Romanian Dendrocoelidae Hallez, 1892 (Platyhelminthes, Tricladida, Dendrocoelidae) Revisited: A Tribute to Radu Codreanu and Doina Balcesco
Simple Summary
Abstract
1. Introduction
2. Methodology
3. Species Inventory
4. The Classical Phylogenetic System, Morphological Types, and Taxa
5. The Morphological Characters of Dendrocoelidae and Their Phylogenetic Value
6. Discussion
7. Conclusions
8. Future Directions of Study
- The status of the described species. Some species may be synonymized, and new species may be described.
- The genus or subgenus level of Paradendrocoelum and Palaeodendrocoelum. In this regard, the species Paradendrocoelum alexandrinae and Palaeodendrocoelum getticum are of particular interest.
- The anophthalmic species Palaeodendrocoelum getticum, Polycladodes voinovi, and Polycladodes affine may support the validation of one of the hypotheses regarding their origin and paleogeographic distribution (the migration route). They can also help clarify eye character, whether it is homologous character (divergent evolution) or analogous (convergent evolution).
- Molecular support for the timing of subterranean colonization. This can be used to validate eye/flagellum evolution hypotheses.
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sluys, R.; Kawakatsu, M.; Riutort, M.; Baguña, J. A new higher classification of planarian flatworms (Platyhelminthes, Tricladida). J. Nat. Hist. 2009, 43, 1763–1777. [Google Scholar] [CrossRef]
- Gourbault, N. Recherches sur les Triclades Paludicoles hypogés. In Mémoires du Muséum National d’Histoire Naturelle Paris; Série A, Tome LXXIII, 249; 1972; pp. + 3. [Google Scholar]
- Stocchino, G.A.; Sluys, R.; Kawakatsu, M.; Sarbu, S.M.; Manconi, R. A new species of freshwater worm (Platyhelminthes, Tricladida, Dendrocoelidae) inhabiting a chemoautotrophic groundwater ecosystem in Romania. Eur. J. Taxon. 2017, 342, 1–21. [Google Scholar] [CrossRef]
- Del Papa, R. Dendrocoelum (Dendrocoelides bennazzii n. sp. From the cave of Stiffe (Abruzzo). Bolletino Zool. 1973, 40, 253–259. [Google Scholar] [CrossRef]
- Kenk, R. The planarians (Turbellaria: Tricladida paludicola) of Lake Ohrid in Macedonia. Smithson. Contrib. Zool. 1978, 280, 1–56. [Google Scholar] [CrossRef][Green Version]
- Villa-Farré, M.; Sluys, R.; Almagro, Í.; Handberg-Thorsager, M.; Romero, R. Freshwater planarians (Platyhelminthes, Tricladida) from the Iberian Peninsula and Greece: Diversity and notes on ecology. Zootaxa 2011, 2779, 1–38. [Google Scholar] [CrossRef]
- Harrath, A.H.; Sluys, R.; Ghlala, A.; Alwasel, S. The first subterranean freshwater planarians from North Africa, with an analysis of adenodactyl structure in the genus Dendrocoelum (Platyhelminthes, Tricladida, Dendrocoelidae). J. Cave Karst Stud. 2012, 74, 48–57. [Google Scholar] [CrossRef]
- Sluys, R. A new, sibling species of cave flatworm from Switzerland (Platyhelminthes, Tricladida, Dendrocoelidae). Rev. Suisse Zool. 2012, 119, 181–188. [Google Scholar] [CrossRef]
- Stocchino, G.A.; Sluys, R.; Marcia, P.; Manconi, R. Subterranean aquatic planarians of Sardinia, with a discussion on the penial flagellum and the bursal canal sphincter in the genus Dendrocoelum (Platyhelminthes, Tricladida, Dendrocoelidae). J. Cave Karst Stud. 2013, 75, 93–112. [Google Scholar] [CrossRef]
- Stocchino, G.A.; Sluys, R.; Montanari, A.; Manconi, R. A new species of stygobiont freshwater planarian (Platyhelminthes, Tricladida) from a chemoautotrophic ecosystem: The Frasassi karst in Italy. Zootaxa 2017, 4323, 547–560. [Google Scholar] [CrossRef]
- Vila-Farré, M.; Brand, J.N.; Boothe, T.; Maren Brockmeyer, M.; Ficze-Schmidt, F.; Grohme, M.A.; Weill, U.; Kluiver, K.H.; Gasiorowski, L.; Kauf, L.; et al. An integrative taxonomic approach reveals unexplored diversity in Croatian planarians. bioRxiv 2025. [Google Scholar] [CrossRef]
- de Beauchamp, P. Nouvelle diagnoses de Triclades obscuricoles, Les Dendrocoelides de Transylvanie. Bull. Soc. Zool. Fr. 1929, 54, 20–28. [Google Scholar]
- de Beauchamp, P. Turbellariés, Hirudinées, Branchiobdellidés. 2e série. Biospeologica LVIII. Arch. Zool. Exp. Gén. 1932, 73, 113–117, 158–163. [Google Scholar]
- de Beauchamp, P. Turbellariés 3e série. Biospeologica LXIX. Arch. Zool. Exp. Gén 1949, 86, 50–65. [Google Scholar]
- Codreanu, R. Polycladodes voinovi, n. sp., nouveau Triclade obscuricole de Roumanie. C. R. Soc. Biol. 1929, 101, 963–966. [Google Scholar]
- Codreanu, R. Dendrocoelum (Dendrocoelides) clujanum n. sp. Nouveau Triclade souterrain de Transylvanie. Anal. Acad. Române Memor. Sect. Stiinț. 1943, 18, 135–158. [Google Scholar]
- Codreanu, R. O nouă tricladă epigee relictă din Defileul Dunării: Palaeodendrocoelum danubialis N.G.; N. SP. Analele Acad. Republicii Pop. Române Ser. Geol. Geogr. Biol. Stiinte Teh. Agric. 1950, 3, 599–622. [Google Scholar]
- Codreanu, R.; Balcesco, D. Sur trois Dendrocoelides aveugle nouveaux des sources du Banat (Roumanie). Rev. Roum. Biol. Série Zool. 1967, 12, 287–294. [Google Scholar]
- Codreanu, R.; Balcesco, D. Sur les rapports entre les sous-genres Paradendrocoelum Kenk 1930 et Dendrocoelides De Beauchamp 1919 d’après les espèces obscuricoles du Banat et de L’Olténie. Rev. Roum. Biol. Zool. 1967, 12, 337–349. [Google Scholar]
- Codreanu, R.; Balcesco, D. Sur deux nouveaux Dendrocoeles hypogés de Roumanie et certains effets de néoténie. Arch. Roum. Path. Exp. Microbiol. 1967, 46, 843–852. [Google Scholar]
- Codreanu, R.; Balcesco, D. Répartition des Dendrocoelides anophtalmes dans les Carpates de Courbure et dans la Plaine Roumaine. Livre Centen. Emile Racovitza 1970, 239–245. [Google Scholar]
- Sluys, R.; Riutort, M. Planarian diversity and phylogeny. In Planarian Regeneration: Methods and Protocols; Rink, J.C., Ed.; Springer Science + Business Media, LLC: Cham, Switzerland, 2018; Volume 1774. [Google Scholar]
- Kuznedelov, K.D.; Novikova, O.A.; Naumova, T.V. Molecular Genetic Typification of Planaria Genus Bdellocephala (Dendrocoelidae, Tricladida, Turbellaria) from Lake Baikal and Estimation of its Specific Variability. 1999. Available online: https://www.researchgate.net/publication/12456102 (accessed on 28 May 2025).
- Kuznedelov, K.D.; Ishida, S.; Nishitani, S.-I. Genetic divergence of Japanese Turbellarians, studied by comparisons of partial 18S rRNA gene sequences I. On representatives on Dendrocoelidae (Platyhelminthes: Tricladida: Paludicola). Zool. Sci. 2000, 17, 491–496. [Google Scholar]
- Riutort, M.; Álvarez-Presas, M.; Lázaro, E.; Solà, E.; Paps, J. Evolutionary history of the Tricladida and the Platyhelminthes: An up-to-date phylogenetic and systematic account. Int. J. Dev. Biol. 2012, 56, 5–17. [Google Scholar] [CrossRef] [PubMed]
- Carranza, S.; Littlewood, D.T.J.; Clough, K.A.; Ruiz-Trillo, I.; Baguña, J.; Riutort, M. A robust molecular phylogeny of the Tricladida (Platyhelminthes: Seriata) with a discussion on morphological synapomorphies. Proc. R. Soc. London Ser. B Biol. Sci. 1998, 265, 631–640. [Google Scholar] [CrossRef] [PubMed]
- Baguñà, J.; Carranza, S.; Paps, J.; Ruiz-Trillo, I.; Riutort, M. Molecular taxonomy and phylogeny of the Tricladida. In Interrelationships of the Platyhelminthes; Littlewood, D.T.J., Bray, R.A., Eds.; SASVC Press: London, UK, 2000; Chapter 6; pp. 49–56. [Google Scholar]
- Álvarez-Presas, M.; Baguñà, J.; Riutort, M. Molecular phylogeny of land and freshwater planarians (Tricladida, Platyhelminthes): From freshwater to land and back. Mol. Phylogenet. Evol. 2008, 47, 555–568. [Google Scholar] [CrossRef] [PubMed]
- Lázaro, E.M.; Sluys, R.; Pala, M.; Stocchino, G.A.; Baguña, J.; Riutort, M. Molecular barcoding and phylogeography of sexual and asexual freshwater planarians of the genus Dugesia in the Western Mediterranean (Platyhelminthes, Tricladida, Dugesiidae). Mol. Phyl. Evol. 2009, 52, 835–845. [Google Scholar] [CrossRef] [PubMed]
- Solà, E.; Sluys, R.; Gritzalis, K.; Riutort, M. Fluvial basin history in the northeastern Mediterranean region underlies dispersal and speciation patterns in the genus Dugesia (Platyhelminthes, Tricladida, Dugesiidae). Mol. Phyl. Evol. 2013, 66, 877–888. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Presas, M.; Riutort, M. Planarian (Platyhelminthes, Tricladida) Diversity and Molecular Markers: A New View of an Old Group. Diversity 2014, 6, 323–338. [Google Scholar] [CrossRef]
- Inoue, K.; Pohl, A.L.; Sei, M.; Lang, B.K.; Berg, D.J. Use of species delimitation approaches to assess biodiversity in freshwater planaria (Platyhelminthes, Tricladida) from desert springs. Aquat. Conserv. Mar. Freshw. Ecosyst. 2020, 30, 209–218. [Google Scholar] [CrossRef]
- Leria, L.; Vila-Farré, M.; Álvarez-Presas, M.; Sánchez-Gracia, A.; Rozas, J.; Sluys, R.; Riutort, M. Cryptic species delineation in freshwater planarians of the genus Dugesia (Platyhelminthes, Tricladida): Extreme intraindividual genetic diversity, morphological stasis, and karyological variability. Mol. Phyl. Evol. 2020, 143, 106496. [Google Scholar] [CrossRef] [PubMed]
- Leria, L.; Riutort, M.; Romero, R.; Ferrer, X.; Vila-Farré, M. Microplate tectonics and environmental factors as distribution drivers in Western Mediterranean freshwater planarians. J. Biogeogr. 2022, 49, 1124–1136. [Google Scholar] [CrossRef]
- Benítez-Álvarez, L.; Leria, L.; Sluys, R.; Leal-Zanchet, A.M.; Riutort, M. Niche modelling and molecular phylogenetics unravel the colonisation biology of three species of the freshwater planarian genus Girardia (Platyhelminthes, Tricladida). Hydrobiologia 2023, 850, 3125–3142. [Google Scholar] [CrossRef]
- Dols-Serrate, D.; Stocchino, G.A.; Nuin-Villabona, P.; Sluys, R.; Riutort, M. New insights into the evolution and biogeography of freshwater planarians on islands in the Tyrrhenian Sea, Western Mediterranean Basin, with the integrative description of a new endemic species from Corsica (Platyhelminthes: Tricladida: Dugesia). Zool. J. Linn. Soc. 2024, 201, zlae080. [Google Scholar] [CrossRef]
- Sluys, R. The evolutionary terrestrialization of planarian flatworms (Platyhelminthes, Tricladida, Geoplanidae): A review and research programme. Zoosyst. Evol. 2019, 95, 543–556. [Google Scholar] [CrossRef]
- Kenk, R. Beiträge zum System der Probursalier (Tricladida paludicola). Zool Anz 1930, 89, 145–162, 289–302. [Google Scholar]
- Ruppert, E.E.; Fox, R.S.; Barnes, R.D. Introduction to Invertebrates. In Invertebrate Zoology, a Functional Evolutionary Approach, 7th ed.; Thomson Brooks/Cole: Pacific Grove, CA, USA, 2004; pp. 1–10. [Google Scholar]
- Sluys, R.; Kawakatsu, M. Towards a phylogenetic classification of dendrocoelid freshwater planarians (Platyhelminthes): A morphological and eclectic approach. J. Zool. Syst. Evol. Res. 2006, 44, 274–284. [Google Scholar] [CrossRef]
- Steinmetz, P.R.H.; Kraus, J.E.M.; Larroux, C.; Hammel, U.J.; Amon-Hassenzahl, A.; Houliston, E.; Wörheide, G.; Nickel, M.; Degnan, B.M.; Technau, U. Independent evolution of striated muscles in cnidarians and bilaterians. Nature 2012, 487, 231–234. [Google Scholar] [CrossRef] [PubMed]
- Ávila, S.P.; Melo, C.; Berning, B.; Sá, N.; Quartau, R.; Rijsdijk, K.F.; Ramalho, R.S.; Cordeiro, R.; De Sá, N.C.; Pimentel, A.; et al. Towards a ‘Sea-Level Sensitive’ dynamic model: Impact of island ontogeny and glacio-eustasy on global patterns of marine island biogeography. Biol. Rev. 2019, 94, 1116–1142. [Google Scholar] [CrossRef] [PubMed]
- Molina, M.D.; Cebrià, F. Decoding stem cells: An overview on planarian stem cell heterogeneity and lineage progression. Biomolecules 2021, 11, 1532. [Google Scholar] [CrossRef] [PubMed]
- Lapan, S.W.; Reddien, P.W. Transcriptome Analysis of the Planarian Eye Identifies ovo as a Specific Regulator of Eye Regeneration. Cell Rep. 2012, 2, 294–307. [Google Scholar] [CrossRef] [PubMed]
- Deochand, M.E.; Birkholz, T.R.; Beane, S.W. Temporal regulation of planarian eye regeneration. Regeneration 2016, 3, 209–221. [Google Scholar] [CrossRef] [PubMed]
- Ivankovic, M.; Haneckova, R.; Thommen, A.; Grohme, M.A.; Vila-Farré, M.; Werner, S.; Rink, C.J. Model systems for regeneration: Planarians. Development 2019, 146, dev167684. [Google Scholar] [CrossRef] [PubMed]
- Ge, X.-Y.; Han, X.; Zhao, Y.-L.; Cui, G.-S.; Yang, Y.-G. An insight into planarian regeneration. Cell Prolif. 2022, 55, e13276. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Liu, Y.; Jin, B.; Dong, Z.; Chen, G.; Liu, D. Djmek is involved in planarian regeneration by regulation of cell proliferation and apoptosis. Biochem. Biophys. Res. Commun. 2020, 532, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Vila-Farré, M.; Rozanski, A.; Ivanković, M.; Cleland, J.; Brand, J.N.; Thalen, F.; Grohme, M.A.; Kannen, S.; Grosbusch, A.L.; Vu, T.-K.H.; et al. Evolutionary dynamics of whole-body regeneration across planarian flatworms. Nat. Ecol. Evol. 2023, 7, 2108–2124. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.Y.; Selck, C.; Friedrich, B.; Lutz, R.; Vila-Farré, M.; Dahl, M.A.; Brandl, H.; Lakshmanaperumal, N.; Henry, I.; Rink, C.J. Reactivating head regrowth in a regeneration-deficient planarian species. Nature 2013, 500, 81–85. [Google Scholar] [CrossRef] [PubMed]
- Porter, M.L.; Sumner-Rooney, L. Evolution in the Dark: Unifying our Understanding of Eye Loss. Integr. Comp. Biol. 2018, 58, 367–371. [Google Scholar] [CrossRef] [PubMed]
- Sumner-Rooney, L.S. The Kingdom of the Blind: Disentangling Fundamental Drivers in the Evolution of Eye Loss. Integr. Comp. Biol. 2018, 58, 372–385. [Google Scholar] [CrossRef] [PubMed]
- Saad, L.O.; Cooke, T.F.; Atabay, K.D.; Reddien, P.W.; Brown, F.D. Reduced adult stem cell fate specification led to eye reduction in cave planarians. Nat. Commun. 2024, 16, 304. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, S.F.; Barresi, M.J. Development and the Environment. In Developmental Biology, 11th ed.; Sinauer New York Oxford University Press: New York, NY, USA, 2018; pp. 763–784. [Google Scholar]
- Mauro, M.; Longo, F.; Valvo, L.V.; Vizzini, A.; Di Grigoli, A.; Radovic, S.; Arizza, V.; Vecchioni, L.; La Paglia, L.; Queiroz, V.; et al. The Use of Environmental DNA as Preliminary Description of Invertebrate Diversity in Three Sicilian Lakes. Animals 2025, 15, 355. [Google Scholar] [CrossRef] [PubMed]
- Dodsworth, S. Genome skimming for next-generation biodiversity analysis. Trends Plant Sci. 2015, 20, 525–527. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Yang, J.; Landis, J.B.; Wang, S.; Jin, L.; Xie, P.; Liu, H.; Yang, J.B.; Yi, T.S. Genome Skimming Contributes to Clarifying Species Limits in Paris Section Axiparis (Melanthiaceae). Front. Plant Sci. 2022, 13, 832034. [Google Scholar] [CrossRef] [PubMed]
- Taite, M.; Fernández-Álvarez, F.Á.; Braid, H.E.; Bush, S.L.; Bolstad, K.; Drewery, J.; Mills, S.; Strugnell, J.M.; Vecchione, M.; Villanueva, R.; et al. Genome skimming elucidates the evolutionary history of Octopoda. Mol. Phyl. Evol. 2023, 182, 107729. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.S.; Chu, C.C.; Lin, J.J.; Chang, C.C.; Wang, C.C.; Wang, C.Y.; Tsui, P.H. Optical coherence tomography: A new strategy to image planarian regeneration. Sci. Rep. 2014, 4, 6316. [Google Scholar] [CrossRef] [PubMed]
- Carbayo, F.; Lenihan, J.W. Micro-computed tomography scan and virtual histological slide data for the land planarian Obama otavioi (Platyhelminthes). GigaScience 2016, 5, s13742-016-0119-4. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.S.; Carbayo, F. X-ray microcomputed tomography applied to the taxonomic study of rare material: Redescriptions of seven of Schirch’s Brazilian species of land planarians (Geoplanidae, Platyhelminthes). ZooKeys 2020, 910, 1–42. [Google Scholar] [CrossRef] [PubMed]
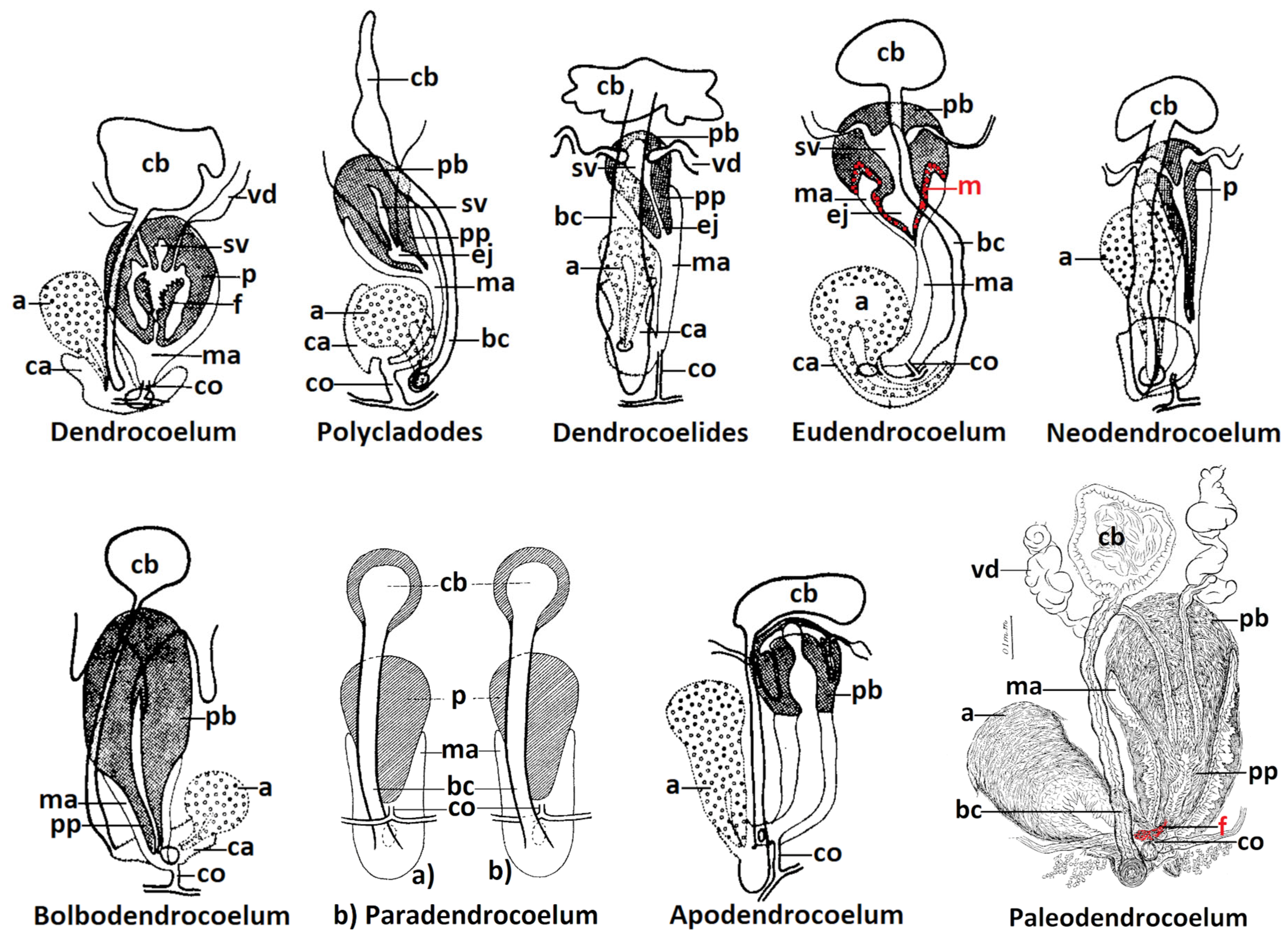

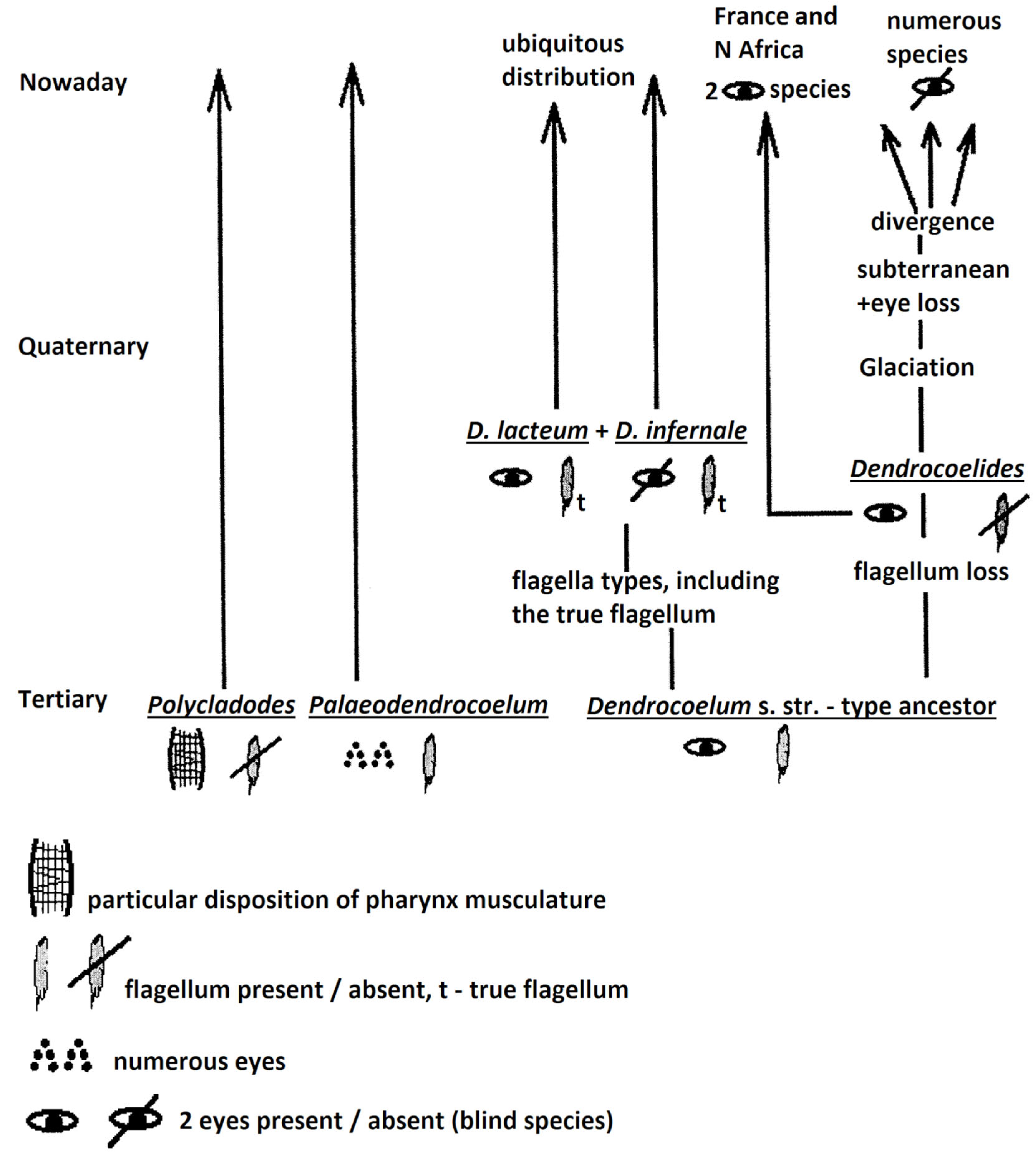

| Species Name (System: Gourbault 1972 and Sluys et al., 2009) | Original Genus/Subgenus (in Primary Reference) | Type Locality/Loc. in Romania | Primary References (Species Original Description) | Literature Source (Used by Author) | Collection Holding Type Specimens |
|---|---|---|---|---|---|
| 1. D. (Dendrocoelides) sphaerophallus (de Beauchamp, 1929) | Dendrocoelides | Pui (Hunedoara, Romania) | Beauchamp 1929 | Beauchamp 1929 [12] | ? |
| 2. D. (Dendrocoelides) chappuisi de Beauchamp, 1932 | Dendrocoelum (Dendrocoelides ?) | Babadag, Tulcea, Romania | Beauchamp 1932 | Beauchamp 1932 [13] | ? |
| 3. D. (Dendrocoelides) clujanum Codreanu, 1943 | Dendrocoelum (Dendrocoelides) | Cluj, Romania | Codreanu 1943 | Codreanu 1943 [16] | ? |
| 4. D. (Dendrocoelides) racovitzai de Beauchamp, 1949 | Dendrocoelum (Dendrocoelides) | Cloșani, Romania | Beauchamp 1949 | Beauchamp 1949 [14] | ? |
| 5. D. (Dendrocoelides) banaticum Codreanu & Balcesco, 1967 | Dendrocoelum (Dendrocoelides) | Oravița, Romania | Codreanu and Balcesco, 1967a | Codreanu and Balcesco, 1967a, b [18,19] | ? |
| 6. D. (Dendrocoelides) atriostrictum Codreanu & Balcesco, 1967 | Dendrocoelum (Dendrocoelides) | Reșița, Semenic Mt., Romania | Codreanu and Balcesco, 1967a | Codreanu and Balcesco, 1967a, b [18,19] | ? |
| 7. D. (Dendrocoelides) debeauchampianum Codreanu & Balcesco, 1967 | Dendrocoelum (Dendrocoelides) | Orșova, Romania | Codreanu and Balcesco, 1967a | Codreanu and Balcesco, 1967a, b [18,19] | ? |
| 8. D. (Dendrocoelides) tismanae Codreanu & Balcesco, 1967 | Dendrocoelum (Dendrocoelides) | Tismana, Romania | Codreanu and Balcesco, 1967b | Codreanu and Balcesco, 1967b [19] | ? |
| 9 D. (Dendrocoelides) stenophallus Codreanu & Balcesco, 1967 | Dendrocoelum (Dendrocoelides) | Sohodol, Romania | Codreanu and Balcesco, 1967b | Codreanu and Balcesco, 1967b [19] | ? |
| 10. D. (Dendrocoelides) orghidani Codreanu & Balcesco, 1967 | Dendrocoelum (Dendrocoelides) | Lipova—Poiana Ruscă Mt., Romania | Codreanu and Balcesco, 1967c | Codreanu and Balcesco, 1967c [20] | ? |
| 11. D. (Dendrocoelides) polymorphum Codreanu & Balcesco, 1967 | Dendrocoelum (Dendrocoelides) | Agigea, Romania | Codreanu and Balcesco, 1967c | Codreanu and Balcesco, 1967c [20] | ? |
| 12. D. (?Paradendreocoelum?) alexandrinae Codreanu & Balcesco, 1970, | Dendrocoelum (Paradendrocoelum) | Vama Buzăului, Romania | Codreanu and Balcesco, 1970 | Codreanu and Balcesco, 1970 [21] | ? |
| 13. D. (Apodendrocoelum) brachyphallus (de Beauchamp, 1929) | Dendrocoelides | Vașcău, Romania | Beauchamp 1929 | Beauchamp 1929 [12] | ? |
| 14. D. (Apodendrocoelum) lipohallus (de Beauchamp, 1929) | Dendrocoelides | Turda, Romania | Beauchamp 1929 | Beauchamp 1929 [12] | ? |
| 15. D. (Palaeodendrocoelum) romanodanubialis (Codreanu, 1949–1950), | Palaeodendrocoelum | Iron Gates on Danube, Romania | Codreanu 1950 | Codreanu 1950 [17] | ? |
| 16. D. (Palaeodendrocoelum) getticum Codreanu & Balcesco, 1970 | Dendrocoelum (Palaeodendrocoelum) | Bucharest, Romania | Codreanu and Balcesco, 1970 | Codreanu and Balcesco, 1970 [21] | ? |
| 17. D. (Eudendrocoelum) botosaneanui del Papa, 1965 | Dendrocoelum (Eudendrocoelum) | Anina, Romania | Del Papa 1965 | Gourbault 1972 [2] | ? |
| 18. Polycladodes album Steinmann, 1910, | Polycladodes | ? Dobrogea, Romania | Steinmann 1910 | Gourbault 1972 [2] | ? |
| 19. Polycladodes voinovi, Codreanu, 1929 | Polycladodes | Sinaia, Romania | Codreanu 1929 | Codreanu 1929 [15] | ? |
| 20. Polycladodes affine (Codreanu & Balcesco, 1970), | Dendrocoelum (Polycladodes) | Unspecified in South Făgăraș Mt., Romania | Codreanu and Balcesco, 1970 | Codreanu and Balcesco, 1970 [21] | ? |
| 21. Dendrocoelum obstinatum Stocchino & Sluys, 2017, Romania, Dobrogea | Dendrocoelum | Movile Cave, Dobrogea, Romania | Stocchino et al. 2017 | Stocchino et al. 2017 [3] | Naturalis Biodiversity Center, University of Sassari |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Babalean, A.F. Romanian Dendrocoelidae Hallez, 1892 (Platyhelminthes, Tricladida, Dendrocoelidae) Revisited: A Tribute to Radu Codreanu and Doina Balcesco. Biology 2025, 14, 887. https://doi.org/10.3390/biology14070887
Babalean AF. Romanian Dendrocoelidae Hallez, 1892 (Platyhelminthes, Tricladida, Dendrocoelidae) Revisited: A Tribute to Radu Codreanu and Doina Balcesco. Biology. 2025; 14(7):887. https://doi.org/10.3390/biology14070887
Chicago/Turabian StyleBabalean, Anda Felicia. 2025. "Romanian Dendrocoelidae Hallez, 1892 (Platyhelminthes, Tricladida, Dendrocoelidae) Revisited: A Tribute to Radu Codreanu and Doina Balcesco" Biology 14, no. 7: 887. https://doi.org/10.3390/biology14070887
APA StyleBabalean, A. F. (2025). Romanian Dendrocoelidae Hallez, 1892 (Platyhelminthes, Tricladida, Dendrocoelidae) Revisited: A Tribute to Radu Codreanu and Doina Balcesco. Biology, 14(7), 887. https://doi.org/10.3390/biology14070887




