Will Wind Turbines Affect the Distribution of Alashan Ground Squirrel? Insights from Large-Scale Wind Farms in China
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Sample Methods
2.3. Burrow Survey
2.4. Environmental Factors
2.5. Data Analysis
2.5.1. Kernel Density Estimation
2.5.2. Spatial Autocorrelation
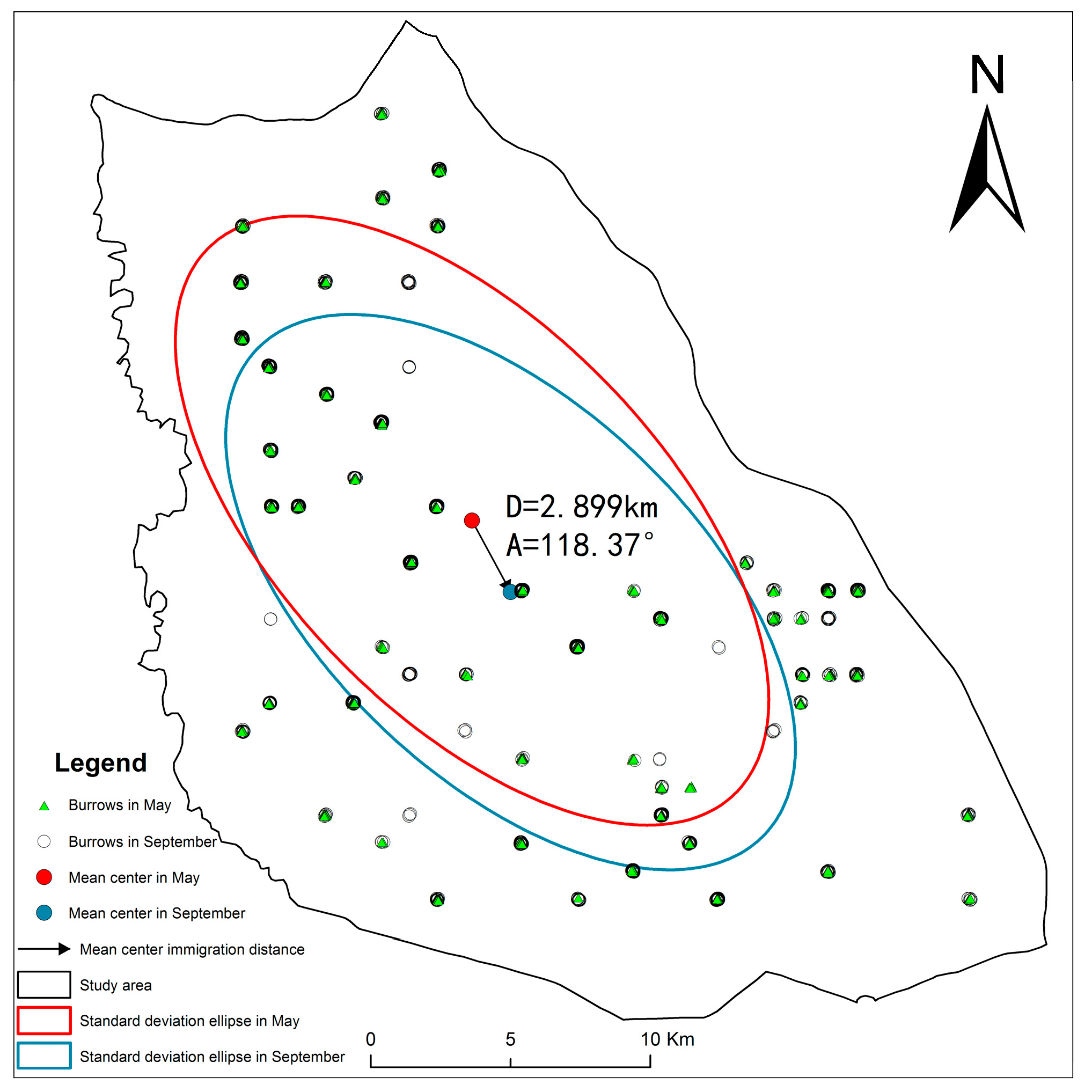
2.5.3. Standard Deviation Ellipse Analysis
2.5.4. Generalized Additive Model and Structural Equation Model
3. Results
3.1. Variation of Alashan Ground Squirrel’s Burrow Density
3.1.1. DEB Distribution in Different Wind Turbine Density Areas
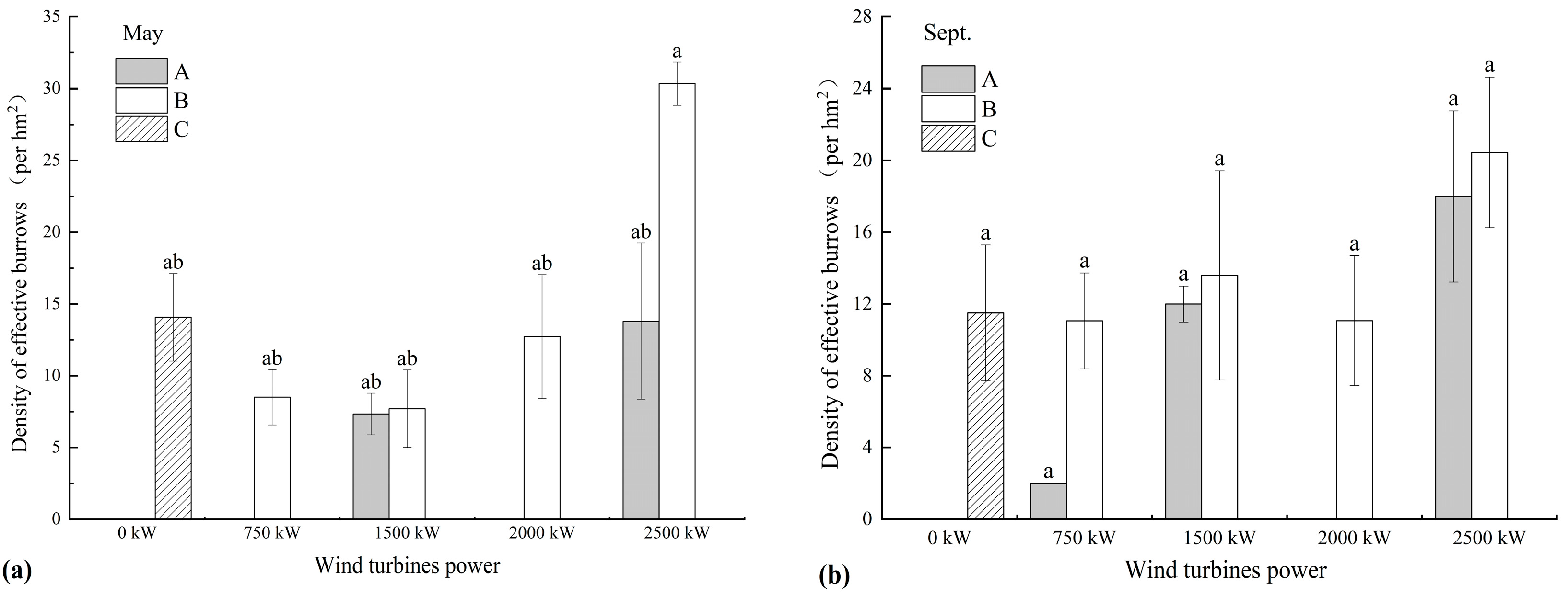
3.1.2. DEB Distribution in Different Wind Turbine Power Areas
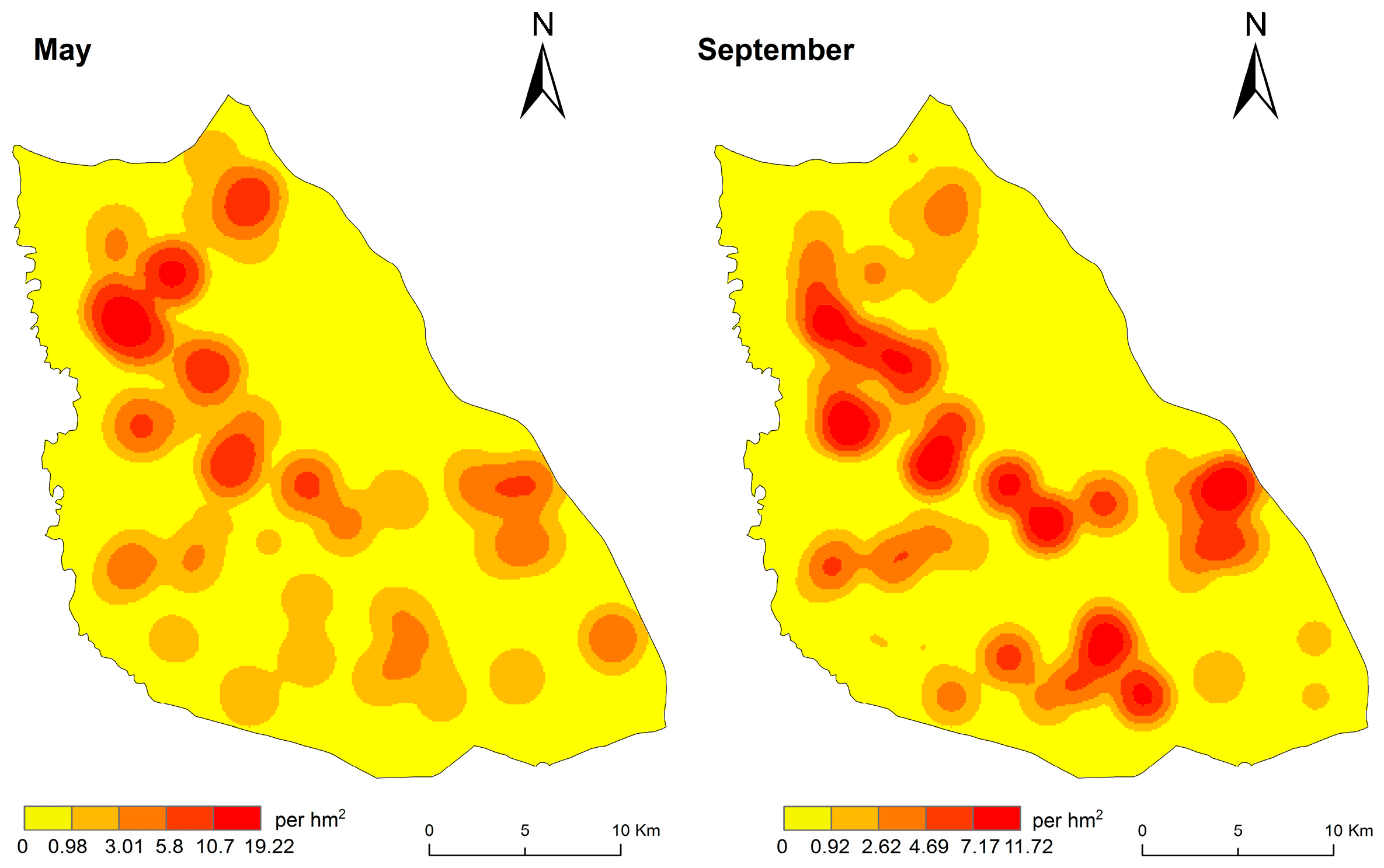
3.2. Spatial Distribution Characteristics of DEB
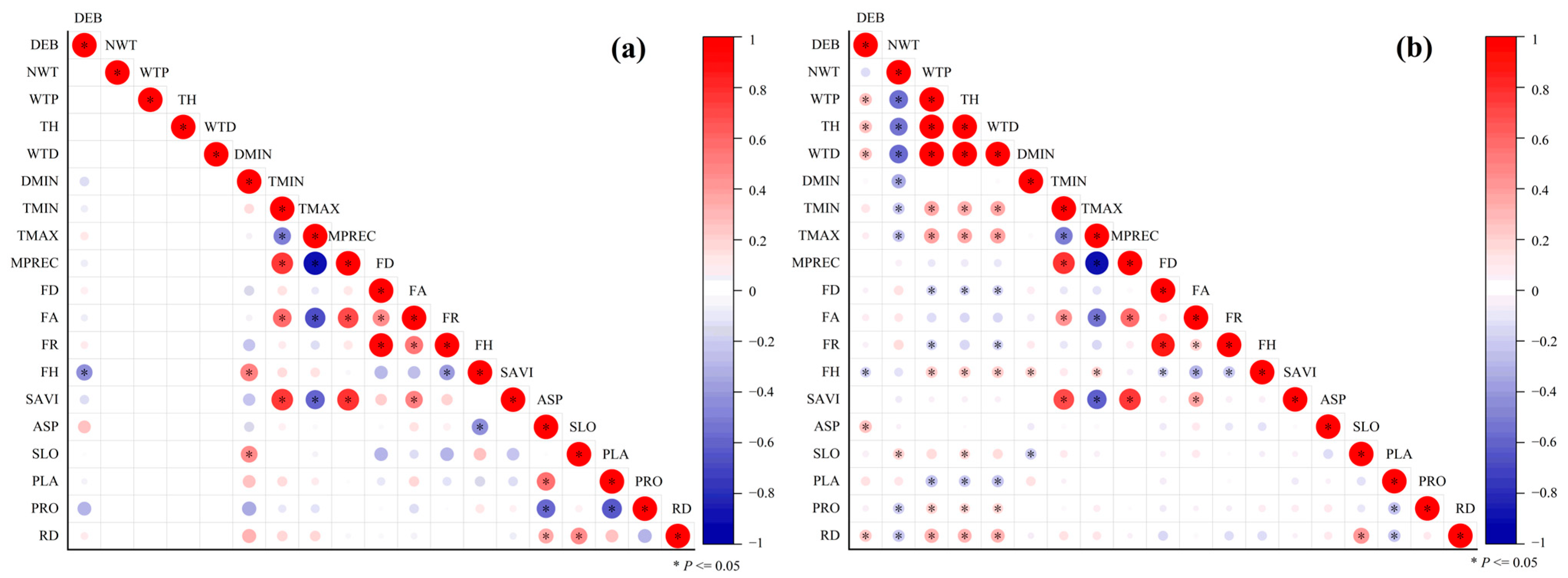
3.3. Influence of Environmental Factors
4. Discussion
4.1. Spatiotemporal Variation Characteristics of the Density of Burrows
4.2. Formation Causes Analysis of the DEB Distribution
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Global Wind Energy Council. Global Wind Report 2024. 2024. Available online: https://www.gwec.net/reports/globalwindreport/2024 (accessed on 26 April 2025).
- European Commission. Commission Sets Out Immediate Actions to Support the European Wind Power Industry. Press Release 2023. Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions. Available online: https://ec.europa.eu/commission/presscorner/detail/en/ip_23_5185 (accessed on 6 May 2025).
- Zhou, H.; Zhang, J.; Zhao, Y. Wind Power Policies in China: Development Themes, Evaluation, and Recommendations. Energy Sci. Eng. 2023, 11, 1044–1059. [Google Scholar] [CrossRef]
- U.S. Department of Energy DOE’s Top Clean Energy Accomplishments in 2024. Available online: https://www.energy.gov/articles/does-top-clean-energy-accomplishments-2024 (accessed on 10 April 2025).
- Jacobson, M.Z.; Delucchi, M.A. Providing All Global Energy with Wind, Water, and Solar Power, Part I: Technologies, Energy Resources, Quantities and Areas of Infrastructure, and Materials. Energy Policy 2011, 39, 1154–1169. [Google Scholar] [CrossRef]
- Jiang, J.; Yang, L.; Li, Z.; Gao, X. Progress in the research on the impact of wind farms on climate and environment. Adv. Earth Sci. 2019, 34, 1038–1049. (In Chinese) [Google Scholar] [CrossRef]
- Smallwood, K.S. Estimating Wind Turbine-Caused Bird Mortality. J. Wildl. Manag. 2007, 71, 2781–2791. [Google Scholar] [CrossRef]
- Cabrera-Cruz, S.A.; Cervantes-Pasqualli, J.; Franquesa-Soler, M.; Muñoz-Jiménez, Ó.; Rodríguez-Aguilar, G.; Villegas-Patraca, R. Estimates of Aerial Vertebrate Mortality at Wind Farms in a Bird Migration Corridor and Bat Diversity Hotspot. Glob. Ecol. Conserv. 2020, 22, e00966. [Google Scholar] [CrossRef]
- Marques, A.T.; Batalha, H.; Rodrigues, S.; Costa, H.; Pereira, M.J.R.; Fonseca, C.; Mascarenhas, M.; Bernardino, J. Understanding Bird Collisions at Wind Farms: An Updated Review on the Causes and Possible Mitigation Strategies. Biol. Conserv. 2014, 179, 40–52. [Google Scholar] [CrossRef]
- Laranjeiro, T.; May, R.; Verones, F. Impacts of Onshore Wind Energy Production on Birds and Bats: Recommendations for Future Life Cycle Impact Assessment Developments. Int. J. Life Cycle Assess 2018, 23, 2007–2023. [Google Scholar] [CrossRef]
- Kunz, T.H.; Arnett, E.B.; Erickson, W.P.; Hoar, A.R.; Johnson, G.D.; Larkin, R.P.; Strickland, M.D.; Thresher, R.W.; Tuttle, M.D. Ecological Impacts of Wind Energy Development on Bats: Questions, Research Needs, and Hypotheses. Front. Ecol. Environ. 2007, 5, 315–324. [Google Scholar] [CrossRef]
- Tang, B.; Wu, D.; Zhao, X.; Zhou, T.; Zhao, W.; Wei, H. The Observed Impacts of Wind Farms on Local Vegetation Growth in Northern China. Remote Sens. 2017, 9, 332. [Google Scholar] [CrossRef]
- Liu, L.; Liu, P.; Yu, J.; Feng, G.; Zhang, Q.; Svenning, J.C. Wind Farms Increase Land Surface Temperature and Reduce Vegetation Productivity in the Inner Mongolia. Geogr. Sustain. 2024, 5, 319–328. [Google Scholar] [CrossRef]
- Wang, G.; Li, G.; Liu, Z. Wind Farms Dry Surface Soil in Temporal and Spatial Variation. Sci. Total Environ. 2023, 857, 159293. [Google Scholar] [CrossRef]
- Santos, M.; Bastos, R.; Travassos, P.; Bessa, R.; Repas, M.; Cabral, J.A. Predicting the Trends of Vertebrate Species Richness as a Response to Wind Farms Installation in Mountain Ecosystems of Northwest Portugal. Ecol. Indic. 2010, 10, 192–205. [Google Scholar] [CrossRef]
- Lintott, P.R.; Richardson, S.M.; Hosken, D.J.; Fensome, S.A.; Mathews, F. Ecological Impact Assessments Fail to Reduce Risk of Bat Casualties at Wind Farms. Curr. Biol. 2016, 26, R1135–R1136. [Google Scholar] [CrossRef] [PubMed]
- Łopucki, R.; Klich, D.; Gielarek, S. Do Terrestrial Animals Avoid Areas Close to Turbines in Functioning Wind Farms in Agricultural Landscapes? Environ. Monit. Assess. 2017, 189, 343. [Google Scholar] [CrossRef] [PubMed]
- Keehn, J.E.; Feldman, C.R. Disturbance Affects Biotic Community Composition at Desert Wind Farms. Wildl. Res. 2018, 45, 383–396. [Google Scholar] [CrossRef]
- Skarin, A.; Sandström, P.; Alam, M. Out of Sight of Wind Turbines—Reindeer Response to Wind Farms in Operation. Ecol. Evol. 2018, 8, 9906–9919. [Google Scholar] [CrossRef]
- Klich, D.; Łopucki, R.; Ścibior, A.; Gołębiowska, D.; Wojciechowska, M. Roe Deer Stress Response to a Wind Farms: Methodological and Practical Implications. Ecol. Indic. 2020, 117, 106658. [Google Scholar] [CrossRef]
- Anoop, V.; Arun, P.R.; Jaypal, R. Do Black-Naped Hares Lepus nigricollis (Mammalia: Lagomorpha: Leporidae) Have Synanthropic Association with Wind Farms? J. Threat. Taxa 2018, 10, 11925. [Google Scholar] [CrossRef]
- Łopucki, R.; Klich, D.; Ścibior, A.; Gołębiowska, D.; Perzanowski, K. Living in Habitats Affected by Wind Turbines May Result in an Increase in Corticosterone Levels in Ground Dwelling Animals. Ecol. Indic. 2018, 84, 165–171. [Google Scholar] [CrossRef]
- Kumara, H.N.; Babu, S.; Rao, G.B.; Mahato, S.; Bhattacharya, M.; Rao, N.V.R.; Tamiliniyan, D.; Parengal, H.; Deepak, D.; Balakrishnan, A.; et al. Responses of Birds and Mammals to Long-Established Wind Farms in India. Sci. Rep. 2022, 12, 1339. [Google Scholar] [CrossRef]
- Velilla, E.; Collinson, E.; Bellato, L.; Berg, M.P.; Halfwerk, W. Vibrational Noise from Wind Energy-turbines Negatively Impacts Earthworm Abundance. Oikos 2021, 130, 844–849. [Google Scholar] [CrossRef]
- Rabin, L.A.; Coss, R.G.; Owings, D.H. The Effects of Wind Turbines on Antipredator Behavior in California Ground Squirrels (Spermophilus beecheyi). Biol. Conserv. 2006, 131, 410–420. [Google Scholar] [CrossRef]
- Agha, M.; Lovich, J.E.; Ennen, J.R.; Augustine, B.; Arundel, T.R.; Murphy, M.O.; Meyer-Wilkins, K.; Bjurlin, C.; Delaney, D.; Briggs, J.; et al. Turbines and Terrestrial Vertebrates: Variation in Tortoise Survivorship Between a Wind Energy Facility and an Adjacent Undisturbed Wildland Area in the Desert Southwest (USA). Environ. Manag. 2015, 56, 332–341. [Google Scholar] [CrossRef] [PubMed]
- Łopucki, R.; Perzanowski, K. Effects of Wind Turbines on Spatial Distribution of the European Hamster. Ecol. Indic. 2018, 84, 433–436. [Google Scholar] [CrossRef]
- Łopucki, R.; Mróz, I. An Assessment of Non-Volant Terrestrial Vertebrates Response to Wind Farms—A Study of Small Mammals. Environ. Monit. Assess. 2016, 188, 122. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Shi, J.; Cao, C. Geographical Division and Classification Management of Rodent Species in Ningxia; Sunshine Publishing: Yinchuan, China, 2019; ISBN 978-7-5525-4710-8. (In Chinese) [Google Scholar][Green Version]
- Stanley, A.P.J.; Roberts, O.; Lopez, A.; Williams, T.; Barker, A. Turbine Scale and Siting Considerations in Wind Plant Layout Optimization and Implications for Capacity Density. Energy Rep. 2022, 8, 3507–3525. [Google Scholar] [CrossRef]
- Jackson, S.F.; Austin, G.E.; Armitage, M.J.S. Surveying Waterbirds Away from Major Waterbodies: Implications for Waterbird Population Estimates in Great Britain. Bird Study 2006, 53, 105–111. [Google Scholar] [CrossRef]
- Bhagia, N.; Rajak, D.R.; Patel, N.K. Improvement in Precision of Crop Acreage Estimation by Remote Sensing Using Frequency Distribution Based Stratification. J. Indian Soc. Remote Sens. 2011, 39, 153–160. [Google Scholar] [CrossRef]
- Li, Z. Identification of Spermophilus Dauricus Burrow Systems and Analysis of Rodent Situation. Chin. J. Control Endem. Dis. 1988, 3, 314. (In Chinese) [Google Scholar][Green Version]
- Hua, R.; Bao, D.; Dong, R.; Tang, Z.; Chu, B.; Hao, Y.; Hua, L. Monitoring of Rodent Damage Areas in Grassland Using Unmanned Aerial Vehicle Remote Sensing Technology. Acta Prataculturae Sin. 2023, 32, 71–82. (In Chinese) [Google Scholar] [CrossRef]
- Esri Hotspot Analysis (Getis-Ord Gi*) (Spatial Statistics). Available online: https://pro.arcgis.com/en/pro-app/latest/tool-reference/spatial-statistics/h-how-hot-spot-analysis-getis-ord-gi-spatial-stati.htm (accessed on 16 May 2025).[Green Version]
- Higdon, R. Generalized Additive Models. In Encyclopedia of Systems Biology; Dubitzky, W., Wolkenhauer, O., Cho, K.H., Yokota, H., Eds.; Springer: New York, NY, USA, 2013; pp. 814–815. ISBN 978-1-4419-9863-7. [Google Scholar][Green Version]
- Zhang, G.; Zhu, A.; Yang, S.; Qin, C.; Xiao, W.; Steve, K.W. Mapping Wildlife Habitat Suitability Using Kernel Density Estimation. Acta Ecol. Sin. 2013, 33, 7590–7600. (In Chinese) [Google Scholar] [CrossRef]
- Fu, C.; Yang, W.; Zhang, J.; Xu, Z.; Tan, B.; Wu, F. Small mammals burrow selection and habitat characteristics in an alpine forest of eastern Tibet Plateau, China. Chin. J. Appl. Ecol. 2016, 27, 1953–1958. [Google Scholar] [CrossRef]
- Ma, T.; Zheng, J.; Wen, A.; Chen, M.; Liu, Z. Group coverage of burrow entrances and distribution characteristics of desert forest-dwelling Rhombomys opimus based on unmanned aerial vehicle (UAV) low-altitude remote sensing: A case study at the southern margin of the Gurbantunggut Desert in Xinjiang. Acta Ecol. Sin. 2018, 38, 953–963. [Google Scholar] [CrossRef]
- Katona, K.; Váczi, O.; Altbäcker, V. Topographic Distribution and Daily Activity of the European Ground Squirrel Population in Bugacpuszta, Hungary. Acta Theriol. 2002, 47, 45–54. [Google Scholar] [CrossRef]
- Fehmi, J.S.; Russo, S.E.; Bartolome, J.W. The Effects of Livestock on California Ground Squirrels (Spermophilus beecheyii). Rangel. Ecol. Manag. 2005, 58, 352–359. [Google Scholar] [CrossRef]
- Meliyo, J.L.; Massawe, B.H.J.; Msanya, B.M.; Kimaro, D.N.; Hieronimo, P.; Mulungu, L.S.; Kihupi, N.I.; Deckers, J.A.; Gulinck, H.; Leirs, H. Landform and Surface Attributes for Prediction of Rodent Burrows in the Western Usambara Mountains, Tanzania. Tanzan. J. Health Res. 2014, 16. [Google Scholar] [CrossRef]
- Qiu, X.; Wei, H.; Guo, S.; Hu, X.; Zhang, Y.; Yang, X.; Shang, W. Effect of Wind Farms on Vegetation in Arid Desert Region. J. Gansu For. Sci. Technol. 2022, 47, 10–15. (In Chinese) [Google Scholar] [CrossRef]
- Jia, X.; Li, G.; Wang, G.; Cao, Y. Effects of Wind Farms on Soil Moisture in Grassland. Arid Land Geogr. 2021, 44, 1125–1134. (In Chinese) [Google Scholar]




| Different Wind Power Zone | Number of Surveyed Grids | Number of Wind Turbines | Tower Height (m) | Wheel Diameter (m) | |
|---|---|---|---|---|---|
| Range | Average Density Mean ± SE (unit/km2) | ||||
| Control | 14 | 0 | 0 | — | — |
| 750 kW | 17 | 1~11 | 4.65 ± 0.55 | 50 | 48.4 |
| 1500 kW | 13 | 1~6 | 3.69 ± 0.40 | 65/70/75/80 | 86/87/97 |
| 2000 kW | 17 | 1~5 | 2.29 ± 0.34 | 80/85/90 | 110/115/120 |
| 2500 kW | 14 | 1~3 | 1.93 ± 0.20 | 100 | 150 |
| Survey Area | Survey Month | FD | FA | FR | FH | SAVI |
|---|---|---|---|---|---|---|
| Control area | May | 0.55 ± 0.18 ns 1 | 33.03 ± 11.26 b | 2.49 ± 0.56 ns | 29.80 ± 18.55 a | 0.17 ± 0.02 b |
| September | 0.62 ± 0.34 ns | 65.88 ± 21.37 a | 2.73 ± 1.19 ns | 29.75 ± 18.77 a | 0.27 ± 0.04 a | |
| Wind farm area | May | 0.62 ± 0.21 ns | 31.94 ± 17.14 b | 2.65 ± 0.57 ns | 19.96 ± 11.18 b | 0.17 ± 0.02 b |
| September | 0.62 ± 0.22 ns | 59.30 ± 21.95 a | 2.68 ± 0.64 ns | 19.26 ± 10.68 b | 0.26 ± 0.04 a |
| Wind Turbine Power Area | Environmental Factor (Deviation Explanation Rate) | AIC Value | Cumulative Deviation Explanation Rate (%) | R2 | F | p | |
|---|---|---|---|---|---|---|---|
| Control area | FH (26.20) + PRO (19.95) 1 | 3973.27 | 46.15 | 0.26 | 4.56 | 0.02 | |
| Wind farm area | May | WTP (14.94) + FH (8.27) + PLA (9.32) + ASP (4.23) | 15,959.31 | 36.76 | 0.24 | 4.51 | <0.001 |
| September | WTP (11.08) + ASP (12.89) + RD (12.64) + PLA (9.39) + FH (4.50) | 9321.93 | 50.50 | 0.31 | 5.03 | <0.001 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Yang, W.; Li, Q.; Zhao, M.; Yang, Y.; Shi, X.; Zhang, D.; Yang, G. Will Wind Turbines Affect the Distribution of Alashan Ground Squirrel? Insights from Large-Scale Wind Farms in China. Biology 2025, 14, 886. https://doi.org/10.3390/biology14070886
Wang Y, Yang W, Li Q, Zhao M, Yang Y, Shi X, Zhang D, Yang G. Will Wind Turbines Affect the Distribution of Alashan Ground Squirrel? Insights from Large-Scale Wind Farms in China. Biology. 2025; 14(7):886. https://doi.org/10.3390/biology14070886
Chicago/Turabian StyleWang, Yuan, Wenbin Yang, Qin Li, Min Zhao, Ying Yang, Xiangfeng Shi, Dazhi Zhang, and Guijun Yang. 2025. "Will Wind Turbines Affect the Distribution of Alashan Ground Squirrel? Insights from Large-Scale Wind Farms in China" Biology 14, no. 7: 886. https://doi.org/10.3390/biology14070886
APA StyleWang, Y., Yang, W., Li, Q., Zhao, M., Yang, Y., Shi, X., Zhang, D., & Yang, G. (2025). Will Wind Turbines Affect the Distribution of Alashan Ground Squirrel? Insights from Large-Scale Wind Farms in China. Biology, 14(7), 886. https://doi.org/10.3390/biology14070886