TLR4 Asp299Gly SNP (rs4986790) Protects from Periodontal Inflammatory Destruction by Altering TLR4 Susceptibility to LPS Stimulation
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Genotyping
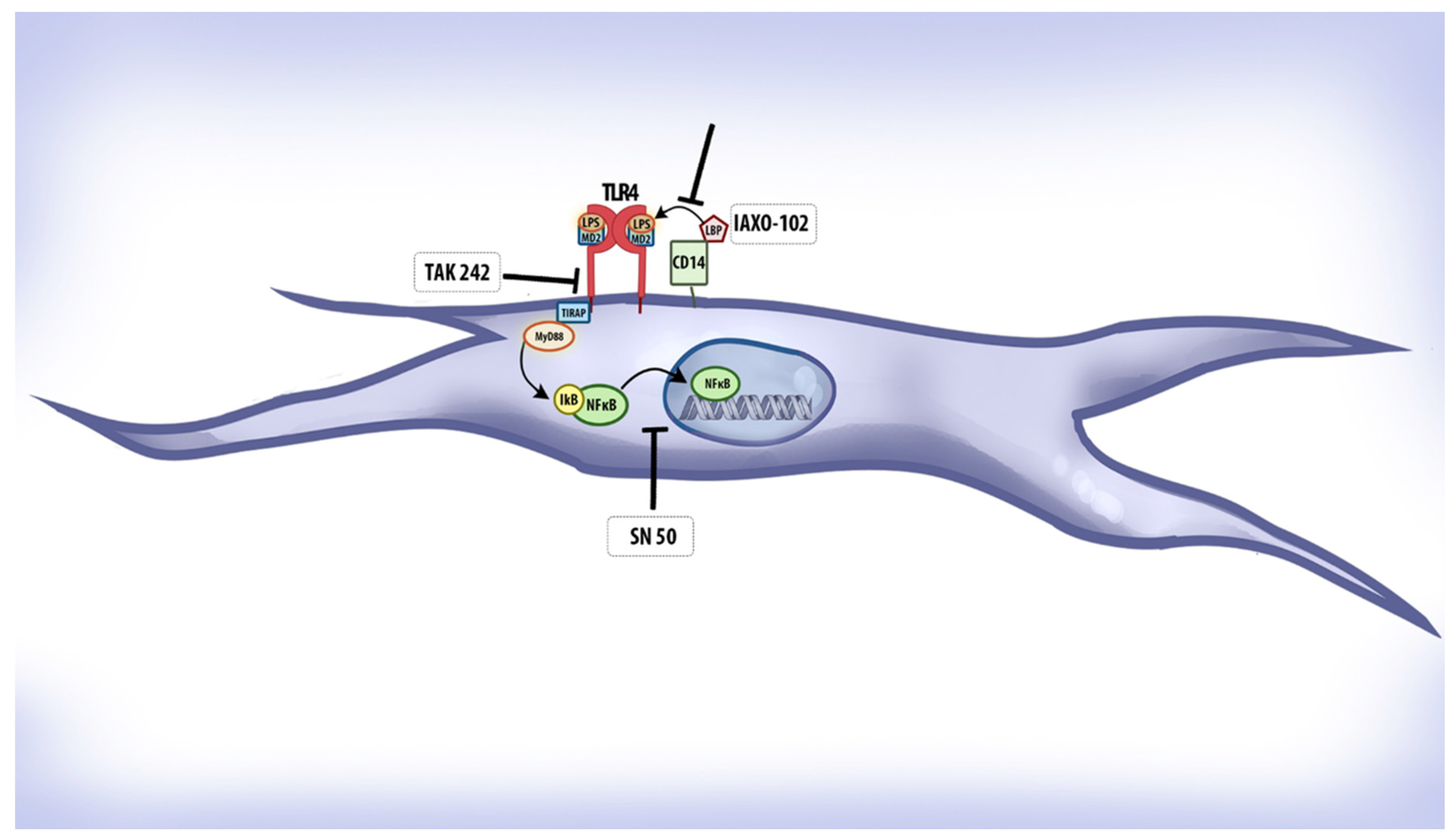
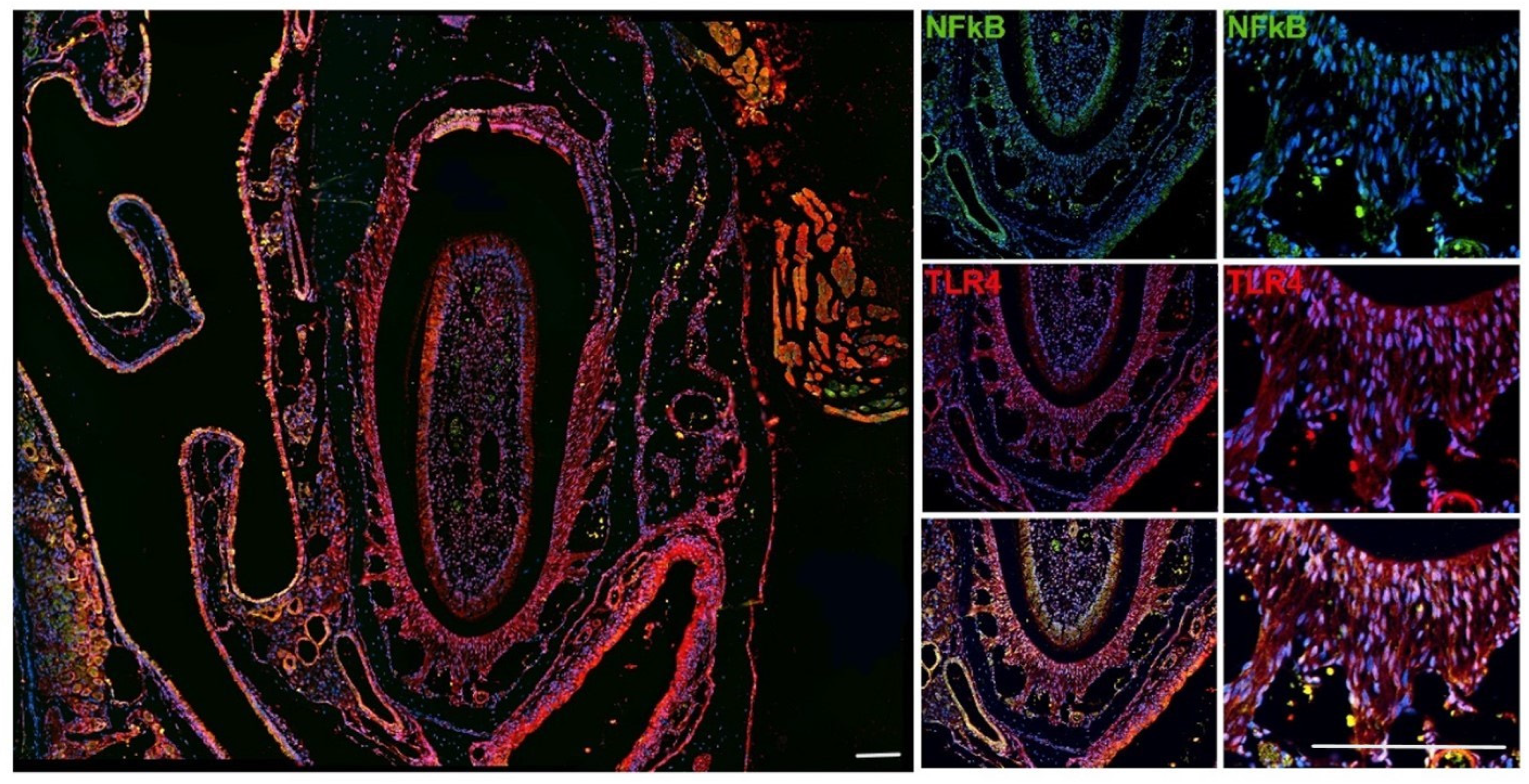
2.3. Immune Localization of TLR4 and NFκB in Mice Tissues
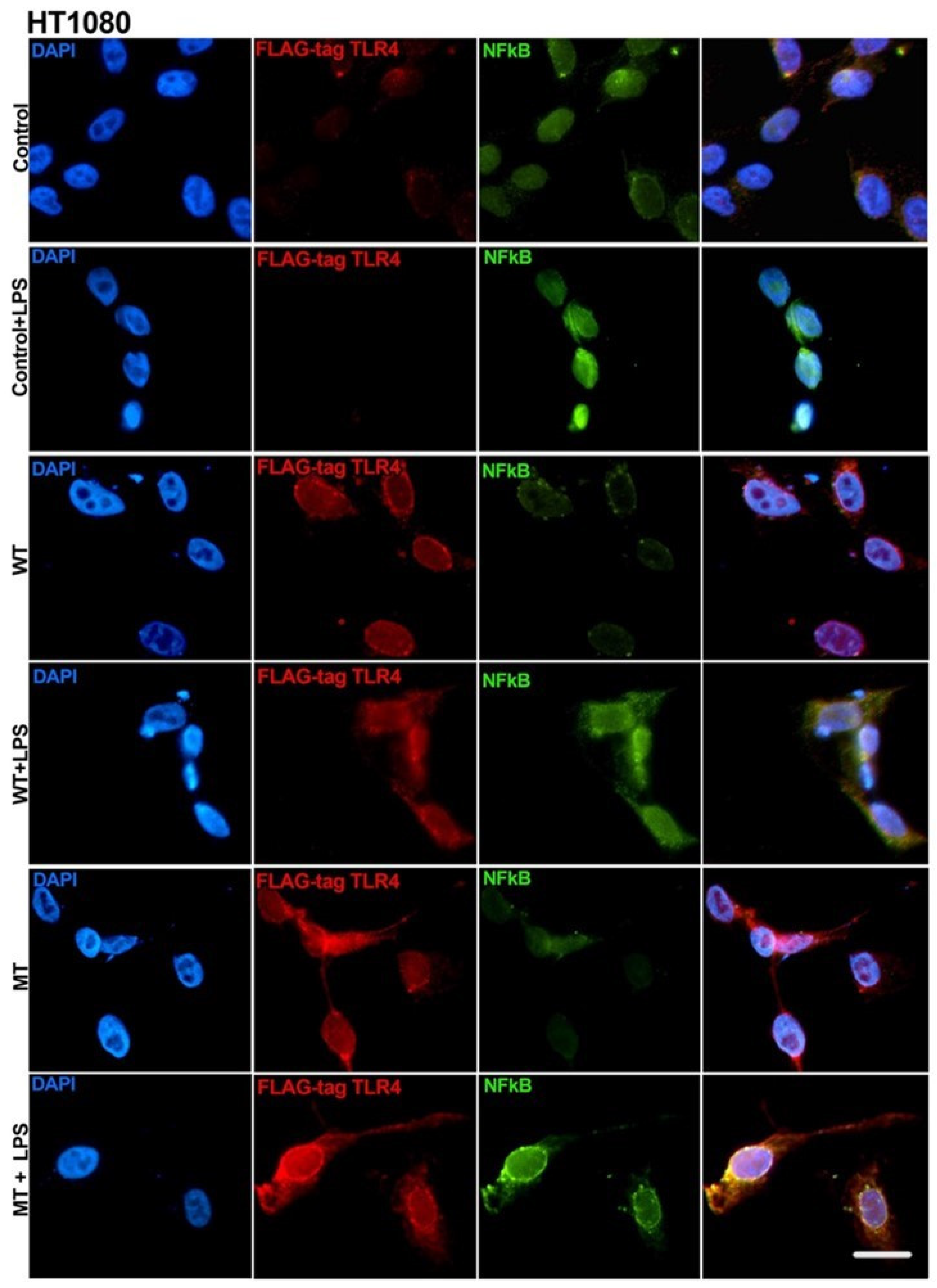
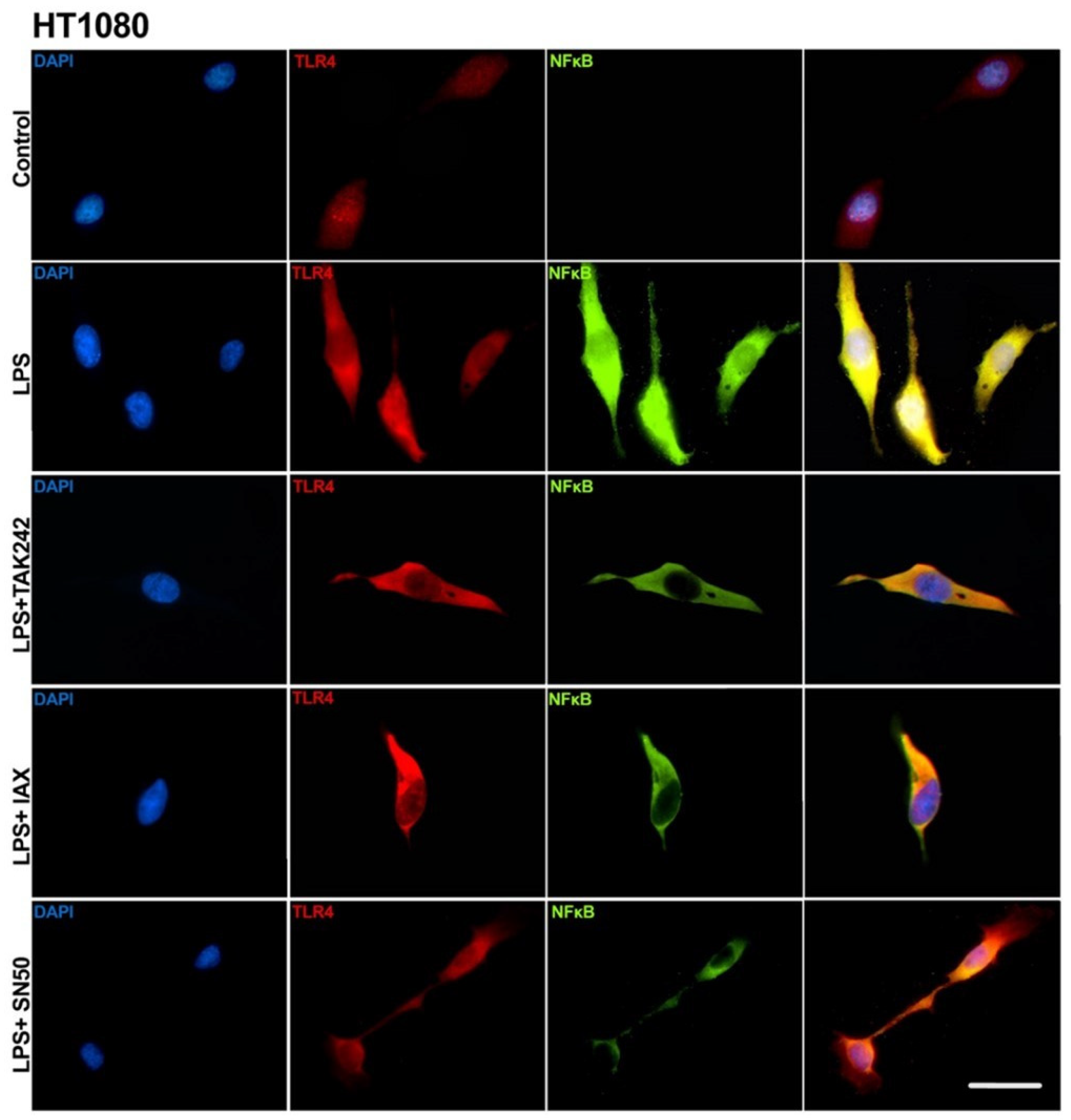
2.4. Transfection of Asp299Gly Variant of TLR4 Receptor
3. Results
3.1. Genotyping
3.2. Immune Localization of TLR4 and NFκB
3.3. Transfection of Asp299Gly Variant of TLR4 Receptor
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Karamifar, K.; Tondari, A.; Saghiri, M.A. Endodontic Periapical Lesion: An Overview on the Etiology, Diagnosis and Current Treatment Modalities. Eur. Endod. J. 2020, 5, 54–67. [Google Scholar] [CrossRef] [PubMed]
- Meyle, J.; Chapple, I. Molecular aspects of the pathogenesis of periodontitis. Periodontol. 2000 2015, 69, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Garlet, G.P. Destructive and protective roles of cytokines in periodontitis: A re-appraisal from host defense and tissue destruction viewpoints. J. Dent. Res. 2010, 89, 1349–1363. [Google Scholar] [CrossRef]
- Cafferata, E.A.; Jerez, A.; Vernal, R.; Monasterio, G.; Pandis, N.; Faggion, C.M. The therapeutic potential of regulatory T lymphocytes in periodontitis: A systematic review. J. Periodontal Res. 2019, 54, 207–217. [Google Scholar] [CrossRef]
- Zhang, J.; Sun, X.; Xiao, L.; Xie, C.; Xuan, D.; Luo, G. Gene polymorphisms and periodontitis. Periodontol. 2000 2011, 56, 102–124. [Google Scholar] [CrossRef] [PubMed]
- Park, B.S.; Lee, J.O. Recognition of lipopolysaccharide pattern by TLR4 complexes. Exp. Mol. Med. 2013, 45, e66. [Google Scholar] [CrossRef] [PubMed]
- Qi, W.; Yang, X.; Ye, N.; Li, S.; Han, Q.; Huang, J.; Wu, B. Gene in the regulation of periodontitis and its molecular mechanism. Exp. Ther. Med. 2019, 18, 1961–1966. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Cao, X.; He, L.; Meng, H.; Yang, B.; Liao, Y. TLR4 polymorphisms may increase susceptibility to periodontitis in Pg-positive individuals. PeerJ 2019, 7, e7828. [Google Scholar] [CrossRef] [PubMed]
- Sellers, R.M.; Payne, J.B.; Yu, F.; LeVan, T.D.; Walker, C.; Mikuls, T.R. TLR4 Asp299Gly polymorphism may be protective against chronic periodontitis. J. Periodontal Res. 2016, 51, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Cavalla, F.; Biguetti, C.C.; Colavite, P.M.; Silveira, E.V.; Martins, W.; Letra, A.; Trombone, A.P.F.; Silva, R.M.; Garlet, G.P. TBX21-1993T/C (rs4794067) polymorphism is associated with increased risk of chronic periodontitis. Virulence 2015, 6, 293–304. [Google Scholar] [CrossRef] [PubMed]
- Garlet, G.P.; Trombone, A.P.F.; Menezes, R.; Letra, A.; Repeke, C.E.; Vieira, A.E.; Martins, W.; das Neves, L.T.; Campanelli, A.P.; dos Santos, C.F.; et al. The use of chronic gingivitis as reference status increases the power and odds of periodontitis genetic studies. J. Clin. Periodontol. 2012, 39, 323–332. [Google Scholar] [CrossRef]
- Vieira, A.R.; Deeley, K.B.; Callahan, N.F.; Noel, J.B.; Anjomshoaa, I.; Carricato, W.M.; Schulhof, L.P.; DeSensi, R.S.; Gandhi, P.; Resick, J.M.; et al. Detection of Streptococcus mutans Genomic DNA in Human DNA Samples Extracted from Saliva and Blood. ISRN Dent. 2011, 2011, 543561. [Google Scholar] [CrossRef] [PubMed]
- Armitage, G.C. Development of a classification system for periodontal diseases and conditions. Ann. Periodontol. 1999, 4, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Feres, M.; Soares, G.M.; Mendes, J.A.; Silva, M.P.; Faveri, M.; Teles, R.; Socransky, S.S.; Figueiredo, L.C. Metronidazole alone or with amoxicillin as adjuncts to non-surgical treatment of chronic periodontitis. J. Clin. Periodontol. 2012, 39, 1149–1158. [Google Scholar] [CrossRef] [PubMed]
- Rider, D.; Furusho, H.; Xu, S.; Trachtenberg, A.J.; Kuo, W.P.; Hirai, K.; Susa, M.; Bahammam, L.; Stashenko, P.; Fujimura, A.; et al. Elevated CD14 and TLR4 signaling deteriorate periapical inflammation in TLR2 deficient mice. Anat. Rec. 2016, 299, 1281–1292. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Loke, J.; Zheng, F.; Hong, F.; Yea, S.; Fukata, M.; Tarocchi, M.; Abar, O.T.; Huang, H.; Sninsky, J.J.; et al. Functional linkage of cirrhosis-predictive SNPs of TLR4 to hepatic stellate cell responses. Hepatology 2009, 49, 960–968. [Google Scholar] [CrossRef] [PubMed]
- Tulic, M.K.; Hurrelbrink, R.J.; Préle, C.M.; Laing, I.A.; Upham, J.W.; Le Souef, P.; Sly, P.D.; Holt, P.G. TLR4 polymorphisms mediate impaired responses to RSV and LPS. J. Immunol. 2007, 179, 132–140. [Google Scholar] [CrossRef]
- Ferwerda, B.; McCall, M.B.B.; Verheijen, K.; Kullberg, B.-J.; van der Ven, A.J.A.M.; van der Meer, J.W.M.; Netea, M.G. Functional consequences of toll-like receptor 4 polymorphisms. Mol. Med. 2008, 14, 346–352. [Google Scholar] [CrossRef]
- Ferwerda, B.; McCall, M.B.B.; Alonso, S.; Giamarellos-Bourboulis, E.J.; Mouktaroudi, M.; Izagirre, N.; Syafruddin, D.; Kibiki, G.; Cristea, T.; Hijmans, A.; et al. TLR4 polymorphisms, infectious diseases, and evolutionary pressure during migration of modern humans. Proc. Natl. Acad. Sci. USA 2007, 104, 16645–16650. [Google Scholar] [CrossRef] [PubMed]
- Rallabhandi, P.; Bell, J.; Boukhvalova, M.S.; Medvedev, A.; Lorenz, E.; Arditi, M.; Hemming, V.G.; Blanco, J.C.G.; Segal, D.M.; Vogel, S.N. Analysis of TLR4 polymorphic variants: New insights into TLR4/MD-2/CD14 stoichiometry, structure, and signaling. J. Immunol. 2006, 177, 322–332. [Google Scholar] [CrossRef] [PubMed]






| Frequency Cases | Frequency Controls | Chi Square | p-Value | OR | SE | 95% CI |
|---|---|---|---|---|---|---|
| 0.01882 | 0.05469 | 7.839 | 0.005113 | 0.3315 | 0.4133 | 0.14–0.75 |
| Frequency Cases | Frequency Controls | Chi Square | p-Value | OR | SE | 95% CI | |
|---|---|---|---|---|---|---|---|
| Exploration (RBP) | 0.01882 | 0.05469 | 7.839 | 0.0051 | 0.3315 | 0.4133 | 0.14–0.75 |
| Validation 1 (PIT) | 0.03226 | 0.05278 | 0.858 | 0.3543 | 0.5982 | 0.5603 | 0.19–1.79 |
| Validation 2 (HOU) | 0.03741 | 0.1208 | 13.27 | 0.0002 | 0.2828 | 0.3656 | 0.13–0.57 |
| Validation 3 (SAO) | 0.05963 | 0.02813 | 3.282 | 0.07 | 2.191 | 0.4429 | 0.91–5.22 |
| Frequency Cases | Frequency Controls | Chi Square | p-Value | OR | SE | 95% CI |
|---|---|---|---|---|---|---|
| 0.03472 | 0.05865 | 7.65 | 0.005678 | 0.5774 | 0.2008 | 0.38–0.85 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cavalla, F.; Biguetti, C.C.; Letra, A.; Silva, R.M.; Vieira, A.R.; Strauss, F.J.; Garlet, G.P. TLR4 Asp299Gly SNP (rs4986790) Protects from Periodontal Inflammatory Destruction by Altering TLR4 Susceptibility to LPS Stimulation. Biology 2025, 14, 894. https://doi.org/10.3390/biology14070894
Cavalla F, Biguetti CC, Letra A, Silva RM, Vieira AR, Strauss FJ, Garlet GP. TLR4 Asp299Gly SNP (rs4986790) Protects from Periodontal Inflammatory Destruction by Altering TLR4 Susceptibility to LPS Stimulation. Biology. 2025; 14(7):894. https://doi.org/10.3390/biology14070894
Chicago/Turabian StyleCavalla, Franco, Claudia C. Biguetti, Ariadne Letra, Renato M. Silva, Alexandre R. Vieira, Franz J. Strauss, and Gustavo P. Garlet. 2025. "TLR4 Asp299Gly SNP (rs4986790) Protects from Periodontal Inflammatory Destruction by Altering TLR4 Susceptibility to LPS Stimulation" Biology 14, no. 7: 894. https://doi.org/10.3390/biology14070894
APA StyleCavalla, F., Biguetti, C. C., Letra, A., Silva, R. M., Vieira, A. R., Strauss, F. J., & Garlet, G. P. (2025). TLR4 Asp299Gly SNP (rs4986790) Protects from Periodontal Inflammatory Destruction by Altering TLR4 Susceptibility to LPS Stimulation. Biology, 14(7), 894. https://doi.org/10.3390/biology14070894






