Glucomannan Accumulation Induced by Exogenous Lanthanum in Amorphophallus konjac: Insights from a Comparative Transcriptome Analysis
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. La (Ш) Treatment
2.3. Determination of Konjac Glucomannan (KGM) Content
2.4. RNA Extraction, cDNA Library Construction, and Transcriptome Sequencing
2.5. Assembly, Data Analysis, and Functional Annotation
2.6. Analysis of Differentially Expressed Genes
2.7. Protein–Protein Interaction (PPI) Network Analysis of KGM Biosynthetic Enzymes and TFs
2.8. Quantitative Real-Time PCR (qRT-PCR) Verification
2.9. Statistical Analysis
3. Results
3.1. KGM Content in Plants Exposed to Different La (Ш) Concentrations
3.2. Assembly and Quality Assessment of Transcriptome Sequencing
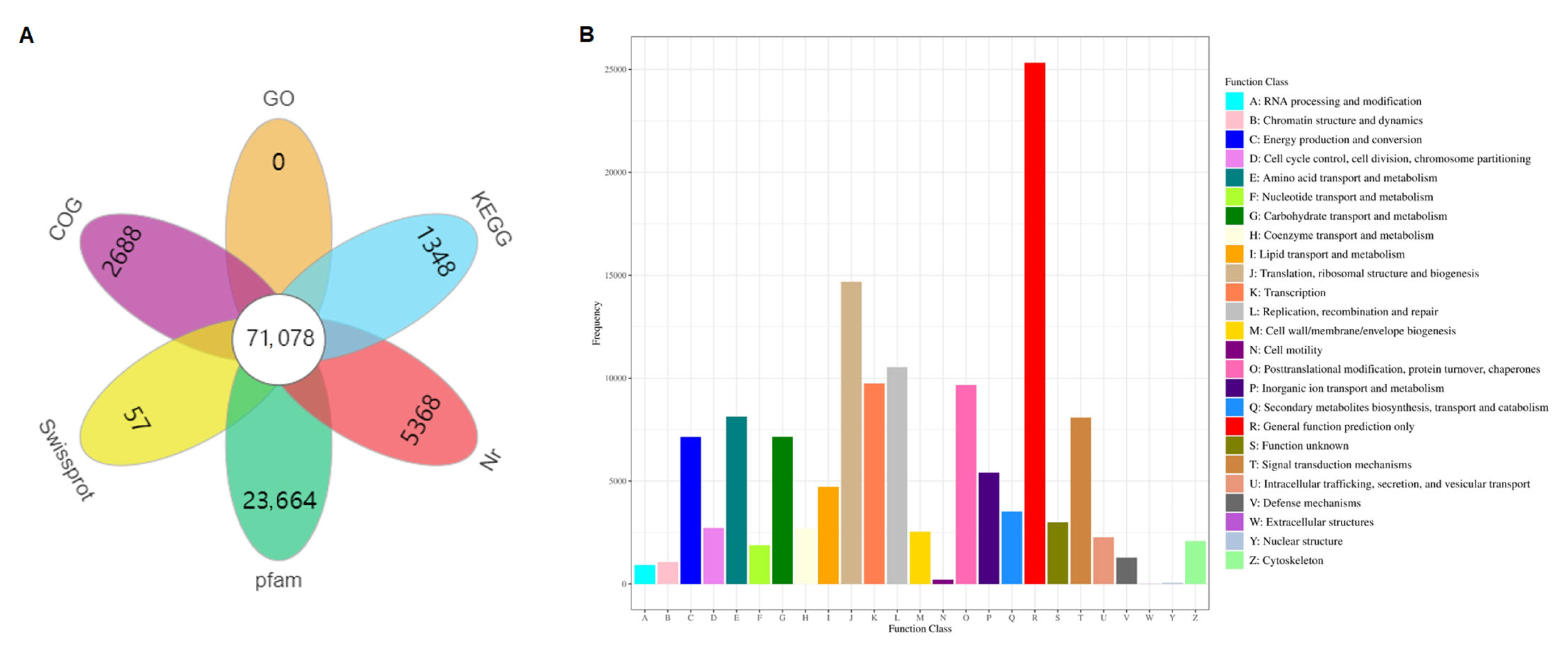
3.3. Functional Annotation and Classification of Unigenes
3.4. Analysis of Differentially Expressed Genes (DEGs)
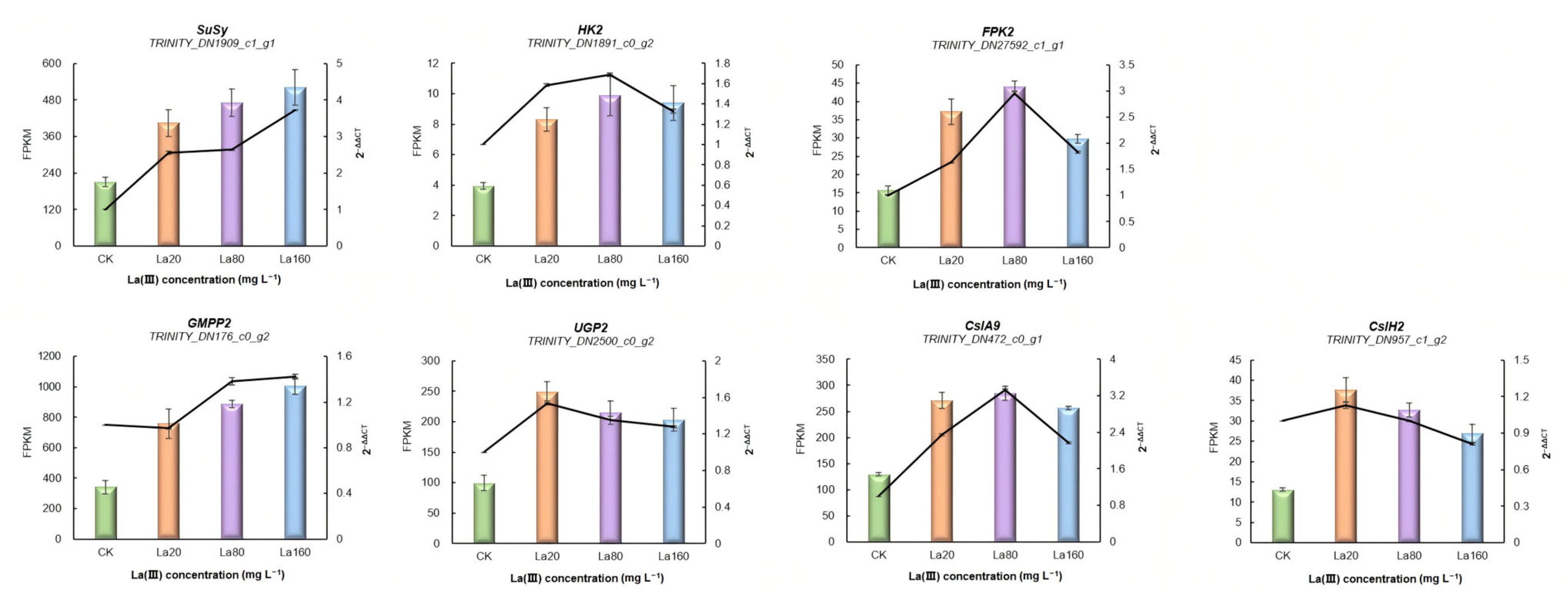
3.5. Identification of KGM Biosynthetic Genes in A. konjac
3.6. Validation of RNA-Seq Results Using qRT-PCR
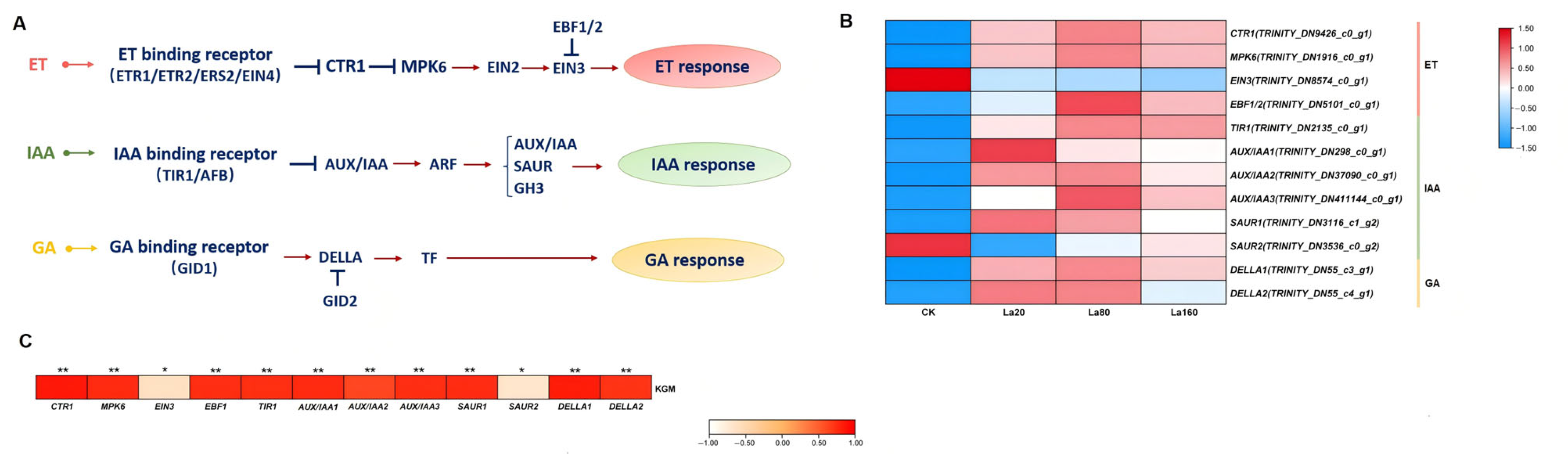
3.7. Plant Hormone Signal Responses to La (Ш)
3.8. TF Responses to La (Ш) Treatment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| La (III) | lanthanum |
| DEG | differentially expressed gene |
| TF | transcription factor |
| KGM | konjac glucomannan |
| A. konjac | Amorphophallus konjac |
| PPI | protein–protein interaction |
| Suc | sucrose |
| Fru | fructose |
| Glc | glucose |
| UDP-Glc | UDP-glucose |
| GDP-man | GDP-mannose |
| Fru-6-P | fructose-6-phosphate |
| Fru-1-P | fructose-1-phosphate |
| Man-6-P | mannose-6-phosphate |
| Man-1-P | mannose-1-phosphate |
| Glc-1-P | glucose-1-phosphate |
| Glc-6-P | glucose-6-phosphate |
| SuSy | sucrose synthase |
| INV | invertase |
| PGI | phosphoglucose isomerase |
| PGM | phosphoglucomutase |
| PMI | phosphomannose isomerase |
| PMM | phosphomannomutase |
| GMPP | GDP-mannose pyrophosphorylase |
| UGP | UDP-glucose pyrophosphorylase |
| AGP | ADP-glucose pyrophosphorylase |
| FRK | fructokinase |
| HK | hexokinase |
| Csls | cellulose synthase-like |
| MSR1 | mannan synthesis-related 1 |
References
- Devaraj, R.D.; Reddy, C.K.; Xu, B. Health-promoting effects of konjac glucomannan and its practical applications: A critical review. Int. J. Biol. Macromol. 2019, 126, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Xue, Z.; Huang, F.; Liu, J.; Ke, Y.; Wei, H.; Gao, P.; Qi, Y.; Yu, L. A high trans-zeatin nucleoside concentration in corms may promote the multileaf growth of Amorphophallus muelleri. Front. Plant Sci. 2022, 13, 964003. [Google Scholar] [CrossRef] [PubMed]
- Liu, P. Konjac; China Agriculture Press: Beijing, China, 2004; pp. 84–91. [Google Scholar]
- Pouchon, C.; Gauthier, J.; Pitteloud, C.; Claudel, C.; Alvarez, N. Phylogenomic study of Amorphophallus (Alismatales; Araceae): When plastid DNA gene sequences help to resolve the backbone subgeneric delineation. J. Syst. Evol. 2023, 61, 64–79. [Google Scholar] [CrossRef]
- Shan, Y.; Li, J.; Zhang, X.; Yu, J. The complete mitochondrial genome of Amorphophallus albus and development of molecular markers for five Amorphophallus species based on mitochondrial DNA. Front. Plant Sci. 2023, 14, 1180417. [Google Scholar] [CrossRef]
- Basak, S.; Singhal, R.S. Composite hydrogels fabricated from konjac glucomannan and gellan gum: Rheological characterization and their potential application in sustainable agriculture. Carbohydr. Polym. 2024, 336, 122091. [Google Scholar] [CrossRef]
- Hong, J.; Shi, Y.; Chen, J.; Mi, M.; Ren, Q.; Zhang, Y.; Shen, M.; Bu, J.; Kang, Y. Konjac glucomannan attenuate high-fat diet-fed obesity through enhancing β-adrenergic-mediated thermogenesis in inguinal white adipose tissue in mice. Glycoconj. J. 2023, 40, 575–586. [Google Scholar] [CrossRef]
- Bu, N.; Zhou, N.; Cao, G.; Mu, R.; Pang, J.; Ma, C.; Wang, L. Konjac glucomannan/carboxymethyl chitosan film embedding gliadin/casein nanoparticles for grape preservation. Int. J. Biol. Macromol. 2023, 249, 126131. [Google Scholar] [CrossRef]
- Zhang, Y.; Aldamarany, W.A.S.; Deng, L.; Zhong, G. Carbohydrate supplementation retains intestinal barrier and ameliorates bacterial translocation in an antibiotic-induced mouse model. Food Funct. 2023, 14, 8186–8200. [Google Scholar] [CrossRef]
- Gao, Z.; Liu, F.; Lan, H.; Jian, T.; Cao, L.; Deng, M.; Wang, L.; Lan, M.; Li, J. Response and mechanisms of Amorphophallus konjac agronomic traits and disease occurrence after biochar application. Sci. Hortic. 2024, 338, 113657. [Google Scholar] [CrossRef]
- Trejo-Téllez, L.I.; Gómez-Merino, F.C. Editorial: Beneficial elements: Novel players in plant biology for innovative crop production, volume II. Front. Plant Sci. 2023, 14, 1303462. [Google Scholar] [CrossRef]
- Li, X.X.; Yu, B.; Dong, Y.Y.; Wang, L.S.; Zhang, S.L.; Shangguan, H.Y.; He, Z.H.; Luo, X.M.; Lai, P.F. Lanthanum chloride enhances the photosynthetic characteristics and increases konjac glucomannan contents in Amorphophallus sinensis Belval. Photosynthetica 2020, 58, 165–173. [Google Scholar] [CrossRef]
- Ben, Y.; Cheng, M.; Liu, Y.; Wang, L.; Yang, Q.; Huang, X.; Zhou, Q. The stimulatory effect and mechanism of low-dose lanthanum on soybean leaf cells. J. Hazard. Mater. 2023, 441, 129924. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Chen, J. Ameliorative effects of lanthanum (Ⅲ) on copper (II) stressed rice (Oryza sativa) and its molecular mechanism revealed by transcriptome profiling. Plant Physiol. Biochem. 2020, 152, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Tong, K.; Yan, L.; Riaz, M.; Gao, G.; Yu, H.; Lu, M.; Niu, Y. Mechanisms of lanthanum-mediated mitigation of salt stress in soybean (Glycine max L.). Physiol. Plant. 2024, 176, e14452. [Google Scholar] [CrossRef]
- Yan, S.; Huang, X.; Zhou, Q. Effect of lanthanum (Ⅲ) on reactive oxygen metabolism of soybean seedlings under supplemental UV-B irradiation. J. Rare Earths 2007, 25, 352–358. [Google Scholar]
- Syrvatka, V.; Rabets, A.; Gromyko, O.; Luzhetskyy, A.; Fedorenko, V. Scandium–microorganism interactions in new biotechnologies. Trends Biotechnol. 2022, 40, 1088–1101. [Google Scholar] [CrossRef]
- He, C.; Feng, Y.; Deng, Y.; Lin, L.; Cheng, S. A systematic review and meta-analysis on the root effects and toxic mechanisms of rare earth elements. Chemosphere 2024, 363, 142951. [Google Scholar] [CrossRef]
- Wang, L.; Li, J.; Zhou, Q.; Yang, G.; Ding, X.L.; Li, X.; Cai, C.X.; Zhang, Z.; Wei, H.Y.; Lu, T.H.; et al. Rare earth elements activate endocytosis in plant cells. Proc. Natl. Acad. Sci. USA 2014, 111, 12936–12941. [Google Scholar] [CrossRef]
- Wang, L.; Wang, W.; Zhou, Q.; Huang, X. Combined effects of lanthanum (Ⅲ) chloride and acid rain on photosynthetic parameters in rice. Chemosphere 2014, 112, 355–361. [Google Scholar] [CrossRef]
- Li, Q. Effects of Rare Earth Lanthanum and Cerium Immersion on the Growth and Rhizosphere Microbial Community of Chinese Cabbage. Master’s Thesis, Henan Agricultural University, Zhengzhou, China, 2024. [Google Scholar]
- Chua, M.; Chan, K.; Hocking, T.J.; Williams, P.A.; Perry, C.J.; Baldwin, T.C. Methodologies for the extraction and analysis of konjac glucomannan from corms of Amorphophallus konjac K. Koch. Carbohydr. Polym. 2012, 87, 2202–2210. [Google Scholar] [CrossRef]
- Fang, X.; Wang, H.; Zhou, X.; Zhang, J.; Xiao, H. Transcriptome reveals insights into biosynthesis of ginseng polysaccharides. BMC Plant Biol. 2022, 22, 594. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Niu, Y.; Wang, Q.; Liu, H.; Jin, Y.; Zhang, S. Cloning and evaluation of reference genes for quantitative real-time PCR analysis in Amorphophallus. PeerJ 2017, 5, e3260. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Zhang, Y.; Feng, C.; Chu, H.; Feng, C.; Wang, H.; Wu, L.; Yin, S.; Liu, C.; Chen, H.; et al. A chromosome level genome assembly of Amorphophallus konjac provides insights into konjac glucomannan biosynthesis. Comput. Struct. Biotechnol. J. 2022, 20, 1002–1011. [Google Scholar] [CrossRef] [PubMed]
- Little, A.; Schwerdt, J.G.; Shirley, N.J.; Khor, S.F.; Neumann, K.; O’Donovan, L.A.; Lahnstein, J.; Collins, H.M.; Henderson, M.; Fincher, G.B.; et al. Revised phylogeny of the cellulose synthase gene superfamily: Insights into cell wall evolution. Plant Physiol. 2018, 177, 1124–1141. [Google Scholar] [CrossRef]
- Miao, H.; Sun, P.; Liu, Q.; Liu, J.; Xu, B.; Jin, Z. The AGPase family proteins in banana: Genome-wide identification, phylogeny, and expression analyses reveal their involvement in the development, ripening, and abiotic/biotic stress responses. Int. J. Mol. Sci. 2017, 18, 1581. [Google Scholar] [CrossRef]
- Chua, M.; Hocking, T.J.; Chan, K.; Baldwin, T.C. Temporal and spatial regulation of glucomannan deposition and mobilization in corms of Amorphophallus konjac (Araceae). Am. J. Bot. 2013, 100, 337–345. [Google Scholar] [CrossRef]
- Wang, Y.L.; Liu, P.Y. Study on the material trends and formation of yield during the growth period of elephant-foot yam (Amorphophallus rivieri Durieu). J. Southwest Agric. Univ. 1990, 12, 471–474. [Google Scholar]
- Li, L.; Yang, M.; Wei, W.; Zhao, J.; Yu, X.; Impaprasert, R.; Wang, J.; Liu, J.; Huang, F.; Srzednicki, G.; et al. Characteristics of Amorphophallus konjac as indicated by its genome. Sci. Rep. 2023, 13, 22684. [Google Scholar] [CrossRef]
- Ye, Z.H.; Zhong, R.; Degola, F. Cell wall biology of the moss Physcomitrium patens. J. Exp. Bot. 2022, 73, 4440–4453. [Google Scholar] [CrossRef]
- Huang, J.; Ma, S.; Zhou, M.; Liu, Z.; Liang, Q. Cytochemical localization and synthesis mechanism of the glucomannan in pseudobulbs of Bletilla striata Reichb. f. Hortic. Res. 2024, 11, uhae092. [Google Scholar] [CrossRef]
- Verhertbruggen, Y.; Yin, L.; Oikawa, A.; Scheller, H.V. Mannan synthase activity in the CSLD family. Plant Signal. Behav. 2014, 6, 1620–1623. [Google Scholar] [CrossRef] [PubMed]
- Pancaldi, F.; van Loo, E.N.; Schranz, M.E.; Trindade, L.M. Genomic architecture and evolution of the cellulose synthase gene superfamily as revealed by phylogenomic analysis. Front. Plant Sci. 2022, 13, 870818. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Chen, T.; Gao, T.; Miao, Z.; Jiang, A.; Shi, L.; Ren, A.; Zhao, M. UDP-glucose pyrophosphorylase influences polysaccharide synthesis, cell wall components, and hyphal branching in Ganoderma lucidum via regulation of the balance between glucose-1-phosphate and UDP-glucose. Fungal Genet. Biol. 2015, 82, 251–263. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wang, L.; Liang, H.; Jin, X.; Wan, J.; Liu, F.; Zhao, K.; Huang, J.; Tian, M. Overexpression of DoUGP enhanced biomass and stress tolerance by promoting polysaccharide accumulation in Dendrobium officinale. Front. Plant Sci. 2020, 11, 533767. [Google Scholar] [CrossRef]
- Wang, Y.; Mortimer, J.C.; Davis, J.; Dupree, P.; Keegstra, K. Identification of an additional protein involved in mannan biosynthesis. Plant J. 2012, 73, 105–117. [Google Scholar] [CrossRef]
- Voiniciuc, C.; Dama, M.; Gawenda, N.; Stritt, F.; Pauly, M. Mechanistic insights from plant heteromannan synthesis in yeast. Proc. Natl. Acad. Sci. USA. 2018, 116, 522–527. [Google Scholar] [CrossRef]
- Burton, R.A.; Johnson, P.E.; Beckles, D.M.; Fincher, G.B.; Jenner, H.L.; Naldrett, M.J.; Denyer, K. Characterization of the genes encoding the cytosolic and plastidial forms of ADP-glucose pyrophosphorylase in wheat endosperm. Plant Physiol. 2002, 130, 1464–1475. [Google Scholar] [CrossRef]
- Shi, H.D.; Zhang, W.Q.; Lu, H.Y.; Zhang, W.Q.; Ye, H.; Liu, D.D. Functional characterization of a starch synthesis-related gene AmAGP in Amorphophallus muelleri. Plant Signal. Behav. 2020, 15, 1805903. [Google Scholar] [CrossRef]
- Zhao, Y.; Yang, M.; Qi, Y.; Gao, P.; Ke, Y.; Liu, J.; Wei, H.; Li, L.; Pan, H.; Huang, F.; et al. Combined analysis of the metabolome and transcriptome sheds new light on the mechanisms of seed maturation in Amorphophallus muelleri. J. Plant Growth Regul. 2024, 43, 4263–4278. [Google Scholar] [CrossRef]
- Baroja-Fernández, E.; Muñoz, F.J.; Montero, M.; Etxeberria, E.; Sesma, M.T.; Ovecka, M.; Bahaji, A.; Ezquer, I.; Li, J.; Prat, S.; et al. Enhancing sucrose synthase activity in transgenic potato (Solanum tuberosum L.) tubers results in increased levels of starch, ADP-glucose and UDP-glucose and total yield. Plant Cell Physiol. 2009, 50, 1651–1662. [Google Scholar] [CrossRef]
- Kim, S.J.; Kim, Y.S.; Yeom, S.J. Phosphate sugar isomerases and their potential for rare sugar bioconversion. J. Microbiol. 2020, 58, 725–733. [Google Scholar] [CrossRef] [PubMed]
- Stiers, K.M.; Muenks, A.G.; Beamer, L.J. Biology, mechanism, and structure of enzymes in the α-D-phosphohexomutase superfamily. Adv. Protein Chem. Struct. Biol. 2017, 109, 265–304. [Google Scholar] [PubMed]
- Xu, J.W.; Ji, S.L.; Li, H.J.; Zhou, J.S.; Duan, Y.Q.; Dang, L.Z.; Mo, M.H. Increased polysaccharide production and biosynthetic gene expressions in a submerged culture of Ganoderma lucidum by the overexpression of the homologous α-phosphoglucomutase gene. Bioprocess Biosyst. Eng. 2015, 38, 399–405. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Zeng, S.; Teixeira da Silva, J.A.; Yu, Z.; Tan, J.; Duan, J. Molecular cloning and functional analysis of the phosphomannomutase (PMM) gene from Dendrobium officinale and evidence for the involvement of an abiotic stress response during germination. Protoplasma 2016, 254, 1693–1704. [Google Scholar] [CrossRef]
- Doello, S.; Forchhammer, K. Phosphoglucomutase comes into the spotlight. J. Exp. Bot. 2023, 74, 1293–1296. [Google Scholar] [CrossRef]
- Sowokinos, J.R.; Spychalla, J.P.; Desborough, S.L. Pyrophosphorylases in Solanum tuberosum (IV. Purification, tissue localization, and physicochemical properties of UDP-glucose pyrophosphorylase). Plant Physiol. 1993, 101, 1073–1080. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, A. Genomic characterization and expression analysis of basic helix-loop-helix (bHLH) family genes in traditional Chinese herb Dendrobium officinale. Plants 2020, 9, 1044. [Google Scholar] [CrossRef]
- Liu, B.; Wang, T.; Liu, L.; Xiao, D.; Yang, Y.; Yuan, L.; Zhang, A.; Xu, K.; Liu, S.; Liu, K.; et al. MYB6/bHLH13-AbSUS2 involved in sugar metabolism regulates root hair initiation of Abies beshanzuensis. New Phytol. 2023, 240, 2386–2403. [Google Scholar] [CrossRef]
- Hussain, A.; Raza, A.; Ameen, A.; Rehman, H.A.; Khawar, H.; Irfan, J.A.; Maqsood, W.; Ali, S.; Khan, N.; Nawaz, M.S.; et al. Research progress of AP2/ERF transcription factor family in important crops. Int. J. Phytopathol. 2022, 11, 135–153. [Google Scholar] [CrossRef]
- Li, P.; Chai, Z.; Lin, P.; Huang, C.; Huang, G.; Xu, L.; Deng, Z.; Zhang, M.; Zhang, Y.; Zhao, X. Genome-wide identification and expression analysis of AP2/ERF transcription factors in sugarcane (Saccharum spontaneum L.). BMC Genom. 2020, 21, 685. [Google Scholar] [CrossRef]
- Mizoi, J.; Shinozaki, K.; Yamaguchi-Shinozaki, K. AP2/ERF family transcription factors in plant abiotic stress responses. Biochim. Biophys. Acta 2012, 1819, 86–96. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Li, S.; Lin, X.; Fang, H.; Shi, Y.; Grierson, D.; Chen, K. Transcription factor CitERF16 is involved in Citrus fruit sucrose accumulation by activating CitSWEET11d. Front. Plant Sci. 2021, 12, 809619. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Li, X. Genome-wide identification of AP2/ERF superfamily genes and their expression during fruit ripening of Chinese jujube. Sci. Rep. 2018, 8, 15612. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Chen, L.; Yang, Y.; Guo, X.; Chen, G.; Xiong, X.; Dong, D.; Li, G. Transcriptome analysis reveals that exogenous ethylene activates immune and defense responses in a high late blight resistant potato genotype. Sci. Rep. 2020, 10, 21294. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Xie, S.; Xiao, Q.; Wei, B.; Zheng, L.; Wang, Y.; Cao, Y.; Zhang, X.; Long, T.; Li, Y.; et al. Sucrose and ABA regulate starch biosynthesis in maize through a novel transcription factor, ZmEREB156. Sci. Rep. 2016, 6, 27590. [Google Scholar] [CrossRef]
- Bari, R.; Jones, J.D.G. Role of plant hormones in plant defense responses. Plant Mol. Biol. 2008, 69, 473–488. [Google Scholar] [CrossRef]
- Zhao, Q.; Guo, H.W. Paradigms and paradox in the ethylene signaling pathway and interaction network. Mol. Plant. 2011, 4, 626–634. [Google Scholar] [CrossRef]
- Xue, H.; Gao, X.; He, P.; Xiao, G. Origin, evolution, and molecular function of DELLA proteins in plants. Crop J. 2022, 10, 287–299. [Google Scholar] [CrossRef]
- Sun, T.P. Gibberellin-GID1-DELLA: A pivotal regulatory module for plant growth and development. Plant Physiol. 2010, 154, 567–570. [Google Scholar] [CrossRef]
- An, F.; Zhang, X.; Zhu, Z.; Ji, Y.; He, W.; Jiang, Z.; Li, M.; Guo, H. Coordinated regulation of apical hook development by gibberellins and ethylene in etiolated Arabidopsis seedlings. Cell Res. 2012, 22, 915–927. [Google Scholar] [CrossRef]
- Achard, P.; Gong, F.; Cheminant, S.; Alioua, M.; Hedden, P.; Genschik, P. The cold-inducible CBF1 factor–dependent signaling pathway modulates the accumulation of the growth-repressing DELLA proteins via its effect on gibberellin metabolism. Plant Cell. 2008, 20, 2117–2129. [Google Scholar] [CrossRef]




| Samples | Raw Reads (Mb) | Raw Bases (Gb) | Clean Reads (Mb) | Aligned Reads (%) | Q30 (%) | GC (%) |
|---|---|---|---|---|---|---|
| CK.1 | 21.47 | 6.44 | 17.90 | 83.38 | 92.83 | 51.03 |
| CK.2 | 21.18 | 6.35 | 16.63 | 78.52 | 92.61 | 49.24 |
| CK.3 | 24.38 | 7.31 | 20.51 | 84.15 | 92.69 | 49.69 |
| La20.1 | 19.75 | 5.93 | 15.83 | 80.11 | 91.68 | 50.94 |
| La20.2 | 21.27 | 6.38 | 17.43 | 81.95 | 92.42 | 52.96 |
| La20.3 | 23.46 | 7.04 | 19.49 | 83.07 | 91.97 | 52.05 |
| La80.1 | 25.72 | 7.72 | 21.21 | 82.45 | 91.47 | 51.93 |
| La80.2 | 25.42 | 7.63 | 20.46 | 80.48 | 92.32 | 52.00 |
| La80.3 | 24.52 | 7.35 | 20.55 | 83.83 | 92.15 | 52.28 |
| La160.1 | 21.22 | 6.36 | 17.55 | 82.73 | 92.01 | 52.52 |
| La160.2 | 31.71 | 9.51 | 25.11 | 79.17 | 92.36 | 52.75 |
| La160.3 | 29.67 | 8.90 | 23.45 | 79.06 | 92.23 | 51.24 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Zeng, Z.; Zhu, S.; Yang, X.; Xuan, X.; Yu, Z. Glucomannan Accumulation Induced by Exogenous Lanthanum in Amorphophallus konjac: Insights from a Comparative Transcriptome Analysis. Biology 2025, 14, 849. https://doi.org/10.3390/biology14070849
Li X, Zeng Z, Zhu S, Yang X, Xuan X, Yu Z. Glucomannan Accumulation Induced by Exogenous Lanthanum in Amorphophallus konjac: Insights from a Comparative Transcriptome Analysis. Biology. 2025; 14(7):849. https://doi.org/10.3390/biology14070849
Chicago/Turabian StyleLi, Xiaoxian, Zhouting Zeng, Siyi Zhu, Xirui Yang, Xiaobo Xuan, and Zhenming Yu. 2025. "Glucomannan Accumulation Induced by Exogenous Lanthanum in Amorphophallus konjac: Insights from a Comparative Transcriptome Analysis" Biology 14, no. 7: 849. https://doi.org/10.3390/biology14070849
APA StyleLi, X., Zeng, Z., Zhu, S., Yang, X., Xuan, X., & Yu, Z. (2025). Glucomannan Accumulation Induced by Exogenous Lanthanum in Amorphophallus konjac: Insights from a Comparative Transcriptome Analysis. Biology, 14(7), 849. https://doi.org/10.3390/biology14070849






