Can We Use CAR-T Cells to Overcome Immunosuppression in Solid Tumours?
Simple Summary
Abstract
1. Introduction
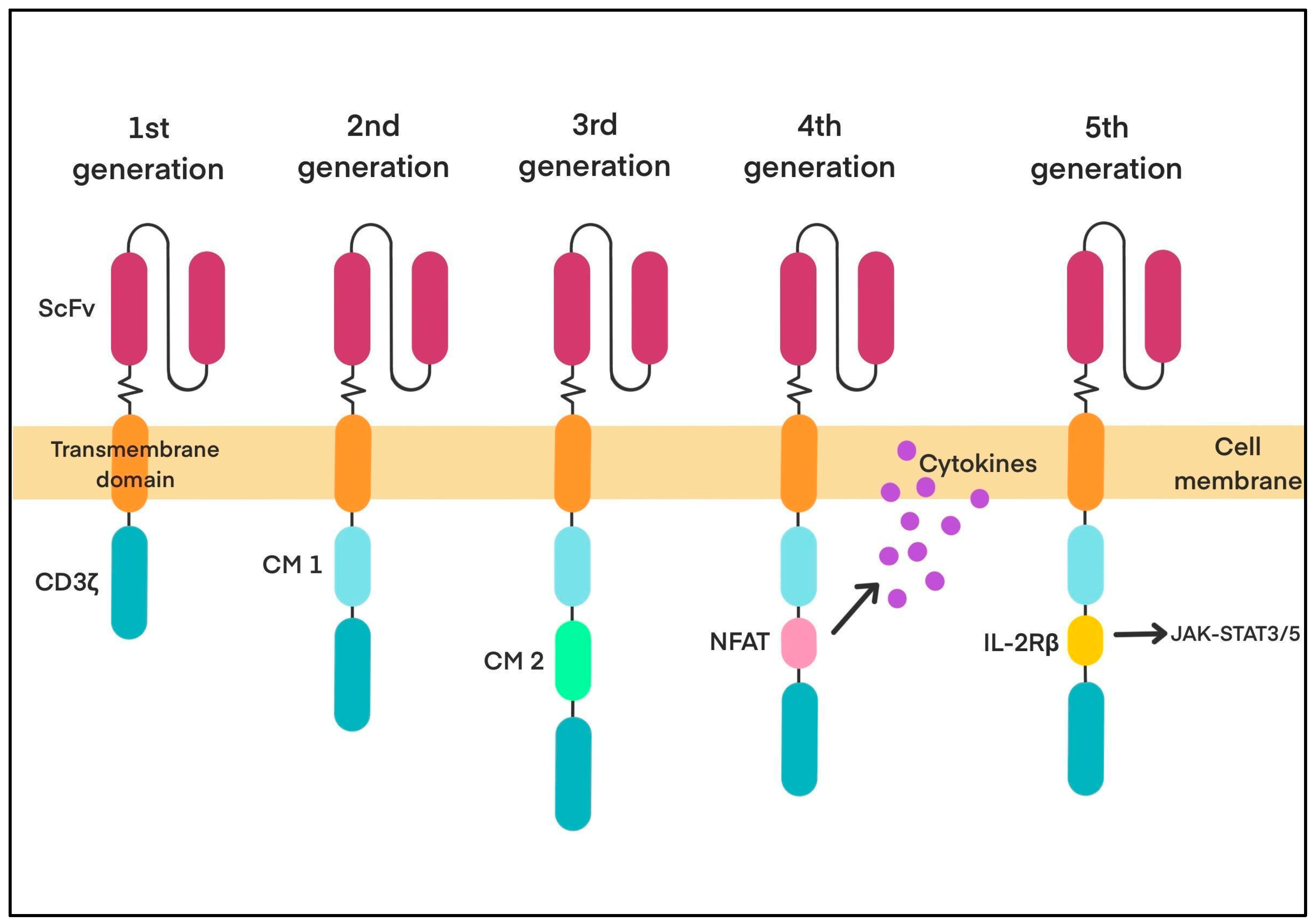
CAR-T-Cell Therapy—Overview and Historical Background
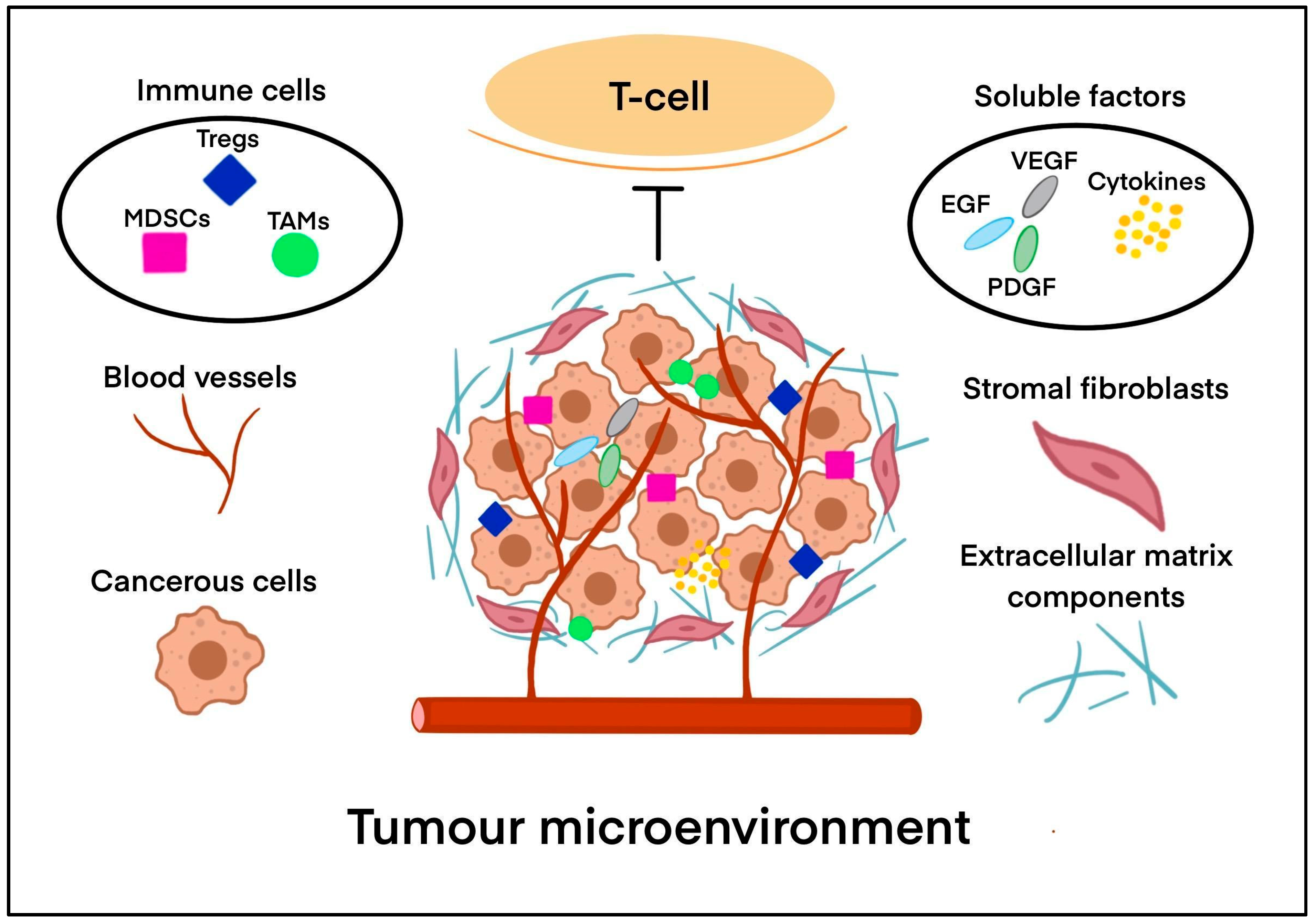
2. The Tumour Microenvironment (TME) in Solid Tumours
2.1. What Makes the TME Immunosuppressive?
2.1.1. Immune Cells
2.1.2. Soluble Factors
2.2. Immune Checkpoint Signalling
2.3. Tumour-Associated Antigens (TAAs)
2.4. Physical and Chemical Barriers
2.5. Extracellular Matrix
3. Why CAR-T Cells over Traditional Chemotherapy or Radiotherapy?
3.1. Toxicities of Chemotherapy
3.2. Toxicities of Radiotherapy
3.3. Toxicities of CAR-T-Cell Therapy
3.4. Short Treatment Duration and Potential for Long-Term Remission
4. Engineering CAR-T Cells to Overcome Immunosuppression
5. Challenges Faced by CAR-T Cells in Solid Tumours
6. Innovative Delivery Approaches
6.1. Administration Methods
6.2. CAR-T-Cell Engineering for Enhanced Penetration
7. Combination Therapies
8. Clinical Advances and Emerging Data
9. Future Directions
9.1. IL-12- and IL-18-Secreting CAR-T Cells
9.2. Hypoxia-Responsive CAR-T Cells
9.3. CAR-T Cells with Radiotherapy
9.4. Nanotechnology in CAR-T Cells
10. Discussion
10.1. Key Findings
10.2. Preclinical Evidence
- Direct killing of tumour cells via granzyme and perforin secretion [1].
- Secondary immune activation via cytokine secretion (IFN-γ, IL-12, IL-18) [137].
- Route of administration: Local delivery generally produced better efficacy and lower systemic toxicity compared to intravenous infusion [102].
- Cytokine profile: IL-18 often induced stronger acute inflammation than IL-12 [138].
- Hypoxia adaptation: CAR-T cells engineered for hypoxic environments showed greater tumour selectivity and safety [139].
- Safety: Certain models reported neurotoxicity (particularly with CNS delivery) or off-target effects linked to antigen expression on healthy tissues [87].
10.3. Clinical Evidence
10.4. Strengths and Limitations of Existing Research
10.5. Implications and Future Directions
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AKI | Acute kidney injury |
| APCs | Antigen-presenting cells |
| BCMA | B-cell maturation antigen |
| CAFs | Cancer-associated fibroblasts |
| CAR | Chimeric antigen receptor |
| CAR-Ms | CAR macrophages |
| CAR-NK | Chimeric antigen receptor natural killer |
| cAMP | Cyclic adenosine monophosphate |
| CD | Cluster of differentiation |
| CLL | Chronic lymphocytic leukaemia |
| cTCRs | Chimeric T-cell receptors |
| CMs | Co-stimulatory molecules |
| CRISPR | Clustered regularly interspaced short palindromic repeat |
| CRS | Cytokine release syndrome |
| CTLs | Cytotoxic T lymphocytes |
| CTLA4 | Cytotoxic T-lymphocyte-associated protein 4 |
| DCs | Dendritic cells |
| DLBCL | Diffuse large B-cell lymphoma |
| DLL3 | Delta-like protein 3 |
| ECM | Extracellular matrix |
| EGF | Epidermal growth factor |
| EMA | European Medicines Agency |
| FAP | Fibroblast activation protein |
| FDA | Food and Drug Administration |
| FSHR | Follicle-stimulating hormone receptor |
| GLUT 1 | Glucose transporter 1 |
| HER2 | Human epidermal growth factor receptor 2 |
| HIFs | Hypoxia-inducible factors |
| HRE | Hypoxia-responsive element |
| HSPGs | Heparan sulfate proteoglycans |
| ICANS | Immune effector cell neurotoxicity syndrome |
| ICOS | Inducible T-cell co-stimulator |
| IL | Interleukin |
| IL13Rα2 | interleukin-13 receptor subunit alpha-2 |
| IMCs | Immature myeloid cells |
| JAK | Janus kinase |
| LAG-3 | Lymphocyte activation gene-3 |
| MDSCs | Myeloid-derived suppressor cells |
| MHC | Major histocompatibility complex |
| MSLN | Mesothelin-directed |
| NFAT | Nuclear factor of activated T-cells |
| NK | Natural killer |
| NKT | Natural killer T |
| OVs | Oncolytic viruses |
| OXPHO | Oxidative phosphorylation |
| PD-1 | Programmed cell death protein 1 |
| PD-L1 | Programmed death ligand 1 |
| PDGF | Platelet-derived growth factor |
| PFS | Progression-free survival |
| RT | Radiotherapy |
| STAT | Signal transducer and activator of transcription |
| TAAs | Tumour-associated antigens |
| TAMs | Tumour-associated macrophages |
| TCR | T-cell receptor |
| TECs | Tumour endothelial cells |
| TIGIT | T-cell immunoreceptor with Ig and ITIM domains |
| TIM-3 | T-cell immunoglobulin and mucin-domain containing-3 |
| TME | Tumour microenvironment |
| TNFR | Tumour necrosis factor receptor |
| TNP | Trinitrophenyl |
| TGF-β | Transforming growth factor beta |
| TRUCK | T-cell redirected for universal cytokine-mediated killing |
| Tregs | Regulatory T cells |
| UniCAR-T | Universal CAR-T |
| VEGF | Vascular endothelial growth factor |
References
- Sterner, R.C.; Sterner, R.M. CAR-T cell therapy: Current limitations and potential strategies. Blood Cancer J. 2021, 11, 69. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Styczyński, J. A brief history of CAR-T cells: From laboratory to the bedside. Acta Haematol. Pol. 2020, 51, 2–5. [Google Scholar] [CrossRef]
- Asmamaw Dejenie, T.; Tiruneh, G.; Medhin, M.; Dessie Terefe, G.; Tadele Admasu, F.; Wale Tesega, W.; Chekol Abebe, E. Current updates on generations, approvals, and clinical trials of CAR T-cell therapy. Hum. Vaccin. Immunother. 2022, 18, 2114254. [Google Scholar] [CrossRef]
- Wrona, E.; Borowiec, M.; Potemski, P. CAR-NK Cells in the Treatment of Solid Tumors. Int. J. Mol. Sci. 2021, 22, 5899. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kuwana, Y.; Asakura, Y.; Utsunomiya, N.; Nakanishi, M.; Arata, Y.; Itoh, S.; Nagase, F.; Kurosawa, Y. Expression of chimeric receptor composed of immunoglobulin-derived V regions and T-cell receptor-derived C regions. Biochem. Biophys. Res. Commun. 1987, 149, 960–968. [Google Scholar] [CrossRef] [PubMed]
- Gross, G.; Waks, T.; Eshhar, Z. Expression of immunoglobulin-T-cell receptor chimeric molecules as functional receptors with antibody-type specificity. Proc. Natl. Acad. Sci. USA 1989, 86, 10024–10028. [Google Scholar] [CrossRef]
- Uscanga-Palomeque, A.C.; Chávez-Escamilla, A.K.; Alvizo-Báez, C.A.; Saavedra-Alonso, S.; Terrazas-Armendáriz, L.D.; Tamez-Guerra, R.S.; Rodríguez-Padilla, C.; Alcocer-González, J.M. CAR-T Cell Therapy: From the Shop to Cancer Therapy. Int. J. Mol. Sci. 2023, 24, 15688. [Google Scholar] [CrossRef] [PubMed]
- Krebs, S.; Chow, K.K.; Yi, Z.; Rodriguez-Cruz, T.; Hegde, M.; Gerken, C.; Ahmed, N.; Gottschalk, S. T cells redirected to interleukin-13Rα2 with interleukin-13 mutein--chimeric antigen receptors have anti-glioma activity but also recognize interleukin-13Rα1. Cytotherapy 2014, 16, 1121–1131. [Google Scholar] [CrossRef]
- Chen, Y.; Yu, Z.; Tan, X.; Jiang, H.; Xu, Z.; Fang, Y.; Han, D.; Hong, W.; Wei, W.; Tu, J. CAR-macrophage: A new immunotherapy candidate against solid tumors. Biomed. Pharmacother. 2021, 139, 111605. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Yang, Y.; Ma, P.; Zha, Y.; Zhang, J.; Lei, A.; Li, N. CAR-macrophage: An extensive immune enhancer to fight cancer. eBioMedicine 2022, 76, 103873. [Google Scholar] [CrossRef] [PubMed]
- Maalej, K.M.; Merhi, M.; Inchakalody, V.P.; Mestiri, S.; Alam, M.; Maccalli, C.; Cherif, H.; Uddin, S.; Steinhoff, M.; Marincola, F.M.; et al. CAR-cell therapy in the era of solid tumour treatment: Current challenges and emerging therapeutic advances. Mol. Cancer 2023, 22, 20. [Google Scholar] [CrossRef]
- Golo, M.; Newman, P.L.H.; Kempe, D.; Biro, M. Mechanoimmunology in the solid tumour microenvironment. Biochem. Soc. Trans. 2024, 52, 1489–1502. [Google Scholar] [CrossRef] [PubMed]
- Giraldo, N.A.; Sanchez-Salas, R.; Peske, J.D.; Vano, Y.; Becht, E.; Petitprez, F.; Validire, P.; Ingels, A.; Cathelineau, X.; Fridman, W.H.; et al. The clinical role of the TME in solid cancer. Br. J. Cancer 2019, 120, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Ni, Y.; Liang, X.; Lin, Y.; An, B.; He, X.; Zhao, X. Mechanisms of tumour resistance to immune checkpoint blockade and combination strategies to overcome resistance. Front. Immunol. 2022, 13, 915094. [Google Scholar] [CrossRef]
- Anderson, N.M.; Simon, M.C. The tumor microenvironment. Curr. Biol. 2020, 30, R921–R925. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- de Visser, K.E.; Joyce, J.A. The evolving tumour microenvironment: From cancer initiation to metastatic outgrowth. Cancer Cell 2023, 41, 374–403. [Google Scholar] [CrossRef] [PubMed]
- Mayer, S.; Milo, T.; Isaacson, A.; Halperin, C.; Miyara, S.; Stein, Y.; Lior, C.; Pevsner-Fischer, M.; Tzahor, E.; Mayo, A.; et al. The tumor microenvironment shows a hierarchy of cell-cell interactions dominated by fibroblasts. Nat. Commun. 2023, 14, 5810. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Baghban, R.; Roshangar, L.; Jahanban-Esfahlan, R.; Seidi, K.; Ebrahimi-Kalan, A.; Jaymand, M.; Kolahian, S.; Javaheri, T.; Zare, P. Tumor microenvironment complexity and therapeutic implications at a glance. Cell Commun. Signal 2020, 18, 59. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kankeu Fonkoua, L.A.; Sirpilla, O.; Sakemura, R.; Siegler, E.L.; Kenderian, S.S. CAR T cell therapy and the tumor microenvironment: Current challenges and opportunities. Mol. Ther. Oncolytics 2022, 25, 69–77. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Xia, X.; Yang, Z.; Lu, Q.; Liu, Z.; Wang, L.; Du, J.; Li, Y.; Yang, D.H.; Wu, S. Reshaping the tumour immune microenvironment to improve CAR-T cell-based cancer immunotherapy. Mol. Cancer 2024, 23, 175. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lindau, D.; Gielen, P.; Kroesen, M.; Wesseling, P.; Adema, G.J. The immunosuppressive tumour network: Myeloid-derived suppressor cells, regulatory T cells and natural killer T cells. Immunology 2013, 138, 105–115. [Google Scholar] [CrossRef]
- Li, C.; Jiang, P.; Wei, S.; Xu, X.; Wang, J. Regulatory T cells in tumour microenvironment: New mechanisms, potential therapeutic strategies and future prospects. Mol. Cancer 2020, 19, 116. [Google Scholar] [CrossRef]
- Escors, D.; Kochan, G. Myeloid-derived suppressor cells and their “inconvenient” plasticity. J. Immunol. Sci. 2018, 2, 42–47. [Google Scholar] [CrossRef]
- Tumino, N.; Fiore, P.F.; Pelosi, A.; Moretta, L.; Vacca, P. Myeloid derived suppressor cells in tumour microenvironment: Interaction with innate lymphoid cells. Semin Immunol. 2022, 61–64, 101668. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Shi, H.; Zhang, B.; Ou, X.; Ma, Q.; Chen, Y.; Shu, P.; Li, D.; Wang, Y. Myeloid-derived suppressor cells as immunosuppressive regulators and therapeutic targets in cancer. Signal Transduct. Target. Ther. 2021, 6, 362. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Groth, C.; Hu, X.; Weber, R.; Fleming, V.; Altevogt, P.; Utikal, J.; Umansky, V. Immunosuppression mediated by myeloid-derived suppressor cells (MDSCs) during tumour progression. Br. J. Cancer 2019, 120, 16–25. [Google Scholar] [CrossRef]
- Lin, Y.; Xu, J.; Lan, H. tumour-associated macrophages in tumour metastasis: Biological roles and clinical therapeutic applications. J. Hematol. Oncol. 2019, 12, 76. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bied, M.; Ho, W.W.; Ginhoux, F.; Blériot, C. Roles of macrophages in tumour development: A spatiotemporal perspective. Cell Mol. Immunol. 2023, 20, 983–992. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Takahashi, H.; Sakakura, K.; Kudo, T.; Toyoda, M.; Kaira, K.; Oyama, T.; Chikamatsu, K. Cancer-associated fibroblasts promote an immunosuppressive microenvironment through the induction and accumulation of protumoral macrophages. Oncotarget 2017, 8, 8633–8647. [Google Scholar] [CrossRef]
- Feng, B.; Wu, J.; Shen, B.; Jiang, F.; Feng, J. Cancer-associated fibroblasts and resistance to anticancer therapies: Status, mechanisms, and countermeasures. Cancer Cell Int. 2022, 22, 166. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yang, S.; Fang, Y.; Ma, Y.; Wang, F.; Wang, Y.; Jia, J.; Yang, Y.; Sun, W.; Zhou, Q.; Li, Z. Angiogenesis and targeted therapy in the tumour microenvironment: From basic to clinical practice. Clin. Transl. Med. 2025, 15, e70313. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wee, P.; Wang, Z. Epidermal Growth Factor Receptor Cell Proliferation Signaling Pathways. Cancers 2017, 9, 52. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Masuda, H.; Zhang, D.; Bartholomeusz, C.; Doihara, H.; Hortobagyi, G.N.; Ueno, N.T. Role of epidermal growth factor receptor in breast cancer. Breast Cancer Res. Treat 2012, 136, 331–345. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pandey, P.; Khan, F.; Upadhyay, T.K.; Seungjoon, M.; Park, M.N.; Kim, B. New insights about the PDGF/PDGFR signaling pathway as a promising target to develop cancer therapeutic strategies. Biomed. Pharmacother. 2023, 161, 114491. [Google Scholar] [CrossRef] [PubMed]
- Lugano, R.; Ramachandran, M.; Dimberg, A. Tumor angiogenesis: Causes, consequences, challenges and opportunities. Cell Mol. Life Sci. 2020, 77, 1745–1770. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Costanza, B.; Umelo, I.A.; Bellier, J.; Castronovo, V.; Turtoi, A. Stromal Modulators of TGF-β in Cancer. J. Clin. Med. 2017, 6, 7. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vanpouille-Box, C.; Formenti, S.C. Dual Transforming Growth Factor-β and Programmed Death-1 Blockade: A Strategy for Immune-Excluded Tumors? Trends Immunol. 2018, 39, 435–437. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Angioni, R.; Sánchez-Rodríguez, R.; Viola, A.; Molon, B. TGF-β in Cancer: Metabolic Driver of the Tolerogenic Crosstalk in the Tumor Microenvironment. Cancers 2021, 13, 401. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, W.; Jin, W.; Hardegen, N.; Lei, K.J.; Li, L.; Marinos, N.; McGrady, G.; Wahl, S.M. Conversion of peripheral CD4+CD25- naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J. Exp. Med. 2003, 198, 1875–1886. [Google Scholar] [CrossRef]
- Shi, X.; Yang, J.; Deng, S.; Xu, H.; Wu, D.; Zeng, Q.; Wang, S.; Hu, T.; Wu, F.; Zhou, H. TGF-β signaling in the tumor metabolic microenvironment and targeted therapies. J. Hematol. Oncol. 2022, 15, 135. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- de Vries, J.E. Immunosuppressive and anti-inflammatory properties of interleukin 10. Ann. Med. 1995, 27, 537–541. [Google Scholar] [CrossRef] [PubMed]
- Huang, A.; Cheng, L.; He, M.; Nie, J.; Wang, J.; Jiang, K. Interleukin-35 on B cell and T cell induction and regulation. J. Inflamm. 2017, 14, 16. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, K.; Huang, A.; Nie, J.; Tan, J.; Xing, S.; Qu, Y.; Jiang, K. IL-35 Regulates the Function of Immune Cells in Tumor Microenvironment. Front. Immunol. 2021, 12, 683332. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Toi, M.; Bando, H.; Ogawa, T.; Muta, M.; Hornig, C.; Weich, H.A. Significance of vascular endothelial growth factor (VEGF)/soluble VEGF receptor-1 relationship in breast cancer. Int. J. Cancer 2002, 98, 14–18. [Google Scholar] [CrossRef] [PubMed]
- Cavazzoni, A.; Digiacomo, G. Role of Cytokines and Other Soluble Factors in tumour Development: Rationale for New Therapeutic Strategies. Cells 2023, 12, 2532. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Qu, R.; Zhao, Y.; Zhang, Y. The mechanism of cytokine regulation of cancer occurrence and development in the tumour microenvironment and its application in cancer treatment: A narrative review. Transl. Cancer Res. 2024, 13, 5649–5663. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Xu, S.; Wang, Q.; Ma, W. Cytokines and soluble mediators as architects of tumour microenvironment reprogramming in cancer therapy. Cytokine Growth Factor. Rev. 2024, 76, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Luo, X.; Xie, Z.; Qiu, J.; Yang, J.; Deng, Y.; Long, R.; Tang, G.; Zhang, C.; Zuo, J. Prospects and challenges of CAR-T cell therapy combined with ICIs. Front. Oncol. 2024, 14, 1368732. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Toor, S.M.; Sasidharan Nair, V.; Decock, J.; Elkord, E. Immune checkpoints in the tumour microenvironment. Semin. Cancer Biol. 2020, 65, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.; Zhang, J.; Chen, S.; Wang, X.; Xi, Q.; Shen, H.; Zhang, R. Immune checkpoint inhibitors: Breakthroughs in cancer treatment. Cancer Biol. Med. 2024, 21, 451–472. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Alsaafeen, B.H.; Ali, B.R.; Elkord, E. Resistance mechanisms to immune checkpoint inhibitors: Updated insights. Mol. Cancer 2025, 24, 20. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Patel, S.A.; Minn, A.J. Combination Cancer Therapy with Immune Checkpoint Blockade: Mechanisms and Strategies. Immunity 2018, 48, 417–433. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Passarelli, A.; Mannavola, F.; Stefania Stucci, L.; Tucci, M.; Silvestris, F. Immune system and melanoma biology: A balance between immunosurveillance and immune escape. Oncotarget 2017, 8, 106132–106142. Available online: https://www.oncotarget.com/article/22190/text/ (accessed on 20 June 2025). [CrossRef]
- Czajka-Francuz, P.; Prendes, M.J.; Mankan, A.; Quintana, Á.; Pabla, S.; Ramkissoon, S.; Jensen, T.J.; Peiró, S.; Severson, E.A.; Achyut, B.R.; et al. Mechanisms of immune modulation in the tumor microenvironment and implications for targeted therapy. Front. Oncol. 2023, 13, 1200646. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, Z.; Han, F.; Du, Y.; Shi, H.; Zhou, W. Hypoxic microenvironment in cancer: Molecular mechanisms and therapeutic interventions. Signal Transduct. Target. Ther. 2023, 8, 70. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tu, V.Y.; Ayari, A.; O’Connor, R.S. Beyond the Lactate Paradox: How Lactate and Acidity Impact T Cell Therapies against Cancer. Antibodies 2021, 10, 25. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Niu, Y.; Mayr, T.; Muders, M.H. Competition for nutrients or cell intrinsic programming?—Metabolic mechanisms behind the tumor promoting immune microenvironment in cancer. Signal Transduct. Target. Ther. 2021, 6, 279. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kalli, M.; Poskus, M.D.; Stylianopoulos, T.; Zervantonakis, I.K. Beyond matrix stiffness: Targeting force-induced cancer drug resistance. Trends Cancer 2023, 9, 937–954. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hu, Q.; Zhu, Y.; Mei, J.; Liu, Y.; Zhou, G. Extracellular matrix dynamics in tumor immunoregulation: From tumor microenvironment to immunotherapy. J. Hematol. Oncol. 2025, 18, 65. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Henke, E.; Nandigama, R.; Ergün, S. Extracellular Matrix in the Tumor Microenvironment and Its Impact on Cancer Therapy. Front. Mol. Biosci. 2020, 6, 160. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Niland, S.; Riscanevo, A.X.; Eble, J.A. Matrix Metalloproteinases Shape the Tumor Microenvironment in Cancer Progression. Int. J. Mol. Sci. 2021, 23, 146. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- McAleer, S. A history of cancer and its treatment: Presidential Address to the Ulster Medical Society. Ulster Med. J. 2022, 91, 124–129. [Google Scholar] [PubMed] [PubMed Central]
- Dona Lemus, O.M.; Cao, M.; Cai, B.; Cummings, M.; Zheng, D. Adaptive Radiotherapy: Next-Generation Radiotherapy. Cancers 2024, 16, 1206. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gianfaldoni, S.; Gianfaldoni, R.; Wollina, U.; Lotti, J.; Tchernev, G.; Lotti, T. An Overview on Radiotherapy: From Its History to Its Current Applications in Dermatology. Open Access Maced. J. Med. Sci. 2017, 5, 521–525. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Olivares-Urbano, M.A.; Griñán-Lisón, C.; Marchal, J.A.; Núñez, M.I. CSC Radioresistance: A Therapeutic Challenge to Improve Radiotherapy Effectiveness in Cancer. Cells 2020, 9, 1651. [Google Scholar] [CrossRef]
- DeVita, V.T., Jr.; Chu, E. A history of cancer chemotherapy. Cancer Res. 2008, 68, 8643–8653. [Google Scholar] [CrossRef] [PubMed]
- Samami, E.; Shahhosseini, Z.; Hamzehgardeshi, Z.; Elyasi, F. Psychological Interventions in Chemotherapy-Induced Nausea and Vomiting in Women with Breast Cancer: A Systematic Review. Iran. J. Med. Sci. 2022, 47, 95–106. [Google Scholar] [PubMed] [PubMed Central]
- Piechotta, V.; Adams, A.; Haque, M.; Scheckel, B.; Kreuzberger, N.; Monsef, I.; Jordan, K.; Kuhr, K.; Skoetz, N. Antiemetics for adults for prevention of nausea and vomiting caused by moderately or highly emetogenic chemotherapy: A network meta-analysis. Cochrane Database Syst Rev. 2021, 11, CD012775. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dahlgren, D.; Sjöblom, M.; Hellström, P.M.; Lennernäs, H. Chemotherapeutics-Induced Intestinal Mucositis: Pathophysiology and Potential Treatment Strategies. Front. Pharmacol. 2021, 12, 681417. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dempke, W.C.M.; Zielinski, R.; Winkler, C.; Silberman, S.; Reuther, S.; Priebe, W. Anthracycline-induced cardiotoxicity—Are we about to clear this hurdle? Eur. J. Cancer 2023, 185, 94–104. [Google Scholar] [CrossRef] [PubMed]
- van den Boogaard, W.M.C.; Komninos, D.S.J.; Vermeij, W.P. Chemotherapy Side-Effects: Not All DNA Damage Is Equal. Cancers 2022, 14, 627. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kim, H.; Hong, B.; Kim, S.; Kang, S.M.; Park, J. Chemotherapy-related cardiotoxicity and its symptoms in patients with breast cancer: A scoping review. Syst. Rev. 2024, 13, 167. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sung, C.Y.W.; Hayase, N.; Yuen, P.S.T.; Lee, J.; Fernandez, K.; Hu, X.; Cheng, H.; Star, R.A.; Warchol, M.E.; Cunningham, L.L. Macrophage depletion protects against cisplatin-induced ototoxicity and nephrotoxicity. Sci Adv. 2024, 10, eadk9878. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Choi, B.D.; Yu, X.; Castano, A.P.; Darr, H.; Henderson, D.B.; Bouffard, A.A.; Larson, R.C.; Scarfò, I.; Bailey, S.R.; Gerhard, G.M.; et al. CRISPR-Cas9 disruption of PD-1 enhances activity of universal EGFRvIII CAR T cells in a preclinical model of human glioblastoma. J. Immunother. Cancer 2019, 7, 304. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhu, L.; Liu, J.; Li, J.; Wang, N.; Zhao, Y.; Su, H. Research progress on HER2-specific chimeric antigen receptor T cells for immunotherapy of solid tumors. Front. Immunol. 2025, 16, 1514994. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rejeski, K.; Subklewe, M.; Locke, F.L. Recognizing, defining, and managing CAR-T hematologic toxicities. Hematol. Am. Soc. Hematol. Educ. Program. 2023, 2023, 198–208. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Abouegylah, M.; Elemary, O.; Munir, A.; Gouda, M.Y.; Arafat, W.O.; Elzawawy, S. Evaluation of the Effect of Axillary Radiotherapy Dose and the Development of Lymphedema in Breast Cancer Patients. Breast Care 2022, 17, 364–370. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, K.; Tepper, J.E. Radiation therapy-associated toxicity: Etiology, management, and prevention. CA Cancer J. Clin. 2021, 71, 437–454. [Google Scholar] [CrossRef] [PubMed]
- Mariotto, A.B.; Rowland, J.H.; Yabroff, K.R.; Scoppa, S.; Hachey, M.; Ries, L.; Feuer, E.J. Long-term survivors of childhood cancers in the United States. Cancer Epidemiol. Biomarkers Prev. 2009, 18, 1033–1040. [Google Scholar] [CrossRef] [PubMed]
- Banfill, K.; Giuliani, M.; Aznar, M.; Franks, K.; McWilliam, A.; Schmitt, M.; Sun, F.; Vozenin, M.C.; Faivre Finn, C.; IASLC Advanced Radiation Technology Committee. Cardiac Toxicity of Thoracic Radiotherapy: Existing Evidence and Future Directions. J. Thorac. Oncol. 2021, 16, 216–227. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lee, D.W.; Santomasso, B.D.; Locke, F.L.; Ghobadi, A.; Turtle, C.J.; Brudno, J.N.; Maus, M.V.; Park, J.H.; Mead, E.; Pavletic, S.; et al. ASTCT Consensus Grading for Cytokine Release Syndrome and Neurologic Toxicity Associated with Immune Effector Cells. Biol. Blood Marrow Transplant. 2019, 25, 625–638. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shimabukuro-Vornhagen, A.; Gödel, P.; Subklewe, M.; Stemmler, H.J.; Schlößer, H.A.; Schlaak, M.; Kochanek, M.; Böll, B.; von Bergwelt-Baildon, M.S. Cytokine release syndrome. J. Immunother. Cancer 2018, 6, 56. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bangolo, A.; Amoozgar, B.; Mansour, C.; Zhang, L.; Gill, S.; Ip, A.; Cho, C. Comprehensive Review of Early and Late Toxicities in CAR T-Cell Therapy and Bispecific Antibody Treatments for Hematologic Malignancies. Cancers 2025, 17, 282. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Baer, B.; Dudley, C.V.; Simons, R.M. Management Principles Associated With Cytokine Release Syndrome. Semin. Oncol. Nurs. 2019, 35, 150931. [Google Scholar] [CrossRef] [PubMed]
- Le, R.Q.; Li, L.; Yuan, W.; Shord, S.S.; Nie, L.; Habtemariam, B.A.; Przepiorka, D.; Farrell, A.T.; Pazdur, R. FDA Approval Summary: Tocilizumab for Treatment of Chimeric Antigen Receptor T Cell-Induced Severe or Life-Threatening Cytokine Release Syndrome. Oncologist 2018, 23, 943–947. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Caimi, P.F.; Pacheco Sanchez, G.; Sharma, A.; Otegbeye, F.; Ahmed, N.; Rojas, P.; Patel, S.; Kleinsorge Block, S.; Schiavone, J.; Zamborsky, K.; et al. Prophylactic Tocilizumab Prior to Anti-CD19 CAR-T Cell Therapy for Non-Hodgkin Lymphoma. Front. Immunol. 2021, 12, 745320. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Morris, E.C.; Neelapu, S.S.; Giavridis, T.; Sadelain, M. Cytokine release syndrome and associated neurotoxicity in cancer immunotherapy. Nat. Rev. Immunol. 2022, 22, 85–96. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lin, M.-Y.; Nam, E.; Shih, R.M.; Shafer, A.; Bouren, A.; Ceja, M.A.; Harris, C.; Khericha, M.; Vo, K.H.; Kim, M.; et al. Chen; Self-regulating CAR-T cells modulate cytokine release syndrome in adoptive T-cell therapy. J. Exp. Med 2024, 221, e20221988. [Google Scholar] [CrossRef] [PubMed]
- De Marco, R.C.; Monzo, H.J.; Ojala, P.M. CAR T Cell Therapy: A Versatile Living Drug. Int. J. Mol. Sci. 2023, 24, 6300. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Korell, F.; Laier, S.; Sauer, S.; Veelken, K.; Hennemann, H.; Schubert, M.L.; Sauer, T.; Pavel, P.; Mueller-Tidow, C.; Dreger, P.; et al. Current Challenges in Providing Good Leukapheresis Products for Manufacturing of CAR-T Cells for Patients with Relapsed/Refractory NHL or ALL. Cells 2020, 9, 1225. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Levine, J.E.; Grupp, S.A.; Pulsipher, M.A.; Dietz, A.C.; Rives, S.; Myers, G.D.; August, K.J.; Verneris, M.R.; Buechner, J.; Laetsch, T.W.; et al. Pooled safety analysis of tisagenlecleucel in children and young adults with B cell acute lymphoblastic leukaemia. J. Immunother. Cancer 2021, 9, e002287. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Worel, N.; Winter, S.; Oesterreicher, S.; Lickefett, B.; Tolios, A.; Wohlfarth, P.; Staber, P.; Rabitsch, W.; Mitterbauer, M.; Rüsing, L.; et al. From tumour Board Decision to Infusion: Timeline of CD19 CAR-T Cell Therapy at the Medical University of Vienna. Transpl. Cell. Ther. 2025, 31, S244. [Google Scholar] [CrossRef]
- Milowsky, M.I.; O’Donnell, P.H.; Hoimes, C.J.; Petrylak, D.P.; Flaig, T.W.; Moon, H.H.; Friedlander, T.W.; Mar, N.; McKay, R.R.; Srinivas, S.; et al. Patient-Reported Outcomes in Patients With Advanced Urothelial Cancer Who Are Ineligible for Cisplatin and Treated With First-Line Enfortumab Vedotin Alone or With Pembrolizumab. J. Clin. Oncol. 2024, 42, 1403–1414. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Neelapu, S.S.; Locke, F.L.; Bartlett, N.L.; Lekakis, L.J.; Miklos, D.B.; Jacobson, C.A.; Braunschweig, I.; Oluwole, O.O.; Siddiqi, T.; Lin, Y.; et al. Axicabtagene Ciloleucel CAR T-Cell Therapy in Refractory Large B-Cell Lymphoma. N. Engl. J. Med. 2017, 377, 2531–2544. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, Y.; An, L.; Huang, R.; Xiong, J.; Yang, H.; Wang, X.; Zhang, X. Strategies to enhance CAR-T persistence. Biomark. Res. 2022, 10, 86. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Maude, S.L.; Laetsch, T.W.; Buechner, J.; Rives, S.; Boyer, M.; Bittencourt, H.; Bader, P.; Verneris, M.R.; Stefanski, H.E.; Myers, G.D.; et al. Tisagenlecleucel in Children and Young Adults with B-Cell Lymphoblastic Leukemia. N. Engl. J. Med. 2018, 378, 439–448. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Xu, J.; Wang, B.-Y.; Yu, S.-H.; Chen, S.-J.; Yang, S.-S.; Liu, R.; Chen, L.-J.; Hou, J.; Chen, Z.; Zhao, W.-H.; et al. Long-term remission and survival in patients with relapsed or refractory multiple myeloma after treatment with LCAR-B38M CAR T cells: 5-year follow-up of the LEGEND-2 trial. J. Hematol. Oncol. 2024, 17, 23. [Google Scholar] [CrossRef] [PubMed]
- Mo, G.; Lee, S.Y.; Coffey, D.G.; Voillet, V.; Kirsch, I.R.; Gottardo, R.; Smythe, K.S.; Yeung, C.C.S.; Greenbaum, A.; Green, D.J.; et al. Long-term Remissions Following CD20-Directed Chimeric Antigen Receptor-Adoptive T-cell Therapy. Blood Cancer Discov. 2024, 5, 258–266. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cappell, K.M.; Sherry, R.M.; Yang, J.C.; Goff, S.L.; Vanasse, D.A.; McIntyre, L.; Rosenberg, S.A.; Kochenderfer, J.N. Long-Term Follow-Up of Anti-CD19 Chimeric Antigen Receptor T-Cell Therapy. J. Clin. Oncol. 2020, 38, 3805–3815. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Locke, F.L.; Hu, Z.-H.; Siddiqi, T.; Jacobson, C.A.; Nikiforow, S.; Ahmed, S.; Miklos, D.B.; Lin, Y.; Lunning, M.A.; Hill, B.T.; et al. Real-World Impact of Time from Leukapheresis to Infusion (Vein-to-Vein Time) in Patients with Relapsed or Refractory (r/r) Large B-Cell Lymphoma (LBCL) Treated with Axicabtagene Ciloleucel. Blood 2022, 140, 7512–7515. [Google Scholar] [CrossRef]
- Grischke, T.K.; Cooperrider, J.H.; Pula, A.; Sneider, A.; Yates, S.; Jakubowiak, A.J.; Derman, B.A. Brain-to-vein and vein-to-vein times and outcomes in CAR T-cell therapy in myeloma. Blood Cancer J. 2025, 15, 47. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hou, B.; Tang, Y.; Li, W.; Zeng, Q.; Chang, D. Efficiency of CAR-T Therapy for Treatment of Solid Tumor in Clinical Trials: A Meta-Analysis. Dis. Markers 2019, 2019, 3425291. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rossi, M.; Breman, E. Engineering strategies to safely drive CAR T-cells into the future. Front. Immunol. 2024, 15, 1411393. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rodriguez-Garcia, A.; Palazon, A.; Noguera-Ortega, E.; Powell, D.J., Jr.; Guedan, S. CAR-T Cells Hit the tumour Microenvironment: Strategies to Overcome tumour Escape. Front. Immunol. 2020, 11, 1109. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gao, T.A.; Chen, Y.Y. Engineering Next-Generation CAR-T Cells: Overcoming tumour Hypoxia and Metabolism. Annu. Rev. Chem. Biomol. Eng. 2022, 13, 193–216. [Google Scholar] [CrossRef] [PubMed]
- McGowan, E.; Lin, Q.; Ma, G.; Yin, H.; Chen, S.; Lin, Y. PD-1 disrupted CAR-T cells in the treatment of solid tumours: Promises and challenges. Biomed. Pharmacother. 2020, 121, 109625. [Google Scholar] [CrossRef]
- Bagley, S.J.; Binder, Z.A.; Lamrani, L.; Marinari, E.; Desai, A.S.; Nasrallah, M.P.; Maloney, E.; Brem, S.; Lustig, R.A.; Kurtz, G.; et al. Repeated peripheral infusions of anti-EGFRvIII CAR T cells in combination with pembrolizumab show no efficacy in glioblastoma: A phase 1 trial. Nat. Cancer 2024, 5, 517–531. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhu, T.; Jiang, G.; Zeng, Q.; Li, Z.; Huang, X. Target delivery of a PD-1-TREM2 scFv by CAR-T cells enhances anti-tumor efficacy in colorectal cancer. Mol. Cancer 2023, 22, 131. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Arjomandnejad, M.; Kopec, A.L.; Keeler, A.M. CAR-T Regulatory (CAR-Treg) Cells: Engineering and Applications. Biomedicines 2022, 10, 287. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Alatrash, G.; Crain, A.K.; Molldrem, J.J. Immune Biology of Allogeneic Hematopoietic Stem Cell Transplantation, 2nd ed.; Chapter 7—Tumour-Associated Antigens; Academic Press: Cambridge, MA, USA, 2019; ISBN 9780128126301. [Google Scholar] [CrossRef]
- Yesevage, T. CAR T-Cell Therapy; New Advances Move On Past Setbacks. GEN Genetic Engineering & Biotechnology News, 15 May 2025. [Google Scholar] [CrossRef]
- Montironi, C.; Muñoz-Pinedo, C.; Eldering, E. Hematopoietic versus Solid Cancers and T Cell Dysfunction: Looking for Similarities and Distinctions. Cancers 2021, 13, 284. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Adusumilli, P.S.; Cherkassky, L.; Villena-Vargas, J.; Colovos, C.; Servais, E.; Plotkin, J.; Jones, D.R.; Sadelain, M. Regional delivery of mesothelin-targeted CAR T cell therapy generates potent and long-lasting CD4-dependent tumour immunity. Sci. Transl. Med. 2014, 6, 261ra151. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ai, K.; Liu, B.; Chen, X.; Huang, C.; Yang, L.; Zhang, W.; Weng, J.; Du, X.; Wu, K.; Lai, P. Optimizing CAR-T cell therapy for solid tumours: Current challenges and potential strategies. J. Hematol. Oncol. 2024, 17, 105. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hadrup, S.; Donia, M.; Thor Straten, P. Effector CD4 and CD8 T cells and their role in the tumor microenvironment. Cancer Microenviron. 2013, 6, 123–133. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Valenza, C.; Guidi, L.; Battaiotto, E.; Trapani, D.; Sartore Bianchi, A.; Siena, S.; Curigliano, G. Targeting HER2 heterogeneity in breast and gastrointestinal cancers. Trends Cancer 2024, 10, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Shao, Q.; Deng, J.; Wu, H.; Huang, Z. HER2-positive gastric cancer: From targeted therapy to CAR-T cell therapy. Front. Immunol. 2025, 16, 1560280. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Khan, S.H.; Choi, Y.; Veena, M.; Lee, J.K.; Shin, D.S. Advances in CAR T cell therapy: Antigen selection, modifications, and current trials for solid tumours. Front. Immunol. 2025, 15, 1489827. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Miao, L.; Zhang, J.; Xu, W.; Qian, Q.; Zhang, G.; Yuan, Q.; Lv, Y.; Zhang, H.; Shen, C.; Wang, W. Global research trends in CAR-T cell therapy for solid tumours: A comprehensive visualization and bibliometric study (2012-2023). Hum. Vaccin. Immunother. 2024, 20, 2338984. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Posey, A.D., Jr.; Young, R.M.; June, C.H. Future perspectives on engineered T cells for cancer. Trends Cancer 2024, 10, 687–695. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Maus, M.V.; Choi, B.D. CAR-T cell therapy for the treatment of adult high-grade gliomas. NPJ Precis. Oncol. 2024, 8, 279. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Milone, M.C.; Xu, J.; Chen, S.J.; Collins, M.A.; Zhou, J.; Powell, D.J., Jr.; Melenhorst, J.J. Engineering enhanced CAR T-cells for improved cancer therapy. Nat. Cancer 2021, 2, 780–793. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fu, R.; Qi, R.; Xiong, H.; Lei, X.; Jiang, Y.; He, J.; Chen, F.; Zhang, L.; Qiu, D.; Chen, Y.; et al. Combination therapy with oncolytic virus and T cells or mRNA vaccine amplifies antitumour effects. Signal Transduct. Target. Ther. 2024, 9, 118. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Aboalela, M.A.; Abdelmoneim, M.; Matsumura, S.; Eissa, I.R.; Bustos-Villalobos, I.; Sibal, P.A.; Orikono, Y.; Takido, Y.; Naoe, Y.; Kasuya, H. Enhancing mesothelin CAR T cell therapy for pancreatic cancer with an oncolytic herpes virus boosting CAR target antigen expression. Cancer Immunol. Immunother. 2025, 74, 202. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, A.X.; Ong, X.J.; D’Souza, C.; Neeson, P.J.; Zhu, J.J. Combining chemotherapy with CAR-T cell therapy in treating solid tumours. Front. Immunol. 2023, 14, 1140541. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Al-Haideri, M.; Tondok, S.B.; Safa, S.H.; Maleki, A.H.; Rostami, S.; Jalil, A.T.; Al-Gazally, M.E.; Alsaikhan, F.; Rizaev, J.A.; Mohammad, T.A.M.; et al. CAR-T cell combination therapy: The next revolution in cancer treatment. Cancer Cell Int. 2022, 22, 365. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, S.; Zhao, Y.; Gao, Y.; Li, F.; Zhang, Y. Targeting metabolism to improve CAR-T cells therapeutic efficacy. Chin. Med. J. 2024, 137, 909–920. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Klysz, D.D.; Fowler, C.; Malipatlolla, M.; Stuani, L.; Freitas, K.A.; Chen, Y.; Meier, S.; Daniel, B.; Sandor, K.; Xu, P.; et al. Inosine induces stemness features in CAR-T cells and enhances potency. Cancer Cell 2024, 42, 266–282.e8. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mackensen, A.; Haanen, J.B.A.G.; Koenecke, C.; Alsdorf, W.; Wagner-Drouet, E.; Borchmann, P.; Heudobler, D.; Ferstl, B.; Klobuch, S.; Bokemeyer, C.; et al. CLDN6-specific CAR-T cells plus amplifying RNA vaccine in relapsed or refractory solid tumours: The phase 1 BNT211-01 trial. Nat. Med. 2023, 29, 2844–2853. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, Y.R.; Lyu, Z.; Shen, X.; Fang, Y.; Yang, L. Boosting CAR-T cell therapy through vaccine synergy. Trends Pharmacol. Sci. 2025, 46, 180–199. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Sferruzza, G.; Yang, L.; Zhou, L.; Chen, S. CAR-T and CAR-NK as cellular cancer immunotherapy for solid tumours. Cell Mol. Immunol. 2024, 21, 1089–1108. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, J.; Jiao, X.; Ma, D.; Fang, Y.; Gao, Q. CAR-T therapy and targeted treatments: Emerging combination strategies in solid tumours. Medicine 2024, 5, 530–549. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, R.; Esmaeili Gouvarchin Ghaleh, H.; Farzanehpour, M.; Dorostkar, R.; Ranjbar, R.; Bolandian, M.; Mirzaei Nodooshan, M.; Ghorbani Alvanegh, A. Combination therapy with CAR T cells and oncolytic viruses: A new era in cancer immunotherapy. Cancer Gene Ther. 2022, 29, 647–660. [Google Scholar] [CrossRef] [PubMed]
- McGrath, K.; Dotti, G. Combining Oncolytic Viruses with Chimeric Antigen Receptor T Cell Therapy. Hum. Gene Ther. 2021, 32, 150–157. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mestermann, K.; Giavridis, T.; Weber, J.; Rydzek, J.; Frenz, S.; Nerreter, T.; Mades, A.; Sadelain, M.; Einsele, H.; Hudecek, M. The tyrosine kinase inhibitor dasatinib acts as a pharmacologic on/off switch for CAR T cells. Sci. Transl. Med. 2019, 11, eaau5907. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Du, B.; Qin, J.; Lin, B.; Zhang, J.; Li, D.; Liu, M. CAR-T therapy in solid tumours. Cancer Cell 2025, 43, 665–679. [Google Scholar] [CrossRef] [PubMed]
- Chmielewski, M.; Abken, H. CAR T Cells Releasing IL-18 Convert to T-Bethigh FoxO1low Effectors that Exhibit Augmented Activity against Advanced Solid tumours. Cell Rep. 2017, 21, 3205–3219. [Google Scholar] [CrossRef] [PubMed]
- Jaspers, J.E.; Khan, J.F.; Godfrey, W.D.; Lopez, A.V.; Ciampricotti, M.; Rudin, C.M.; Brentjens, R.J. IL-18-secreting CAR T cells targeting DLL3 are highly effective in small cell lung cancer models. J. Clin. Invest 2023, 133, e166028. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhu, X.; Chen, J.; Li, W.; Xu, Y.; Shan, J.; Hong, J.; Zhao, Y.; Xu, H.; Ma, J.; Shen, J.; et al. Hypoxia-Responsive CAR-T Cells Exhibit Reduced Exhaustion and Enhanced Efficacy in Solid tumours. Cancer Res. 2024, 84, 84–100. [Google Scholar] [CrossRef] [PubMed]
- Kosti, P.; Opzoomer, J.W.; Larios-Martinez, K.I.; Henley-Smith, R.; Scudamore, C.L.; Okesola, M.; Taher, M.Y.M.; Davies, D.M.; Muliaditan, T.; Larcombe-Young, D.; et al. Hypoxia-sensing CAR T cells provide safety and efficacy in treating solid tumours. Cell Rep. Med. 2021, 2, 100227. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hatae, R.; Kyewalabye, K.; Yamamichi, A.; Chen, T.; Phyu, S.; Chuntova, P.; Nejo, T.; Levine, L.S.; Spitzer, M.H.; Okada, H. Enhancing CAR-T cell metabolism to overcome hypoxic conditions in the brain tumour microenvironment. JCI Insight 2024, 9, e177141. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hovhannisyan, L.; Riether, C.; Aebersold, D.M.; Medová, M.; Zimmer, Y. CAR T cell-based immunotherapy and radiation therapy: Potential, promises and risks. Mol. Cancer 2023, 22, 82. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhong, L.; Li, Y.; Muluh, T.A.; Wang, Y. Combination of CAR-T cell therapy and radiotherapy: Opportunities and challenges in solid tumours (Review). Oncol. Lett. 2023, 26, 281. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Qin, V.M.; Haynes, N.M.; D’Souza, C.; Neeson, P.J.; Zhu, J.J. CAR-T Plus Radiotherapy: A Promising Combination for Immunosuppressive tumours. Front. Immunol. 2022, 12, 813832. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fan, M.; Zheng, J.; Huang, Y.; Lu, M.; Shang, Z.; Du, M. Nanoparticle-mediated universal CAR-T therapy. Int. J. Pharm. 2024, 666, 124779. [Google Scholar] [CrossRef] [PubMed]
- Dana, N.; Dabiri, A.; Najafi, M.B.; Rahimi, A.; Ishaghi, S.M.M.; Shariati, L.; Shao, M.; Borzacchiello, A.; Rahimmanesh, I.; Makvandi, P. Advances in bioengineered CAR T/NK cell therapy for glioblastoma: Overcoming immunosuppression and nanotechnology-based strategies for enhanced CAR T/NK cell therapy. Bioeng. Transl. Med. 2024, 10, e10716. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kang, X.; Mita, N.; Zhou, L.; Wu, S.; Yue, Z.; Babu, R.J.; Chen, P. Nanotechnology in Advancing Chimeric Antigen Receptor T Cell Therapy for Cancer Treatment. Pharmaceutics 2024, 16, 1228. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mi, J.; Ye, Q.; Min, Y. Advances in Nanotechnology Development to Overcome Current Roadblocks in CAR-T Therapy for Solid tumours. Front. Immunol. 2022, 13, 849759. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sharma, P.; Allison, J.P. The future of immune checkpoint therapy. Science 2015, 348, 56–61. [Google Scholar] [CrossRef] [PubMed]
- FDA Guidance for Industry: Preclinical Assessment of Investigational Cellular and Gene Therapy Products; EMA/CAT/852292/2018 Rev.1. Available online: https://www.fda.gov/media/87564/download (accessed on 11 March 2025).
- Zushin, P.H.; Mukherjee, S.; Wu, J.C. FDA Modernization Act 2.0: Transitioning beyond animal models with human cells, organoids, and AI/ML-based approaches. J. Clin. Invest 2023, 133, e175824. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, Y.; Zhou, H.; Ju, S.; Dong, X.; Zheng, C. The solid tumour microenvironment and related targeting strategies: A concise review. Front. Immunol. 2025, 16, 1563858. [Google Scholar] [CrossRef]

| Toxicity Type | Chemotherapy | Radiotherapy | CAR-T Therapy |
|---|---|---|---|
| Gastrointestinal toxicity | Nausea (96.5%) [42], emesis (acute, delayed, anticipatory), malnutrition, acid–base imbalance [34,35], diarrhoea, and mucositis [36]. | Nausea, emesis, and diarrhoea [43]. | Nausea [47,48]. |
| Cardiotoxicity | Chronic heart failure, hypertension, arrhythmias, and cardiomyopathy [37]. | Valvular heart disease (60%) [45], myocarditis, and fibrosis [43,45,46]. | In severe cases of CRS [47,48]. |
| Nephrotoxicity | Acute kidney injury and chronic renal failure risk [38,40]. | Radiation-induced kidney injury [74]. | Low incidence of acute kidney injury [75]. |
| Haematological toxicity | Myelosuppression, anaemia, and thrombocytopenia [41]. | Marrow suppression (depending on site) [43]. | Cytopenias * and CRS-related systemic inflammation [47]. |
| Neurological toxicity | Polyneuropathies [41]. | Late cognitive impairment [43]. | ICANS: confusion, aphasia, and seizures [49]. |
| Immunologic toxicity | Hypersensitivity reactions [33]. | Dermatitis and pneumonitis from local immune effects [43]. | CRS (50–90%) [47,48]. |
| Other | Alopecia (98.6%), anorexia (97.9%), and dysgeusia (97.2%) [42]. | Lymphoedema (breast cancer radiotherapy: 20.4%; overall: 42.2%) [44], alopecia, and dermatitis [43]. | Fever [47,48]. |
| Reversibility | Chronic or irreversible (e.g., cardiotoxicity, neuropathy) [37,40]. | Acute symptoms (reversible); late fibrosis and cardiac effects may persist [43]. | Mostly reversible; long-term organ damage risk [49]. |
| Challenge | Findings | CAR-T Strategy | Clinical/Preclinical Results | Risks and Limitations | References |
|---|---|---|---|---|---|
| Antigen heterogeneity | Solid tumours lack unique, uniformly expressed antigens; shared expression with healthy tissues increases toxicity risks | — | — | High risk of off-tumour toxicity; antigen escape | [116] |
| HER2-targeted CAR-T in gastric cancer (preclinical) | HER2 identified as a viable target; CAR-Ts show selective cytotoxicity | HER2-specific CAR-T cell | Eliminated HER2+ patient-derived cells; strong activity in xenograft models; MHC-independent apoptosis induction | Limited to HER2+ cancers; need for careful patient selection | [117] |
| HER2-targeted CAR-T in gastric cancer (clinical) | Evaluation of safety and efficacy in HER2+ advanced cancer patients | HER2-specific CAR-T cell | 1 partial response (4.5 mo); 5 stable disease cases (median PFS: 4.8 mo) | 1 patient developed severe GI bleeding | [117] |
| EGFR-targeted CAR-T in NSCLC (phase I trial 1) | Investigated efficacy in EGFR-expressing NSCLC | EGFR-specific CAR-T cell | 4 partial responses (2–4 mo); 8 stable disease; manageable grade 3 AEs; median PFS: 3 mo; OS: 4.9 mo | Short duration of response; toxicity manageable but present | [118] |
| EGFR-targeted CAR-T in NSCLC (phase I trial 2) | Follow-up in refractory/relapsed NSCLC | EGFR-specific CAR-T cell | 1 durable partial response (>13 mo); 6 stable disease; median PFS: 7.13 mo; OS: 15.63 mo; all AEs were manageable | Small sample size; early-phase trial | [118] |
| General clinical landscape | Solid tumours still pose major hurdles due to TME and immune evasion | Various under investigation | Some promising early results in HER2+ and EGFR+ cancers | No major breakthroughs yet; still investigational | [119] |
| Approach | Mechanism | Key Outcomes | Phase of Study | References |
|---|---|---|---|---|
| Checkpoint Inhibitors (e.g., anti-PD-1/PD-L1) | Restore CAR-T function by reversing T-cell exhaustion and improving persistence | Enhanced CAR-T proliferation, persistence, and antitumour response in early studies | Clinical trial | [107] |
| Oncolytic Viruses | Induce tumour cell lysis, release tumour antigens, promote inflammation, and recruit T cells into cold tumours | OV + CAR-T shows superior tumour control and survival in solid tumour models | Preclinical model, early clinical trials | [123,134] |
| Low-dose Chemotherapy/Radiotherapy | Lymphodepletion reduces suppressive immune cells (Tregs, MDSCs) and prepares niche for CAR-T expansion | Improved CAR-T engraftment and synergy in trials combining radiotherapy with EGFRvIII-targeted CAR-T cell | Clinical trial | [125,126] |
| Metabolic Checkpoint Inhibitors | Inhibit IDO and A2A receptors to reduce kynurenine and adenosine-mediated T-cell suppression | Enhanced CAR-T function under metabolic stress; adenosine deaminase overexpression improves antitumour activity | Preclinical model clinical trials | [127,128] |
| RNA/mRNA Vaccines | Boost CAR-T and endogenous T-cell immunity; promote epitope spreading; overcome antigen escape | Amplifying RNA vaccines improve CAR-T expansion and responses; mRNA boosters expand polyclonal immunity | Clinical trial | [129,130,131] |
| Synthetic Notch/Logic-Gated CARs | Conditional activation improves specificity and reduces off-tumour toxicity | Enhanced discrimination between tumour and healthy tissue; precise response control | Preclinical models, early clinical trial | [133] |
| Kinase Inhibitors (e.g., Dasatinib) | Reversible CAR-T inhibition allows functional recovery and prevents exhaustion | Dasatinib temporarily rests CAR-T cells and improves their long-term function | Preclinical model | [135] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gwadera, J.; Grajewski, M.; Chowaniec, H.; Gucia, K.; Michoń, J.; Mikulicz, Z.; Knast, M.; Pujanek, P.; Tołkacz, A.; Murawa, A.; et al. Can We Use CAR-T Cells to Overcome Immunosuppression in Solid Tumours? Biology 2025, 14, 1035. https://doi.org/10.3390/biology14081035
Gwadera J, Grajewski M, Chowaniec H, Gucia K, Michoń J, Mikulicz Z, Knast M, Pujanek P, Tołkacz A, Murawa A, et al. Can We Use CAR-T Cells to Overcome Immunosuppression in Solid Tumours? Biology. 2025; 14(8):1035. https://doi.org/10.3390/biology14081035
Chicago/Turabian StyleGwadera, Julia, Maksymilian Grajewski, Hanna Chowaniec, Kasper Gucia, Jagoda Michoń, Zofia Mikulicz, Małgorzata Knast, Patrycja Pujanek, Amelia Tołkacz, Aleksander Murawa, and et al. 2025. "Can We Use CAR-T Cells to Overcome Immunosuppression in Solid Tumours?" Biology 14, no. 8: 1035. https://doi.org/10.3390/biology14081035
APA StyleGwadera, J., Grajewski, M., Chowaniec, H., Gucia, K., Michoń, J., Mikulicz, Z., Knast, M., Pujanek, P., Tołkacz, A., Murawa, A., & Dobosz, P. (2025). Can We Use CAR-T Cells to Overcome Immunosuppression in Solid Tumours? Biology, 14(8), 1035. https://doi.org/10.3390/biology14081035






