C-Terminal Modification Contributes the Antibacterial Activity of a Cecropin-like Region of Heteroscorpine-1 from Scorpion Venom
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Peptide Design
2.2. Peptide Synthesis
2.3. Antimicrobial Activity Assay
2.4. Hemolytic Activity Assay
2.5. In Silico Prediction of Hemolytic Activity
2.6. Membrane Disruption Property Assay
2.7. DNA-Binding Assay
2.8. Molecular Docking
3. Results
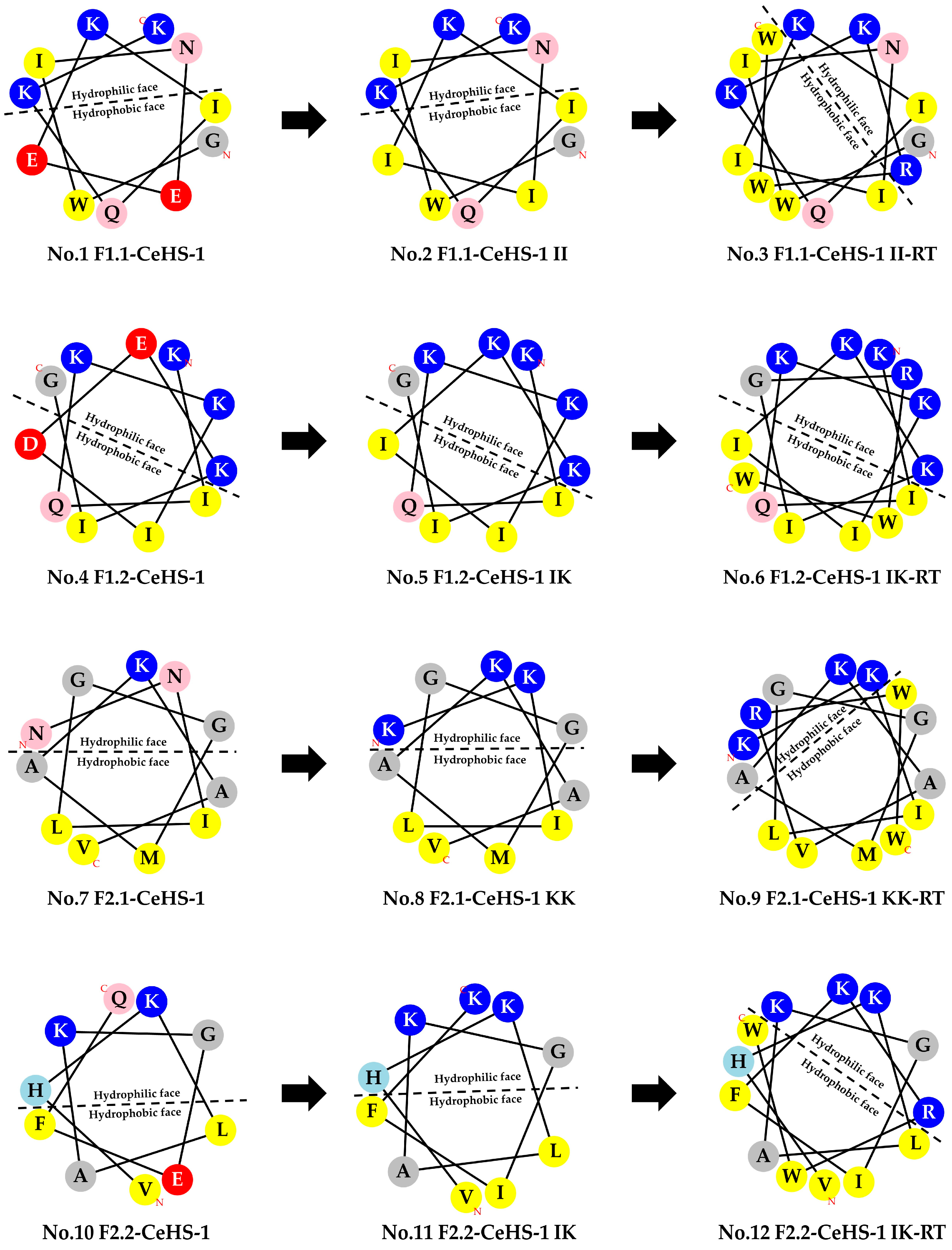
3.1. Peptides Design and Physicochemical Properties
3.2. Antimicrobial Activity
3.3. Hemolytic Activity
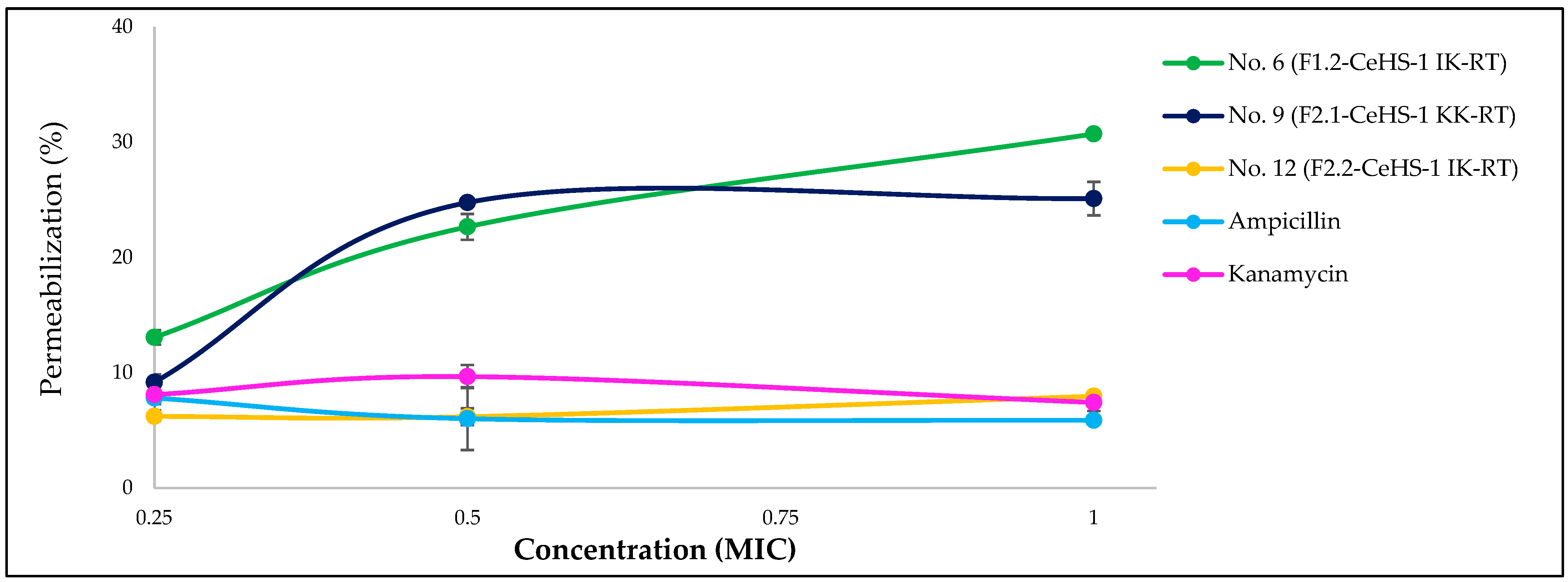
3.4. Membrane Disruption Property
3.5. DNA-Binding Property
3.6. Molecular Docking for DNA-Binding Property
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mwangi, J.; Kamau, P.M.; Thuku, R.C.; Lai, R. Design Methods for Antimicrobial Peptides with Improved Performance. Zool. Res. 2023, 44, 1095–1114. [Google Scholar] [CrossRef]
- Antimicrobial Resistance Collaborators. Global Burden of Bacterial Antimicrobial Resistance in 2019: A Systematic Analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- de Kraker, M.E.A.; Stewardson, A.J.; Harbarth, S. Will 10 Million People Die a Year Due to Antimicrobial Resistance by 2050? PLoS Med. 2016, 13, e1002184. [Google Scholar] [CrossRef] [PubMed]
- Ventola, C.L. The Antibiotic Resistance Crisis: Part 1: Causes and Threats. Pharm. Ther. 2015, 40, 277–283. [Google Scholar]
- Dong, C.; Li, M.; Zhang, R.; Lu, W.; Xu, L.; Liu, J.; Chu, X. The Expression of Antibacterial Peptide Turgencin A in Pichia Pastoris and an Analysis of Its Antibacterial Activity. Molecules 2023, 28, 5405. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Jiang, C. Antimicrobial Peptides: Structure, Mechanism, and Modification. Eur. J. Med. Chem. 2023, 255, 115377. [Google Scholar] [CrossRef]
- da Cunha, N.B.; Cobacho, N.B.; Viana, J.F.C.; Lima, L.A.; Sampaio, K.B.O.; Dohms, S.S.M.; Ferreira, A.C.R.; de la Fuente-Núñez, C.; Costa, F.F.; Franco, O.L.; et al. The next Generation of Antimicrobial Peptides (AMPs) as Molecular Therapeutic Tools for the Treatment of Diseases with Social and Economic Impacts. Drug Discov. Today 2017, 22, 234–248. [Google Scholar] [CrossRef]
- Watkins, R.R.; Bonomo, R.A. Overview: Global and Local Impact of Antibiotic Resistance. Infect. Dis. Clin. N. Am. 2016, 30, 313–322. [Google Scholar] [CrossRef]
- Ma, X.; Wang, Q.; Ren, K.; Xu, T.; Zhang, Z.; Xu, M.; Rao, Z.; Zhang, X. A Review of Antimicrobial Peptides: Structure, Mechanism of Action, and Molecular Optimization Strategies. Fermentation 2024, 10, 540. [Google Scholar] [CrossRef]
- Asensio-Calavia, P.; González-Acosta, S.; Otazo-Pérez, A.; López, M.R.; Morales-delaNuez, A.; Pérez de la Lastra, J.M. Teleost Piscidins-in Silico Perspective of Natural Peptide Antibiotics from Marine Sources. Antibiotics 2023, 12, 855. [Google Scholar] [CrossRef]
- Sinha, R.; Shukla, P. Antimicrobial Peptides: Recent Insights on Biotechnological Interventions and Future Perspectives. Protein Pept. Lett. 2019, 26, 79–87. [Google Scholar] [CrossRef]
- Huerta-Cantillo, J.; Navarro-García, F. Properties and Design of Antimicrobial Peptides as Potential Tools against Pathogens and Malignant Cells. Investig. Discapac. 2016, 5, 96–115. [Google Scholar]
- Rungsa, P.; Peigneur, S.; Jangpromma, N.; Klaynongsruang, S.; Tytgat, J.; Daduang, S. In Silico and in Vitro Structure-Activity Relationship of Mastoparan and Its Analogs. Molecules 2022, 27, 561. [Google Scholar] [CrossRef] [PubMed]
- Tajer, L.; Paillart, J.C.; Dib, H.; Sabatier, J.M.; Fajloun, Z.; Abi Khattar, Z. Molecular Mechanisms of Bacterial Resistance to Antimicrobial Peptides in the Modern Era: An Updated Review. Microorganisms 2024, 12, 1259. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.-H.; Hall, K.N.; Aguilar, M.-I. Antimicrobial Peptide Structure and Mechanism of Action: A Focus on the Role of Membrane Structure. Curr. Top. Med. Chem. 2016, 16, 25–39. [Google Scholar] [CrossRef] [PubMed]
- Gagat, P.; Ostrówka, M.; Duda-Madej, A.; Mackiewicz, P. Enhancing Antimicrobial Peptide Activity through Modifications of Charge, Hydrophobicity, and Structure. Int. J. Mol. Sci. 2024, 25, 10821. [Google Scholar] [CrossRef]
- Hilpert, K.; McLeod, B.; Yu, J.; Elliott, M.R.; Rautenbach, M.; Ruden, S.; Bürck, J.; Muhle-Goll, C.; Ulrich, A.S.; Keller, S.; et al. Short Cationic Antimicrobial Peptides Interact with ATP. Antimicrob. Agents Chemother. 2010, 54, 4480–4483. [Google Scholar] [CrossRef]
- Gaspar, D.; Veiga, A.S.; Castanho, M.A.R.B. From Antimicrobial to Anticancer Peptides. a Review. Front. Microbiol. 2013, 4, 294. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Jang, J.H.; Kim, S.C.; Cho, J.H. De Novo Generation of Short Antimicrobial Peptides with Enhanced Stability and Cell Specificity. J. Antimicrob. Chemother. 2014, 69, 121–132. [Google Scholar] [CrossRef]
- Li, J.; Koh, J.-J.; Liu, S.; Lakshminarayanan, R.; Verma, C.S.; Beuerman, R.W. Membrane Active Antimicrobial Peptides: Translating Mechanistic Insights to Design. Front. Neurosci. 2017, 11, 73. [Google Scholar] [CrossRef]
- Huan, Y.; Kong, Q.; Mou, H.; Yi, H. Antimicrobial Peptides: Classification, Design, Application and Research Progress in Multiple Fields. Front. Microbiol. 2020, 11, 582779. [Google Scholar] [CrossRef]
- Mikut, R.; Ruden, S.; Reischl, M.; Breitling, F.; Volkmer, R.; Hilpert, K. Improving Short Antimicrobial Peptides despite Elusive Rules for Activity. Biochim. Biophys. Acta 2016, 1858, 1024–1033. [Google Scholar] [CrossRef] [PubMed]
- Mangoni, M.L.; Shai, Y. Short Native Antimicrobial Peptides and Engineered Ultrashort Lipopeptides: Similarities and Differences in Cell Specificities and Modes of Action. Cell. Mol. Life Sci. 2011, 68, 2267–2280. [Google Scholar] [CrossRef] [PubMed]
- Sharma, L.; Bisht, G.S. Short Antimicrobial Peptides: Therapeutic Potential and Recent Advancements. Curr. Pharm. Des. 2023, 29, 3005–3017. [Google Scholar] [CrossRef] [PubMed]
- Nell, M.J.; Tjabringa, G.S.; Wafelman, A.R.; Verrijk, R.; Hiemstra, P.S.; Drijfhout, J.W.; Grote, J.J. Development of Novel LL-37 Derived Antimicrobial Peptides with LPS and LTA Neutralizing and Antimicrobial Activities for Therapeutic Application. Peptides 2006, 27, 649–660. [Google Scholar] [CrossRef]
- de Breij, A.; Riool, M.; Cordfunke, R.A.; Malanovic, N.; de Boer, L.; Koning, R.I.; Ravensbergen, E.; Franken, M.; van der Heijde, T.; Boekema, B.K.; et al. The Antimicrobial Peptide SAAP-148 Combats Drug-Resistant Bacteria and Biofilms. Sci. Transl. Med. 2018, 10, eaan4044. [Google Scholar] [CrossRef]
- Erviana, R.; Saengkun, Y.; Rungsa, P.; Jangpromma, N.; Tippayawat, P.; Klaynongsruang, S.; Daduang, J.; Daduang, S. Novel Antimicrobial Peptides from a Cecropin-like Region of Heteroscorpine-1 from Heterometrus Laoticus Venom with Membrane Disruption Activity. Molecules 2021, 26, 5872. [Google Scholar] [CrossRef]
- Torres, M.D.T.; Sothiselvam, S.; Lu, T.K.; de la Fuente-Nunez, C. Peptide Design Principles for Antimicrobial Applications. J. Mol. Biol. 2019, 431, 3547–3567. [Google Scholar] [CrossRef]
- Lee, J.-K.; Park, S.-C.; Hahm, K.-S.; Park, Y. Antimicrobial HPA3NT3 Peptide Analogs: Placement of Aromatic Rings and Positive Charges Are Key Determinants for Cell Selectivity and Mechanism of Action. Biochim. Biophys. Acta 2013, 1828, 443–454. [Google Scholar] [CrossRef]
- Cerovský, V.; Hovorka, O.; Cvacka, J.; Voburka, Z.; Bednárová, L.; Borovicková, L.; Slaninová, J.; Fucík, V. Melectin: A Novel Antimicrobial Peptide from the Venom of the Cleptoparasitic Bee Melecta Albifrons. Chembiochem 2008, 9, 2815–2821. [Google Scholar] [CrossRef]
- Anunthawan, T.; Yaraksa, N.; Phosri, S.; Theansungnoen, T.; Daduang, S.; Dhiravisit, A.; Thammasirirak, S. Improving the Antibacterial Activity and Selectivity of an Ultra Short Peptide by Hydrophobic and Hydrophilic Amino Acid Stretches. Bioorg. Med. Chem. Lett. 2013, 23, 4657–4662. [Google Scholar] [CrossRef]
- Schmidtchen, A.; Pasupuleti, M.; Mörgelin, M.; Davoudi, M.; Alenfall, J.; Chalupka, A.; Malmsten, M. Boosting Antimicrobial Peptides by Hydrophobic Oligopeptide End Tags. J. Biol. Chem. 2009, 284, 17584–17594. [Google Scholar] [CrossRef]
- Pasupuleti, M.; Schmidtchen, A.; Chalupka, A.; Ringstad, L.; Malmsten, M. End-Tagging of Ultra-Short Antimicrobial Peptides by W/F Stretches to Facilitate Bacterial Killing. PLoS ONE 2009, 4, e5285. [Google Scholar] [CrossRef]
- Gan, B.H.; Gaynord, J.; Rowe, S.M.; Deingruber, T.; Spring, D.R. The Multifaceted Nature of Antimicrobial Peptides: Current Synthetic Chemistry Approaches and Future Directions. Chem. Soc. Rev. 2021, 50, 7820–7880. [Google Scholar] [CrossRef]
- de Santana, C.J.C.; Pires Júnior, O.R.; Fontes, W.; Palma, M.S.; Castro, M.S. Mastoparans: A Group of Multifunctional α-Helical Peptides with Promising Therapeutic Properties. Front. Mol. Biosci. 2022, 9, 824989. [Google Scholar] [CrossRef]
- Mangoni, M.L.; Fiocco, D.; Barra, D.; Simmaco, M. Bombinins. In Handbook of Biologically Active Peptides; Academic Press: Cambridge, MA, USA, 2006; pp. 333–337. [Google Scholar] [CrossRef]
- Shahmiri, M.; Mechler, A. The Role of C-Terminal Amidation in the Mechanism of Action of the Antimicrobial Peptide Aurein 1.2. Eurobiotech J. 2020, 4, 25–31. [Google Scholar] [CrossRef]
- Van Wyk, R.J.; Serem, J.C.; Oosthuizen, C.B.; Semenya, D.; Serian, M.; Lorenz, C.D.; Mason, A.J.; Bester, M.J.; Gaspar, A.R.M. Carboxy-Amidated AamAP1-Lys Has Superior Conformational Flexibility and Accelerated Killing of Gram-Negative Bacteria. Biochemistry 2025, 64, 841–859. [Google Scholar] [CrossRef]
- Nikookar Golestani, R.; Ghods, E.; Rostamian, M.; Madanchi, H.; Talebi, A.F. Investigating the Antimicrobial Activity, Cytotoxicity, and Action Mechanism of Acylated and Amidated Derivatives of AurH1 Antifungal Peptide. BMC Microbiol. 2023, 23, 332. [Google Scholar] [CrossRef] [PubMed]
- Salazar-Hernández, D.; Juárez-González, V.R.; Bustamante, V.H.; Martínez-Martínez, L.L.; Ramírez, V.; Balleza, D.; Quintero-Hernández, V. Conformational Flexibility and Net Charge Are Key Determinants for the Antimicrobial Activity of Peptide Uy234 Against Multidrug-Resistant Bacteria. Int. J. Pept. Res. Ther. 2024, 30, 79. [Google Scholar] [CrossRef]
- Roy, A.; Bharadvaja, N. Venom-Derived Bioactive Compounds as Potential Anticancer Agents: A Review. Int. J. Pept. Res. Ther. 2021, 27, 129–147. [Google Scholar] [CrossRef]
- Kawakami, H.; Goto, S.G.; Murata, K.; Matsuda, H.; Shigeri, Y.; Imura, T.; Inagaki, H.; Shinada, T. Isolation of Biologically Active Peptides from the Venom of Japanese Carpenter Bee, Xylocopa Appendiculata. J. Venom. Anim. Toxins Incl. Trop. Dis. 2017, 23, 29. [Google Scholar] [CrossRef] [PubMed]
- Bordon, K.D.C.F.; Cologna, C.T.; Fornari-Baldo, E.C.; Pinheiro-Júnior, E.L.; Cerni, F.A.; Amorim, F.G.; Anjolette, F.A.P.; Cordeiro, F.A.; Wiezel, G.A.; Cardoso, I.A.; et al. From Animal Poisons and Venoms to Medicines: Achievements, Challenges and Perspectives in Drug Discovery. Front. Pharmacol. 2020, 11, 1132. [Google Scholar] [CrossRef]
- Garvey, M. Antimicrobial Peptides Demonstrate Activity against Resistant Bacterial Pathogens. Infect. Dis. Rep. 2023, 15, 454–469. [Google Scholar] [CrossRef]
- Dijksteel, G.S.; Ulrich, M.M.W.; Middelkoop, E.; Boekema, B.K.H.L. Review: Lessons Learned from Clinical Trials Using Antimicrobial Peptides (AMPs). Front. Microbiol. 2021, 12, 616979. [Google Scholar] [CrossRef] [PubMed]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in Vitro Evaluating Antimicrobial Activity: A Review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef]
- Al-Ani, I.; Zimmermann, S.; Reichling, J.; Wink, M. Pharmacological Synergism of Bee Venom and Melittin with Antibiotics and Plant Secondary Metabolites against Multi-Drug Resistant Microbial Pathogens. Phytomedicine 2015, 22, 245–255. [Google Scholar] [CrossRef]
- Mondragón, A.; Harrison, S.C. The Phage 434 CroOR1 Complex at 2.5 Å Resolution. J. Mol. Biol. 1991, 219, 321–334. [Google Scholar] [CrossRef]
- Rozek, T.; Wegener, K.L.; Bowie, J.H.; Olver, I.N.; Carver, J.A.; Wallace, J.C.; Tyler, M.J. The Antibiotic and Anticancer Active Aurein Peptides from the Australian Bell Frogs Litoria Aurea and Litoria Raniformis the Solution Structure of Aurein 1.2. Eur. J. Biochem. 2000, 267, 5330–5341. [Google Scholar] [CrossRef]
- Qi, X.; Zhou, C.; Li, P.; Xu, W.; Cao, Y.; Ling, H.; Ning Chen, W.; Ming Li, C.; Xu, R.; Lamrani, M.; et al. Novel Short Antibacterial and Antifungal Peptides with Low Cytotoxicity: Efficacy and Action Mechanisms. Biochem. Biophys. Res. Commun. 2010, 398, 594–600. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos Cabrera, M.P.; De Souza, B.M.; Fontana, R.; Konno, K.; Palma, M.S.; De Azevedo, W.F.; Ruggiero Neto, J. Conformation and Lytic Activity of Eumenine Mastoparan: A New Antimicrobial Peptide from Wasp Venom. J. Pept. Res. 2004, 64, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Prates, M.V.; Sforça, M.L.; Regis, W.C.B.; Leite, J.R.S.A.; Silva, L.P.; Pertinhez, T.A.; Araújo, A.L.T.; Azevedo, R.B.; Spisni, A.; Bloch, C. The NMR-Derived Solution Structure of a New Cationic Antimicrobial Peptide from the Skin Secretion of the Anuran Hyla Punctata. J. Biol. Chem. 2004, 279, 13018–13026. [Google Scholar] [CrossRef]
- Wu, S.; Nie, Y.; Zeng, X.C.; Cao, H.; Zhang, L.; Zhou, L.; Yang, Y.; Luo, X.; Liu, Y. Genomic and Functional Characterization of Three New Venom Peptides from the Scorpion Heterometrus Spinifer. Peptides 2014, 53, 30–41. [Google Scholar] [CrossRef]
- Liu, R.; Liu, H.; Ma, Y.; Wu, J.; Yang, H.; Ye, H.; Lai, R. There Are Abundant Antimicrobial Peptides in Brains of Two Kinds of Bombina Toads. J. Proteome Res. 2011, 10, 1806–1815. [Google Scholar] [CrossRef]
- Csordás, A.; Michl, H. Isolierung Und Strukturaufklärung Eines Hämolytisch Wirkenden Polypeptides Aus Dem Abwehrsekret Europäischer Unken. Monatsh Chem. 1970, 101, 182–189. [Google Scholar] [CrossRef]
- Yang, X.; Lee, W.-H.; Zhang, Y. Extremely Abundant Antimicrobial Peptides Existed in the Skins of Nine Kinds of Chinese Odorous Frogs. J. Proteome Res. 2012, 11, 306–319. [Google Scholar] [CrossRef]
- King, J.D.; Al-Ghaferi, N.; Abraham, B.; Sonnevend, A.; Leprince, J.; Nielsen, P.F.; Conlon, J.M. Pentadactylin: An Antimicrobial Peptide from the Skin Secretions of the South American Bullfrog Leptodactylus Pentadactylus. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2005, 141, 393–397. [Google Scholar] [CrossRef]
- Thompson, A.H.; Bjourson, A.J.; Orr, D.F.; Shaw, C.; McClean, S. A Combined Mass Spectrometric and CDNA Sequencing Approach to the Isolation and Characterization of Novel Antimicrobial Peptides from the Skin Secretions of Phyllomedusa Hypochondrialis Azurea. Peptides 2007, 28, 1331–1343. [Google Scholar] [CrossRef]
- Bradford, A.M.; Raftery, M.J.; Bowie, J.H.; Tyler, M.J.; Wallace, J.C.; Adams, G.W.; Severini, C. Novel Uperin Peptides from the Dorsal Glands of the Australian Flood-Plain Toadlet Uperoleia Inundata. Aust. J. Chem. Int. J. Chem. Sci. 1996, 49, 475–484. [Google Scholar] [CrossRef]
- Ouyang, X.; Li, B.; Yang, T.; Yang, Y.; Ba, Z.; Zhang, J.; Yang, P.; Liu, Y.; Wang, Y.; Zhao, Y.; et al. High Therapeutic Index α-Helical AMPs and Their Therapeutic Potential on Bacterial Lung and Skin Wound Infections. ACS Infect. Dis. 2024, 10, 3138–3157. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Watson, K.M.; Peterkofsky, A.; Buckheit, R.W. Identification of Novel Human Immunodeficiency Virus Type 1-Inhibitory Peptides Based on the Antimicrobial Peptide Database. Antimicrob. Agents Chemother. 2010, 54, 1343–1346. [Google Scholar] [CrossRef] [PubMed]
- Monincová, L.; Buděšínský, M.; Slaninová, J.; Hovorka, O.; Cvačka, J.; Voburka, Z.; Fučík, V.; Borovičková, L.; Bednárová, L.; Straka, J.; et al. Novel Antimicrobial Peptides from the Venom of the Eusocial Bee Halictus Sexcinctus (Hymenoptera: Halictidae) and Their Analogs. Amino Acids 2010, 39, 763–775. [Google Scholar] [CrossRef]
- Hong, W.; Li, T.; Song, Y.; Zhang, R.; Zeng, Z.; Han, S.; Zhang, X.; Wu, Y.; Li, W.; Cao, Z. Inhibitory Activity and Mechanism of Two Scorpion Venom Peptides against Herpes Simplex Virus Type 1. Antivir. Res. 2014, 102, 1–10. [Google Scholar] [CrossRef]
- Yang, X.; Hu, Y.; Xu, S.; Hu, Y.; Meng, H.; Guo, C.; Liu, Y.; Liu, J.; Yu, Z.; Wang, H. Identification of Multiple Antimicrobial Peptides from the Skin of Fine-Spined Frog, Hylarana Spinulosa (Ranidae). Biochimie 2013, 95, 2429–2436. [Google Scholar] [CrossRef] [PubMed]
- Richter, A.; Sutherland, D.; Ebrahimikondori, H.; Babcock, A.; Louie, N.; Li, C.; Coombe, L.; Lin, D.; Warren, R.L.; Yanai, A.; et al. Associating Biological Activity and Predicted Structure of Antimicrobial Peptides from Amphibians and Insects. Antibiotics 2022, 11, 1710. [Google Scholar] [CrossRef]
- Lee, J.; Kang, H.K.; Cheong, H.; Park, Y. A Novel Antimicrobial Peptides From Pine Needles of Pinus Densiflora Sieb. et Zucc. Against Foodborne Bacteria. Front. Microbiol. 2021, 12, 662462. [Google Scholar] [CrossRef]
- Gause, G.F.; Brazhnikova, M.G. Gramicidin S and Its Use in the Treatment of Infected Wounds. Nature 1944, 154, 703. [Google Scholar] [CrossRef]
- Cantisani, M.; Leone, M.; Mignogna, E.; Kampanaraki, K.; Falanga, A.; Morelli, G.; Galdiero, M.; Galdiero, S. Structure-Activity Relations of Myxinidin, an Antibacterial Peptide Derived from the Epidermal Mucus of Hagfish. Antimicrob. Agents Chemother. 2013, 57, 5665–5673. [Google Scholar] [CrossRef] [PubMed]
- Sandhu, G.; Morrow, M.R.; Booth, V. Roles of Histidine Charge and Cardiolipin in Membrane Disruption by Antimicrobial Peptides Gaduscidin-1 and Gaduscidin-2. Biochim. Biophys. Acta (BBA)-Biomembr. 2020, 1862, 183444. [Google Scholar] [CrossRef] [PubMed]
- Benfield, A.H.; Henriques, S.T. Mode-of-Action of Antimicrobial Peptides: Membrane Disruption vs. Intracellular Mechanisms. Front. Med. Technol. 2020, 2, 610997. [Google Scholar] [CrossRef]
- Rathinakumar, R.; Walkenhorst, W.F.; Wimley, W.C. Broad-Spectrum Antimicrobial Peptides by Rational Combinatorial Design and High-Throughput Screening: The Importance of Interfacial Activity. J. Am. Chem. Soc. 2009, 131, 7609–7617. [Google Scholar] [CrossRef]
- Sani, M.A.; Henriques, S.T.; Weber, D.; Separovic, F. Bacteria May Cope Differently from Similar Membrane Damage Caused by the Australian Tree Frog Antimicrobial Peptide Maculatin 1.1. J. Biol. Chem. 2015, 290, 19853–19862. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, D.; Mohan, M.; Borade, D.M.; Swami, O.C. Ampicillin: Rise Fall and Resurgence. J. Clin. Diagn. Res. 2014, 8, ME01. [Google Scholar] [CrossRef] [PubMed]
- Mingeot-Leclercq, M.P.; Glupczynski, Y.; Tulkens, P.M. Aminoglycosides: Activity and Resistance. Antimicrob. Agents Chemother. 1999, 43, 727–737. [Google Scholar] [CrossRef]
- Erviana, R.; Saengkun, Y.; Rungsa, P.; Jangpromma, N.; Mustofa; Daduang, S. The Recombinant Expression and Antimicrobial Activity Determination of Cecropin-like Part of Heteroscorpine-1 from Heterometrus Laoticus. Biodiversitas 2022, 23, 5646–5653. [Google Scholar] [CrossRef]
- Le, C.-F.; Fang, C.-M.; Sekaran, S.D. Intracellular Targeting Mechanisms by Antimicrobial Peptides. Antimicrob. Agents Chemother. 2017, 61, 10-1128. [Google Scholar] [CrossRef]
- Zhang, J.; Movahedi, A.; Wei, Z.; Sang, M.; Wu, X.; Wang, M.; Wei, H.; Pan, H.; Yin, T.; Zhuge, Q. High-Level SUMO-Mediated Fusion Expression of ABP-DHC-Cecropin A from Multiple Joined Genes in Escherichia coli. Anal. Biochem. 2016, 509, 15–23. [Google Scholar] [CrossRef]
- Chubukov, V.; Mukhopadhyay, A.; Petzold, C.J.; Keasling, J.D.; Martín, H.G. Synthetic and Systems Biology for Microbial Production of Commodity Chemicals. NPJ Syst. Biol. Appl. 2016, 2, 16009. [Google Scholar] [CrossRef] [PubMed]



| Peptide | Origin | Sequence | AA | Identity (%) | APD ID | Ref. | |||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CeHS-1 | G | W | I | N | E | E | K | - | I | Q | K | K | I | D | E | K | - | I | G | N | N | I | L | G | - | - | - | G | - | - | - | M | A | - | K | - | A | V | - | V | H | K | - | L | A | K | G | - | - | E | - | F | - | Q | 37 | ||||
| F1-CeHS-1 | This work | G | W | I | N | E | E | K | - | I | Q | K | K | I | D | E | K | - | I | G | |||||||||||||||||||||||||||||||||||||||
| Aurein 1.1 | Litoria aurea | G | - | - | - | L | F | D | - | I | I | K | K | I | A | E | S | - | I | - | 13 | 41.18 | AP00012 | [49] | |||||||||||||||||||||||||||||||||||
| P11-5 | Synthetic peptide | G | - | - | - | - | - | K | - | L | F | K | K | I | L | - | K | - | I | L | 11 | 41.18 | AP03683 | [50] | |||||||||||||||||||||||||||||||||||
| Mastoparan-AF | Anterhynchium flavomarginatum micado | - | - | I | N | L | L | K | - | I | A | K | G | I | I | - | K | - | S | L | 14 | 41.18 | AP01517 | [51] | |||||||||||||||||||||||||||||||||||
| P11-6 | Synthetic peptide | - | - | - | - | - | K | K | L | I | - | K | K | I | L | - | K | - | I | L | 11 | 38.89 | AP03684 | [50] | |||||||||||||||||||||||||||||||||||
| Hylaseptin P1 | Hyla punctata | G | - | I | - | L | D | A | - | I | A | - | K | I | A | - | K | A | A | G | 14 | 38.89 | AP01249 | [52] | |||||||||||||||||||||||||||||||||||
| Spiniferin | Heterometrus spinifer | - | - | I | L | G | E | - | - | I | W | K | G | I | - | - | K | D | I | L | 13 | 38.89 | AP02551 | [53] | |||||||||||||||||||||||||||||||||||
| F2-CeHS-1 | This work | N | N | I | L | G | - | - | - | G | - | - | - | M | A | - | K | - | A | V | - | V | H | K | - | L | A | K | G | - | - | E | - | F | - | Q | 21 | ||||||||||||||||||||||
| Maximin 31 | Bombina maxima | - | G | I | - | G | - | - | - | G | A | L | L | S | A | G | K | S | A | - | - | - | L | K | G | L | A | K | G | L | A | E | H | F | - | - | 25 | 43.33 | AP01732 | [54] | |||||||||||||||||||
| Bombinin | Bombina variegata L | - | G | I | - | - | - | - | - | G | A | L | - | S | A | - | K | G | A | - | - | - | L | K | G | L | A | K | G | L | A | E | H | F | A | N | 24 | 42.86 | AP00049 | [55] | |||||||||||||||||||
| Nigrocin-1-OW3 | Odorrana wuchuanensis | - | G | I | L | G | N | I | V | G | - | - | - | M | - | G | K | K | - | V | - | V | C | G | - | L | - | S | G | L | C | 21 | 41.67 | AP01883 | [56] | ||||||||||||||||||||||||
| Ocellatin-P1 | Leptodactylus pentadactylus | - | G | L | L | D | T | L | K | G | - | - | - | A | A | - | K | N | - | V | - | V | - | G | S | L | A | S | K | V | M | E | - | - | L | - | 25 | 40.00 | AP00540 | [57] | |||||||||||||||||||
| Phylloseptin-H11 | Phyllomedusa hypochondrialis | F | L | S | L | - | - | I | P | - | - | - | - | H | A | I | - | N | A | V | G | V | H | - | - | - | A | K | - | - | - | - | H | F | - | - | 19 | 39.13 | AP00972 | [58] | |||||||||||||||||||
| Uperin 3.6 | Uperoleia inundata | G | V | I | - | A | A | - | K | K | - | V | - | V | N | V | - | L | - | K | N | L | - | - | - | F | 17 | 38.10 | AP00325 | [59] | |||||||||||||||||||||||||||||
| Peptide | Origin | Sequence | AA | Identity (%) | APD ID | Ref. | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F1-CeHS-1 | G | - | W | - | I | N | E | E | K | - | I | Q | K | K | I | D | E | - | - | K | - | I | G | 17 | ||||
| F1.1-CeHS-1 | This work | G | - | W | - | I | N | E | E | K | - | I | Q | K | K | 11 | ||||||||||||
| IK2 | Synthetic peptide | G | - | - | - | I | - | I | K | K | - | I | I | K | K | I | 10 | 50.00 | AP04801 | [60] | ||||||||
| B6 | Synthetic peptide | G | I | W | S | D | L | A | E | - | - | I | I | K | K | F | 13 | 42.86 | AP03851 | [61] | ||||||||
| Halictine 2 | Halictus sexcinctus | G | K | W | - | M | S | L | L | K | - | I | L | K | 12 | 38.46 | AP01923 | [62] | ||||||||||
| F1.2-CeHS-1 | This work | - | K | - | I | Q | K | K | I | D | E | - | - | K | - | I | G | 11 | ||||||||||
| IK2 | Synthetic peptide | - | G | - | I | I | K | K | I | I | K | - | - | K | - | I | - | 10 | 54.55 | AP04801 | [60] | |||||||
| P11-6 | Synthetic peptide | K | K | L | I | - | K | K | I | - | L | - | - | K | - | I | L | 11 | 53.85 | AP03684 | [50] | |||||||
| Hp1036 | Heterometrus petersii | - | - | - | I | L | G | K | I | W | E | G | I | K | S | I | F | 13 | 42.86 | AP02334 | [63] | |||||||
| Peptide | Origin | Sequence | AA | Identity (%) | APD ID | Ref. | |||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F2-CeHS-1 | N | N | I | - | - | L | - | G | - | - | G | - | M | A | - | - | K | A | V | V | H | K | - | L | - | - | A | K | - | G | E | F | Q | - | 21 | ||||
| F2.1-CeHS-1 | This work | N | N | I | - | - | L | G | - | - | G | - | M | A | - | - | K | A | V | 11 | |||||||||||||||||||
| Temporin-SN2 | Hylarana spinulosa | - | F | I | T | G | L | I | G | - | - | G | L | M | - | - | - | K | A | L | 13 | 46.67 | AP02273 | [64] | |||||||||||||||
| Temporin-SN3 | Hylarana spinulosa | - | F | I | S | G | L | I | G | - | - | G | L | M | - | - | - | K | A | L | 13 | 46.67 | AP02274 | [64] | |||||||||||||||
| OdMa4 | Odorrana margaretae | - | G | I | - | - | L | S | G | L | L | G | - | - | A | G | K | K | I | V | C | 15 | 41.18 | AP03539 | [65] | ||||||||||||||
| F2.2-CeHS-1 | This work | V | H | K | - | L | - | - | A | K | - | G | E | F | Q | - | 10 | ||||||||||||||||||||||
| PN5 | Pinus densiflora | - | F | K | F | L | - | - | A | R | T | G | K | F | L | 11 | 41.67 | AP03449 | [66] | ||||||||||||||||||||
| Gramicidin S | Bacillus brevis | V | - | K | - | L | F | P | V | K | - | - | L | F | Q | - | 10 | 41.69 | AP02243 | [67] | |||||||||||||||||||
| MD4K | Myxine glutinosa L. | G | I | H | K | I | L | - | - | - | K | Y | G | K | P | S | - | 12 | 38.46 | AP04009 | [68] | ||||||||||||||||||
| No. | Peptide Name | Amino Acid Sequence | AA | Net Charge * | %H | H | µH |
|---|---|---|---|---|---|---|---|
| 1 | F1.1-CeHS-1 | GWINEEKIQKK-NH2 | 11 | +2 | 22.27 | 0.071 | 0.215 |
| 2 | F1.1-CeHS-1 II | GWINIIKIQKK-NH2 | 11 | +4 | 45.45 | 0.515 | 0.497 |
| 3 | F1.1-CeHS-1 II-RT | GWINIIKIQKKRWW-NH2 | 14 | +5 | 50.00 | 0.654 | 0.461 |
| 4 | F1.2-CeHS-1 | KIQKKIDEKIG-NH2 | 11 | +3 | 22.27 | −0.017 | 0.609 |
| 5 | F1.2-CeHS-1 IK | KIQKKIIKKIG-NH2 | 11 | +6 | 36.36 | 0.185 | 0.691 |
| 6 | F1.2-CeHS-1 IK-RT | KIQKKIIKKIGRWW-NH2 | 14 | +7 | 42.86 | 0.394 | 0.814 |
| 7 | F2.1-CeHS-1 | NNILGGMAKAV-NH2 | 11 | +2 | 54.55 | 0.398 | 0.575 |
| 8 | F2.1-CeHS-1 KK | KKILGGMAKAV-NH2 | 11 | +4 | 55.55 | 0.327 | 0.618 |
| 9 | F2.1-CeHS-1 KK-RT | KKILGGMAKAVRWW-NH2 | 14 | +5 | 57.14 | 0.506 | 0.622 |
| 10 | F2.2-CeHS-1 | VHKLAKGEFQ-NH2 | 10 | +2 | 40.00 | 0.231 | 0.394 |
| 11 | F2.2-CeHS-1 IK | VHKLAKGIFK-NH2 | 10 | +4 | 50.00 | 0.398 | 0.697 |
| 12 | F2.2-CeHS-1 IK-RT | VHKLAKGIFKRWW-NH2 | 13 | +5 | 53.85 | 0.575 | 0.654 |
| No. | Peptide Name | S. aureus | E. coli | K. pneumoniae | P. aeruginosa | ||||
|---|---|---|---|---|---|---|---|---|---|
| MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | ||
| 2 | F1.1-CeHS-1 II | ND | ND | ND | ND | ND | ND | ND | ND |
| 3 | F1.1-CeHS-1 II-RT | ND | ND | 128 | 256 | ND | ND | ND | ND |
| 5 | F1.2-CeHS-1 IK | ND | ND | ND | ND | ND | ND | ND | ND |
| 6 | F1.2-CeHS-1 IK-RT | 32 | 64 | 8 | 8 | 256 | 256 | 16 | 32 |
| 8 | F2.1-CeHS-1 KK | ND | ND | ND | ND | ND | ND | ND | ND |
| 9 | F2.1-CeHS-1 KK-RT | 64 | 128 | 8 | 32 | 128 | 128 | 64 | 128 |
| 11 | F2.2-CeHS-1 IK | ND | ND | ND | ND | ND | ND | ND | ND |
| 12 | F2.2-CeHS-1 IK-RT | 32 | 32 | 4 | 16 | 256 | 256 | 32 | 64 |
| Positive control | |||||||||
| Ampicillin | 8 | 8 | 4 | 32 | >256 | >256 | 128 | 256 | |
| No. | Peptide Name | PROB |
|---|---|---|
| 3 | F1.1-CeHS-1 II-RT | 0.556 |
| 6 | F1.2-CeHS-1 IK-RT | 0.007 |
| 9 | F2.1-CeHS-1 KK-RT | 0.262 |
| 12 | F2.2-CeHS-1 IK-RT | 0.010 |
| No | Peptide Name | Binding Energy (Kcal/mol) | RMSD | Chemical Bond Interaction (Å)/Nucleotide Composition | ||
|---|---|---|---|---|---|---|
| H-Bonds ● Conventional H-Bond ● Carbon H-Bond ● Pi-Donor H-bond | Charge ● Salt Bridge; Charge-Charge ● Charge-Charge ● π-Cation ● π-Anion | Hydrophobic Interactions | ||||
| ● Alkyl ● π-alkyl ●π-π shaped | ||||||
| 2 | F1.1 CeHS-1 II | −37.6 ± 4.5 | −5.2 ± 1.0 | Trp2●P/●P, Ile3●D, Lys7●P/●P, Lys10●G/●T/●T/●D, Lys11●P | Trp2●P, Lys7●P/●P, Lys11●P | N.D |
| 3 | F1.1 CeHS-1 II-RT | −71.8 ± 3.5 | −9.0 ± 0.8 | Lys7●P, Gln9●P, Lys10●D/●A/●C/●T | Trp2●●P, Lys7●P/●P, Lys11●P/●P, | Trp14●T |
| 5 | F1.2-CeHS-1 IK | −47.3 ± 10.9 | −7.4 ± 0.6 | Lys5●●T, Lys9●A/●C/●A/●D, Ile10●D | Lys1●P/●P, Lys4●P | N.D |
| 6 | F1.2-CeHS-1 IK-RT | −80.0 ± 6.3 | −8.7 ± 0.1 | Lys5●T/●D, Lys9●C/●T, Arg12●P, Trp13●P, Trp14●P | Lys1●●P, Lys4●P, Lys8●P/●P, Arg12●●●P/●●P, | N.D |
| 8 | F2.1-CeHS-1 KK | −41.7 ± 12.7 | −2.3 ± 1.4 | Lys1●P, Lys2●C/●P/●D, Gly6●D, Lys9●T/●C/●C/●●D | Lys1●●P/●P | N.D |
| 9 | F2.1-CeHS-1 KK-RT | −79.4 ± 7.3 | −5.9 ± 0.3 | Lys1●P, Lys2●T/●A, Lys9●A | Lys1●P/●●P, Lys2●C/●P, Lys9●P/●P, Arg12●P/●P, Trp14●P | Lys2●●A/●A, Lys9●A |
| 11 | F2.2-CeHS-1 IK | −42.8 ± 3.7 | −5.0 ± 0.9 | His2●T, Lys6●T/●G/●D, Lys10●T/●D/●P | Val1●P, Lys3●●P/●P, Phe9●P | N.D |
| 12 | F2.2-CeHS-1 IK-RT | −69.3 ± 2.9 | −4.7 ± 0.9 | His2●P, Lys6●P, Lys10●P, Trp13●A | Val1●P, His2●P, Lys6●P/●P, Lys10●P/●P, Trp12●●P | Val1●A, Ala5●●A, Phe9●A/●A, Trp13●●A/●A |
| Positive control | ||||||
| RT2 | −81.7 ± 15.0 | 1.0 ± 0.9 | ||||
| KT2 | −90.5 ± 14.6 | 2.1 ± 1.2 | ||||
| Negative control | ||||||
| Magainin 2 | −59.4 ± 9.9 | 10.6 ± 0.5 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saengkun, Y.; Klamrak, A.; Janpan, P.; Rahman, S.S.; Erviana, R.; Puangmalai, N.; Jangpromma, N.; Daduang, J.; Daduang, S.; Areemit, J. C-Terminal Modification Contributes the Antibacterial Activity of a Cecropin-like Region of Heteroscorpine-1 from Scorpion Venom. Biology 2025, 14, 1044. https://doi.org/10.3390/biology14081044
Saengkun Y, Klamrak A, Janpan P, Rahman SS, Erviana R, Puangmalai N, Jangpromma N, Daduang J, Daduang S, Areemit J. C-Terminal Modification Contributes the Antibacterial Activity of a Cecropin-like Region of Heteroscorpine-1 from Scorpion Venom. Biology. 2025; 14(8):1044. https://doi.org/10.3390/biology14081044
Chicago/Turabian StyleSaengkun, Yutthakan, Anuwatchakij Klamrak, Piyapon Janpan, Shaikh Shahinur Rahman, Rima Erviana, Nawan Puangmalai, Nisachon Jangpromma, Jureerut Daduang, Sakda Daduang, and Jringjai Areemit. 2025. "C-Terminal Modification Contributes the Antibacterial Activity of a Cecropin-like Region of Heteroscorpine-1 from Scorpion Venom" Biology 14, no. 8: 1044. https://doi.org/10.3390/biology14081044
APA StyleSaengkun, Y., Klamrak, A., Janpan, P., Rahman, S. S., Erviana, R., Puangmalai, N., Jangpromma, N., Daduang, J., Daduang, S., & Areemit, J. (2025). C-Terminal Modification Contributes the Antibacterial Activity of a Cecropin-like Region of Heteroscorpine-1 from Scorpion Venom. Biology, 14(8), 1044. https://doi.org/10.3390/biology14081044






