Exploring the Potential of Extracellular Vesicles from Atlantic Cod (Gadus morhua L.) Serum and Mucus for Wound Healing In Vitro
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
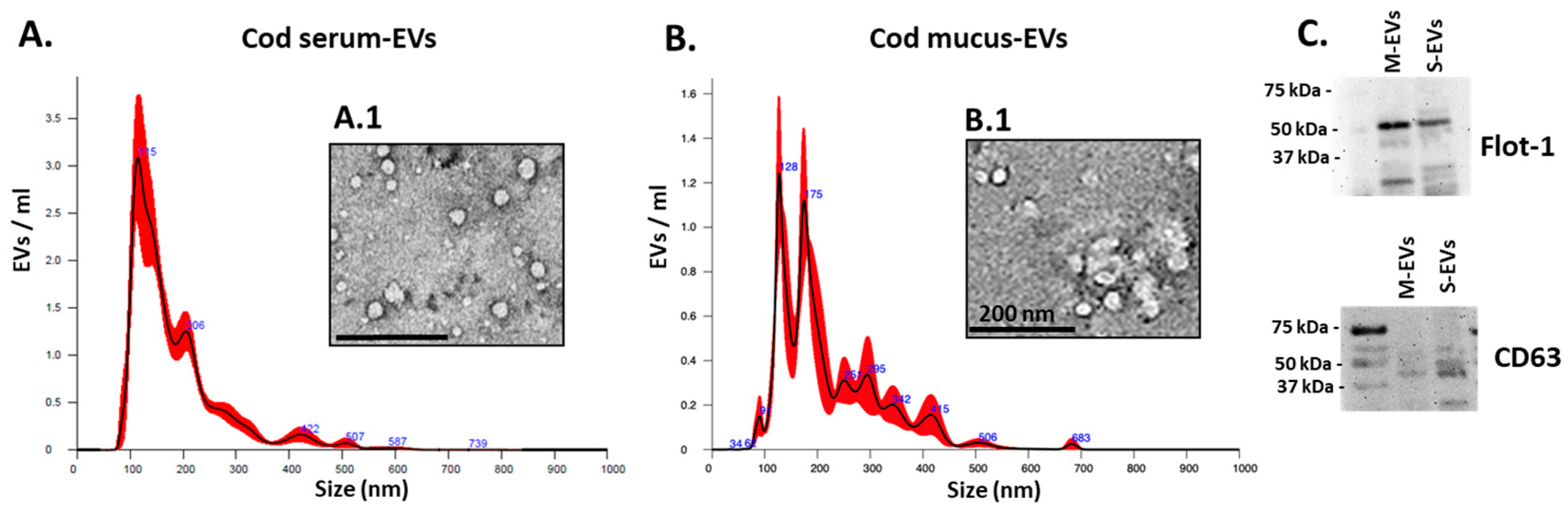
2.1. Cod Serum and Mucus Extracellular Vesicle Preparation and Characterisation
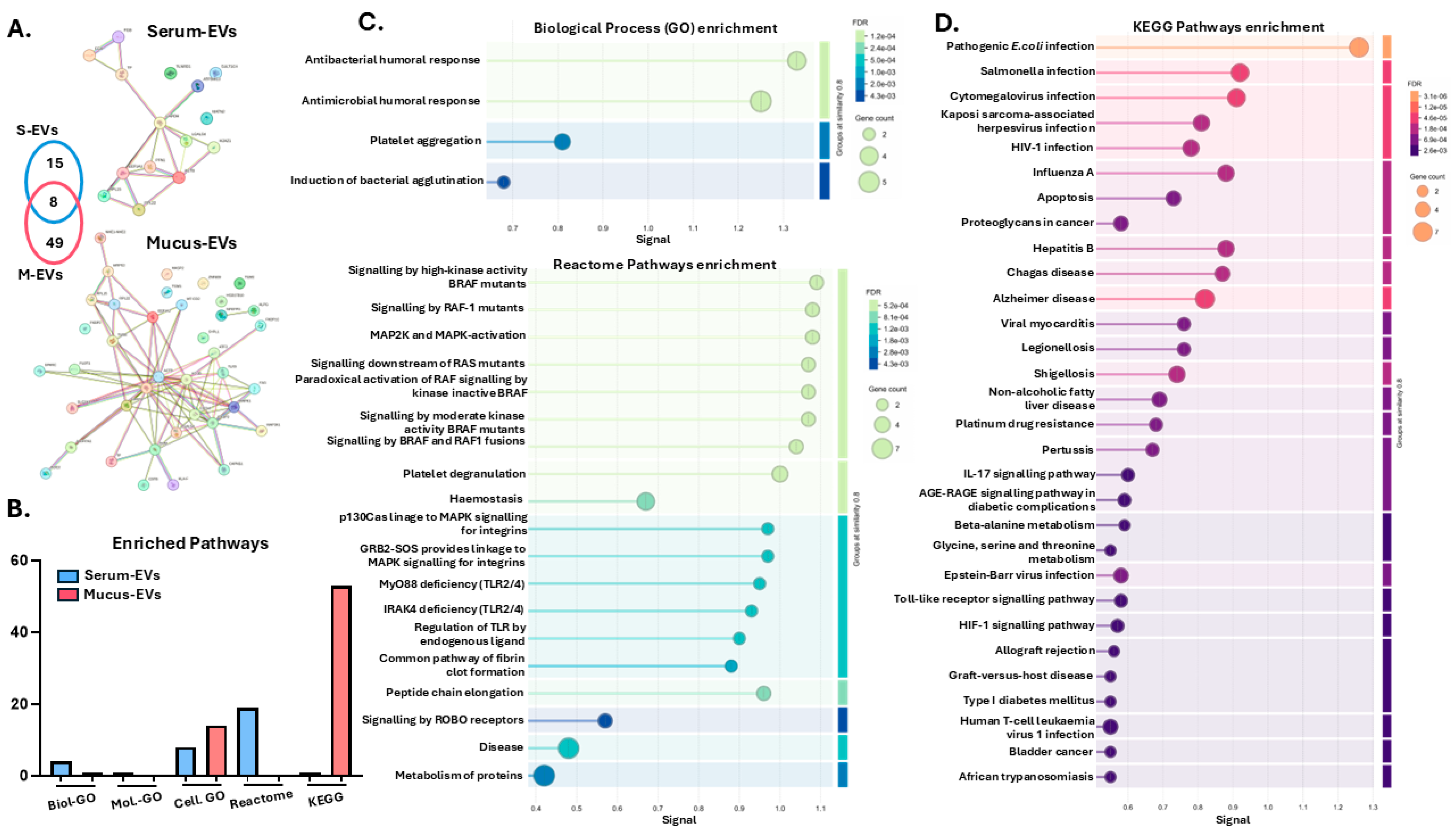
2.2. EV Protein Cargo Analysis by Liquid Chromatography with Tandem Mass Spectrometry (LC-MS/MS); Protein–Protein Interaction Network and Pathway Enrichment Analysis
2.3. In Vitro Application of Atlantic Cod Serum EVs and Mucus EVs on In Vitro Wound Healing Models
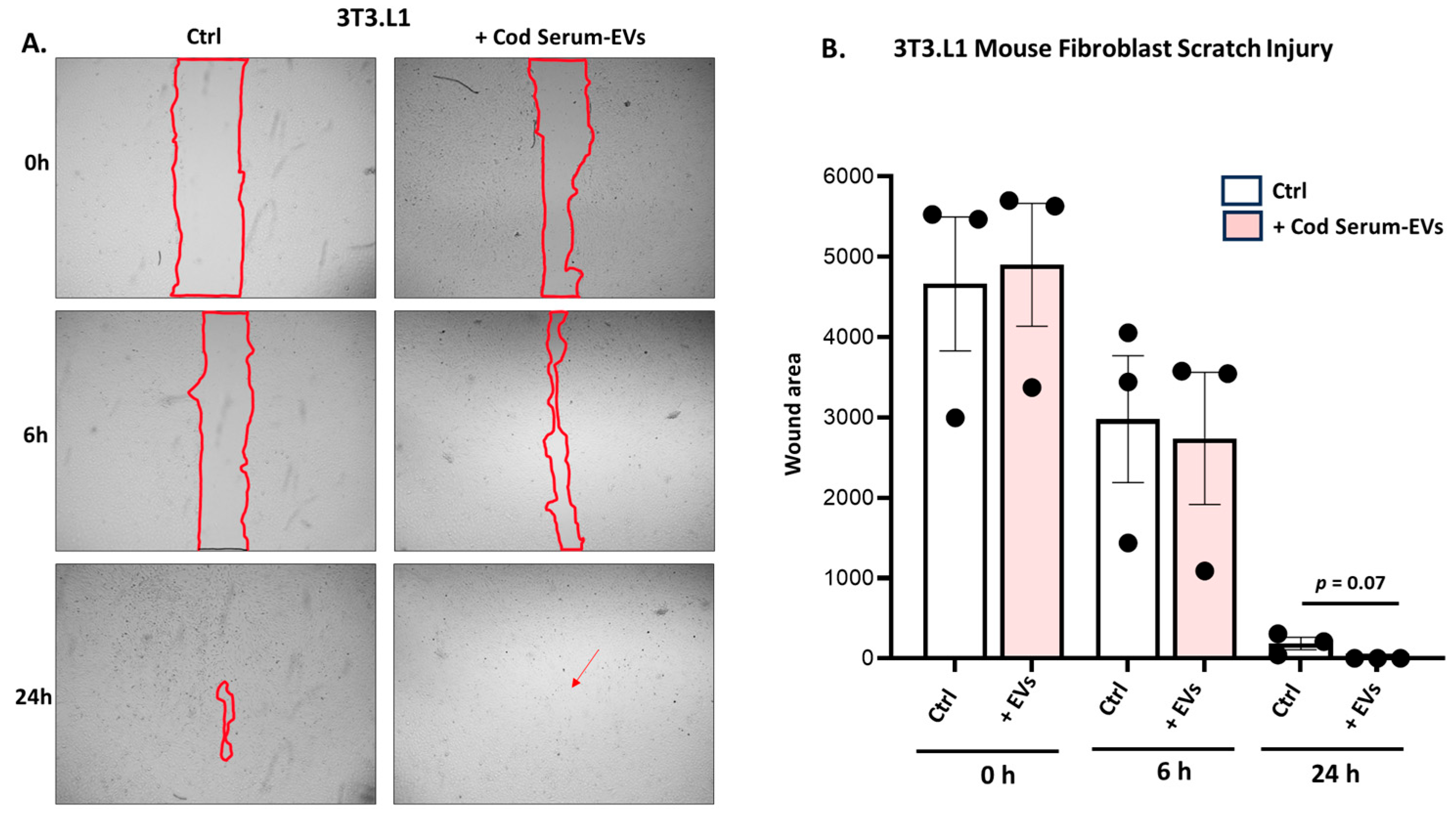
2.3.1. Mouse Fibroblast (3T3-L1) Scratch Injury Model
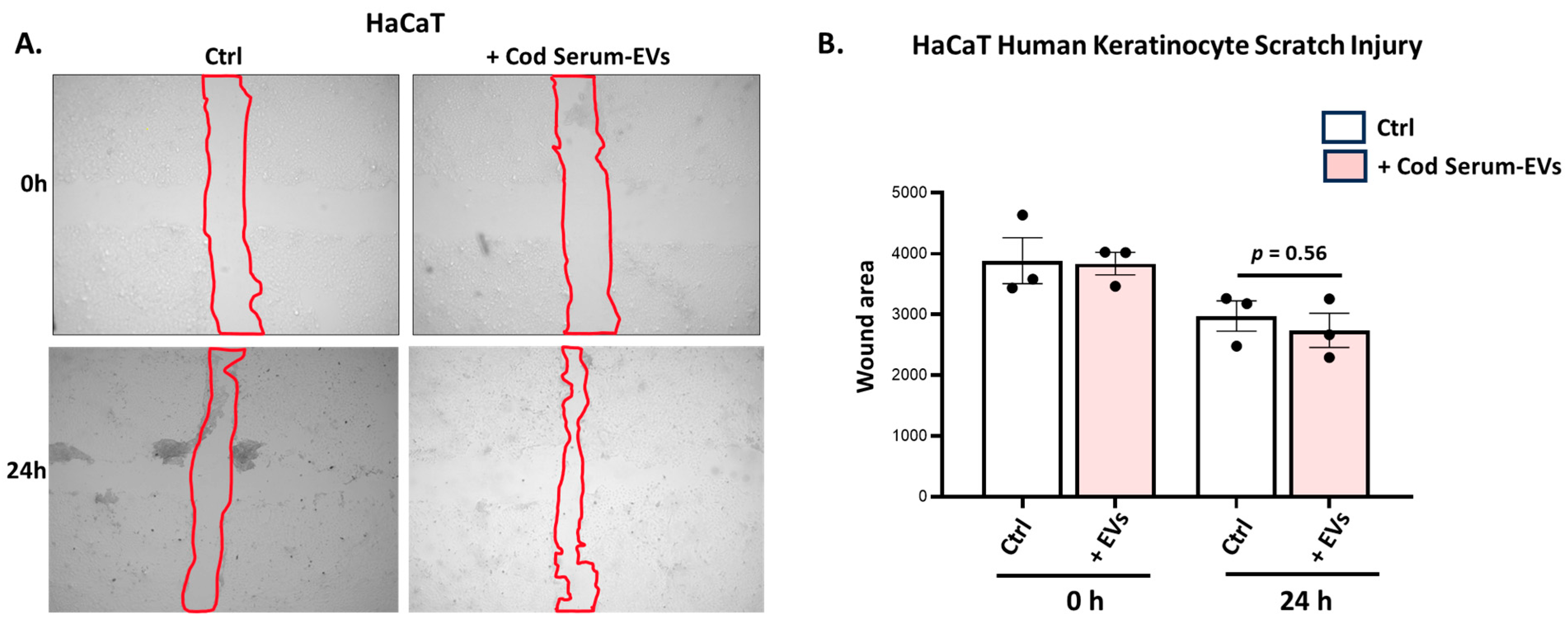
2.3.2. Human Keratinocytes (HaCaT) Scratch Injury Model
2.3.3. Human Dermal Fibroblasts (HDFa) Scratch Injury Model
2.4. Immunocytochemistry for FGF-2 and Vimentin Staining on HDFa Cells Following Cod EV Treatment
2.5. Statistical Analysis
3. Results
3.1. Cod Serum EV and Mucus EV Characterisation
3.2. LC-MS/MS Analysis of Cod Serum EVs and Mucus EV Protein Cargoes
3.3. Protein–Protein Interaction Network and Pathway Enrichment Analysis of Cod Serum EV and Mucus EV Proteomes
3.4. Application of Cod Serum EVs and Mucus EVs on Fibroblast and Keratinocyte In Vitro Wound Healing Scratch Assays
3.4.1. Application of Cod Serum EVs on Mouse Fibroblast (3T3.L1) Scratch Assay
3.4.2. Application of Cod Serum EVs on Human Immortalized Keratinocyte (HaCat) Scratch Assays
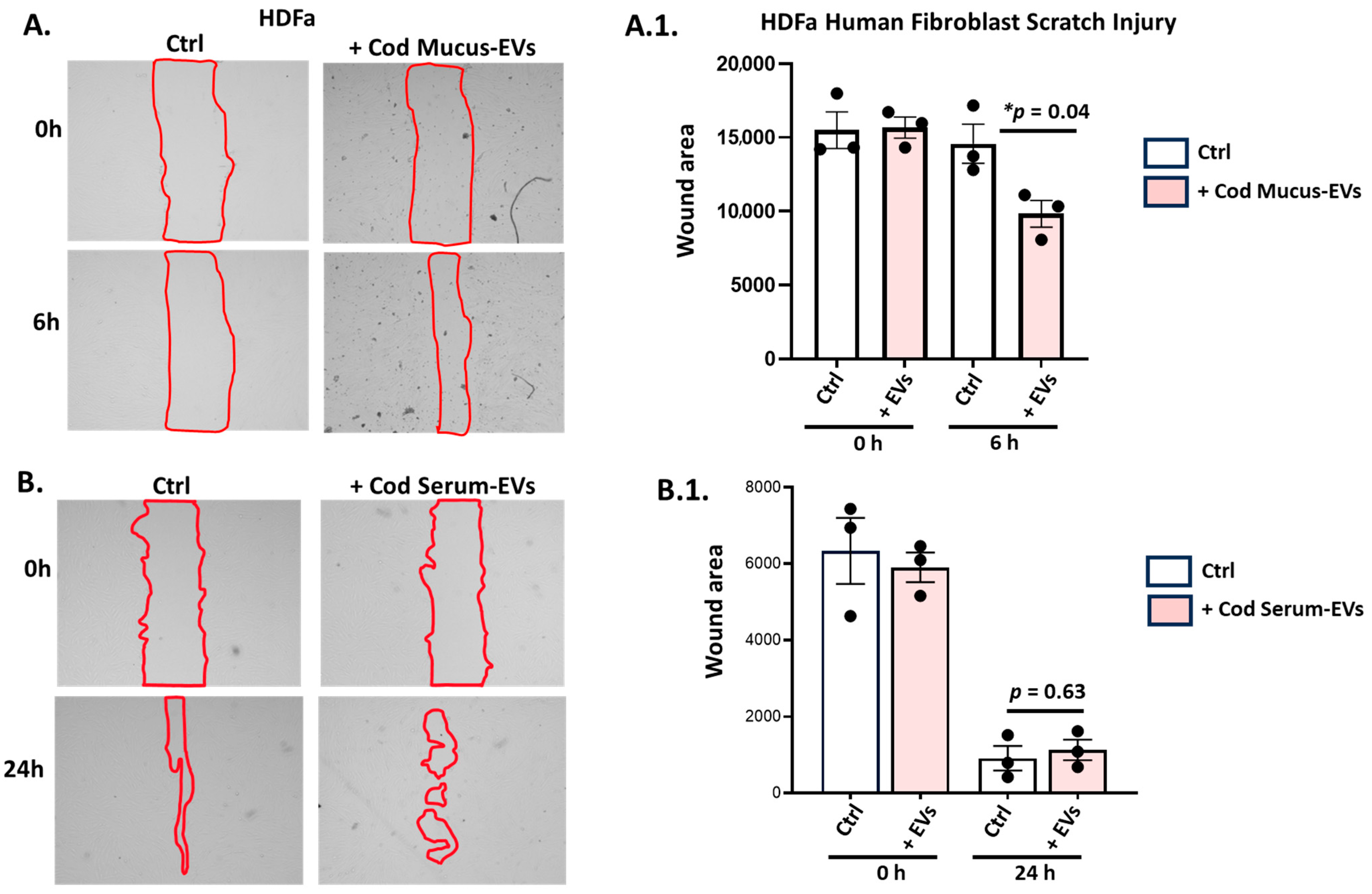
3.4.3. Application of Cod Serum EVs and Mucus EVs on the Human Dermal Fibroblast (HDFa) Scratch Assay
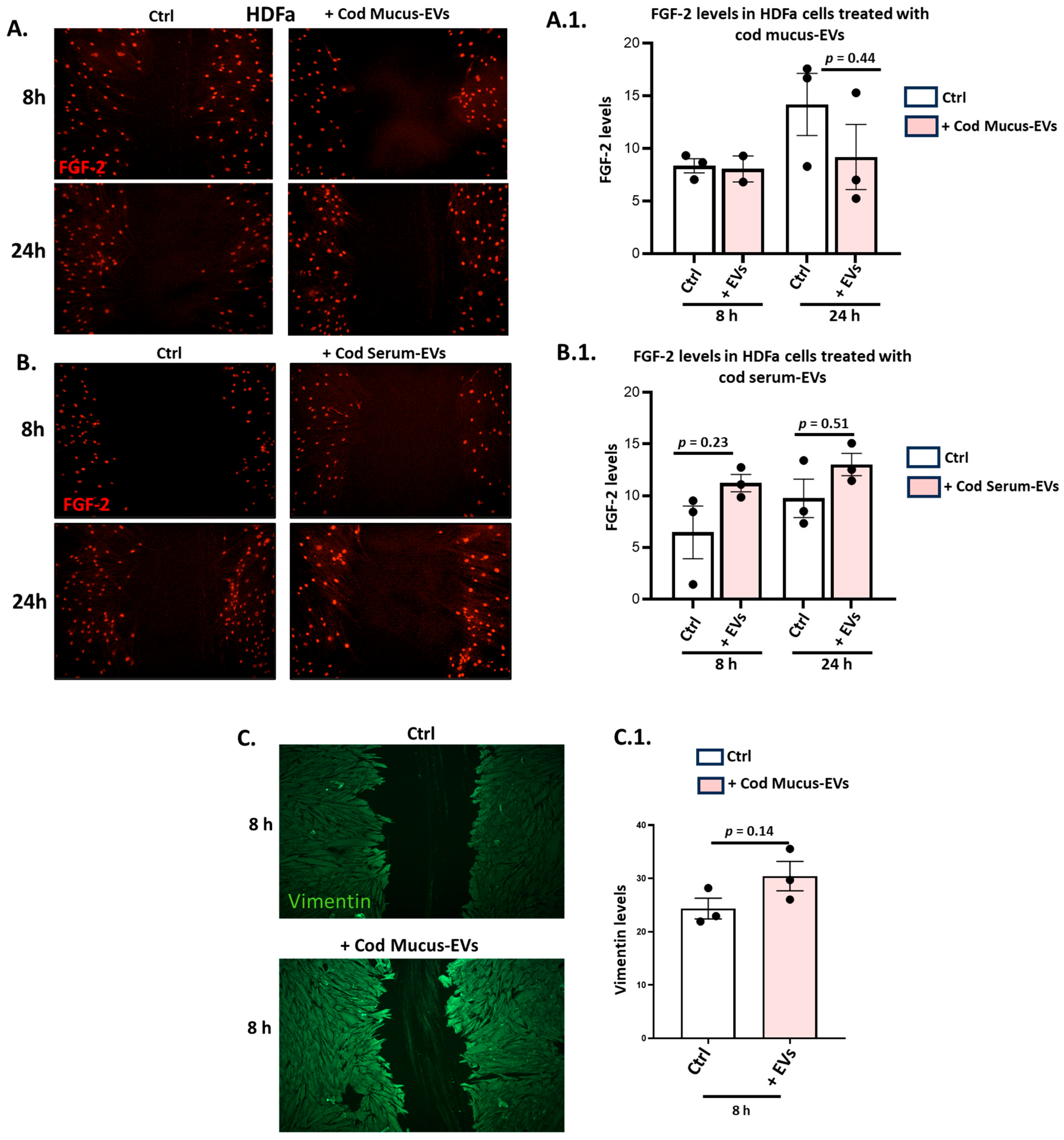
3.5. Effects of Cod Serum EVs and Mucus EVs on FGF-2 and Vimentin in the HDFa Scratch Injury Model
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Grada, A.; Mervis, J.; Falanga, V. Research Techniques Made Simple: Animal Models of Wound Healing. J. Investig. Dermatol. 2018, 138, 2095–2105.e1. [Google Scholar] [CrossRef] [PubMed]
- Alves, A.L.; Marques, A.L.P.; Martins, E.; Silva, T.H.; Reis, R.L. Cosmetic Potential of Marine Fish Skin Collagen. Cosmetics 2017, 4, 39. [Google Scholar] [CrossRef]
- Sousa, R.O.; Alves, A.L.; Carvalho, D.N.; Martins, E.; Oliveira, C.; Silva, T.H.; Reis, R.L. Acid and enzymatic extraction of collagen from Atlantic cod (Gadus morhua) swim bladders envisaging health-related applications. J. Biomater. Sci. Polym. Ed. 2020, 31, 20–37. [Google Scholar] [CrossRef] [PubMed]
- Furtado, M.; Chen, L.; Chen, Z.; Chen, A.; Cui, W. Development of fish collagen in tissue regeneration and drug delivery. Eng. Regen. 2022, 3, 217–231. [Google Scholar] [CrossRef]
- Gudmundsdóttir, A.; Hilmarsson, H.; Stefansson, B. Potential use of Atlantic cod trypsin in biomedicine. Biomed. Res. Int. 2013, 2013, 749078. [Google Scholar] [CrossRef] [PubMed]
- Khazaeli, P.; Alaei, M.; Khaksarihadad, M.; Ranjbar, M. Preparation of PLA/chitosan nanoscaffolds containing cod liver oil and experimental diabetic wound healing in male rats study. J. Nanobiotechnol. 2020, 18, 176. [Google Scholar] [CrossRef] [PubMed]
- Kirsner, R.S.; Margolis, D.J.; Baldursson, B.T.; Petursdottir, K.; Davidsson, O.B.; Weir, D.; Lantis, J.C., 2nd. Fish skin grafts compared to human amnion/chorion membrane allografts: A double-blind, prospective, randomized clinical trial of acute wound healing. Wound Repair Regen. 2020, 28, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Kotronoulas, A.; Jónasdóttir, H.S.; Sigurðardóttir, R.S.; Halldórsson, S.; Haraldsson, G.G.; Rolfsson, Ó. Wound healing grafts: Omega-3 fatty acid lipid content differentiates the lipid profiles of acellular Atlantic cod skin from traditional dermal substitutes. J. Tissue Eng. Regen. Med. 2020, 14, 441–451. [Google Scholar] [CrossRef] [PubMed]
- Kotronoulas, A.; de Lomana, A.L.G.; Karvelsson, S.T.; Heijink, M.; Stone Ii, R.; Giera, M.; Rolfsson, O. Lipid mediator profiles of burn wound healing: Acellular cod fish skin grafts promote the formation of EPA and DHA derived lipid mediators following seven days of treatment. Prostaglandins Leukot. Essent. Fatty Acids 2021, 175, 102358. [Google Scholar] [CrossRef] [PubMed]
- Lange, S.; Kraev, I.; Magnadóttir, B.; Dodds, A.W. Complement component C4-like protein in Atlantic cod (Gadus morhua L.)—Detection in ontogeny and identification of post-translational deimination in serum and extracellular vesicles. Dev. Comp. Immunol. 2019, 101, 103437. [Google Scholar] [CrossRef] [PubMed]
- Magnadóttir, B.; Kraev, I.; Guðmundsdóttir, S.; Dodds, A.W.; Lange, S. Extracellular vesicles from cod (Gadus morhua L.) mucus contain innate immune factors and deiminated protein cargo. Dev. Comp. Immunol. 2019, 99, 103397. [Google Scholar] [CrossRef] [PubMed]
- Magnadóttir, B.; Uysal-Onganer, P.; Kraev, I.; Dodds, A.W.; Guðmundsdóttir, S.; Lange, S. Extracellular vesicles, deiminated protein cargo and microRNAs are novel serum biomarkers for environmental rearing temperature in Atlantic cod (Gadus morhua L.). Aquacult. Rep. 2020, 16, 100245. [Google Scholar] [CrossRef]
- Ding, J.Y.; Chen, M.J.; Wu, L.F.; Shu, G.F.; Fang, S.J.; Li, Z.Y.; Chu, X.R.; Li, X.K.; Wang, Z.G.; Ji, J.S. Mesenchymal stem cell-derived extracellular vesicles in skin wound healing: Roles, opportunities and challenges. Mil. Med. Res. 2023, 10, 36. [Google Scholar] [CrossRef] [PubMed]
- De Jong, O.G.; Van Balkom, B.W.; Schiffelers, R.M.; Bouten, C.V.; Verhaar, M.C. Extracellular vesicles: Potential roles in regenerative medicine. Front. Immunol. 2014, 5, 608. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.; Jung, Y.; Seo, J.; Bae, Y.; Kim, H.S.; Jeong, W. Roles of extracellular vesicles from mesenchymal stem cells in regeneration. Mol. Cells. 2024, 47, 100151. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Wang, H.; Wang, X.; Yang, Y.; Zhong, K.; Zhang, X.; Li, H. MSC-EVs and UCB-EVs promote skin wound healing and spatial transcriptome analysis. Sci. Rep. 2025, 15, 4006. [Google Scholar] [CrossRef] [PubMed]
- Keshtkar, S.; Azarpira, N.; Ghahremani, M.H. Mesenchymal stem cell-derived extracellular vesicles: Novel frontiers in regenerative medicine. Stem Cell Res. Ther. 2018, 9, 63. [Google Scholar] [CrossRef] [PubMed]
- Casado-Díaz, A.; Quesada-Gómez, J.M.; Dorado, G. Extracellular Vesicles Derived From Mesenchymal Stem Cells (MSC) in Regenerative Medicine: Applications in Skin Wound Healing. Front. Bioeng. Biotechnol. 2020, 8, 146. [Google Scholar] [CrossRef] [PubMed]
- Sisa, C.; Kholia, S.; Naylor, J.; Herrera Sanchez, M.B.; Bruno, S.; Deregibus, M.C.; Camussi, G.; Inal, J.M.; Lange, S.; Hristova, M. Mesenchymal Stromal Cell Derived Extracellular Vesicles Reduce Hypoxia-Ischaemia Induced Perinatal Brain Injury. Front. Physiol. 2019, 10, 282. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Ma, L.; Lin, P.; Dai, Z.; Lu, Z.; Yan, L.; Zhou, C.; Qian, Z.J.; Hong, P.; Li, C. Extracellular vesicles derived from Pinctada martensii mucus regulate skin inflammation via the NF-κB/NLRP3/MAPK pathway. Biochem. Biophys. Res. Commun. 2022, 634, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.K.; Choi, Y.C.; Cho, S.H.; Choi, J.S.; Cho, Y.W. The Antioxidant Effect of Small Extracellular Vesicles Derived from Aloe vera Peels for Wound Healing. Tissue Eng. Regen. Med. 2021, 18, 561–571. [Google Scholar] [CrossRef] [PubMed]
- Álvarez, S.; Contreras-Kallens, P.; Aguayo, S.; Ramírez, O.; Vallejos, C.; Ruiz, J.; Carrasco-Gallardo, E.; Troncoso-Vera, S.; Morales, B.; Schuh, C.M.A.P. Royal jelly extracellular vesicles promote wound healing by modulating underlying cellular responses. Mol. Ther. Nucleic Acids 2023, 31, 541–552. [Google Scholar] [CrossRef] [PubMed]
- Yun, Y.R.; Won, J.E.; Jeon, E.; Lee, S.; Kang, W.; Jo, H.; Jang, J.H.; Shin, U.S.; Kim, H.W. Fibroblast growth factors: Biology, function, and application for tissue regeneration. J. Tissue Eng. 2010, 2010, 218142. [Google Scholar] [CrossRef] [PubMed]
- Koike, Y.; Yozaki, M.; Utani, A.; Murota, H. Fibroblast growth factor 2 accelerates the epithelial-mesenchymal transition in keratinocytes during wound healing process. Sci. Rep. 2020, 10, 18545. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.; Shen, Y.; Mohanasundaram, P.; Lindström, M.; Ivaska, J.; Ny, T.; Eriksson, J.E. Vimentin coordinates fibroblast proliferation and keratinocyte differentiation in wound healing via TGF-β-Slug signaling. Proc. Natl. Acad. Sci. USA 2016, 113, E4320-7. [Google Scholar] [CrossRef] [PubMed]
- Ostrowska-Podhorodecka, Z.; Ding, I.; Norouzi, M.; McCulloch, C.A. Impact of Vimentin on Regulation of Cell Signaling and Matrix Remodeling. Front. Cell Dev. Biol. 2022, 10, 869069. [Google Scholar] [CrossRef] [PubMed]
- Magnadóttir, B.; Kraev, I.; Dodds, A.W.; Lange, S. The Proteome and Citrullinome of Hippoglossus hippoglossus Extracellular Vesicles-Novel Insights into Roles of the Serum Secretome in Immune, Gene Regulatory and Metabolic Pathways. Int. J. Mol. Sci. 2021, 22, 875. [Google Scholar] [CrossRef] [PubMed]
- Welsh, J.A.; Goberdhan, D.C.I.; O’Driscoll, L.; Buzas, E.I.; Blenkiron, C.; Bussolati, B.; Cai, H.; Di Vizio, D.; Driedonks, T.A.P.; Erdbrügger, U. Minimal information for studies of extracellular vesicles (MISEV2023): From basic to advanced approaches. J. Extracell. Vesicles 2024, 13, e12404. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Chen, Q.; Zhang, Q.; Tian, W.; Chen, T.; Liu, Z. Therapeutic potential of adipose-derived stem cell extracellular vesicles: From inflammation regulation to tissue repair. Stem Cell Res. Ther. 2024, 15, 249. [Google Scholar] [CrossRef] [PubMed]
- Carney, R.P.; Mizenko, R.R.; Bozkurt, B.T.; Lowe, N.; Henson, T.; Arizzi, A.; Wang, A.; Tan, C.; George, S.C. Harnessing extracellular vesicle heterogeneity for diagnostic and therapeutic applications. Nat. Nanotechnol. 2025, 20, 14–25. [Google Scholar] [CrossRef] [PubMed]
- Antich-Rosselló, M.; Forteza-Genestra, M.A.; Monjo, M.; Ramis, J.M. Platelet-Derived Extracellular Vesicles for Regenerative Medicine. Int. J. Mol. Sci. 2021, 22, 8580. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhao, M.; Liu, S.; Guo, J.; Lu, Y.; Cheng, J.; Liu, J. Macrophage-derived extracellular vesicles: Diverse mediators of pathology and therapeutics in multiple diseases. Cell Death Dis. 2020, 11, 924. [Google Scholar] [CrossRef] [PubMed]
- Burnouf, T.; Chou, M.L.; Lundy, D.J.; Chuang, E.Y.; Tseng, C.L.; Goubran, H. Expanding applications of allogeneic platelets, platelet lysates, and platelet extracellular vesicles in cell therapy, regenerative medicine, and targeted drug delivery. J. Biomed. Sci. 2023, 30, 79. [Google Scholar] [CrossRef] [PubMed]
- Trigo, C.M.; Rodrigues, J.S.; Camões, S.P.; Solá, S.; Miranda, J.P. Mesenchymal stem cell secretome for regenerative medicine: Where do we stand? J. Adv. Res. 2025, 70, 103–124. [Google Scholar] [CrossRef] [PubMed]
- Lotfy, A.; AboQuella, N.M.; Wang, H. Mesenchymal stromal/stem cell (MSC)-derived exosomes in clinical trials. Stem Cell Res. Ther. 2023, 14, 66. [Google Scholar] [CrossRef] [PubMed]
- van Herwijnen, M.J.; Zonneveld, M.I.; Goerdayal, S.; Nolte-’t Hoen, E.N.; Garssen, J.; Stahl, B.; Maarten Altelaar, A.F.; Redegeld, F.A.; Wauben, M.H. Comprehensive Proteomic Analysis of Human Milk-derived Extracellular Vesicles Unveils a Novel Functional Proteome Distinct from Other Milk Components. Mol. Cell Proteom. 2016, 15, 3412–3423. [Google Scholar] [CrossRef] [PubMed]
- Tong, L.; Hao, H.; Zhang, Z.; Lv, Y.; Liang, X.; Liu, Q.; Liu, T.; Gong, P.; Zhang, L.; Cao, F.; et al. Milk-derived extracellular vesicles alleviate ulcerative colitis by regulating the gut immunity and reshaping the gut microbiota. Theranostics 2021, 11, 8570–8586. [Google Scholar] [CrossRef] [PubMed]
- Zonneveld, M.I.; van Herwijnen, M.J.C.; Fernandez-Gutierrez, M.M.; Giovanazzi, A.; de Groot, A.M.; Kleinjan, M.; van Capel, T.M.M.; Sijts, A.J.A.M.; Taams, L.S.; Garssen, J.; et al. Human milk extracellular vesicles target nodes in interconnected signalling pathways that enhance oral epithelial barrier function and dampen immune responses. J. Extracell. Vesicles 2021, 10, e12071. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Kim, D.E.; Han, G.; Lim, N.R.; Kim, E.H.; Jang, Y.; Cho, H.; Jang, H.; Kim, K.H.; Kim, S.H.; et al. Harnessing the Natural Healing Power of Colostrum: Bovine Milk-Derived Extracellular Vesicles from Colostrum Facilitating the Transition from Inflammation to Tissue Regeneration for Accelerating Cutaneous Wound Healing. Adv. Healthc. Mater. 2022, 11, e2102027. [Google Scholar] [CrossRef] [PubMed]
- Savcı, Y.; Kırbaş, O.K.; Bozkurt, B.T.; Abdik, E.A.; Taşlı, P.N.; Şahin, F.; Abdik, H. Grapefruit-derived extracellular vesicles as a promising cell-free therapeutic tool for wound healing. Food Funct. 2021, 12, 5144–5156. [Google Scholar] [CrossRef] [PubMed]
- Urzì, O.; Cafora, M.; Ganji, N.R.; Tinnirello, V.; Gasparro, R.; Raccosta, S.; Manno, M.; Corsale, A.M.; Conigliaro, A.; Pistocchi, A.; et al. Lemon-derived nanovesicles achieve antioxidant and anti-inflammatory effects activating the AhR/Nrf2 signaling pathway. iScience 2023, 26, 107041. [Google Scholar] [CrossRef] [PubMed]
- Jayathilaka, E.H.T.T.; Edirisinghe, S.L.; Oh, C.; Nikapitiya, C.; De Zoysa, M. Exosomes from bacteria (Streptococcus parauberis) challenged olive flounder (Paralichthys olivaceus): Isolation, molecular characterization, wound healing, and regeneration activities. Fish. Shellfish Immunol. 2023, 137, 108777. [Google Scholar] [CrossRef] [PubMed]
- Star, B.; Nederbragt, A.J.; Jentoft, S.; Grimholt, U.; Malmstrøm, M.; Gregers, T.F.; Rounge, T.B.; Paulsen, J.; Solbakken, M.H.; Sharma, A.; et al. The genome sequence of Atlantic cod reveals a unique immune system. Nature 2011, 477, 207–210. [Google Scholar] [CrossRef] [PubMed]
- Rajan, B.; Lokesh, J.; Kiron, V.; Brinchmann, M.F. Differentially expressed proteins in the skin mucus of Atlantic cod (Gadus morhua) upon natural infection with Vibrio anguillarum. BMC Vet. Res. 2013, 9, 103. [Google Scholar] [CrossRef] [PubMed]
- Magnadóttir, B.; Crispin, M.; Royle, L.; Colominas, C.; Harvey, D.J.; Dwek, R.A.; Rudd, P.M. The carbohydrate moiety of serum IgM from Atlantic cod (Gadus morhua L.). Fish. Shellfish Immunol. 2002, 12, 209–227. [Google Scholar] [CrossRef] [PubMed]
- Magnadóttir, B.; Lange, S. Is Apolipoprotein A-I a regulating protein for the complement system of cod (Gadus morhua L.)? Fish. Shellfish Immunol. 2004, 16, 265–269. [Google Scholar] [CrossRef] [PubMed]
- Magnadóttir, B.; Lange, S.; Steinarsson, A.; Gudmundsdóttir, S. The ontogenic development of innate immune parameters of cod (Gadus morhua L.). Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2004, 139, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Magnadottir, B.; Gudmundsdottir, S.; Gudmundsdottir, B.K.; Helgason, S. Natural antibodies of cod (Gadus morhua L.): Specificity, activity and affinity. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2009, 154, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Magnadottir, B.; Audunsdottir, S.S.; Bragason, B.T.; Gisladottir, B.; Jonsson, Z.O.; Gudmundsdottir, S. The acute phase response of Atlantic cod (Gadus morhua): Humoral and cellular response. Fish. Shellfish Immunol. 2011, 30, 1124–1130. [Google Scholar] [CrossRef] [PubMed]
- Magnadottir, B.; Lange, S.; Gudmundsdottir, S.; Bøgwald, J.; Dalmo, R.A. Ontogeny of humoral immune parameters in fish. Fish. Shellfish Immunol. 2005, 19, 429–439. [Google Scholar] [CrossRef] [PubMed]
- Magnadóttir, B.; Hayes, P.; Gísladóttir, B.; Bragason, B.Þ.; Hristova, M.; Nicholas, A.P.; Guðmundsdóttir, S.; Lange, S. Pentraxins CRP-I and CRP-II are post-translationally deiminated and differ in tissue specificity in cod (Gadus morhua L.) ontogeny. Dev. Comp. Immunol. 2018, 87, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Magnadóttir, B.; Hayes, P.; Hristova, M.; Bragason, B.T.; Nicholas, A.P.; Dodds, A.W.; Guðmundsdóttir, S.; Lange, S. Post-translational protein deimination in cod (Gadus morhua L.) ontogeny novel roles in tissue remodelling and mucosal immune defences? Dev. Comp. Immunol. 2018, 87, 157–170. [Google Scholar] [CrossRef] [PubMed]
- Lange, S.; Bambir, S.; Dodds, A.W.; Magnadóttir, B. The ontogeny of complement component C3 in Atlantic cod (Gadus morhua L.) -an immunohistochemical study. Fish. Shellfish Immunol. 2004, 16, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Lange, S.; Dodds, A.W.; Gudmundsdóttir, S.; Bambir, S.H.; Magnadóttir, B. The ontogenic transcription of complement component C3 and Apolipoprotein A-I tRNA in Atlantic cod (Gadus morhua L.)—A role in development and homeostasis? Dev. Comp. Immunol. 2005, 29, 1065–1077. [Google Scholar] [CrossRef] [PubMed]
- Audunsdottir, S.S.; Magnadottir, B.; Gisladottir, B.; Jonsson, Z.O.; Bragason, B.T. The acute phase response of cod (Gadus morhua L.): Expression of immune response genes. Fish. Shellfish Immunol. 2012, 32, 360–367. [Google Scholar] [CrossRef] [PubMed]
- Widholm, H.; Lundbäck, A.S.; Daggfeldt, A.; Magnadottir, B.; Warr, G.W.; Pilström, L. Light chain variable region diversity in Atlantic cod (Gadus morhua L.). Dev. Comp. Immunol. 1999, 23, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Bjørnestad, S.A.; Solbakken, M.H.; Krokene, P.; Thiede, B.; Hylland, K.; Jakobsen, K.S.; Jentoft, S.; Bakke, O.; Progida, C. The Atlantic Cod MHC I compartment has the properties needed for cross-presentation in the absence of MHC II. Sci. Rep. 2024, 14, 25404. [Google Scholar] [CrossRef] [PubMed]
- Györkei, Á.; Johansen, F.E.; Qiao, S.W. Systematic characterization of immunoglobulin loci and deep sequencing of the expressed repertoire in the Atlantic cod (Gadus morhua). BMC Genom. 2024, 25, 663. [Google Scholar] [CrossRef] [PubMed]
- López-Porras, A.; Jonsson, A.; Qiao, S.W.; Johansen, F.E. Sustained T cell-independent type 2 antibody response in a naturally MHC II-deficient teleost fish. Dev. Comp. Immunol. 2025, 164, 105330. [Google Scholar] [CrossRef] [PubMed]
- Guslund, N.C.; Solbakken, M.H.; Brieuc, M.S.O.; Jentoft, S.; Jakobsen, K.S.; Qiao, S.W. Single-Cell Transcriptome Profiling of Immune Cell Repertoire of the Atlantic Cod Which Naturally Lacks the Major Histocompatibility Class II System. Front. Immunol. 2020, 11, 559555. [Google Scholar] [CrossRef] [PubMed]
- Seth, N.; Chopra, D.; Lev-Tov, H. Fish Skin Grafts with Omega-3 for Treatment of Chronic Wounds: Exploring the Role of Omega-3 Fatty Acids in Wound Healing and A Review of Clinical Healing Outcomes. Surg. Technol. Int. 2022, 40, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Posner, K.M.; Bakus, C.; Sodha, S. Rapid Healing of Necrotizing Fasciitis Using the Kerecis Fish Skin Xenograft: A Clinical Case Report. Cureus 2024, 16, e73060. [Google Scholar] [CrossRef] [PubMed]
- Steinberger, E.E.; Vogt, A.Z.; Tan, J.F. Skin and Bone: Intact Fish Skin to Reconstruct Traumatic Orbital Floor and Wall Defects. Ophthalmic Plast. Reconstr. Surg. 2024, 40, e78–e80. [Google Scholar] [CrossRef] [PubMed]
- Cerceo, J.R.; Malkoc, A.; Nguyen, A.; Daoud, A.; Wong, D.T.; Woodward, B. Management of Large Full-Thickness Burns Using Kerecis™ Acellular Fish Skin Graft and ReCell™ Autologous Skin Cell Suspension: A Case Report of Two Patients with Large Surface Area Burns. Cureus 2024, 16, e71101. [Google Scholar] [CrossRef] [PubMed]
- Rajan, B.; Kiron, V.; Fernandes, J.M.; Brinchmann, M.F. Localization and functional properties of two galectin-1 proteins in Atlantic cod (Gadus morhua) mucosal tissues. Dev. Comp. Immunol. 2013, 40, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Stafford, J.L.; Belosevic, M. Transferrin and the innate immune response of fish: Identification of a novel mechanism of macrophage activation. Dev. Comp. Immunol. 2003, 27, 539–554. [Google Scholar] [CrossRef] [PubMed]
- Mohd-Padil, H.; Mohd-Adnan, A.; Gabaldón, T. Phylogenetic analyses uncover a novel clade of transferrin in nonmammalian vertebrates. Mol. Biol. Evol. 2013, 30, 894–905. [Google Scholar] [CrossRef] [PubMed]
- Vasta, G.R.; Ahmed, H.; Du, S.; Henrikson, D. Galectins in teleost fish: Zebrafish (Danio rerio) as a model species to address their biological roles in development and innate immunity. Glycoconj. J. 2004, 21, 503–521. [Google Scholar] [CrossRef] [PubMed]
- Sciacchitano, S.; Lavra, L.; Morgante, A.; Ulivieri, A.; Magi, F.; De Francesco, G.P.; Bellotti, C.; Salehi, L.B.; Ricci, A. Galectin-3: One Molecule for an Alphabet of Diseases, from A to Z. Int. J. Mol. Sci. 2018, 19, 379. [Google Scholar] [CrossRef] [PubMed]
- McLeod, K.; Walker, J.T.; Hamilton, D.W. Galectin-3 regulation of wound healing and fibrotic processes: Insights for chronic skin wound therapeutics. J. Cell Commun. Signal. 2018, 12, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Talapatra, S.; Wagner, J.D.; Thompson, C.B. Elongation factor-1 alpha is a selective regulator of growth factor withdrawal and ER stress-induced apoptosis. Cell Death Differ. 2002, 9, 856–861. [Google Scholar] [CrossRef] [PubMed]
- Khacho, M.; Mekhail, K.; Pilon-Larose, K.; Pause, A.; Côté, J.; Lee, S. eEF1A is a novel component of the mammalian nuclear protein export machinery. Mol. Biol. Cell. 2008, 19, 5296–5308. [Google Scholar] [CrossRef] [PubMed]
- Davey, R.J.; Moens, P.D. Profilin: Many facets of a small protein. Biophys. Rev. 2020, 12, 827–849. [Google Scholar] [CrossRef] [PubMed]
- Pandey, S.; Anshu, T.; Maharana, K.C.; Sinha, S. Molecular insights into diabetic wound healing: Focus on Wnt/β-catenin and MAPK/ERK signaling pathways. Cytokine 2025, 191, 156957. [Google Scholar] [CrossRef] [PubMed]
- D’Arpa, P.; Leung, K.P. Toll-Like Receptor Signaling in Burn Wound Healing and Scarring. Adv. Wound Care 2017, 6, 330–343. [Google Scholar] [CrossRef] [PubMed]
- Putra, A.; Ibrahim, S.; Muhar, A.M.; Kuntardjo, N.; Dirja, B.T.; Pasongka, Z.; Tunru, I.S. Topical gel of mesenchymal stem cells-conditioned medium under TNF-α precondition accelerates wound closure healing in full-thickness skin defect animal model. J. Med. Life 2022, 15, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Gawronska-Kozak, B.; Machcinska-Zielinska, S.; Walendzik, K.; Kopcewicz, M.; Pääkkönen, M.; Wisniewska, J. Hypoxia and Foxn1 alter the proteomic signature of dermal fibroblasts to redirect scarless wound healing to scar-forming skin wound healing in Foxn1−/− mice. BMC Biol. 2024, 22, 193. [Google Scholar] [CrossRef] [PubMed]
- Mu, X.; Gu, R.; Tang, M.; Wu, X.; He, W.; Nie, X. IL-17 in wound repair: Bridging acute and chronic responses. Cell Commun. Signal. 2024, 22, 288. [Google Scholar] [CrossRef] [PubMed]
- Escuin-Ordinas, H.; Liu, Y.; Sun, L.; Hugo, W.; Dimatteo, R.; Huang, R.R.; Krystofinski, P.; Azhdam, A.; Lee, J.; Comin-Anduix, B.; et al. Wound healing with topical BRAF inhibitor therapy in a diabetic model suggests tissue regenerative effects. PLoS ONE 2021, 16, e0252597. [Google Scholar] [CrossRef] [PubMed]
- Basharat, S.; Malik, S.; Samad, H.U.A.; Murtaza, M.A. Diving deep into healing: The promising role of fish skin in wound recovery. Wounds 2024, 36, 274–280. [Google Scholar] [CrossRef] [PubMed]
- Iida, C.; Ohsawa, S.; Taniguchi, K.; Yamamoto, M.; Morata, G.; Igaki, T. JNK-mediated Slit-Robo signaling facilitates epithelial wound repair by extruding dying cells. Sci. Rep. 2019, 9, 19549. [Google Scholar] [CrossRef] [PubMed]
- Tong, M.; Jun, T.; Nie, Y.; Hao, J.; Fan, D. The Role of the Slit/Robo Signaling Pathway. J. Cancer 2019, 10, 2694–2705. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.T.; Chen, Y.C.; Tsai, F.Y.; Judy, C.P.; Adler, C.E. Pluripotent Stem Cell Plasticity is Sculpted by a Slit-Independent Robo Pathway in a Regenerative Animal. bioRxiv 2025. [Google Scholar] [CrossRef] [PubMed]
- Grazul-Bilska, A.T.; Johnson, M.L.; Bilski, J.J.; Redmer, D.A.; Reynolds, L.P.; Abdullah, A.; Abdullah, K.M. Wound healing: The role of growth factors. Drugs Today 2003, 39, 787–800. [Google Scholar] [CrossRef] [PubMed]
- Coelho-Rato, L.S.; Parvanian, S.; Modi, M.K.; Eriksson, J.E. Vimentin at the core of wound healing. Trends Cell Biol. 2024, 34, 239–254. [Google Scholar] [CrossRef] [PubMed]
- Gansevoort, M.; Wentholt, S.; Li Vecchi, G.; de Vries, M.; Versteeg, E.M.M.; Boekema, B.K.H.L.; Choppin, A.; Barritault, D.; Chiappini, F.; van Kuppevelt, T.H.; et al. Next-Generation Biomaterials for Wound Healing: Development and Evaluation of Collagen Scaffolds Functionalized with a Heparan Sulfate Mimic and Fibroblast Growth Factor 2. J. Funct. Biomater. 2025, 16, 51. [Google Scholar] [CrossRef] [PubMed]
- Paganini, C.; Capasso Palmiero, U.; Pocsfalvi, G.; Touzet, N.; Bongiovanni, A.; Arosio, P. Scalable Production and Isolation of Extracellular Vesicles: Available Sources and Lessons from Current Industrial Bioprocesses. Biotechnol. J. 2019, 14, e1800528. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
D’Alessio, S.; Kraev, I.; Magnadóttir, B.; Lange, S. Exploring the Potential of Extracellular Vesicles from Atlantic Cod (Gadus morhua L.) Serum and Mucus for Wound Healing In Vitro. Biology 2025, 14, 870. https://doi.org/10.3390/biology14070870
D’Alessio S, Kraev I, Magnadóttir B, Lange S. Exploring the Potential of Extracellular Vesicles from Atlantic Cod (Gadus morhua L.) Serum and Mucus for Wound Healing In Vitro. Biology. 2025; 14(7):870. https://doi.org/10.3390/biology14070870
Chicago/Turabian StyleD’Alessio, Stefania, Igor Kraev, Bergljót Magnadóttir, and Sigrun Lange. 2025. "Exploring the Potential of Extracellular Vesicles from Atlantic Cod (Gadus morhua L.) Serum and Mucus for Wound Healing In Vitro" Biology 14, no. 7: 870. https://doi.org/10.3390/biology14070870
APA StyleD’Alessio, S., Kraev, I., Magnadóttir, B., & Lange, S. (2025). Exploring the Potential of Extracellular Vesicles from Atlantic Cod (Gadus morhua L.) Serum and Mucus for Wound Healing In Vitro. Biology, 14(7), 870. https://doi.org/10.3390/biology14070870







