. Pineal Res. 2019, 66, e12548. [Google Scholar] [CrossRef]
- Hu, Y.; Xiong, Y.; Zha, K.; Tao, R.; Chen, L.; Xue, H.; Yan, C.; Lin, Z.; Endo, Y.; Cao, F.; et al. Melatonin Promotes Bmscs Osteoblastic Differentiation and Relieves Inflammation by Suppressing the Nf-Κb Pathways. Stem Cells Int. 2023, 2023, 7638842. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Xu, T.Y.; Yu, A.X.; Liang, J.L.; Zhou, Y.S.; Sun, H.Z.; Dai, Y.L.; Liu, J.; Yu, P. The Role of Ferroptosis in Osteoporosis and Advances in Chinese Herbal Interventions. Biology 2025, 14, 367. [Google Scholar] [CrossRef]
- Wang, X.; Ma, H.; Sun, J.; Zheng, T.; Zhao, P.; Li, H.; Yang, M. Mitochondrial Ferritin Deficiency Promotes Osteoblastic Ferroptosis Via Mitophagy in Type 2 Diabetic Osteoporosis. Biol. Trace Elem. Res. 2022, 200, 298–307. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.J.; Ikeda, M.; Ide, T.; Hur, K.Y.; Lee, M.S. Mitochondrial Event as an Ultimate Step in Ferroptosis. Cell Death Discov. 2022, 8, 414. [Google Scholar] [CrossRef]
- Hou, L.; Wang, G.; Zhang, X.; Lu, F.; Xu, J.; Guo, Z.; Lin, J.; Zheng, Z.; Liu, H.; Hou, Y.; et al. Mitoquinone Alleviates Osteoarthritis Progress by Activating the Nrf2-Parkin Axis. iScience 2023, 26, 107647. [Google Scholar] [CrossRef]
| Signaling Pathways | Osteoblast Function | Osteoclast Function | Ferroptosis Mechanism | Reference |
|---|---|---|---|---|
| Wnt/β-catenin | Inhibits differentiation and mineralization | Not directly involved | Ferroptosis inhibits β-catenin nuclear translocation, reducing osteogenic activity | [102] |
| TGF-β/BMP | Inhibits osteogenic differentiation | No clear association | Ferroptosis downregulates BMP signaling, reducing bone matrix synthesis | [103] |
| Nrf2 pathway | Promote differentiation and proliferation | Inhibits production and activity | Ferroptosis suppresses Nrf2 pathway, exacerbating oxidative stress damage | [104,105,106] |
| NF-κB pathway | Not directly involved | Enhances osteoclast production | ROS activates NF-κB in osteoclasts | [107,108] |
| MAPK pathway | Not directly involved | Promotes osteoclast differentiation and bone resorption | ROS activates p38/JNK to promote osteoclastogenesis | [107,108] |
| PI3K/Akt/mTOR | Promotes survival and differentiation | Promotes survival and differentiation | Iron overload inhibits PI3K/Akt/mTOR in osteoblasts, reducing survival and differentiation; in osteoclasts, it activates PI3K/Akt/mTOR, enhancing survival and bone resorption | [108,109] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, H.; Ru, K.; Liu, S.; Zhu, C.; Qian, A.; Chen, Z. Targeting Ferroptosis: Emerging Insights into Osteoporosis Mechanisms. Biology 2025, 14, 1062. https://doi.org/10.3390/biology14081062
Yang H, Ru K, Liu S, Zhu C, Qian A, Chen Z. Targeting Ferroptosis: Emerging Insights into Osteoporosis Mechanisms. Biology. 2025; 14(8):1062. https://doi.org/10.3390/biology14081062
Chicago/Turabian StyleYang, Hailing, Kang Ru, Shuai Liu, Chunyu Zhu, Airong Qian, and Zhihao Chen. 2025. "Targeting Ferroptosis: Emerging Insights into Osteoporosis Mechanisms" Biology 14, no. 8: 1062. https://doi.org/10.3390/biology14081062
APA StyleYang, H., Ru, K., Liu, S., Zhu, C., Qian, A., & Chen, Z. (2025). Targeting Ferroptosis: Emerging Insights into Osteoporosis Mechanisms. Biology, 14(8), 1062. https://doi.org/10.3390/biology14081062






