Increasing Sea Surface Temperatures Driving Widespread Tropicalization in South Atlantic Pelagic Fisheries
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Catch Data, Thermal Preferences, and Climatic Data
2.2. Catch Composition Analysis
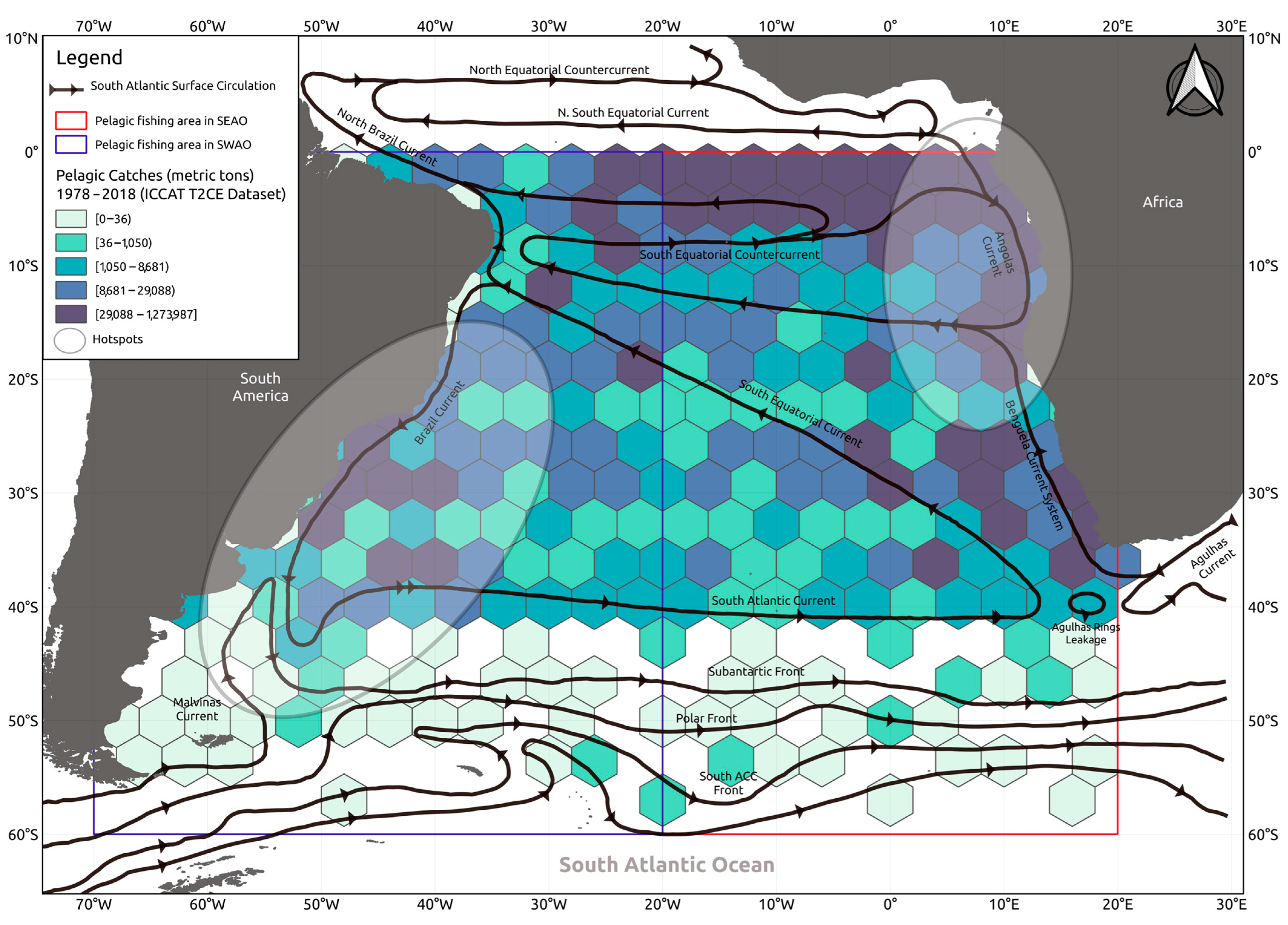
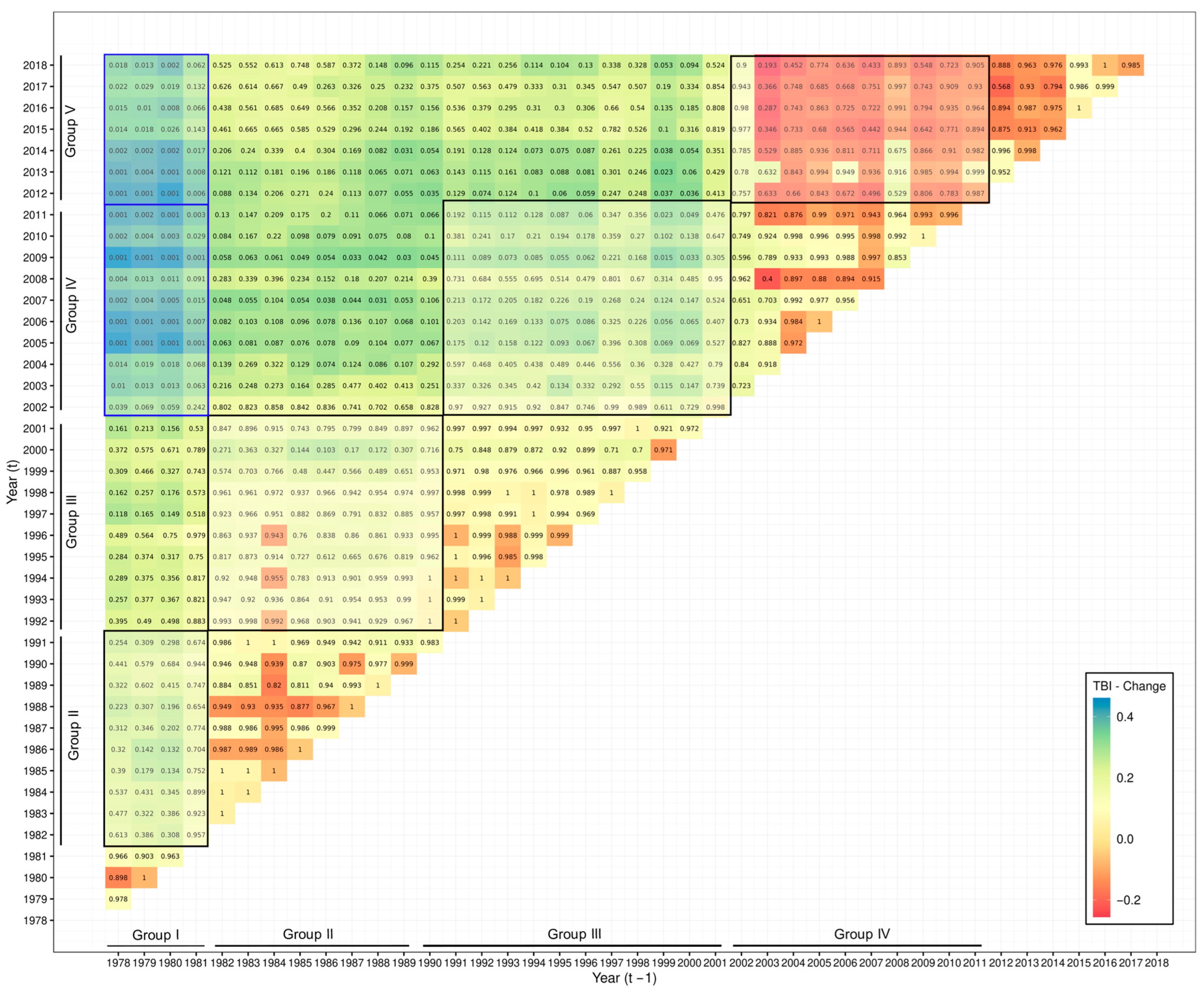
3. Results
3.1. Catch Composition and Thermal Preferences
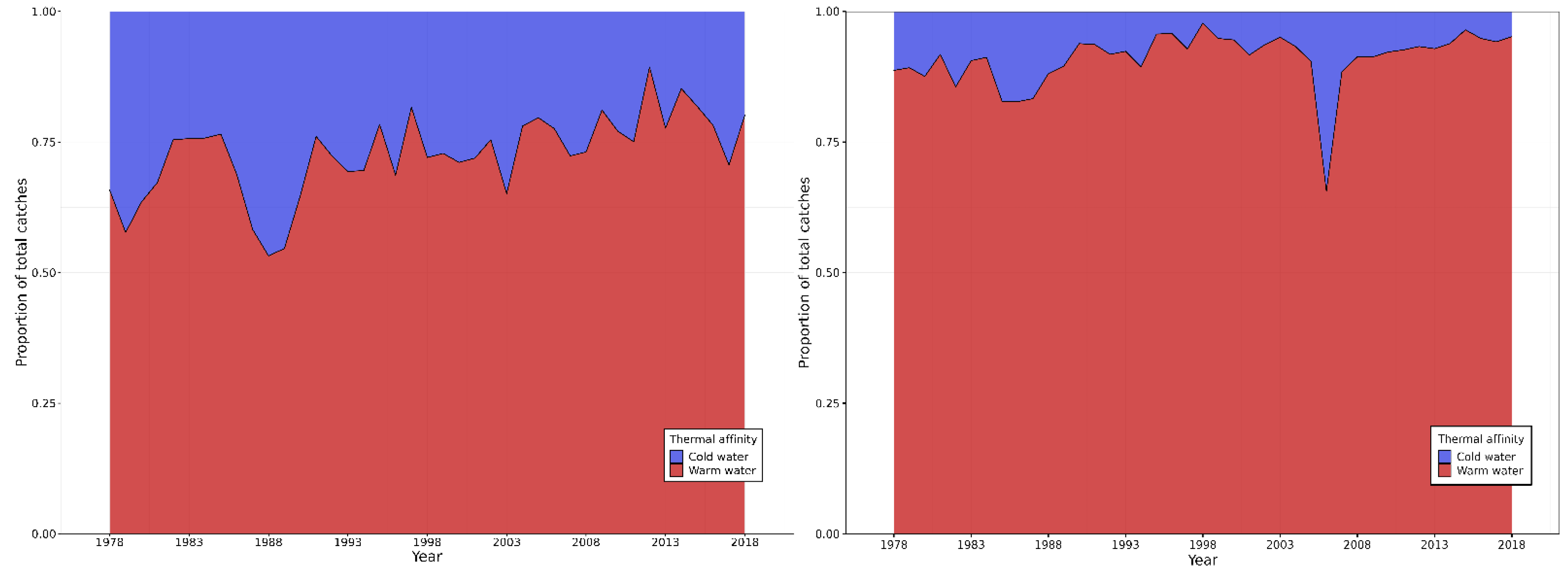
3.2. Mean Temperature of the Catches
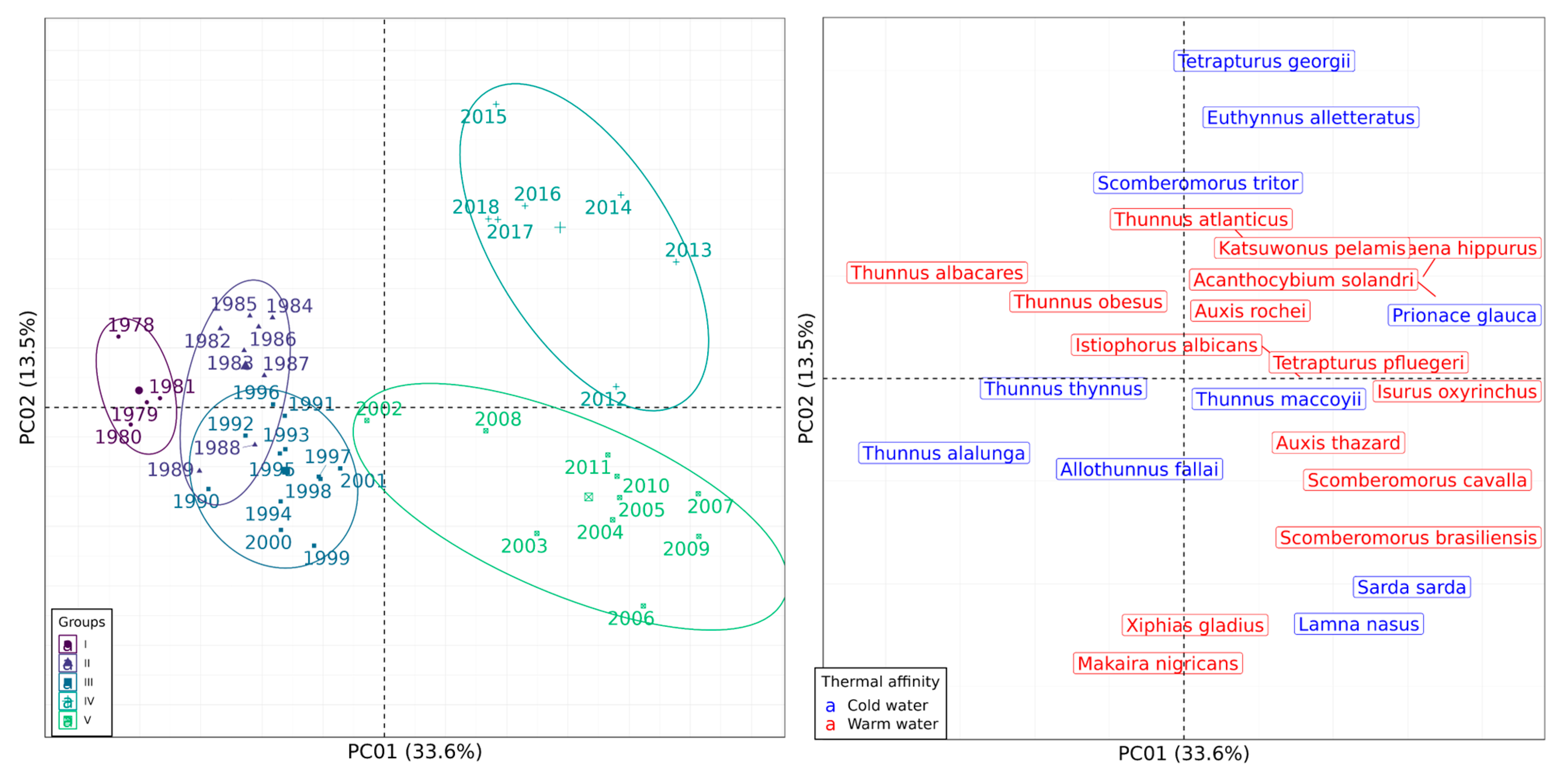
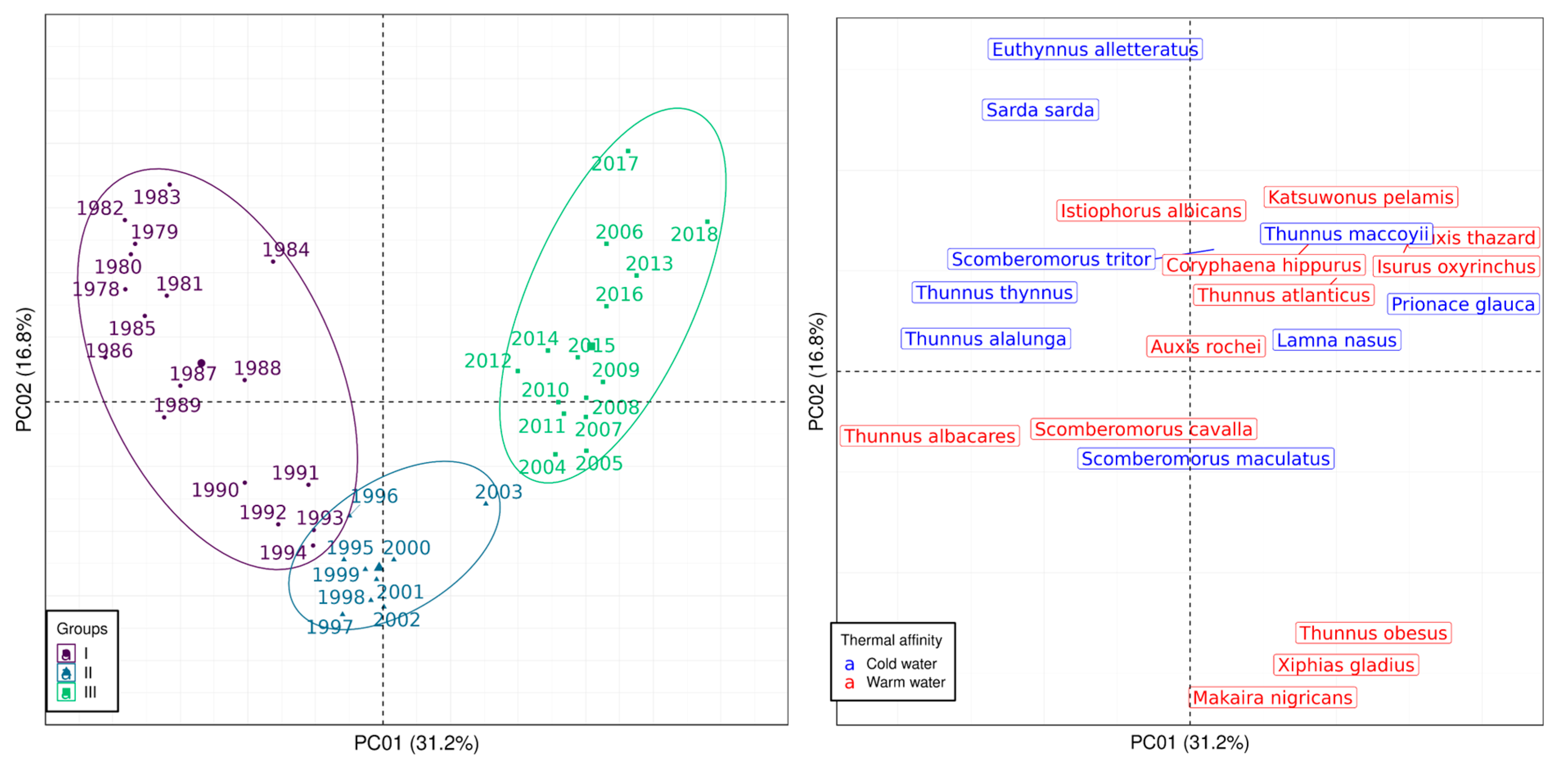
3.3. Catch Composition Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. The State of World Fisheries and Aquaculture. Contributing to Food Security and Nutrition for All; FAO: Rome, Italy, 2016. [Google Scholar]
- Hicks, C.C.; Cohen, P.J.; Graham, N.A.J.; Nash, K.L.; Allison, E.H.; D’Lima, C.; Mills, D.J.; Roscher, M.; Thilsted, S.H.; Thorne-Lyman, A.L.; et al. Harnessing global fisheries to tackle micronutrient deficiencies. Nature 2019, 574, 95–98. [Google Scholar] [CrossRef]
- Halpern, B.S.; Walbridge, S.; Selkoe, K.A.; Kappel, C.V.; Micheli, F.; D’Agrosa, C.; Bruno, J.F.; Casey, K.S.; Ebert, C.; Fox, H.E.; et al. A global map of human impact on marine ecosystems. Science 2018, 319, 948–952. [Google Scholar] [CrossRef]
- Monllor-Hurtado, A.; Pennino, M.G.; Sanchez-Lizaso, J.L. Shift in tuna catches due to ocean warming. PLoS ONE 2017, 12, e0178196. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Abraham, J.; Trenberth, K.E.; Boyer, T.; Mann, M.E.; Zhu, J.; Wang, F.; Yu, F.; Locarnini, R.; Fasullo, J.; et al. New Record Ocean Temperatures and Related Climate Indicators in 2023. Adv. Atmos. Sci. 2024, 41, 1068–1082. [Google Scholar] [CrossRef]
- Fu, W.; Randerson, J.; Moore, J.K. Climate change impacts on net primary production (NPP) and export production (EP) regulated by increasing stratification and phytoplankton community structure in the CMIP5 models. Biogeosciences 2016, 13, 5151–5170. [Google Scholar] [CrossRef]
- Schmidtko, S.; Stramma, J.; Visbeck, M. Decline in global oceanic oxygen content during the past five decades. Nature 2017, 542, 335–339. [Google Scholar] [CrossRef]
- IPCC. Summary for Policymakers. In IPCC Special Report on the Ocean and Cryosphere in a Changing Climate; Pörtner, H.-O., Roberts, D.C., Masson-Delmotte, V., Zhai, P., Tignor, M., Poloczanska, E., Mintenbeck, K., Nicolai, M., Okem, A., Petzold, J., et al., Eds.; University Printing House: Cambridge, UK, 2019; pp. 3–46. [Google Scholar]
- Caesar, L.; McCarthy, G.D.; Thornalley, D.J.R.; Cahill, N.; Rahmstorf, S. Current Atlantic Meridional Overturning Circulation weakest in last millennium. Nat. Geosci. 2021, 14, 118–120. [Google Scholar] [CrossRef]
- Jennings, S.; Mélin, F.; Blanchard, J.L.; Forster, R.M.; Dulvy, N.K.; Wilson, R.W. Global-scale predictions of community and ecosystem properties from simple ecological theory. Proc. R. Soc. B 2008, 275, 1375–1383. [Google Scholar] [CrossRef] [PubMed]
- Blanchard, J.L.; Jennings, S.; Holmes, R.; Harle, J.; Merino, G.; Allen, J.I.; Holt, J.; Dulvy, N.K.; Barange, M. Potential consequences of climate change for primary production and fish production in large marine ecosystems. Phil. Trans. R. Soc. B 2012, 367, 2979–2989. [Google Scholar] [CrossRef]
- Poloczanska, E.S.; Brown, C.J.; Sydeman, W.J.; Kiessling, W.; Schoeman, D.S. Global imprint of climate change on marine life. Nat. Clim. Change 2013, 3, 919–925. [Google Scholar] [CrossRef]
- Poloczanska, E.S.; Burrows, M.T.; Brown, C.J.; García Molinos, J.; Halpern, B.S.; Hoegh-guldberg, O.; Kappel, C.V.; Moore, P.J.; Richardson, A.J.; Schoeman, D.S.; et al. Responses of Marine Organisms to Climate Change across Oceans. Front. Mar. Sci. 2016, 3, 62. [Google Scholar] [CrossRef]
- Cardoso, L.G.; Sant’Ana, R.; Freire, M.A.; Weigert, S.C.; Poubel, M.; Bezerra, N.A.; Rodrigues, L.S. Poleward catch displacement of blackfin tuna Thunnus atlanticus in the southwestern Atlantic Ocean: Possible effect of increasing water temperatures. Fish Manag Ecol. 2024, 31, e12697. [Google Scholar] [CrossRef]
- Schroeder, R.; Petermann, A.; Correia, A.T. The History of the Brazilian Sardine (Sardinella brasiliensis) Between Two Fishery Collapses: An Ecosystem Modeling Approach to Study Its Life Cycle. Biology 2025, 14, 13. [Google Scholar] [CrossRef]
- Cheung, W.L.; Lam, V.W.Y.; Sarmiento, G.L.; Kearney, K.; Watson, R.; Pauly, D. Projecting global marine biodiversity impacts under climate change scenarios. Fish Fish. 2009, 10, 235–251. [Google Scholar] [CrossRef]
- Pecl, G.T.; Araújo, M.B.; Bell, J.D.; Blanchard, J.; Bonebrake, T.C.; Chen, I.-C.; Clark, T.D.; Colwell, R.K.; Danielsen, F.; Evengård, B.; et al. Biodiversity redistribution under climate change: Impacts on ecosystems and human well-being. Science 2017, 355, eaai9214. [Google Scholar] [CrossRef]
- Hillebrand, H.; Brey, T.; Gutt, J.; Hagen, W.; Metfies, K.; Meyer, B.; Lewandowska, A. Climate Change: Warming Impacts on Marine Biodiversity. In Handbook on Marine Environment Protection; Salomon, M., Markus, T., Eds.; Springer: Cham, Switzerland, 2018. [Google Scholar] [CrossRef]
- Chaudhary, C.; Richardson, A.J.; Schoeman, D.S.; Costello, M.J. Global warming is causing a more pronounced dip in marine species richness around the equator. Proc. Natl. Acad. Sci. USA 2021, 118, e2015094118. [Google Scholar] [CrossRef] [PubMed]
- Hu, N.; Bourdeau, P.E.; Harlos, C.; Liu, Y.; Hollander, J. Meta-analysis reveals variance in tolerance to climate change across marine trophic levels. Sci. Total Environ. 2022, 827, 154244. [Google Scholar] [CrossRef] [PubMed]
- Crona, B.I.; Wassénius, E.; Jonell, M.; Koehn, J.Z.; Short, R.; Tigchelaar, M.; Daw, T.M.; Golden, C.D.; Gephart, J.A.; Allison, E.H.; et al. Four ways blue foods can help achieve food system ambitions across nations. Nature 2023, 616, 104–112. [Google Scholar] [CrossRef]
- FAO. The State of World Fisheries and Aquaculture 2022. Towards Blue Transformation; FAO: Rome, Italy, 2022. [Google Scholar]
- Bornatowski, H.; Angelini, R.; Coll, M.; Barreto, R.R.P.; Amorim, A.F. Ecological role and historical trends of large pelagic predators in a subtropical marine ecosystem of the South Atlantic. Rev. Fish Biol. Fish. 2017, 27, 697–713. [Google Scholar] [CrossRef]
- Petrik, C.M.; Stock, C.A.; Andersen, K.H.; van Denderen, P.D.; Watson, J.R. Large pelagic fish are most sensitive to climate change despite pelagification of ocean food webs. Front. Mar. Sci. 2020, 7, 588482. [Google Scholar] [CrossRef]
- Erauskin-Extramiana, M.; Arrizabalaga, H.; Hobday, A.J.; Cabré, A.; Ibaibarriga, L.; Arregui, I.; Murua, H.; Chust, G. Large-scale distribution of tuna species in a warming ocean. Glob. Change Biol. 2019, 25, 2043–2060. [Google Scholar] [CrossRef] [PubMed]
- Schmid, C.; Siedler, G.; Zenk, W. Dynamics of Intermediate Water Circulation in the Subtropical South Atlantic. J. Phys. Oceanogr. 2000, 30, 3191–3211. [Google Scholar] [CrossRef]
- Stramma, L. Geostrophic transport of the South Equatorial Current in the Atlantic. J. Mar. Res. 2001, 49, 281–294. [Google Scholar] [CrossRef]
- Rodrigues, R. Circulação Atmosférica e Oceânica. In Introdução às Ciências do Mar; Castello, J.P., Krug, L.C., Eds.; Textos: Pelotas, Brasil, 2015. [Google Scholar]
- Franco, B.C.; Defeo, O.; Piola, A.R.; Barreiro, M.; Yang, H.; Ortega, L.; Gianelli, I.; Castello, J.P.; Vera, C.; Buratti, C.; et al. Climate change impacts on the atmospheric circulation, ocean and fisheries in the southwest South Atlantic Ocean: A review. Clim. Change 2020, 162, 2359–2377. [Google Scholar] [CrossRef]
- Souza, R.B.; Pezzi, L.P. Processos Regionais de Interação Oceano-Atmosfera no Atlântico Sul. In Fronteiras do Conhecimento em Ciências do Mar; Lana, P.C., Castello, J.P., Eds.; Ed. FURG: Rio Grande, Brasil, 2020. [Google Scholar]
- Roch, M.; Brandt, P.; Schmidtko, S.; Velho, F.V.; Ostrowski, M. Southeastern Tropical Atlantic Changing From Subtropical to Tropical Conditions. Front. Mar. Sci. 2021, 8, 748383. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, H.; Cao, J.; Yu, W.; Chen, X. Long-Term Variability in the Southwest Atlantic Marine Fishery Ecosystems in Relation to Climate Change. Fish. Oceanogr. 2025, 34, e12721. [Google Scholar] [CrossRef]
- Marcello, F.; Wainer, I.; Rodrigues, R.R. South Atlantic Subtropical Gyre Late Twentieth Century Changes. J. Geophys. Res. Ocean. 2018, 123, 5194–5209. [Google Scholar] [CrossRef]
- Yang, H.; Lohmann, G.; Krebs-Kanzow, U.; Ionita, M.; Shi, X.; Sidorenko, D.; Gong, X.; Chen, X.; Gowan, E.J. Poleward shift of the major ocean gyres detected in a warming climate. Geophys. Res. Lett. 2020, 47, e2019GL085868. [Google Scholar] [CrossRef]
- Lumpkin, R.; Garzoli, S. Interannual to decadal changes in the western South Atlantic’s surface circulation. J. Geophys. Res. Oceans. 2011, 16, C01014. [Google Scholar] [CrossRef]
- Artana, C.; Provost, C.; Lellouche, J.-M.; Rio, M.-H.; Ferrai, R.; Sennéchael, N. The Malvinas Current at the Confluence with the Brazil Current: Inferences from 25 Years of Mercator Ocean Reanalysis. J. Geophys. Res. Oceans. 2019, 124, 7178–7200. [Google Scholar] [CrossRef]
- Hobday, A.J.; Pecl, G.T. Identification of global marine hotspots: Sentinels for change and vanguards for adaptation action. Rev. Fish. Biol. Fish. 2014, 24, 415–425. [Google Scholar] [CrossRef]
- Popova, E.; Yool, A.; Byfield, V.; Cochrane, K.; Coward, A.C.; Salim, S.S.; Gasalla, M.A.; Henson, S.A.; Hobday, A.J.; Pecl, G.T.; et al. From global to regional and back again: Common climate stressors of marine ecosystems relevant for adaptation across five ocean warming hotspots. Glob. Change Biol. 2016, 22, 2038–2053. [Google Scholar] [CrossRef]
- Gianelli, I.; Ortega, L.; Marín, Y.; Piola, A.R.; Defeo, O. Evidence of ocean warming in Uruguay’s fisheries landings: The mean temperature of the catch approach. Mar. Ecol. Prog. Ser. 2019, 625, 115–125. [Google Scholar] [CrossRef]
- Cheung, W.L.; Watson, R.; Pauly, D. Signature of ocean warming in global fisheries catch. Nature 2013, 497, 365–369. [Google Scholar] [CrossRef] [PubMed]
- Perez, J.A.A.; Sant’Ana, R. Tropicalization of demersal megafauna in the western South Atlantic since 2013. Commun. Earth Environ. 2022, 3, 227. [Google Scholar] [CrossRef]
- Talley, L.D.; Pickard, G.L.; Emery, W.J.; Swift, J.H. Pacific Ocean. In Descriptive Physical Oceanography: An Introduction, 6th ed.; Academic Press: London, UK, 2011. [Google Scholar]
- Arvedlund, M. First records of unusual marine fish distributions—Can they predict climate changes? J. Mar. Biol. Assoc. UK 2009, 89, 863–866. [Google Scholar] [CrossRef]
- Schroeder, R.; Sant’Ana, R.; Lima, A.O.S.; Dallabona, J.A.; Delabary, G.S.; Gavazzoni, L.; Oliveira, L.d.; Laaf, Y.d.O.; Travassos, P. A Crusade Throughout the World’s Oceans: Genetic Evidence of the Southern Bluefin Tuna Thunnus maccoyii and the Pacific Bluefin Tuna Thunnus orientalis in Brazilian Waters. Biology 2025, 14, 340. [Google Scholar] [CrossRef]
- Tomanek, L. Variation in the heat shock response and its implication for predicting the effect of global climate change on species’ biogeographical distribution ranges and metabolic costs. J. Exp. Biol. 2010, 213, 971–979. [Google Scholar] [CrossRef]
- van Denderen, D.; Gislason, H.; van den Heuvel, J.; Andersen, K.H. Global analysis of fish growth rates shows weaker responses to temperature than metabolic predictions. Global Ecol. Biogeogr. 2020, 29, 2203–2213. [Google Scholar] [CrossRef]
- Lynch, P.D.; Shertzer, K.W.; Cortés, E.; Latour, R.J. Abundance trends of highly migratory species in the Atlantic Ocean: Accounting for water temperature profiles. ICES J. Mar. Sci. 2018, 75, 1427–1438. [Google Scholar] [CrossRef]
- Boyce, D.G.; Tittensor, D.P.; Worm, B. Effects of temperature on global patterns of tuna and billfish richness. Mar. Ecol. Prog. Ser. 2008, 355, 267–276. [Google Scholar] [CrossRef]
- Blank, J.M.; Morrissette, J.M.; Landeira-Ferandez, A.M.; Blackwell, S.B.; Williams, T.D.; Block, B.A. In situ cardiac performance of Pacific bluefin tuna hearts in response to acute temperature change. J. Exp. Biol. 2004, 207, 881–890. [Google Scholar] [CrossRef]
- Block, B.A.; Teo, S.L.H.; Walli, A.; Boustany, A.; Stokesbury, M.J.W.; Farwell, C.J.; Weng, K.C.; Dewar, H.; Williams, T.D. Electronic tagging and population structure of Atlantic bluefin tuna. Nature 2005, 434, 1121–1127. [Google Scholar] [CrossRef]
- Laurs, R.M. The North Pacific Albacore: An Important Visitor to California Current Waters; CalCOFI Rep.: Long Beach, CA, USA, 1983. [Google Scholar]
- Travassos, P.; Frédou, F.; Frédou, T.; Amorim, A.; Pimenta, E.; Hazin, F.; da Silva, G.B.; Andrade, H.A.; Leite, N.; Lessa, R.; et al. Projeto de Apoio Técnico-Científico ao Desenvolvimento da Pesca de Atuns e Afins no Brasil (Chamada MCTI/MPA/CNPq 22/2015—Linha Temática II—Atuns e afins); Universidade Federal Rural de Pernambuco: Recife, Brazil, 2022. [Google Scholar]
- Froese, R.; Pauly, D. Fish Base. World Wide Web Electronic Publication. 2022. Available online: www.fishbase.org (accessed on 1 April 2022).
- Tsikliras, A.C.; Stergiou, K.I. Mean temperature of the catch increases quickly in the Mediterranean Sea. Mar. Ecol. Prog. Ser. 2014, 515, 281–284. [Google Scholar] [CrossRef]
- Tsikliras, A.C.; Stergiou, K.I. Mean temperature of the catch (MTC) in the Greek Seas based on landings and survey data. Front. Mar. Sci. 2015, 2, 23. [Google Scholar] [CrossRef]
- Liang, C.; Xian, W.; Pauly, D. Impacts of Ocean Warming on China’s Fisheries Catches: An Application of “Mean Temperature of the Catch” Concept. Front. Mar. Sci. 2018, 5, 26. [Google Scholar] [CrossRef]
- Kangur, K.; Tammiksaar, E.; Pauly, D. Using the “mean temperature of the catch” to assess fish community responses to warming in a temperate lake. Environ. Biol. Fishes. 2022, 105, 883–894. [Google Scholar] [CrossRef]
- Dimarchopoulou, D.; Tsikliras, A.C.; Stergiou, K.I. Responses in fisheries catch data to a warming ocean along a latitudinal gradient in the western Pacific Ocean. Ecol. Indic. 2022, 125, 107585. [Google Scholar] [CrossRef]
- Alajmi, F.; Al-Husaini, M.; Al-Sarawi, H.; Pauly, D. Mean temperature of the catch index can be masked by changes in catch composition unrelated to ocean warming. Fish Fish. 2023, 24, 1411–1422. [Google Scholar] [CrossRef]
- Keskin, Ç.; Pauly, D. Changes in the “mean temperature of the catch”: Application of a new index to Turkish fisheries. Acta Adriat. 2014, 55, 259–272. [Google Scholar] [CrossRef]
- Schwarzkopf, F.U.; Biastoch, A.; Böning, C.W.; Chanut, J.; Durgadoo, J.V.; Getzlaff, K.; Harlaß, J.; Rieck, J.K.; Roth, C.; Scheinert, M.M.; et al. The INALT family—A set of high-resolution nests for the Agulhas Current system within global NEMO ocean/sea-ice configurations. Geosci. Model Dev. 2019, 12, 3329–3355. [Google Scholar] [CrossRef]
- Wood, S.N. Generalized Additive Models: An Introduction with R, 2nd ed.; Chapman & Hall/CRC: New York, NY, USA, 2017. [Google Scholar]
- Wood, S.N. Thin plate regression splines. J. R. Stat. Soc. Ser. B (Stat. Methodol.) 2003, 65, 95–114. [Google Scholar] [CrossRef]
- Tredennick, A.T.; Hooker, G.; Ellner, S.P.; Adler, P.B.A. practical guide to selecting models for exploration, inference, and prediction in ecology. Ecology 2021, 102, e03336. [Google Scholar] [CrossRef] [PubMed]
- Zeileis, A. dynlm: Dynamic Linear Regression, R Package, version 0.3-6; R Foundation for Statistical Computing: Vienna, Austria, 2006; Available online: https://CRAN.R-project.org/package=dynlm (accessed on 1 April 2022).
- Pfaff, B. Analysis of Integrated and Cointegrated Time Series with R, 2nd ed.; Springer: New York, NY, USA, 2008. [Google Scholar] [CrossRef]
- Therneau, T.M.; Atkinson, B. mvpart: Multivariate Partitioning. R Package, Version 1.6-2; R Foundation for Statistical Computing: Vienna, Austria, 2013; Available online: https://CRAN.R-project.org/package=mvpart (accessed on 1 April 2022).
- Legendre, P.; Legendre, L. Numerical Ecology, 3rd ed.; Elsevier Science BV: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Legendre, P.; De Cáceres, M. Beta diversity as the variance of community data: Dissimilarity coefficients and partitioning. Ecol. Lett. 2013, 16, 951–963. [Google Scholar] [CrossRef] [PubMed]
- Dray, S.; Bauman, D.; Blanchet, G.; Borcard, D.; Clappe, S.; Guenard, G.; Jombart, T.; Larocque, G.; Legendre, P.; Madi, N.; et al. adespatial: Multivariate Multiscale Spatial Analysis, R Package, version 0.3-14; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://CRAN.R-project.org/package=adespatial (accessed on 1 April 2022).
- Legendre, P. A temporal beta-diversity index to identify sites that have changed in exceptional ways in space-time surveys. Ecol. Evol. 2019, 9, 3500–3514. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2024; Available online: https://www.R-project.org/ (accessed on 1 April 2022).
- Riskas, K.A.; Tiwari, M. An overview of fisheries and sea turtle bycatch along the Atlantic coast of Africa. Munibe Monogr. Nat. Ser. 2013, 1, 71–82. [Google Scholar] [CrossRef]
- Rosenberg, A.A.; Fogarty, M.J.; Cooper, A.B.; Dickey-Collas, M.; Fulton, E.A.; Gutiérrez, N.L.; Hyde, K.J.W.; Kleisner, K.M.; Kristiansen, T.; Longo, C.; et al. Developing New Approaches to Global Stock Status Assessment and Fishery Production Potential of the Seas. FAO Fisheries and Aquaculture Circular No. 1086; FAO: Rome, Italy, 2014; 175p. [Google Scholar]
- Megrey, B.A.; Hare, J.A.; Stockhausen, W.T.; Dommasnes, A.; Gjøsæter, H.; Overholtz, W.; Gaichas, S.; Skaret, G.; Falk-Petersen, J.; Link, J.S.; et al. A Cross-Ecosystem Comparison of Spatial and Temporal Patterns of Covariation in the Recruitment of Functionally Analogous Fish Stocks. Prog. Oceanogr. 2009, 81, 63–92. [Google Scholar] [CrossRef]
- Ward, P.; Hindmarsh, S. An overview of historical changes in the fishing gear and practices of pelagic longliners, with particular reference to Japan’s Pacific fleet. Rev. Fish. Biol. Fish. 2007, 17, 501–516. [Google Scholar] [CrossRef]
- Crespo, G.O.; Dunn, D.C.; Reygondeau, G.; Boerder, K.; Worm, B.; Cheung, W.; Tittensor, D.P.; Halpin, P.N. The environmental niche of the global high seas pelagic longline fleet. Sci. Adv. 2018, 4, eaat3681. [Google Scholar] [CrossRef]
- Dell’Apa, A.; Boenish, R.; Fujita, R.; Kleisner, K. Effects of climate change and variability on large pelagic fish in the Northwest Atlantic Ocean: Implications for improving climate resilient management for pelagic longline fisheries. Front. Mar. Sci. 2023, 10, 1206911. [Google Scholar] [CrossRef]
- Lehodey, P.; Bertignac, M.; Hampton, J.; Lewis, A.; Picaut, J. El Niño Southern Oscillation and tuna in the western Pacific. Nature 1997, 389, 715–717. [Google Scholar] [CrossRef]
- Ravier, C.; Fromentin, J.M. Are the long-term fluctuations in Atlantic bluefin tuna (Thunnus thynnus) population related to environmental changes? Fish. Oceanogr. 2004, 13, 145–160. [Google Scholar] [CrossRef]
- Chang, Y.-L.; Sun, C.-L.; Chen, Y.; Yeh, S.-Z.; DiNardo, G.; Su1, N.-J. Modelling the impacts of environmental variation on the habitat suitability of swordfish, Xiphias gladius, in the equatorial Atlantic Ocean. ICES J. Mar. Sci. 2013, 70, 1000–1012. [Google Scholar] [CrossRef]
- Skubel, R.A.; Kirtman, B.P.; Fallows, C.; Hammerschlag, N. Patterns of long-term climate variability and predation rates by a marine apex predator, the white shark Carcharodon carcharias. Mar. Ecol. Progr. Ser. 2018, 587, 129–139. [Google Scholar] [CrossRef]
- Hofmann, G.E.; Todgham, A.E. Living in the now: Physiological mechanisms to tolerate a rapidly changing environment. Annu Rev Physiol. 2010, 72, 127–145. [Google Scholar] [CrossRef] [PubMed]
- Lahellec, G.; Daurès, F.; Lehuta, S. Revealing the adaptation strategies of pelagic fleets in the Bay of Biscay by combining fishery data and fishers’ knowledge. ICES J. Mar. Sci. 2024, 82, fsae171. [Google Scholar] [CrossRef]
- Lamine, B.E.; Schickele, A.; Guidetti, P.; Allemand, D.; Hilmi, N.; Raybaud, V. Redistribution of fisheries catch potential in Mediterranean and North European waters under climate change scenarios. Sci. Total Environ. 2023, 879, 163055. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.D.; Ota, Y.; Sumaila, U.R.; Cisneros-Montemayor, A.M.; Cheung, W.W.L. Adaptation strategies to climate change in marine systems. Glob. Change Biol. 2017, 24, e1–e14. [Google Scholar] [CrossRef]
- Wilkinson, J. Fish: A Global Value Chain Driven onto the Rocks. Sociol. Rural. 2006, 46, 85–170. [Google Scholar] [CrossRef]
- Coll, M.; Bellido, J.M.; Pennino, M.G.; Albo-Puigserver, M.; Báez, J.C.; Christensen, V.; Corrales, X.; Fernández-Corredor, E.; Giménez, J.; Julià, L.; et al. Retrospective analysis of the pelagic ecosystem of the Western Mediterranean Sea: Drivers, changes and effects. Sci. Total Environ. 2024, 907, 167790. [Google Scholar] [CrossRef]
- Andrews, A.J.; Pampoulie, C.; Di Natale, A.; Addis, P.; Bernal-Casasola, D.; Aniceti, V.; Carenti, G.; Gómez-Fernández, V.; Chosson, V.; Ughi, A.; et al. Exploitation shifted trophic ecology and habitat preferences of Mediterranean and Black Sea bluefin tuna over centuries. Fish Fish. 2023, 24, 1461–1477. [Google Scholar] [CrossRef]
- ICCAT. Executive Summary of the Atlantic Albacore Stock Assessment. Advice to the ICCAT Commission; ICCAT: Madrid, Spain, 2020. [Google Scholar]
- Matano, R.P.; Palma, E.D.; Piola, A.R. The influence of the Brazil and Malvinas Currents on the Southwestern Atlantic Shelf circulation. Ocean Sci. 2010, 6, 983–995. [Google Scholar] [CrossRef]
- Rodrigues, R.R.; Artana, C.; Gonçalves Neto, A.; Frölicher, T.L.; Keenlyside, N.; Hobday, A.J.; Burger, F.A.; Bernardo, P.S.; Araújo, J. Extreme compound events in the equatorial and South Atlantic. Nat. Commun. 2025, 16, 3183. [Google Scholar] [CrossRef]
- Imbol Koungue, R.A.; Brandt, P.; Lübbecke, J.; Prigent, A.; Martins, M.S.; Rodrigues, R.R. The 2019 Benguela Niño. Front. Mar. Sci. 2021, 8, 800103. [Google Scholar] [CrossRef]
- Hardman-Mountford, N.J.; Richardson, A.J.; Agenbag, J.J.; Hagen, E.; Nykjaer, L.; Shillington, F.A.; Villacastin, C. Ocean climate of the South East Atlantic observed from satellite data and wind models. Progr. Oceanogr 2003, 59, 181–221. [Google Scholar] [CrossRef]
- Punzón, A.; López-López, L.; González-Irusta, J.M.; Preciado, I.; Hidalgo, M.; Serrano, A.; Tel, E.; Somavilla, R.; Polo, J.; Blanco, M.; et al. Tracking the effect of temperature in marine demersal fish communities. Ecol. Indic. 2021, 12, 107142. [Google Scholar] [CrossRef]
- Sumby, J.; Haward, M.; Fulton, E.A.; Pecl, G.T. Hot fish: The response to climate change by regional fisheries bodies. Mar. Pol. 2021, 123, 104284. [Google Scholar] [CrossRef]
- Tashman, L.J. Out-of-sample tests of forecasting accuracy: An analysis and review. Int. J. Forecast. 2000, 16, 437–450. [Google Scholar] [CrossRef]
- Bergmeir, C.; Benítez, J.M. On the use of cross-validation for time series predictor evaluation. Inf. Sci. 2012, 191, 192–213. [Google Scholar] [CrossRef]


| Ocean Side | MTC Range (°C) | MTC/yr (°C) | p-Value | r2 | Adj. r2 |
|---|---|---|---|---|---|
| SWAO | 24.59–25.68 | 0.0120 | 0.00004 | 0.3586 | 0.3422 |
| SEAO | 25.19–25.93 | 0.0042 | 0.0201 | 0.1309 | 0.1087 |
| Ocean Side | SST Range (°C) | SST/yr (°C) | p-Value | r2 | Adj. r2 |
| SWAO | 23.38–24.01 | 0.0079 | 0.000002 | 0.4477 | 0.4336 |
| SEAO | 22.19–22.96 | 0.0080 | 0.0001 | 0.3186 | 0.3012 |
| Ocean Side | BCt Range (Sv) | BCt/yr (Sv) | p-Value | r2 | Adj. r2 |
| SWAO | −30.10–−14.19 | 0.366 | 0.0003 | 0.450 | 0.4255 |
| SEAO | - | - | - | - | - |
| SAO Side | Species | Thermal Affinity | Slope | p-Value | Slope Change (%) |
|---|---|---|---|---|---|
| SWAO | 0.0120 | 0.000035 | |||
| Prionace glauca | Cold | 0.0209 | 0.000000002 | 74.378 | |
| Thunnus albacares | Warm | 0.0171 | 0.000001 | 43.192 | |
| Thunnus obesus | Warm | 0.0167 | 0.000003 | 39.583 | |
| Scomberomorus brasiliensis | Warm | 0.0109 | 0.000141 | −8.929 | |
| Katsuwonus pelamis | Warm | 0.0090 | 0.007880 | −25.070 | |
| Thunnus alalunga | Cold | −0.0115 | 0.00000000037 | −196.328 | |
| SEAO | 0.0042 | 0.0201 | |||
| Thunnus albacares | Warm | 0.0097 | 0.0003 | 132.573 | |
| Prionace glauca | Cold | 0.0067 | 0.0001 | 60.373 | |
| Katsuwonus pelamis | Warm | 0.0051 | 0.0455 | 21.649 | |
| Scomberomorus tritor | Cold | 0.0046 | 0.0010 | 10.319 | |
| Thunnus maccoyii | Cold | 0.0045 | 0.0123 | 8.786 | |
| Isurus oxyrinchus | Warm | 0.0043 | 0.0171 | 2.648 | |
| Thunnus obesus | Warm | 0.0039 | 0.0406 | −5.952 | |
| Auxis thazard | Warm | 0.0038 | 0.0353 | −9.865 | |
| Euthynnus alletteratus | Cold | 0.0034 | 0.0524 | −17.728 | |
| Thunnus alalunga | Cold | −0.0024 | 0.0856 | −158.109 |
| SAO Side | Variable | Time Lag (yr) | Slope | SE | p-Value | r2 | AIC |
|---|---|---|---|---|---|---|---|
| SWAO | SST | 0 | 0.4570 | 0.2603 | 0.0869 | 0.0733 | 1.04 |
| 1 | 0.3588 | 0.2676 | 0.1879 | 0.0452 | 2.59 | ||
| 2 | 0.5761 | 0.2513 | 0.0277 | 0.1244 | −3.54 | ||
| 3 | 0.5283 | 0.2640 | 0.0530 | 0.1001 | −2.95 | ||
| 4 | 0.8378 | 0.2581 | 0.0026 | 0.2314 | −8.30 | ||
| BCt | 0 | 0.02325 | 0.00751 | 0.00511 | 0.2940 | −20.69 | |
| 1 | 0.01101 | 0.00847 | 0.20619 | 0.0685 | −14.70 | ||
| 2 | 0.00749 | 0.00851 | 0.38849 | 0.0340 | −13.69 | ||
| 3 | 0.01670 | 0.00850 | 0.06281 | 0.1553 | −15.09 | ||
| 4 | 0.00961 | 0.00933 | 0.31505 | 0.0504 | −12.69 | ||
| SEAO | SST | 0 | 0.1970 | 0.1256 | 0.1249 | 0.0593 | −43.56 |
| 1 | 0.1138 | 0.1312 | 0.3913 | 0.0194 | −39.99 | ||
| 2 | 0.2461 | 0.1312 | 0.0686 | 0.0868 | −40.81 | ||
| 3 | 0.0966 | 0.1462 | 0.5132 | 0.0120 | −36.26 | ||
| 4 | 0.3428 | 0.1388 | 0.0186 | 0.1483 | −39.66 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sant’Ana, R.; Thá, D.; Henry, L.-A.; Schroeder, R.; Perez, J.A.A. Increasing Sea Surface Temperatures Driving Widespread Tropicalization in South Atlantic Pelagic Fisheries. Biology 2025, 14, 1039. https://doi.org/10.3390/biology14081039
Sant’Ana R, Thá D, Henry L-A, Schroeder R, Perez JAA. Increasing Sea Surface Temperatures Driving Widespread Tropicalization in South Atlantic Pelagic Fisheries. Biology. 2025; 14(8):1039. https://doi.org/10.3390/biology14081039
Chicago/Turabian StyleSant’Ana, Rodrigo, Daniel Thá, Lea-Anne Henry, Rafael Schroeder, and José Angel Alvarez Perez. 2025. "Increasing Sea Surface Temperatures Driving Widespread Tropicalization in South Atlantic Pelagic Fisheries" Biology 14, no. 8: 1039. https://doi.org/10.3390/biology14081039
APA StyleSant’Ana, R., Thá, D., Henry, L.-A., Schroeder, R., & Perez, J. A. A. (2025). Increasing Sea Surface Temperatures Driving Widespread Tropicalization in South Atlantic Pelagic Fisheries. Biology, 14(8), 1039. https://doi.org/10.3390/biology14081039






