Immunological Factors in Recurrent Pregnancy Loss: Mechanisms, Controversies, and Emerging Therapies
Simple Summary
Abstract
1. Introduction
2. Background: Immune Mechanisms in Pregnancy and RPL
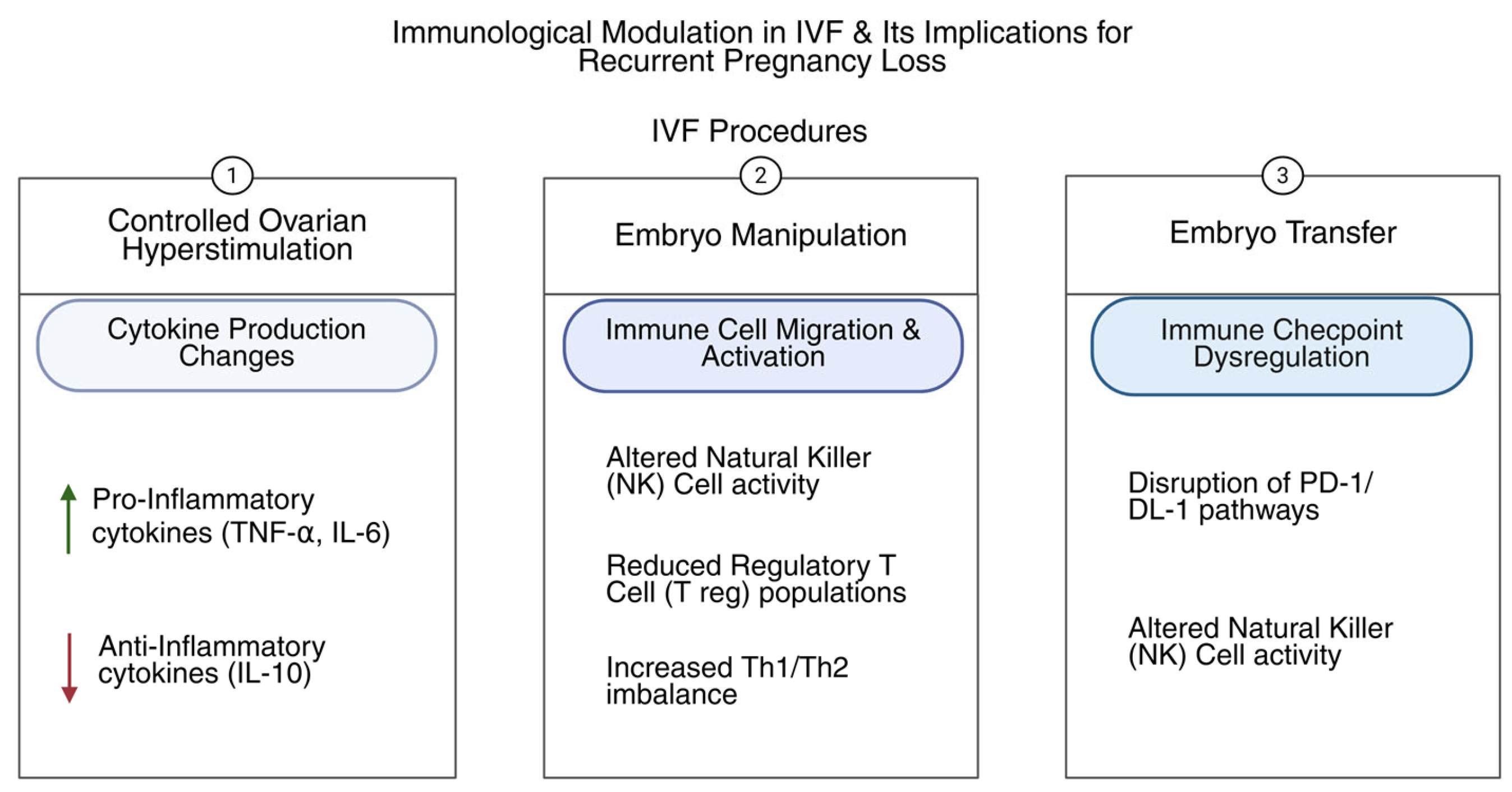
3. Immunological Mechanisms in RPL During IVF
3.1. Maternal–Fetal Immune Tolerance
3.2. Innate Immunity and NK Cells
3.3. Adaptive Immunity and Autoimmunity
3.4. Inflammatory Cytokines and Chemokines
3.5. Genetic and Epigenetic Influences on Immune Regulation
3.6. Uterine Microbiome and Endometrial Receptivity
4. Controversies in Immunological Assessment and Treatment
4.1. Diagnostic Challenges
4.2. Debate over the Role of Immune Dysregulation
4.3. Therapeutic Controversies
5. Emerging Therapies and Future Directions
5.1. Novel Immunomodulatory Agents
5.2. Cell-Based Therapies
5.3. Personalized Immunotherapy
5.4. Immunogenetics and Biomarker Discovery
5.5. Non-Invasive Immune Monitoring
5.6. Ongoing Clinical Trials and Research Gaps
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Eshre Guideline Group on RPL; Bender Atik, R.; Christiansen, O.B.; Elson, J.; Kolte, A.M.; Lewis, S.; Middeldorp, S.; McHeik, S.; Peramo, B.; Quenby, S.; et al. ESHRE guideline: Recurrent pregnancy loss: An update in 2022. Hum. Reprod. Open 2023, 2023, hoad002. [Google Scholar] [CrossRef]
- Sadeghi, M.R. ART Strategy for Treatment of Recurrent Pregnancy Loss: Isn’t It Better to Forget? J. Reprod. Infertil. 2016, 17, 191. [Google Scholar] [PubMed]
- Arnadottir, G.A.; Jonsson, H.; Hartwig, T.S.; Gruhn, J.R.; Moller, P.L.; Gylfason, A.; Westergaard, D.; Chan, A.C.; Oddsson, A.; Stefansdottir, L.; et al. Sequence diversity lost in early pregnancy. Nature 2025, 642, 672–681. [Google Scholar] [CrossRef]
- Roosan, D.; Padua, P.; Khan, R.; Khan, H.; Verzosa, C.; Wu, Y. Effectiveness of ChatGPT in clinical pharmacy and the role of artificial intelligence in medication therapy management. J. Am. Pharm. Assoc. 2024, 64, 422–428.e428. [Google Scholar] [CrossRef]
- Mackay, A.; Taylor, S.; Glass, B. Inequity of Access: Scoping the Barriers to Assisted Reproductive Technologies. Pharmacy 2023, 11, 17. [Google Scholar] [CrossRef]
- Garmendia, J.V.; De Sanctis, C.V.; Hajduch, M.; De Sanctis, J.B. Exploring the Immunological Aspects and Treatments of Recurrent Pregnancy Loss and Recurrent Implantation Failure. Int. J. Mol. Sci. 2025, 26, 1295. [Google Scholar] [CrossRef]
- Potiris, A.; Perros, P.; Drakaki, E.; Mavrogianni, D.; Machairiotis, N.; Sfakianakis, A.; Karampitsakos, T.; Vrachnis, D.; Antonakopoulos, N.; Panagopoulos, P.; et al. Investigating the Association of Assisted Reproduction Techniques and Adverse Perinatal Outcomes. J. Clin. Med. 2024, 13, 328. [Google Scholar] [CrossRef]
- Balasundaram, P.; Farhana, A. Immunology at the Maternal-Fetal Interface; StatPearls: Treasure Island, FL, USA, 2025. [Google Scholar]
- Geldenhuys, J.; Rossouw, T.M.; Lombaard, H.A.; Ehlers, M.M.; Kock, M.M. Disruption in the Regulation of Immune Responses in the Placental Subtype of Preeclampsia. Front. Immunol. 2018, 9, 1659. [Google Scholar] [CrossRef]
- Vani, V.; Vasan, S.S.; Adiga, S.K.; Varsha, S.R.; Seshagiri, P.B. Molecular regulators of human blastocyst development and hatching: Their significance in implantation and pregnancy outcome. Am. J. Reprod. Immunol. 2023, 89, e13635. [Google Scholar] [CrossRef]
- Cherouveim, P.; Mavrogianni, D.; Drakaki, E.; Potiris, A.; Zikopoulos, A.; Papamentzelopoulou, M.; Kouvoutsaki, K.; Machairiotis, N.; Karampitsakos, T.; Skentou, C.; et al. ANRIL rs4977574 Gene Polymorphism in Women with Recurrent Pregnancy Loss. J. Clin. Med. 2023, 12, 5944. [Google Scholar] [CrossRef]
- Patronia, M.M.; Potiris, A.; Mavrogianni, D.; Drakaki, E.; Karampitsakos, T.; Machairoudias, P.; Topis, S.; Zikopoulos, A.; Vrachnis, D.; Moustakli, E.; et al. The Expression of microRNAs and Their Involvement in Recurrent Pregnancy Loss. J. Clin. Med. 2024, 13, 3361. [Google Scholar] [CrossRef]
- Motlagh Asghari, K.; Novinbahador, T.; Mehdizadeh, A.; Zolfaghari, M.; Yousefi, M. Revolutionized attitude toward recurrent pregnancy loss and recurrent implantation failure based on precision regenerative medicine. Heliyon 2024, 10, e39584. [Google Scholar] [CrossRef] [PubMed]
- Uta, C.; Tirziu, A.; Zimbru, E.L.; Zimbru, R.I.; Georgescu, M.; Haidar, L.; Panaitescu, C. Alloimmune Causes of Recurrent Pregnancy Loss: Cellular Mechanisms and Overview of Therapeutic Approaches. Medicina 2024, 60, 1896. [Google Scholar] [CrossRef] [PubMed]
- Caliendo, A.M.; Gilbert, D.N.; Ginocchio, C.C.; Hanson, K.E.; May, L.; Quinn, T.C.; Tenover, F.C.; Alland, D.; Blaschke, A.J.; Bonomo, R.A.; et al. Better tests, better care: Improved diagnostics for infectious diseases. Clin. Infect. Dis. 2013, 57 (Suppl. S3), S139–S170. [Google Scholar] [CrossRef]
- Nash, A.; Aghlara-Fotovat, S.; Hernandez, A.; Scull, C.; Veiseh, O. Clinical translation of immunomodulatory therapeutics. Adv. Drug Deliv. Rev. 2021, 176, 113896. [Google Scholar] [CrossRef]
- Bashiri, A.; Halper, K.I.; Orvieto, R. Recurrent Implantation Failure-update overview on etiology, diagnosis, treatment and future directions. Reprod. Biol. Endocrinol. 2018, 16, 121. [Google Scholar] [CrossRef] [PubMed]
- Guan, D.; Sun, W.; Gao, M.; Chen, Z.; Ma, X. Immunologic insights in recurrent spontaneous abortion: Molecular mechanisms and therapeutic interventions. Biomed. Pharmacother. 2024, 177, 117082. [Google Scholar] [CrossRef]
- Cao, C.; Bai, S.; Zhang, J.; Sun, X.; Meng, A.; Chen, H. Understanding recurrent pregnancy loss: Recent advances on its etiology, clinical diagnosis, and management. Med. Rev. 2022, 2, 570–589. [Google Scholar] [CrossRef]
- Moustakli, E.; Zikopoulos, A.; Skentou, C.; Katopodis, P.; Domali, E.; Potiris, A.; Stavros, S.; Zachariou, A. Impact of Reductive Stress on Human Infertility: Underlying Mechanisms and Perspectives. Int. J. Mol. Sci. 2024, 25, 1802. [Google Scholar] [CrossRef]
- Ma, J.; Gao, W.; Li, D. Recurrent implantation failure: A comprehensive summary from etiology to treatment. Front. Endocrinol. 2022, 13, 1061766. [Google Scholar] [CrossRef]
- Zhang, S.; Lin, H.; Kong, S.; Wang, S.; Wang, H.; Wang, H.; Armant, D.R. Physiological and molecular determinants of embryo implantation. Mol. Aspects Med. 2013, 34, 939–980. [Google Scholar] [CrossRef]
- Pantos, K.; Grigoriadis, S.; Maziotis, E.; Pistola, K.; Xystra, P.; Pantou, A.; Kokkali, G.; Pappas, A.; Lambropoulou, M.; Sfakianoudis, K.; et al. The Role of Interleukins in Recurrent Implantation Failure: A Comprehensive Review of the Literature. Int. J. Mol. Sci. 2022, 23, 2198. [Google Scholar] [CrossRef]
- Sfakianoudis, K.; Rapani, A.; Grigoriadis, S.; Pantou, A.; Maziotis, E.; Kokkini, G.; Tsirligkani, C.; Bolaris, S.; Nikolettos, K.; Chronopoulou, M.; et al. The Role of Uterine Natural Killer Cells on Recurrent Miscarriage and Recurrent Implantation Failure: From Pathophysiology to Treatment. Biomedicines 2021, 9, 1425. [Google Scholar] [CrossRef] [PubMed]
- Kanter, J.; Gordon, S.M.; Mani, S.; Sokalska, A.; Park, J.Y.; Senapati, S.; Huh, D.D.; Mainigi, M. Hormonal stimulation reduces numbers and impairs function of human uterine natural killer cells during implantation. Hum. Reprod. 2023, 38, 1047–1059. [Google Scholar] [CrossRef] [PubMed]
- Mattar, C.N.Z.; Chew, W.L.; Lai, P.S. Embryo and fetal gene editing: Technical challenges and progress toward clinical applications. Mol. Ther. Methods Clin. Dev. 2024, 32, 101229. [Google Scholar] [CrossRef]
- Duan, H.; Deng, W.; Kzhyshkowska, J.; Chen, D.; Zhang, S. Macrophage at maternal-fetal Interface: Perspective on pregnancy and related disorders. Placenta 2025. [Google Scholar] [CrossRef]
- Stavros, S.; Panagopoulos, P.; Machairiotis, N.; Potiris, A.; Mavrogianni, D.; Sfakianakis, A.; Drakaki, E.; Christodoulaki, C.; Panagiotopoulos, D.; Sioutis, D.; et al. Association between cytokine polymorphisms and recurrent pregnancy loss: A review of current evidence. Int. J. Gynaecol. Obstet. 2024, 167, 45–57. [Google Scholar] [CrossRef]
- Moffett, A.; Colucci, F. Uterine NK cells: Active regulators at the maternal-fetal interface. J. Clin. Investig. 2014, 124, 1872–1879. [Google Scholar] [CrossRef]
- PrabhuDas, M.; Piper, J.M.; Jean-Philippe, P.; Lachowicz-Scroggins, M. Immune Regulation, Maternal Infection, Vaccination, and Pregnancy Outcome. J. Womens Health 2021, 30, 199–206. [Google Scholar] [CrossRef]
- Jiang, X.; Wang, H. Macrophage subsets at the maternal-fetal interface. Cell Mol. Immunol. 2020, 17, 889–891. [Google Scholar] [CrossRef]
- Wei, R.; Lai, N.; Zhao, L.; Zhang, Z.; Zhu, X.; Guo, Q.; Chu, C.; Fu, X.; Li, X. Dendritic cells in pregnancy and pregnancy-associated diseases. Biomed. Pharmacother. 2021, 133, 110921. [Google Scholar] [CrossRef] [PubMed]
- Jameel, S.; Bhuwalka, R.; Begum, M.; Bonu, R.; Jahan, P. Circulating levels of cytokines (IL-6, IL-10 and TGF- beta) and CD4(+)CD25(+)FOXP3(+)Treg cell population in recurrent pregnancy loss. Reprod. Biol. 2024, 24, 100842. [Google Scholar] [CrossRef] [PubMed]
- Ozbey, G.; Tanriverdi, E.S.; Cakir, A.; Yilmaz, E. Investigation of the Relationship Between IL-17, IL-27, IL-2 Blood Levels in Spontaneous Abortion and Healthy Pregnant Women. Life 2025, 15, 326. [Google Scholar] [CrossRef]
- Ding, J.; Maxwell, A.; Adzibolosu, N.; Hu, A.; You, Y.; Liao, A.; Mor, G. Mechanisms of immune regulation by the placenta: Role of type I interferon and interferon-stimulated genes signaling during pregnancy. Immunol. Rev. 2022, 308, 9–24. [Google Scholar] [CrossRef]
- Peng, X.; Chinwe Oluchi-Amaka, I.; Kwak-Kim, J.; Yang, X. A comprehensive review of the roles of T-cell immunity in preeclampsia. Front. Immunol. 2025, 16, 1476123. [Google Scholar] [CrossRef]
- Sultana, S.; Nallari, P.; Ananthapur, V. Recurrent pregnancy loss (RPL): An overview. J. Womens Health Dev. 2020, 3, 302–315. [Google Scholar] [CrossRef]
- Catamo, E.; Zupin, L.; Segat, L.; Celsi, F.; Crovella, S. HLA-G and susceptibility to develop celiac disease. Hum. Immunol. 2015, 76, 36–41. [Google Scholar] [CrossRef]
- Andersen, L.H.J.; Sanz Martinez, R.; Dai, Y.; Eriksen, J.O.; Gerlach, M.K.; Larsen, L.G.; Macklon, N.S.; Juul Hare, K.; Sandelin, A.; Nielsen, H.S.; et al. Upregulation of immune genes in the proliferative phase endometrium enables classification into women with recurrent pregnancy loss versus controls. Hum. Reprod. 2025, 40, 1045–1065. [Google Scholar] [CrossRef]
- Saito, S.; Nakashima, A.; Shima, T.; Tsuda, S. Pregnancy depends on a delicate balance of immune activation and regulation. Explor. Immunol. 2021, 1, 461–478. [Google Scholar] [CrossRef]
- Morelli, S.S.; Mandal, M.; Goldsmith, L.T.; Kashani, B.N.; Ponzio, N.M. The maternal immune system during pregnancy and its influence on fetal development. Res. Rep. Biol. 2015, 171–189. [Google Scholar] [CrossRef]
- Zhang, X.; Wei, H. Role of Decidual Natural Killer Cells in Human Pregnancy and Related Pregnancy Complications. Front. Immunol. 2021, 12, 728291. [Google Scholar] [CrossRef]
- Canella, P.; Barini, R.; Carvalho, P.O.; Razolli, D.S. Lipid emulsion therapy in women with recurrent pregnancy loss and repeated implantation failure: The role of abnormal natural killer cell activity. J. Cell Mol. Med. 2021, 25, 2290–2296. [Google Scholar] [CrossRef]
- Bustamante, J.G.; Goyal, A.; Rout, P.; Singhal, M. Antiphospholipid Syndrome; StatPearl: Treasure Island, FL, USA, 2025. [Google Scholar]
- Graham, J.J.; Longhi, M.S.; Heneghan, M.A. T helper cell immunity in pregnancy and influence on autoimmune disease progression. J. Autoimmun. 2021, 121, 102651. [Google Scholar] [CrossRef]
- Yockey, L.J.; Iwasaki, A. Interferons and Proinflammatory Cytokines in Pregnancy and Fetal Development. Immunity 2018, 49, 397–412. [Google Scholar] [CrossRef] [PubMed]
- Sonehara, K.; Yano, Y.; Naito, T.; Goto, S.; Yoshihara, H.; Otani, T.; Ozawa, F.; Kitaori, T.; Biobank Japan, P.; Matsuda, K.; et al. Common and rare genetic variants predisposing females to unexplained recurrent pregnancy loss. Nat. Commun. 2024, 15, 5744. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Xiong, Y.; Qu, B.; Bao, A.; Zhang, Y. DNA Methylation and Recurrent Pregnancy Loss: A Mysterious Compass? Front. Immunol. 2021, 12, 738962. [Google Scholar] [CrossRef] [PubMed]
- Cai, R.; Yang, Q.; Liao, Y.; Qin, L.; Han, J.; Gao, R. Immune Treatment Strategies in Unexplained Recurrent Pregnancy Loss. Am. J. Reprod. Immunol. 2025, 93, e70060. [Google Scholar] [CrossRef]
- Gunther, V.; Allahqoli, L.; Watrowski, R.; Maass, N.; Ackermann, J.; von Otte, S.; Alkatout, I. Vaginal Microbiome in Reproductive Medicine. Diagnostics 2022, 12, 1948. [Google Scholar] [CrossRef]
- Blazheva, S.; Pachkova, S.; Bodurska, T.; Ivanov, P.; Blazhev, A.; Lukanov, T.; Konova, E. Unlocking the Uterine Code: Microbiota, Immune Cells, and Therapy for Recurrent Reproductive Failure. Microorganisms 2024, 12, 547. [Google Scholar] [CrossRef]
- Balla, B.; Illes, A.; Tobias, B.; Piko, H.; Beke, A.; Sipos, M.; Lakatos, P.; Kosa, J.P. The Role of the Vaginal and Endometrial Microbiomes in Infertility and Their Impact on Pregnancy Outcomes in Light of Recent Literature. Int. J. Mol. Sci. 2024, 25, 3227. [Google Scholar] [CrossRef]
- Achilli, C.; Duran-Retamal, M.; Saab, W.; Serhal, P.; Seshadri, S. The role of immunotherapy in in vitro fertilization and recurrent pregnancy loss: A systematic review and meta-analysis. Fertil. Steril. 2018, 110, 1089–1100. [Google Scholar] [CrossRef]
- Bagkou Dimakou, D.; Tamblyn, J.; Justin, C.; Coomarasamy, A.; Richter, A. Diagnosis and management of idiopathic recurrent pregnancy loss (RPL): Current immune testing and immunomodulatory treatment practice in the United Kingdom. J. Reprod. Immunol. 2022, 153, 103662. [Google Scholar] [CrossRef]
- Turesheva, A.; Aimagambetova, G.; Ukybassova, T.; Marat, A.; Kanabekova, P.; Kaldygulova, L.; Amanzholkyzy, A.; Ryzhkova, S.; Nogay, A.; Khamidullina, Z.; et al. Recurrent Pregnancy Loss Etiology, Risk Factors, Diagnosis, and Management. Fresh Look into a Full Box. J. Clin. Med. 2023, 12, 4074. [Google Scholar] [CrossRef] [PubMed]
- Fukui, A.; Kamoi, M.; Funamizu, A.; Fuchinoue, K.; Chiba, H.; Yokota, M.; Fukuhara, R.; Mizunuma, H. NK cell abnormality and its treatment in women with reproductive failures such as recurrent pregnancy loss, implantation failures, preeclampsia, and pelvic endometriosis. Reprod. Med. Biol. 2015, 14, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Brusch, A. The Significance of Anti-Beta-2-Glycoprotein I Antibodies in Antiphospholipid Syndrome. Antibodies 2016, 5, 16. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Chu, D.; Kalantar-Zadeh, K.; George, J.; Young, H.A.; Liu, G. Cytokines: From Clinical Significance to Quantification. Adv. Sci. 2021, 8, e2004433. [Google Scholar] [CrossRef]
- Bagkou Dimakou, D.; Tamblyn, J.; Lissauer, D.; Richter, A. Evaluation of peripheral NK tests offered to women with recurrent pregnancy loss and a search for novel candidate biomarkers. J. Reprod. Immunol. 2025, 169, 104522. [Google Scholar] [CrossRef]
- Ali, S.; Majid, S.; Niamat Ali, M.; Taing, S.; El-Serehy, H.A.; Al-Misned, F.A. Evaluation of etiology and pregnancy outcome in recurrent miscarriage patients. Saudi J. Biol. Sci. 2020, 27, 2809–2817. [Google Scholar] [CrossRef]
- Ahmad, H.I.; Jabbar, A.; Mushtaq, N.; Javed, Z.; Hayyat, M.U.; Bashir, J.; Naseeb, I.; Abideen, Z.U.; Ahmad, N.; Chen, J. Immune Tolerance vs. Immune Resistance: The Interaction Between Host and Pathogens in Infectious Diseases. Front. Vet. Sci. 2022, 9, 827407. [Google Scholar] [CrossRef]
- Chaplin, D.D. Overview of the immune response. J. Allergy Clin. Immunol. 2010, 125, S3–S23. [Google Scholar] [CrossRef]
- Kalra, A.; Mackay, O.; Thomas-Jones, E.; Solomon, T.; Foscarini-Craggs, P. Does the Use of Intravenous Immunoglobulin Improve Clinical Outcomes in Adults With Autoimmune Encephalitis? A Systematic Review. Brain Behav. 2025, 15, e70491. [Google Scholar] [CrossRef]
- Baschieri, L.; Antonelli, A.; Nardi, S.; Alberti, B.; Lepri, A.; Canapicchi, R.; Fallahi, P. Intravenous immunoglobulin versus corticosteroid in treatment of Graves’ ophthalmopathy. Thyroid 1997, 7, 579–585. [Google Scholar] [CrossRef]
- Kumar, P.; Marron, K.; Harrity, C. Intralipid therapy and adverse reproductive outcome: Is there any evidence? Reprod. Fertil. 2021, 2, 173–186. [Google Scholar] [CrossRef] [PubMed]
- Skeith, L. Anticoagulating patients with high-risk acquired thrombophilias. Blood 2018, 132, 2219–2229. [Google Scholar] [CrossRef] [PubMed]
- De Jong, P.G.; Kaandorp, S.; Di Nisio, M.; Goddijn, M.; Middeldorp, S. Aspirin and/or heparin for women with unexplained recurrent miscarriage with or without inherited thrombophilia. Cochrane Database Syst. Rev. 2014, 2014, CD004734. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Yuan, Y.; Liu, H.; Lu, Q.; Mu, R. Glucocorticoids Improve the Pregnancy Rate and Outcome in Women With Unexplained Positive Autoantibodies: A Systematic Review and Meta-Analysis. Front. Med. 2022, 9, 819406. [Google Scholar] [CrossRef]
- D’Ippolito, S.; Gavi, F.; Granieri, C.; De Waure, C.; Giuliano, S.; Cosentino, F.; Tersigni, C.; Scambia, G.; Di Simone, N. Efficacy of Corticosteroids in Patients With Recurrent Pregnancy Loss: A Systematic Review and Meta-Analysis. Am. J. Reprod. Immunol. 2025, 93, e70037. [Google Scholar] [CrossRef]
- Habets, D.H.J.; Pelzner, K.; Wieten, L.; Spaanderman, M.E.A.; Villamor, E.; Al-Nasiry, S. Intravenous immunoglobulins improve live birth rate among women with underlying immune conditions and recurrent pregnancy loss: A systematic review and meta-analysis. Allergy Asthma Clin. Immunol. 2022, 18, 23. [Google Scholar] [CrossRef]
- Han, E.J.; Lee, H.N.; Kim, M.K.; Lyu, S.W.; Lee, W.S. Efficacy of intralipid administration to improve in vitro fertilization outcomes: A systematic review and meta-analysis. Clin. Exp. Reprod. Med. 2021, 48, 203–210. [Google Scholar] [CrossRef]
- Tang, Y.; Tong, X. Efficacy Evaluation of Aspirin Plus Prednisone or Prednisolone in IVF/RIF Patients: A Systematic Review and Meta-Analysis. Clin. Exp. Obstet. Gynecol. 2024, 51, 187. [Google Scholar] [CrossRef]
- Ichikawa, T.; Watanabe, T.; Kubota, Y.; Matsuda, S.; Shigemi, D.; Kasano, S.; Yokote, R.; Yonezawa, M.; Ouchi, N.; Negishi, Y.; et al. Impact of heparin-aspirin therapy in patients with recurrent pregnancy loss characterized by thrombophilia resistant to low-dose aspirin therapy: A retrospective study. Reprod. Med. Biol. 2025, 24, e12643. [Google Scholar] [CrossRef]
- Yousefi, M.; Ahmadian-Heris, J.; Danaii, S.; Abdolmohammadi-Vahid, S.; Aghebati-Maleki, L. Recent Advances in Immunotherapeutic Approaches for Recurrent Reproductive Failure. In IVF Technologies and Infertility–Current Practices and New Perspectives; Vladimirov, I.K., Ed.; IntechOpen: Rijeka, Croatia, 2022. [Google Scholar]
- Sharma, P.; Goswami, S.; Raychaudhuri, D.; Siddiqui, B.A.; Singh, P.; Nagarajan, A.; Liu, J.; Subudhi, S.K.; Poon, C.; Gant, K.L.; et al. Immune checkpoint therapy-current perspectives and future directions. Cell 2023, 186, 1652–1669. [Google Scholar] [CrossRef]
- Romanowska-Prochnicka, K.; Felis-Giemza, A.; Olesinska, M.; Wojdasiewicz, P.; Paradowska-Gorycka, A.; Szukiewicz, D. The Role of TNF-alpha and Anti-TNF-alpha Agents during Preconception, Pregnancy, and Breastfeeding. Int. J. Mol. Sci. 2021, 22, 2922. [Google Scholar] [CrossRef] [PubMed]
- Duricova, D.; Dvorakova, E.; Hradsky, O.; Mitrova, K.; Durilova, M.; Kozeluhova, J.; Kohout, P.; Zarubova, K.; Bronsky, J.; Hradska, N.; et al. Safety of Anti-TNF-Alpha Therapy During Pregnancy on Long-term Outcome of Exposed Children: A Controlled, Multicenter Observation. Inflamm. Bowel Dis. 2019, 25, 789–796. [Google Scholar] [CrossRef] [PubMed]
- McGeachy, M.J.; Cua, D.J.; Gaffen, S.L. The IL-17 Family of Cytokines in Health and Disease. Immunity 2019, 50, 892–906. [Google Scholar] [CrossRef] [PubMed]
- Kampan, N.C.; Xiang, S.D.; McNally, O.M.; Stephens, A.N.; Quinn, M.A.; Plebanski, M. Immunotherapeutic Interleukin-6 or Interleukin-6 Receptor Blockade in Cancer: Challenges and Opportunities. Curr. Med. Chem. 2018, 25, 4785–4806. [Google Scholar] [CrossRef]
- Vilotic, A.; Nacka-Aleksic, M.; Pirkovic, A.; Bojic-Trbojevic, Z.; Dekanski, D.; Jovanovic Krivokuca, M. IL-6 and IL-8: An Overview of Their Roles in Healthy and Pathological Pregnancies. Int. J. Mol. Sci. 2022, 23, 4574. [Google Scholar] [CrossRef]
- Pilat, N.; Sprent, J. Treg Therapies Revisited: Tolerance Beyond Deletion. Front. Immunol. 2020, 11, 622810. [Google Scholar] [CrossRef]
- Tang, C.; Hu, W. The role of Th17 and Treg cells in normal pregnancy and unexplained recurrent spontaneous abortion (URSA): New insights into immune mechanisms. Placenta 2023, 142, 18–26. [Google Scholar] [CrossRef]
- Amini, L.; Kaeda, J.; Fritsche, E.; Roemhild, A.; Kaiser, D.; Reinke, P. Clinical adoptive regulatory T Cell therapy: State of the art, challenges, and prospective. Front. Cell Dev. Biol. 2022, 10, 1081644. [Google Scholar] [CrossRef]
- Rungsiwiwut, R.; Virutamasen, P.; Pruksananonda, K. Mesenchymal stem cells for restoring endometrial function: An infertility perspective. Reprod. Med. Biol. 2021, 20, 13–19. [Google Scholar] [CrossRef]
- Montgomery, L.; Larbi, A. Monitoring Immune Responses to Vaccination: A Focus on Single-Cell Analysis and Associated Challenges. Vaccines 2025, 13, 420. [Google Scholar] [CrossRef]
- Yang, Y.; Zhao, Y.; Liu, X.; Huang, J. Artificial intelligence for prediction of response to cancer immunotherapy. Semin. Cancer Biol. 2022, 87, 137–147. [Google Scholar] [CrossRef]
- Goetz, L.H.; Schork, N.J. Personalized medicine: Motivation, challenges, and progress. Fertil. Steril. 2018, 109, 952–963. [Google Scholar] [CrossRef]
- Jalilvand, A.; Yari, K.; Heydarpour, F. Role of Polymorphisms on the Recurrent Pregnancy Loss: A Systematic Review, Meta-analysis and Bioinformatic Analysis. Gene 2022, 844, 146804. [Google Scholar] [CrossRef] [PubMed]
- Ratre, P.; Thareja, S.; Mishra, P.K. Identification of cell-free circulating epigenomic biomarkers for early diagnosis and response to therapies in breast cancer patients. Int. Rev. Cell Mol. Biol. 2025, 391, 95–134. [Google Scholar] [CrossRef] [PubMed]
- Felekkis, K.; Papaneophytou, C. The Circulating Biomarkers League: Combining miRNAs with Cell-Free DNAs and Proteins. Int. J. Mol. Sci. 2024, 25, 3403. [Google Scholar] [CrossRef] [PubMed]
- Yadav, R.; Singh, A.V.; Kushwaha, S.; Chauhan, D.S. Emerging role of exosomes as a liquid biopsy tool for diagnosis, prognosis & monitoring treatment response of communicable & non-communicable diseases. Indian. J. Med. Res. 2024, 159, 163–180. [Google Scholar] [CrossRef]
- Adhit, K.K.; Wanjari, A.; Menon, S.; K, S. Liquid Biopsy: An Evolving Paradigm for Non-invasive Disease Diagnosis and Monitoring in Medicine. Cureus 2023, 15, e50176. [Google Scholar] [CrossRef]
- Benjamin-Davalos, S.; Koroleva, M.; Allen, C.L.; Ernstoff, M.S.; Shu, S. Co-Isolation of Cytokines and Exosomes: Implications for Immunomodulation Studies. Front. Immunol. 2021, 12, 638111. [Google Scholar] [CrossRef]
- Essola, J.M.; Zhang, M.; Yang, H.; Li, F.; Xia, B.; Mavoungou, J.F.; Hussain, A.; Huang, Y. Exosome regulation of immune response mechanism: Pros and cons in immunotherapy. Bioact. Mater. 2024, 32, 124–146. [Google Scholar] [CrossRef]
- Abdolmohammadi-Vahid, S.; Danaii, S.; Hamdi, K.; Jadidi-Niaragh, F.; Ahmadi, M.; Yousefi, M. Novel immunotherapeutic approaches for treatment of infertility. Biomed. Pharmacother. 2016, 84, 1449–1459. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, Z.; Teng, X. New advances in the treatment of thin endometrium. Front. Endocrinol. 2024, 15, 1269382. [Google Scholar] [CrossRef]
- Mei, Y.; Lin, Y.; Chen, Y.; Zheng, J.; Ke, X.; Liang, X.; Wang, F. Preimplantation genetic testing for aneuploidy optimizes reproductive outcomes in recurrent reproductive failure: A systematic review. Front. Med. 2024, 11, 1233962. [Google Scholar] [CrossRef]
- Ebrahimi, F.; Omidvar-Mehrabadi, A.; Shahbazi, M.; Mohammadnia-Afrouzi, M. Innate and adaptive immune dysregulation in women with recurrent implantation failure. J. Reprod. Immunol. 2024, 164, 104262. [Google Scholar] [CrossRef] [PubMed]
- Sathish, J.G.; Sethu, S.; Bielsky, M.C.; de Haan, L.; French, N.S.; Govindappa, K.; Green, J.; Griffiths, C.E.; Holgate, S.; Jones, D.; et al. Challenges and approaches for the development of safer immunomodulatory biologics. Nat. Rev. Drug Discov. 2013, 12, 306–324. [Google Scholar] [CrossRef] [PubMed]
- Odendaal, J.; Quenby, S.; Sammaritano, L.; Macklon, N.; Branch, D.W.; Rosenwaks, Z. Immunologic and rheumatologic causes and treatment of recurrent pregnancy loss: What is the evidence? Fertil. Steril. 2019, 112, 1002–1012. [Google Scholar] [CrossRef] [PubMed]

| Immune Component | Role in Normal Pregnancy | Dysregulation Associated with RPL and IVF |
|---|---|---|
| Uterine Natural Killer (uNK) Cells [29] | Promote trophoblast invasion and vascular remodeling; maintain immune tolerance at the maternal–fetal interface | Increased cytotoxicity or abnormal activation linked to implantation failure and miscarriage |
| Regulatory T Cells (Tregs) [30] | Suppress maternal immune response to fetal antigens; maintain tolerance | Reduced number/function leads to loss of immune tolerance, increased inflammation |
| Macrophages [31] | Tissue remodeling, phagocytosis of apoptotic cells, immune regulation | Altered polarization (M1/M2 Macrophages imbalance) contributes to pro-inflammatory environment |
| Dendritic Cells [32] | Antigen presentation and immune modulation | Dysregulated antigen presentation can provoke immune rejection of the fetus |
| Cytokines (e.g., IL-10, TGF-β) [33] | Anti-inflammatory cytokines support tolerance and placental development | Decreased levels shift balance toward pro-inflammatory cytokines (e.g., TNF-α, IL-17) implicated in pregnancy loss |
| Cytokines (e.g., TNF-α, IL-17) [34] | Typically regulated to prevent excessive inflammation | Elevated levels promote cytotoxicity and tissue damage leading to miscarriage |
| Therapy | Proposed Mechanism | Evidence Summary | Limitations/Challenges |
|---|---|---|---|
| Corticosteroids [68,69] | Immunosuppression | Some benefit in small studies | Side effects; inconsistent efficacy |
| IVIG [70] | Immunomodulation | Positive results in some trials | High cost; lack of large RCTs |
| Intralipid [71] | Suppression of NK cell activity | Experimental; mixed clinical results | Limited evidence; experimental status |
| Aspirin [72] | Anticoagulant, immune modulation | Useful in thrombophilia and APS | Limited to specific patient groups |
| Heparin [73] | Anticoagulant, immune modulation | Beneficial in APS and thrombophilia | Restricted to diagnosed cases; bleeding risk |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moustakli, E.; Potiris, A.; Zikopoulos, A.; Drakaki, E.; Arkoulis, I.; Skentou, C.; Tsakiridis, I.; Dagklis, T.; Drakakis, P.; Stavros, S. Immunological Factors in Recurrent Pregnancy Loss: Mechanisms, Controversies, and Emerging Therapies. Biology 2025, 14, 877. https://doi.org/10.3390/biology14070877
Moustakli E, Potiris A, Zikopoulos A, Drakaki E, Arkoulis I, Skentou C, Tsakiridis I, Dagklis T, Drakakis P, Stavros S. Immunological Factors in Recurrent Pregnancy Loss: Mechanisms, Controversies, and Emerging Therapies. Biology. 2025; 14(7):877. https://doi.org/10.3390/biology14070877
Chicago/Turabian StyleMoustakli, Efthalia, Anastasios Potiris, Athanasios Zikopoulos, Eirini Drakaki, Ioannis Arkoulis, Charikleia Skentou, Ioannis Tsakiridis, Themistoklis Dagklis, Peter Drakakis, and Sofoklis Stavros. 2025. "Immunological Factors in Recurrent Pregnancy Loss: Mechanisms, Controversies, and Emerging Therapies" Biology 14, no. 7: 877. https://doi.org/10.3390/biology14070877
APA StyleMoustakli, E., Potiris, A., Zikopoulos, A., Drakaki, E., Arkoulis, I., Skentou, C., Tsakiridis, I., Dagklis, T., Drakakis, P., & Stavros, S. (2025). Immunological Factors in Recurrent Pregnancy Loss: Mechanisms, Controversies, and Emerging Therapies. Biology, 14(7), 877. https://doi.org/10.3390/biology14070877









