Stage-Specific Impacts of Climate Change on Greater White-Fronted Geese Along the East Asian Flyway
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
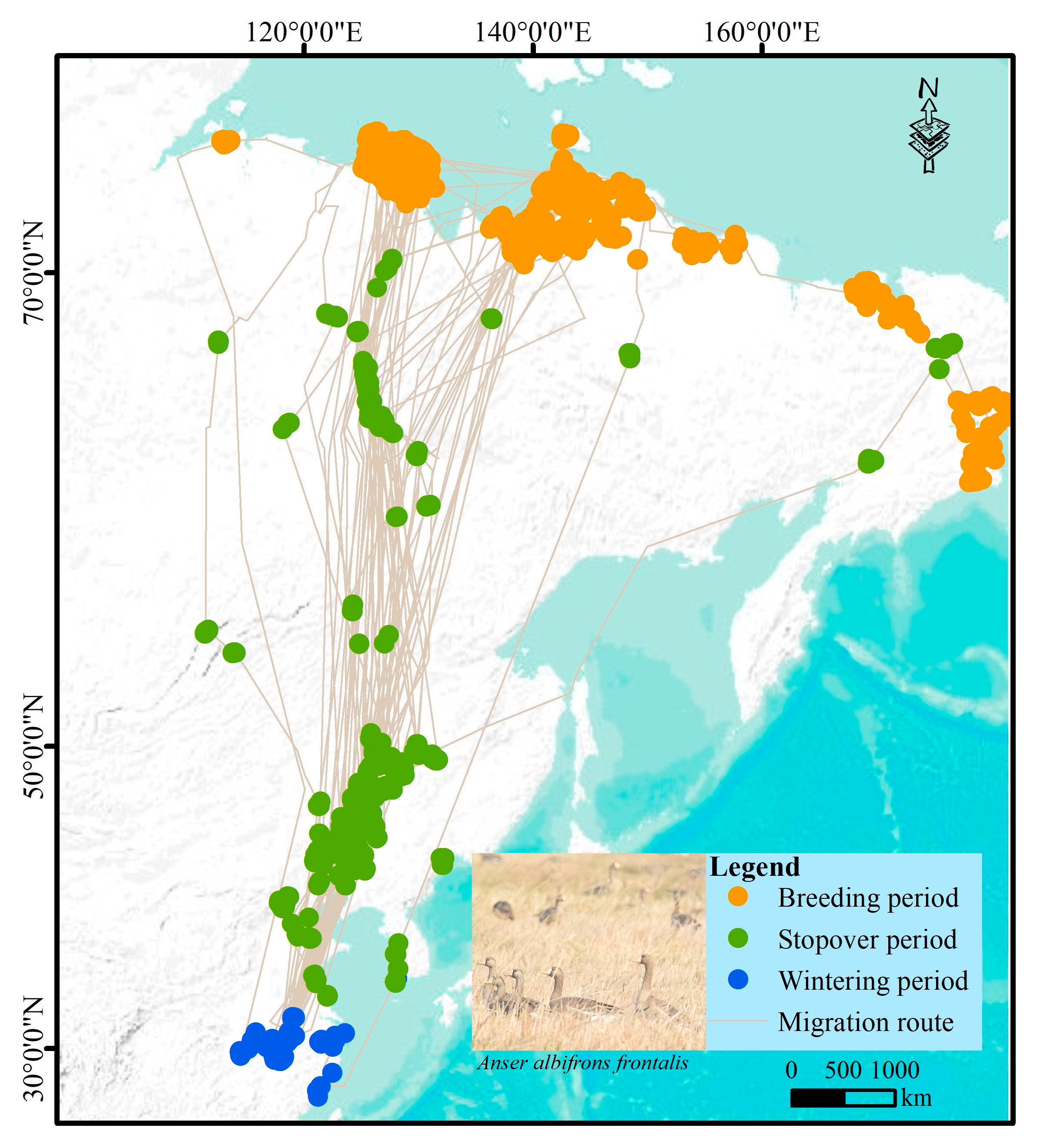
2.1. Occurrence Data
2.2. Environmental Data
2.3. SDM Analysis
2.4. Analysis of Habitat Change Under Climate Change
2.5. Statistical Analysis
3. Results
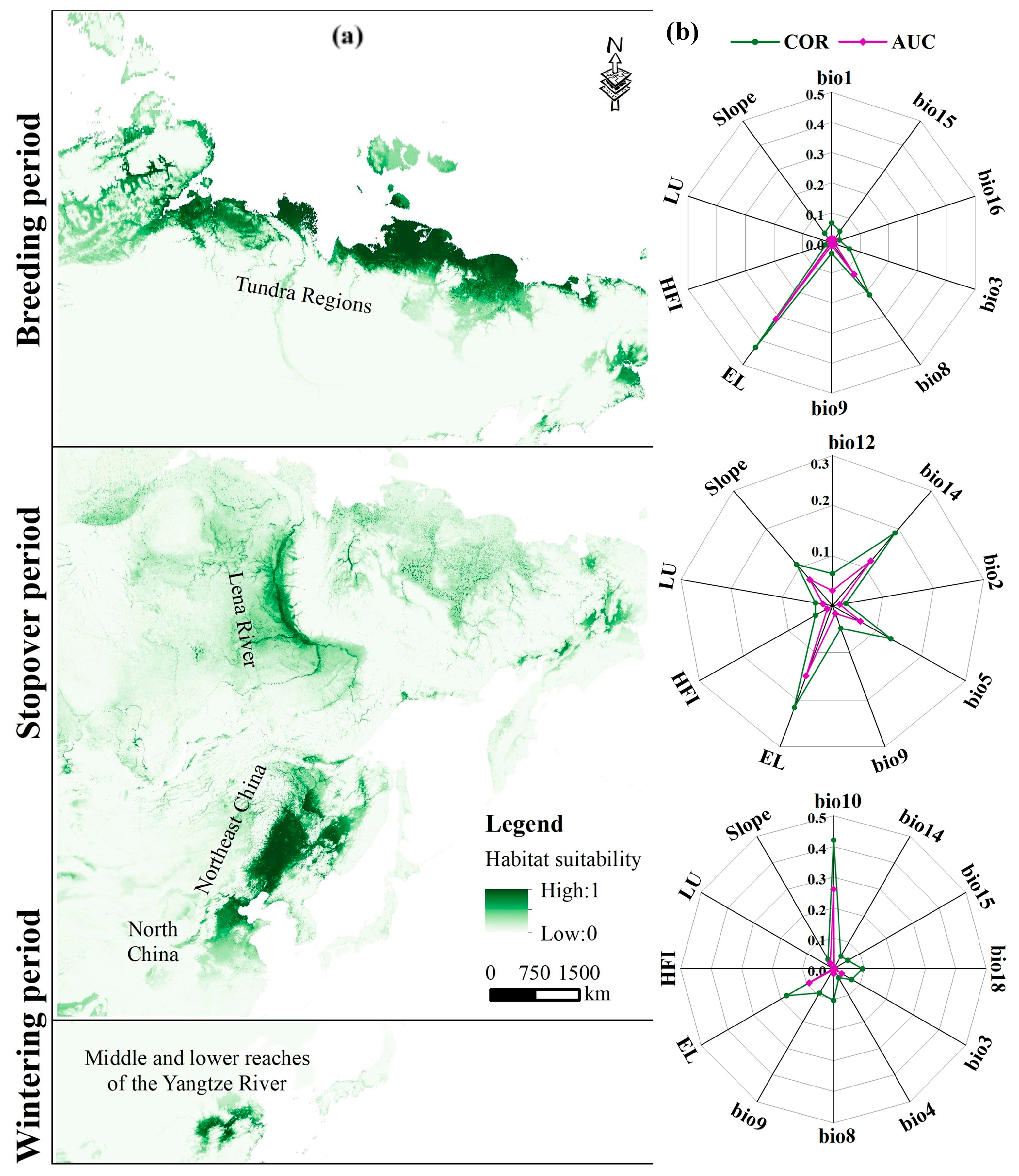
3.1. Environmental Drivers of Breeding, Stopover, and Wintering Habitat Distribution
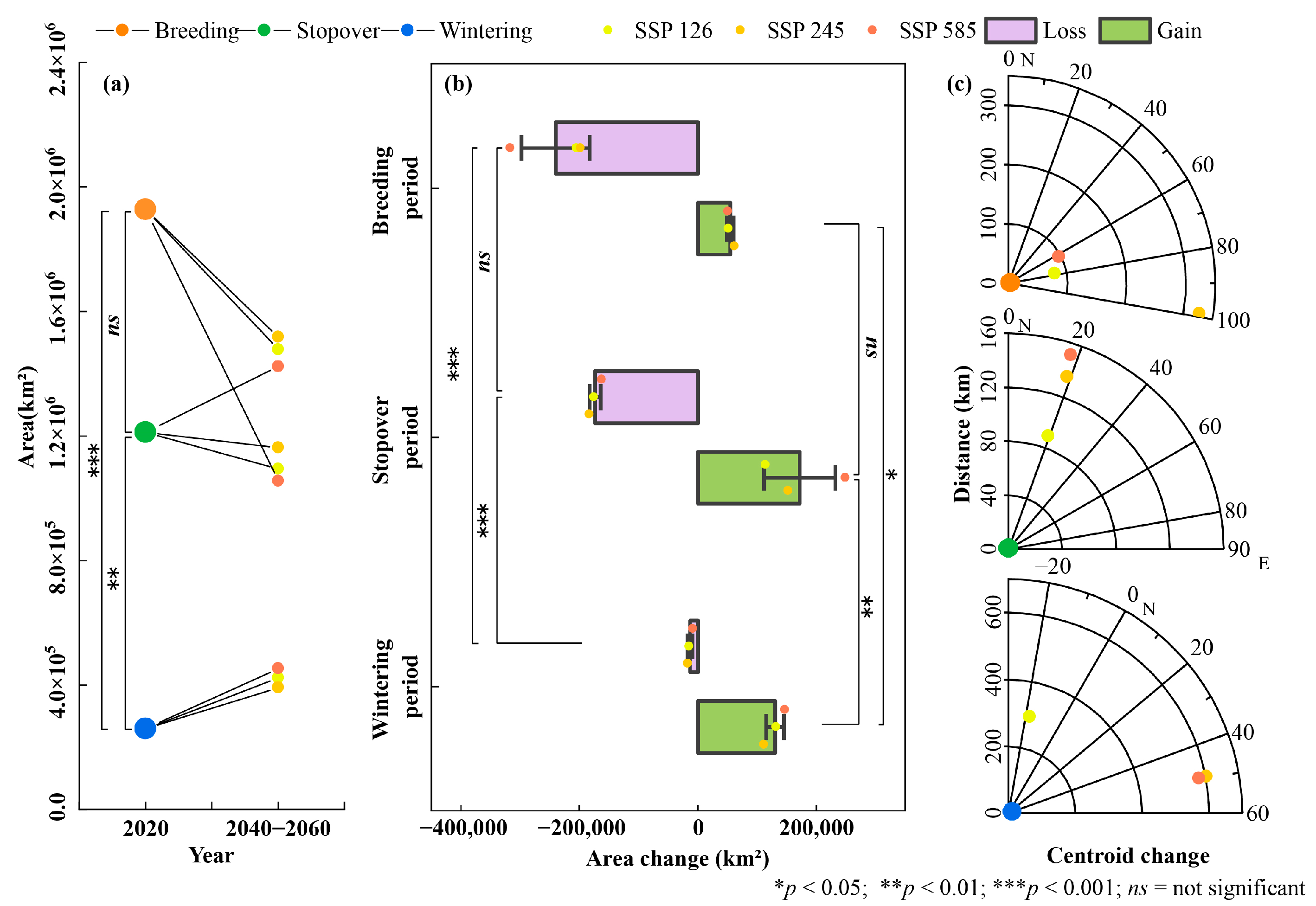
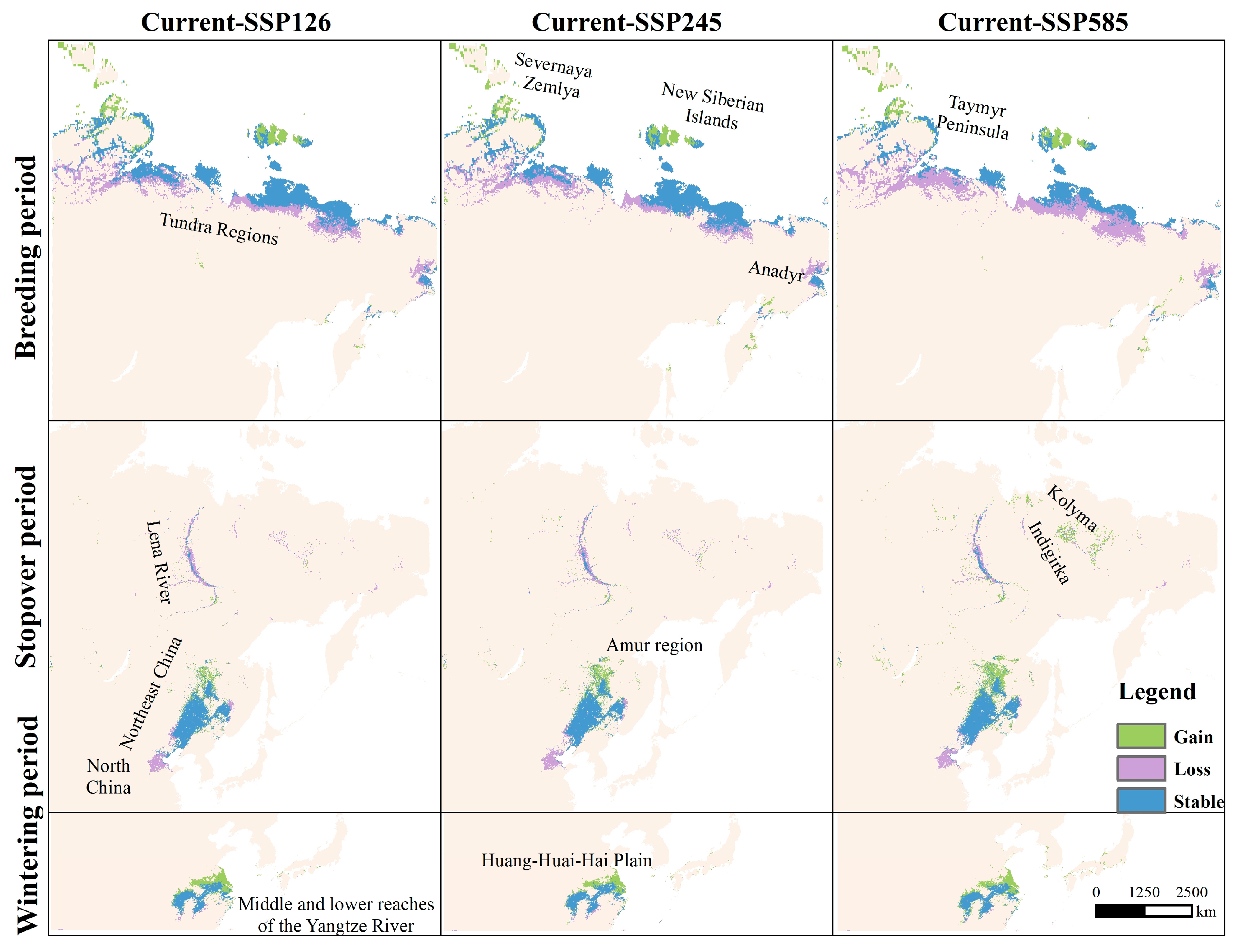
3.2. Changes in Area and Distribution of Suitable Habitat Under Future Climate Change
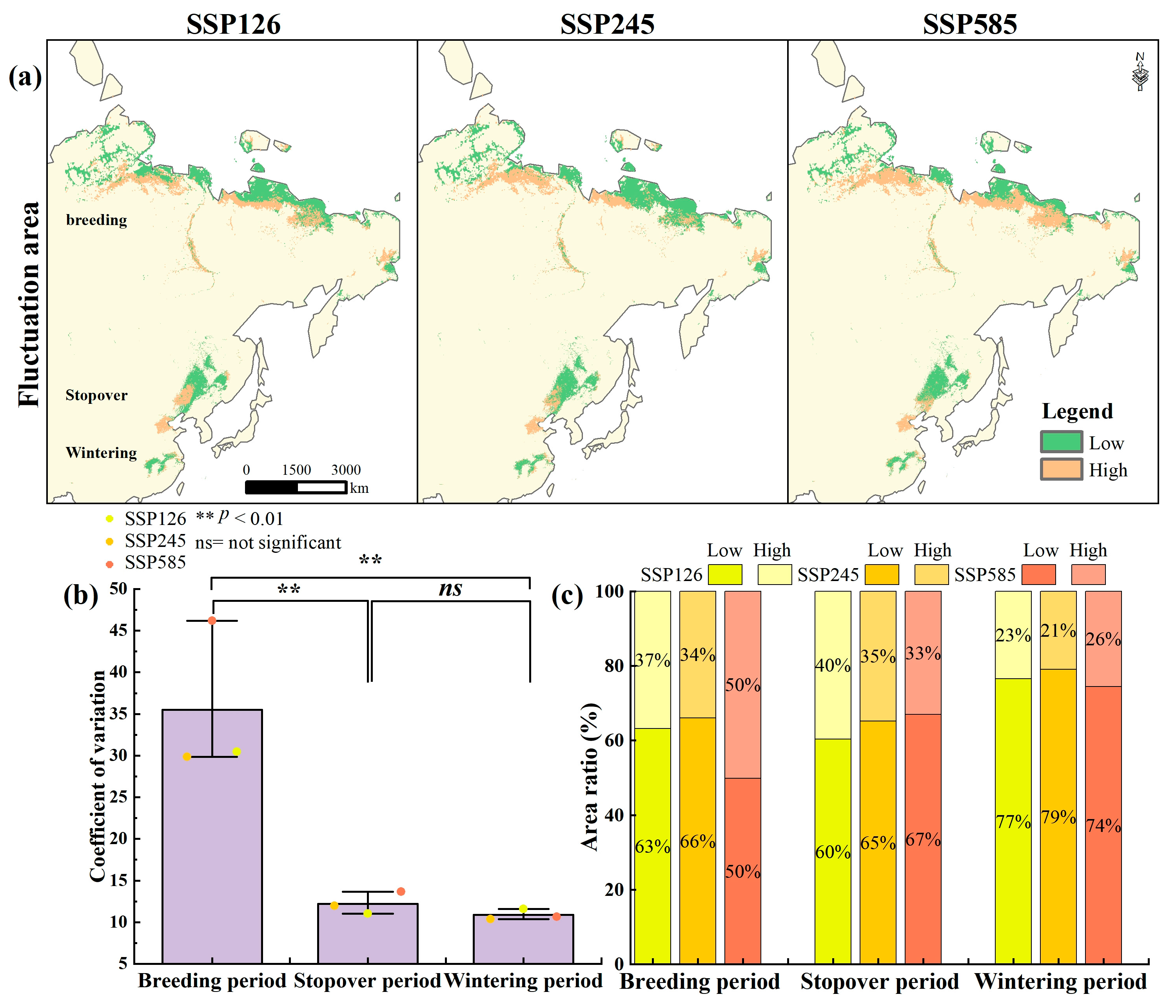
3.3. Habitat Suitability Fluctuation Under Future Climate Change
4. Discussion
4.1. Habitat Distribution and Key Environmental Factors
4.2. Effects of Climate Change on Habitat Distribution and Stability
4.3. Conservation Strategies for Greater White-Fronted Geese
4.4. Limitations and Future Work
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| GWFG | Greater White-fronted Goose |
| SDM | Species distribution model |
| GLM | Generalized Linear Model |
| BRT | Boosted Regression Trees |
| RF | Random Forest |
| SVM | Support Vector Machine |
| AUC | Area Under the Curve |
| TSS | True Skill Statistic |
References
- Arias, P.; Bellouin, N.; Coppola, E.; Jones, R.; Krinner, G.; Marotzke, J.; Naik, V.; Palmer, M.; Plattner, G.-K.; Rogelj, J. Climate Change 2021: The physical science basis. In Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; technical summary; The Intergovernmental Panel on Climate Change: Geneva, Switzerland, 2021. [Google Scholar]
- Hugonnet, R.; McNabb, R.; Berthier, E.; Menounos, B.; Nuth, C.; Girod, L.; Farinotti, D.; Huss, M.; Dussaillant, I.; Brun, F. Accelerated global glacier mass loss in the early twenty-first century. Nature 2021, 592, 726–731. [Google Scholar] [CrossRef]
- Perkins-Kirkpatrick, S.; Lewis, S. Increasing trends in regional heatwaves. Nat. Commun. 2020, 11, 3357. [Google Scholar] [CrossRef]
- Ripple, W.J.; Wolf, C.; Gregg, J.W.; Rockström, J.; Mann, M.E.; Oreskes, N.; Lenton, T.M.; Rahmstorf, S.; Newsome, T.M.; Xu, C.; et al. The 2024 state of the climate report: Perilous times on planet Earth. BioScience 2024, 74, 812–824. [Google Scholar] [CrossRef]
- Bastazini, V.A.G.; Galiana, N.; Hillebrand, H.; Estiarte, M.; Ogaya, R.; Penuelas, J.; Sommer, U.; Montoya, J.M. The impact of climate warming on species diversity across scales: Lessons from experimental meta-ecosystems. Glob. Ecol. Biogeogr. 2021, 30, 1545–1554. [Google Scholar] [CrossRef] [PubMed]
- Hughes, L. Biological consequences of global warming: Is the signal already apparent? Trends Ecol. Evol. 2000, 15, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Saunders, S.P.; Meehan, T.D.; Michel, N.L.; Bateman, B.L.; DeLuca, W.; Deppe, J.L.; Grand, J.; LeBaron, G.S.; Taylor, L.; Westerkam, H. Unraveling a century of global change impacts on winter bird distributions in the eastern United States. Glob. Change Biol. 2022, 28, 2221–2235. [Google Scholar] [CrossRef]
- Nuijten, R.J.; Wood, K.A.; Haitjema, T.; Rees, E.C.; Nolet, B.A. Concurrent shifts in wintering distribution and phenology in migratory swans: Individual and generational effects. Glob. Change Biol. 2020, 26, 4263–4275. [Google Scholar] [CrossRef]
- Garcia, R.A.; Cabeza, M.; Rahbek, C.; Araújo, M.B. Multiple dimensions of climate change and their implications for biodiversity. Science 2014, 344, 1247579. [Google Scholar] [CrossRef]
- Habibullah, M.S.; Din, B.H.; Tan, S.-H.; Zahid, H. Impact of climate change on biodiversity loss: Global evidence. Environ. Sci. Pollut. Res. 2022, 29, 1073–1086. [Google Scholar] [CrossRef]
- Northrup, J.M.; Rivers, J.W.; Yang, Z.; Betts, M.G. Synergistic effects of climate and land-use change influence broad-scale avian population declines. Glob. Change Biol. 2019, 25, 1561–1575. [Google Scholar] [CrossRef] [PubMed]
- Runge, C.A.; Watson, J.E.; Butchart, S.H.; Hanson, J.O.; Possingham, H.P.; Fuller, R.A. Protected areas and global conservation of migratory birds. Science 2015, 350, 1255–1258. [Google Scholar] [CrossRef]
- Zhang, Y.; Na, X.; Li, W. Impacts of climate changes on the potential habitat suitability of Grus japonensis on migration routes. Ecol. Indic. 2024, 166, 112462. [Google Scholar] [CrossRef]
- Qiu, J.; Zhang, Y.; Ma, J. Wetland habitats supporting waterbird diversity: Conservation perspective on biodiversity-ecosystem functioning relationship. J. Environ. Manag. 2024, 357, 120663. [Google Scholar] [CrossRef]
- Xu, Y.; Si, Y.; Takekawa, J.; Liu, Q.; Prins, H.H.T.; Yin, S.; Prosser, D.J.; Gong, P.; de Boer, W.F. A network approach to prioritize conservation efforts for migratory birds. Conserv. Biol. 2019, 34, 416–426. [Google Scholar] [CrossRef]
- Hitch, A.T.; Leberg, P.L. Breeding Distributions of North American Bird Species Moving North as a Result of Climate Change. Conserv. Biol. 2006, 21, 534–539. [Google Scholar] [CrossRef]
- Parmesan, C. Ecological and evolutionary responses to recent climate change. Annu. Rev. Ecol. Evol. Syst. 2006, 37, 637–669. [Google Scholar] [CrossRef]
- Tomkiewicz, S.M.; Fuller, M.R.; Kie, J.G.; Bates, K.K. Global positioning system and associated technologies in animal behaviour and ecological research. Philos. Trans. R. Soc. B Biol. Sci. 2010, 365, 2163–2176. [Google Scholar] [CrossRef]
- Williams, H.J.; Taylor, L.A.; Benhamou, S.; Bijleveld, A.I.; Clay, T.A.; de Grissac, S.; Demšar, U.; English, H.M.; Franconi, N.; Gómez-Laich, A. Optimizing the use of biologgers for movement ecology research. J. Anim. Ecol. 2020, 89, 186–206. [Google Scholar] [CrossRef] [PubMed]
- Wilmers, C.C.; Nickel, B.; Bryce, C.M.; Smith, J.A.; Wheat, R.E.; Yovovich, V. The golden age of bio-logging: How animal-borne sensors are advancing the frontiers of ecology. Ecology 2015, 96, 1741–1753. [Google Scholar] [CrossRef]
- Guisan, A.; Thuiller, W. Predicting species distribution: Offering more than simple habitat models. Ecol. Lett. 2005, 8, 993–1009. [Google Scholar] [CrossRef] [PubMed]
- Araújo, M.B.; New, M. Ensemble forecasting of species distributions. Trends Ecol. Evol. 2007, 22, 42–47. [Google Scholar] [CrossRef] [PubMed]
- De la Cruz, A.; Numa, C. Habitat availability decline for waterbirds in a sensitive wetland: Climate change impact on the Ebro Delta. Ecol. Model. 2024, 498, 110896. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Z.; Zhang, J.; Zhu, B.; Tian, J. Projection of the potential distribution of suitable habitats for Siberian crane (Grus leucogeranus) in the middle and lower reaches of the Yangtze River basin. Front. Earth Sci. 2023, 11, 1193677. [Google Scholar] [CrossRef]
- Zurell, D.; Zimmermann, N.E.; Brun, P. The niche through time: Considering phenology and demographic stages in plant distribution models. J. Ecol. 2024, 112, 1926–1939. [Google Scholar] [CrossRef]
- International, B. Anser albifrons. The IUCN Red List of Threatened Species 2022; e.T22679881A213839615; The International Union for Conservation of Nature: Gland, Switzerland, 2022. [Google Scholar] [CrossRef]
- Wang, C.; Xia, S.; Yu, X.; Wen, L. Responses to extreme drought in wintering waterbirds: A multi-species approach. Front. Zool 2025, 22, 3. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Xu, F.; Cole, E.F.; Sheldon, B.C.; de Boer, W.F.; Wielstra, B.; Fu, H.; Gong, P.; Si, Y. Spatially heterogeneous shifts in vegetation phenology induced by climate change threaten the integrity of the avian migration network. Glob Chang. Biol 2024, 30, e17148. [Google Scholar] [CrossRef]
- Fan, R.; Lei, J.; Wu, E.; Lu, C.; Jia, Y.; Zeng, Q.; Lei, G. Species Distribution Modeling of the Breeding Site Distribution and Conservation Gaps of Lesser White-Fronted Goose in Siberia under Climate Change. Land 2022, 11, 1946. [Google Scholar] [CrossRef]
- Wauchope, H.S.; Shaw, J.D.; Varpe, Ø.; Lappo, E.G.; Boertmann, D.; Lanctot, R.B.; Fuller, R.A. Rapid climate-driven loss of breeding habitat for Arctic migratory birds. Glob. Change Biol. 2016, 23, 1085–1094. [Google Scholar] [CrossRef]
- Deng, X.; Zhao, Q.; Zhang, J.; Kölzsch, A.; Solovyeva, D.; Bysykatova-Harmey, I.; Xu, Z.; Kruckenberg, H.; Cao, L.; Fox, A.D. Contrasting habitat use and conservation status of Chinese-wintering and other Eurasian Greater White-fronted Goose (Anser albifrons) populations. Avian Res. 2021, 12, 71. [Google Scholar] [CrossRef]
- Iverson, A.R.; Schaefer, J.L.; Skalos, S.M.; Hawkins, C.E. Global positioning system (GPS) and platform transmitter terminal (PTT) tags reveal fine-scale migratory movements of small birds: A review highlights further opportunities for hypothesis-driven research. Ornithol. Appl. 2023, 125, duad014. [Google Scholar] [CrossRef]
- Garriga, J.; Palmer, J.R.; Oltra, A.; Bartumeus, F. Expectation-maximization binary clustering for behavioural annotation. PLoS ONE 2016, 11, e0151984. [Google Scholar] [CrossRef]
- Deng, X.; Zhao, Q.; Fang, L.; Xu, Z.; Wang, X.; He, H.; Cao, L.; Fox, A.D. Spring migration duration exceeds that of autumn migration in Far East Asian Greater White-fronted Geese (Anser albifrons). Avian Res. 2019, 10, 19. [Google Scholar] [CrossRef]
- Kölzsch, A.; Müskens, G.J.D.M.; Kruckenberg, H.; Glazov, P.; Weinzierl, R.; Nolet, B.A.; Wikelski, M. Towards a new understanding of migration timing: Slower spring than autumn migration in geese reflects different decision rules for stopover use and departure. Oikos 2016, 125, 1496–1507. [Google Scholar] [CrossRef]
- Hernandez, P.A.; Graham, C.H.; Master, L.L.; Albert, D.L. The effect of sample size and species characteristics on performance of different species distribution modeling methods. Ecography 2006, 29, 773–785. [Google Scholar] [CrossRef]
- Venter, O.; Sanderson, E.W.; Magrach, A.; Allan, J.R.; Beher, J.; Jones, K.R.; Possingham, H.P.; Laurance, W.F.; Wood, P.; Fekete, B.M.; et al. Global terrestrial Human Footprint maps for 1993 and 2009. Sci. Data 2016, 3, 160067. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Lu, Y.; Fang, Y.; Xin, X.; Li, L.; Li, W.; Jie, W.; Zhang, J.; Liu, Y.; Zhang, L. The Beijing climate center climate system model (BCC-CSM): The main progress from CMIP5 to CMIP6. Geosci. Model Dev. 2019, 12, 1573–1600. [Google Scholar] [CrossRef]
- De Marco, P.; Nóbrega, C.C. Evaluating collinearity effects on species distribution models: An approach based on virtual species simulation. PLoS ONE 2018, 13, e0202403. [Google Scholar] [CrossRef]
- Naimi, B.; Araújo, M.B. sdm: A reproducible and extensible R platform for species distribution modelling. Ecography 2016, 39, 368–375. [Google Scholar] [CrossRef]
- Ahmadi, M.; Nawaz, M.A.; Asadi, H.; Hemami, M.R.; Naderi, M.; Shafapourtehrany, M.; Shabani, F. Protecting alpine biodiversity in the Middle East from climate change: Implications for high-elevation birds. Divers. Distrib. 2024, 30, e13826. [Google Scholar] [CrossRef]
- Jiménez-Valverde, A.; Lobo, J.M. Threshold criteria for conversion of probability of species presence to either–or presence–absence. Acta Oecologica 2007, 31, 361–369. [Google Scholar] [CrossRef]
- Liang, J.; Gao, X.; Zeng, G.; Hua, S.; Zhong, M.; Li, X.; Li, X. Coupling Modern Portfolio Theory and Marxan enhances the efficiency of Lesser White-fronted Goose’s (Anser erythropus) habitat conservation. Sci. Rep. 2018, 8, 214. [Google Scholar] [CrossRef]
- Brown, C.E. Applied Multivariate Statistics in Geohydrology and Related Sciences; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Ely, C.R.; Fox, A.D.; Alisauskas, R.T.; Andreev, A.; Bromley, R.G.; Degtyarev, A.G.; Ebbinge, B.; Gurtovaya, E.N.; Kerbes, R.; Kondratyev, A.V.; et al. Circumpolar variation in morphological characteristics of Greater White-fronted GeeseAnser albifrons. Bird Study 2010, 52, 104–119. [Google Scholar] [CrossRef]
- Si, Y.; Xu, Y.; Xu, F.; Li, X.; Zhang, W.; Wielstra, B.; Wei, J.; Liu, G.; Luo, H.; Takekawa, J.; et al. Spring migration patterns, habitat use, and stopover site protection status for two declining waterfowl species wintering in China as revealed by satellite tracking. Ecol. Evol. 2018, 8, 6280–6289. [Google Scholar] [CrossRef]
- Zhang, W.; Li, X.; Yu, L.; Si, Y. Multi-scale habitat selection by two declining East Asian waterfowl species at their core spring stopover area. Ecol. Indic. 2018, 87, 127–135. [Google Scholar] [CrossRef]
- Zhang, Y.; Prins, H.H.T.; Cao, L.; Zhao, M.; Boer, W.F.d. Variation in Elevation and Sward Height Facilitate Coexistence of Goose Species through Allometric Responses in Wetlands. Waterbirds 2016, 39, 34–44. [Google Scholar] [CrossRef][Green Version]
- Zhang, J.; Deng, X.; Zhao, Q.; Solovyeva, D.; Kölzsch, A.; Bysykatova-Harmey, I.; Xu, Z.; Xie, Y.; Kruckenberg, H.; Cao, L.; et al. Biogeographical variation in migratory patterns of palearctic breeding Greater White-fronted Geese. J. Biogeogr. 2023, 51, 215–229. [Google Scholar] [CrossRef]
- Bradie, J.; Leung, B. A quantitative synthesis of the importance of variables used in MaxEnt species distribution models. J. Biogeogr. 2016, 44, 1344–1361. [Google Scholar] [CrossRef]
- Li, X.; Si, Y.; Ji, L.; Gong, P. Dynamic response of East Asian Greater White-fronted Geese to changes of environment during migration: Use of multi-temporal species distribution model. Ecol. Model. 2017, 360, 70–79. [Google Scholar] [CrossRef]
- Lameris, T.K.; Scholten, I.; Bauer, S.; Cobben, M.M.P.; Ens, B.J.; Nolet, B.A. Potential for an Arctic-breeding migratory bird to adjust spring migration phenology to Arctic amplification. Glob. Change Biol. 2017, 23, 4058–4067. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Cai, Y.; Li, B.; Chen, J. Managing Wetland Habitats for Waterbirds: An International Perspective. Wetlands 2009, 30, 15–27. [Google Scholar] [CrossRef]
- Gillings, S.; Balmer, D.E.; Fuller, R.J. Directionality of recent bird distribution shifts and climate change in Great Britain. Glob. Change Biol. 2015, 21, 2155–2168. [Google Scholar] [CrossRef]
- Martins, P.M.; Anderson, M.J.; Sweatman, W.L.; Punnett, A.J. Significant shifts in latitudinal optima of North American birds. Proc. Natl. Acad. Sci. USA 2024, 121, e2307525121. [Google Scholar] [CrossRef]
- Pearson, R.G.; Phillips, S.J.; Loranty, M.M.; Beck, P.S.A.; Damoulas, T.; Knight, S.J.; Goetz, S.J. Shifts in Arctic vegetation and associated feedbacks under climate change. Nat. Clim. Change 2013, 3, 673–677. [Google Scholar] [CrossRef]
- Klaassen, M. Relationships between migration and breeding strategies in arctic breeding birds. In Avian Migration; Springer: Berlin/Heidelberg, Germany, 2003; pp. 237–249. [Google Scholar]
- Li, J.; Dong, W.; Yan, Z. Changes of climate extremes of temperature and precipitation in summer in eastern China associated with changes in atmospheric circulation in East Asia during 1960–2008. Chin. Sci. Bull. 2012, 57, 1856–1861. [Google Scholar] [CrossRef]
- Liu, Y.; Long, H. Land use transitions and their dynamic mechanism: The case of the Huang-Huai-Hai Plain. J. Geogr. Sci. 2016, 26, 515–530. [Google Scholar] [CrossRef]
- Xu, W.; Fan, X.; Ma, J.; Pimm, S.L.; Kong, L.; Zeng, Y.; Li, X.; Xiao, Y.; Zheng, H.; Liu, J.; et al. Hidden Loss of Wetlands in China. Curr. Biol. 2019, 29, 3065–3071.e3062. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Wang, X.; Cao, L.; Fox, A.D. Why Chinese wintering geese hesitate to exploit farmland. IBIS 2018, 160, 703–705. [Google Scholar] [CrossRef]
- Callaghan, C.T.; Chase, J.M.; McGlinn, D.J. Anthropogenic habitat modification causes nonlinear multiscale bird diversity declines. Ecography 2024, 2024, e06759. [Google Scholar] [CrossRef]
- Groves, C.R.; Game, E.T.; Anderson, M.G.; Cross, M.; Enquist, C.; Ferdana, Z.; Girvetz, E.; Gondor, A.; Hall, K.R.; Higgins, J. Incorporating climate change into systematic conservation planning. Biodivers. Conserv. 2012, 21, 1651–1671. [Google Scholar] [CrossRef]
- Edney, A.J.; Wood, M.J. Applications of digital imaging and analysis in seabird monitoring and research. IBIS 2020, 163, 317–337. [Google Scholar] [CrossRef]
- Oliver, R.Y.; Ellis, D.P.; Chmura, H.E.; Krause, J.S.; Pérez, J.H.; Sweet, S.K.; Gough, L.; Wingfield, J.C.; Boelman, N.T. Eavesdropping on the Arctic: Automated bioacoustics reveal dynamics in songbird breeding phenology. Sci. Adv. 2018, 4, eaaq1084. [Google Scholar] [CrossRef]
- Smith, P.A.; McKinnon, L.; Meltofte, H.; Lanctot, R.B.; Fox, A.D.; Leafloor, J.O.; Soloviev, M.; Franke, A.; Falk, K.; Golovatin, M.; et al. Status and trends of tundra birds across the circumpolar Arctic. Ambio 2020, 49, 732–748. [Google Scholar] [CrossRef] [PubMed]
- Si, Y.; Xu, F.; Wei, J.; Zhang, L.; Murray, N.; Yang, R.; Ma, K.; Gong, P. A systematic network-based migratory bird monitoring and protection system is needed in China. Sci. Bull. 2021, 66, 955–957. [Google Scholar] [CrossRef]
- Si, Y.; Wei, J.; Wu, W.; Zhang, W.; Hou, L.; Yu, L.; Wielstra, B. Reducing human pressure on farmland could rescue China’s declining wintering geese. Mov. Ecol. 2020, 8, 35. [Google Scholar] [CrossRef] [PubMed]
- Streicher, U.; Crudge, B. Muong La proposed Species and Habitat Conservation Area, Vietnam. Oryx 2012, 46, 479. [Google Scholar] [CrossRef][Green Version]
- Teng, J.; Xia, S.; Liu, Y.; Yu, X.; Duan, H.; Xiao, H.; Zhao, C. Assessing habitat suitability for wintering geese by using Normalized Difference Water Index (NDWI) in a large floodplain wetland, China. Ecol. Indic. 2021, 122, 107260. [Google Scholar] [CrossRef]
- Kim, E.-J.; Hur, W.-H.; Kim, H.-J.; Choi, Y.-S.; Kim, D.; Lee, W.-S.; Han, S.; Joo, H.; Choi, C.-Y. Population trend and spatio-temporal distribution of Greater White-fronted (Anser albifrons) and Bean Geese (Anser fabalis) in Korea. Avian Res. 2024, 15, 100214. [Google Scholar] [CrossRef]
| Migration Stage | Variable Code | Variable Name | Unit |
|---|---|---|---|
| Breeding period | bio1 | Annual mean temperature | °C |
| bio15 | Precipitation seasonality (coefficient of variation) | % | |
| bio16 | Precipitation of wettest quarter | mm | |
| bio3 | Isothermality (bio2/bio7) (×100) | / | |
| bio8 | Mean temperature of wettest quarter | °C | |
| bio9 | Mean temperature of driest quarter | °C | |
| EL | Elevation | m | |
| HFI | Human footprint index | / | |
| LU | Land use | / | |
| Slope | Slope | % | |
| Stopover period | bio12 | Annual precipitation | mm |
| bio14 | Precipitation of driest month | mm | |
| bio2 | Mean diurnal range (mean of monthly (max temp − min temp)) | °C | |
| bio5 | Max temperature of warmest month | °C | |
| bio9 | Mean temperature of driest quarter | °C | |
| EL | Elevation | m | |
| HFI | Human footprint index | / | |
| LU | Land use | / | |
| Slope | Slope | % | |
| Wintering period | bio10 | Mean temperature of warmest quarter | °C |
| bio14 | Precipitation of driest month | mm | |
| bio15 | Precipitation seasonality (coefficient of variation) | % | |
| bio18 | Precipitation of warmest quarter | mm | |
| bio3 | Isothermality (bio2/bio7) (×100) | / | |
| bio4 | Temperature seasonality (standard deviation × 100) | °C | |
| bio8 | Mean temperature of wettest quarter | °C | |
| bio9 | Mean temperature of driest quarter | °C | |
| EL | Elevation | m | |
| HFI | Human footprint index | / | |
| LU | Land use | / | |
| Slope | Slope | % |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.; Xia, S.; Yu, X.; Duan, H.; Qi, G. Stage-Specific Impacts of Climate Change on Greater White-Fronted Geese Along the East Asian Flyway. Biology 2025, 14, 1050. https://doi.org/10.3390/biology14081050
Wang C, Xia S, Yu X, Duan H, Qi G. Stage-Specific Impacts of Climate Change on Greater White-Fronted Geese Along the East Asian Flyway. Biology. 2025; 14(8):1050. https://doi.org/10.3390/biology14081050
Chicago/Turabian StyleWang, Chunxiao, Shaoxia Xia, Xiubo Yu, Houlang Duan, and Guang Qi. 2025. "Stage-Specific Impacts of Climate Change on Greater White-Fronted Geese Along the East Asian Flyway" Biology 14, no. 8: 1050. https://doi.org/10.3390/biology14081050
APA StyleWang, C., Xia, S., Yu, X., Duan, H., & Qi, G. (2025). Stage-Specific Impacts of Climate Change on Greater White-Fronted Geese Along the East Asian Flyway. Biology, 14(8), 1050. https://doi.org/10.3390/biology14081050







