Revealing the Improving Effect and Molecular Mechanism of L-Clausenamide in Combating the Acute Lung Injury: Insights from Network Pharmacology, Molecular Docking, and In Vitro Validation
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. ADME Analysis and Target Prediction of L-Clausenamide
2.2. The Collection of the Acute Lung Injury and ROS-Related Targets
2.3. Identification of Overlapping Anti-Acute Lung Injury Targets of L-Clausenamide
2.4. Protein–Protein Interaction (PPI) Analysis
2.5. Network Construction of PPI Analysis and the Drug–Compound–Target–Disease Network
2.6. Screening the Core Targets and Central Targets of L-Clausenamide
2.7. GO and KEGG Enrichment Analysis
2.8. Molecular Docking
2.9. Materials
2.10. Cell Culture and Treatment
2.11. Cell Viability Determination
2.12. ROS Measurement
2.13. Membrane Potential Measurement
2.14. Mitochondrial Membrane Potential Measurement
2.15. Intracellular ATP Determination
2.16. Apoptotic Cell Measurement and Mitochondrial Morphology Observation
2.17. Microscope and Image Analysis
2.18. SPR Analysis
2.19. Western Blot Analysis
2.20. Statistical Analysis
3. Results
3.1. The ADME Analysis of L-Clausenamide
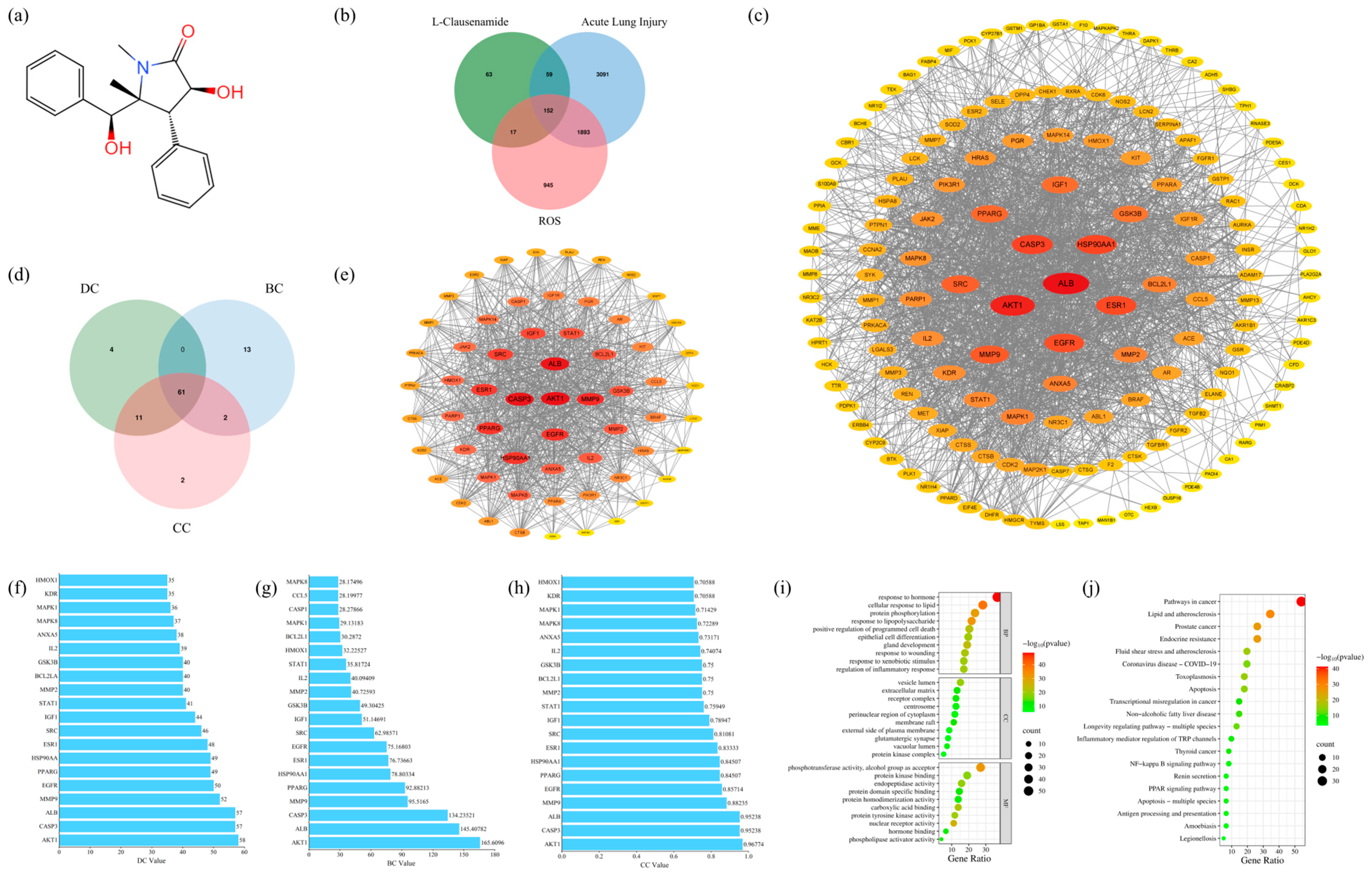
3.2. The Anti-Acute Lung Injury Targets of L-Clausenamide
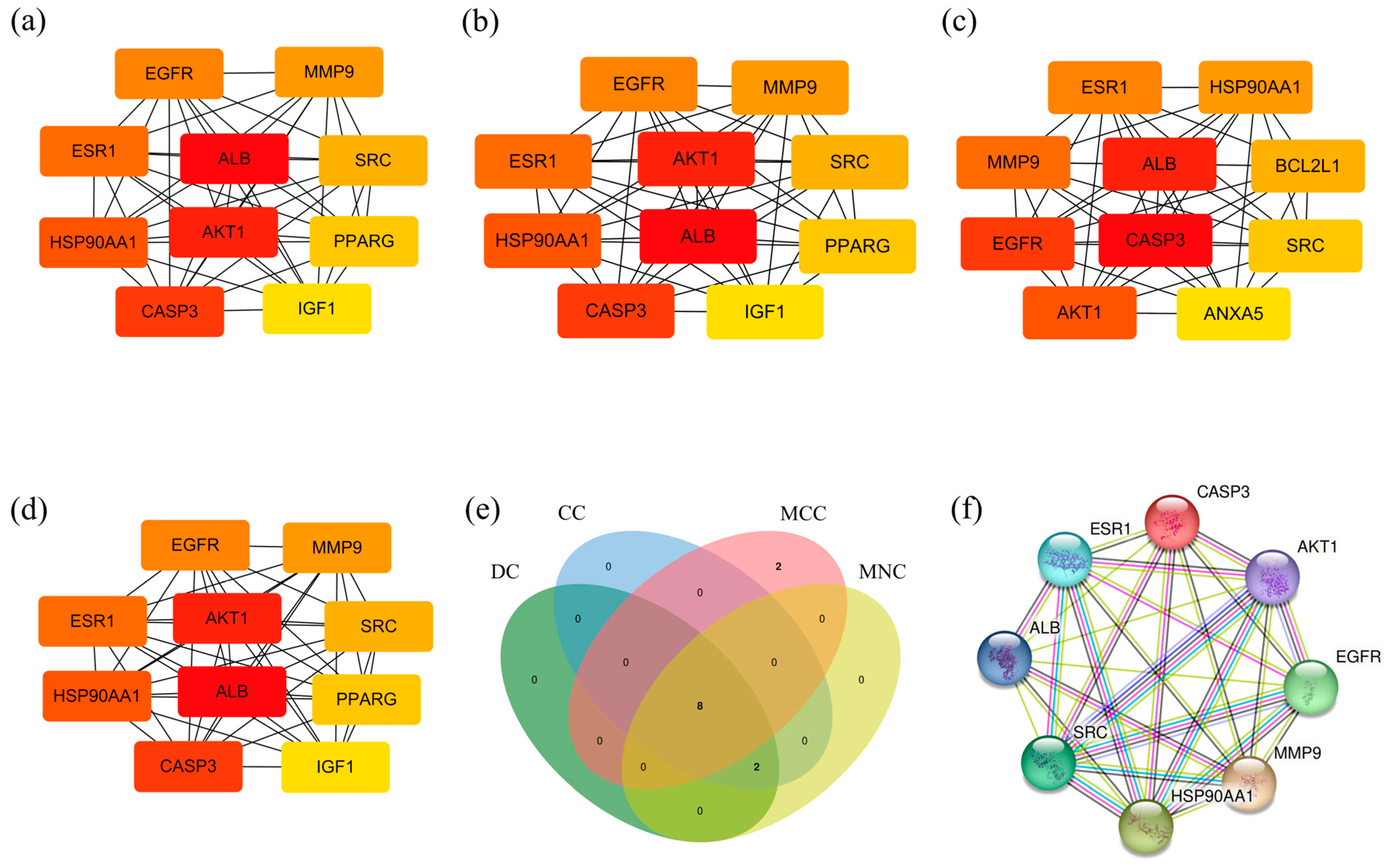
3.3. Protein–Protein Interaction (PPI) Analysis and Core Target Screening
3.4. Result of GO and KEGG Enrichment Analysis
3.5. Central Target Screening
3.6. Result of Molecular Docking
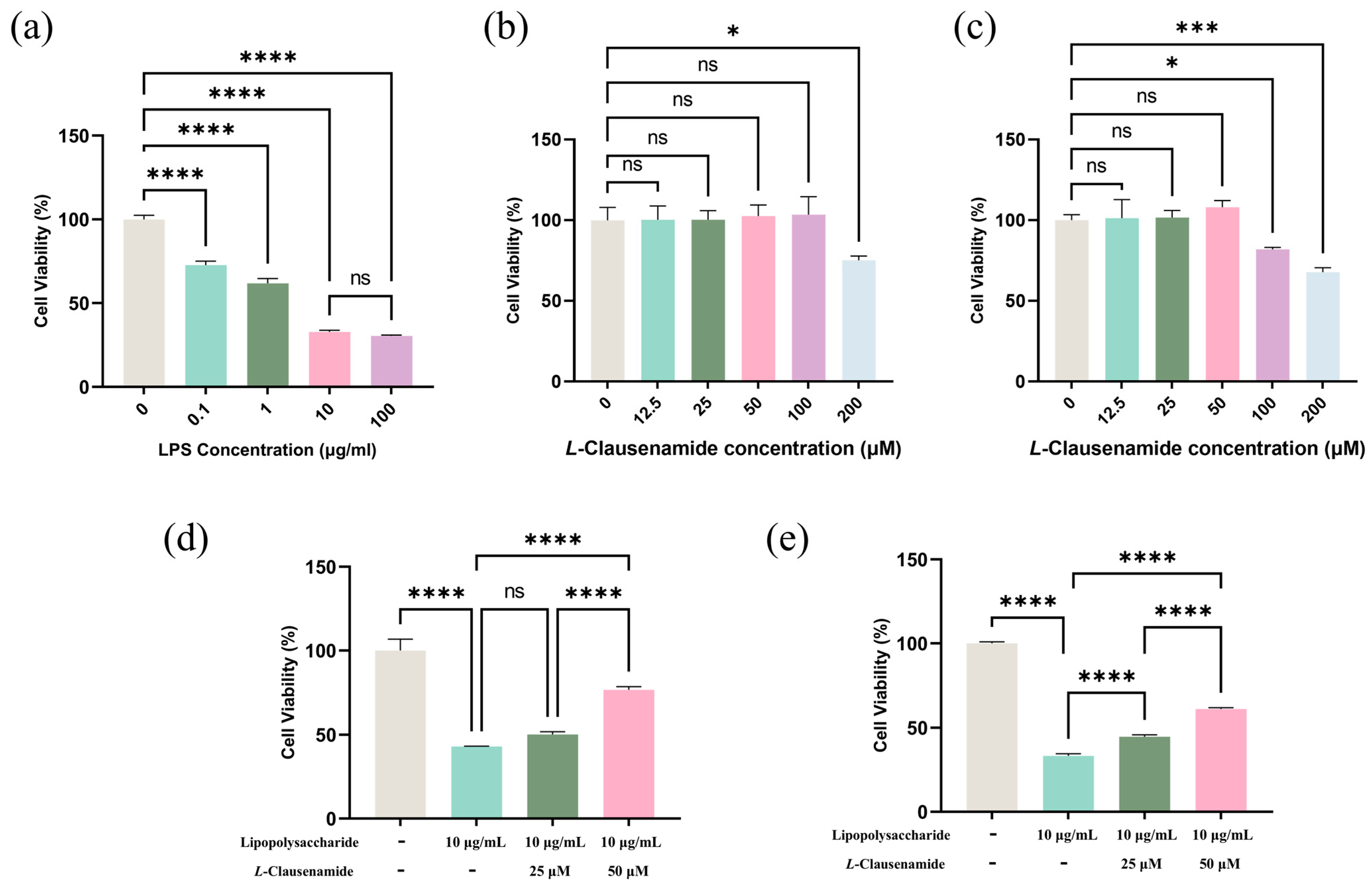
3.7. L-Clausenamide Inhibits LPS-Facilitated Cell Viability Decrease
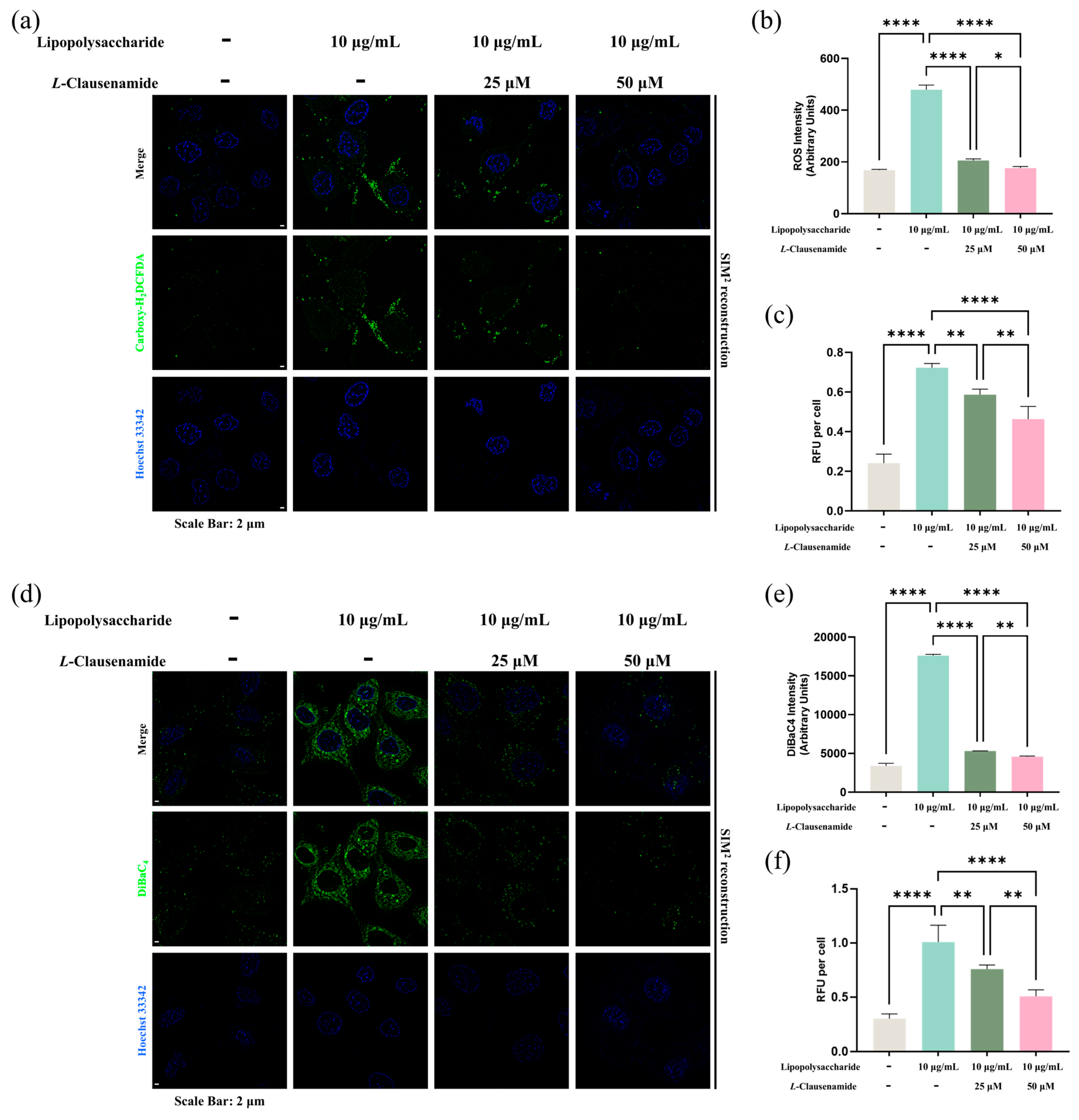
3.8. L-Clausenamide Inhibits LPS-Facilitated Intracellular ROS Accumulation
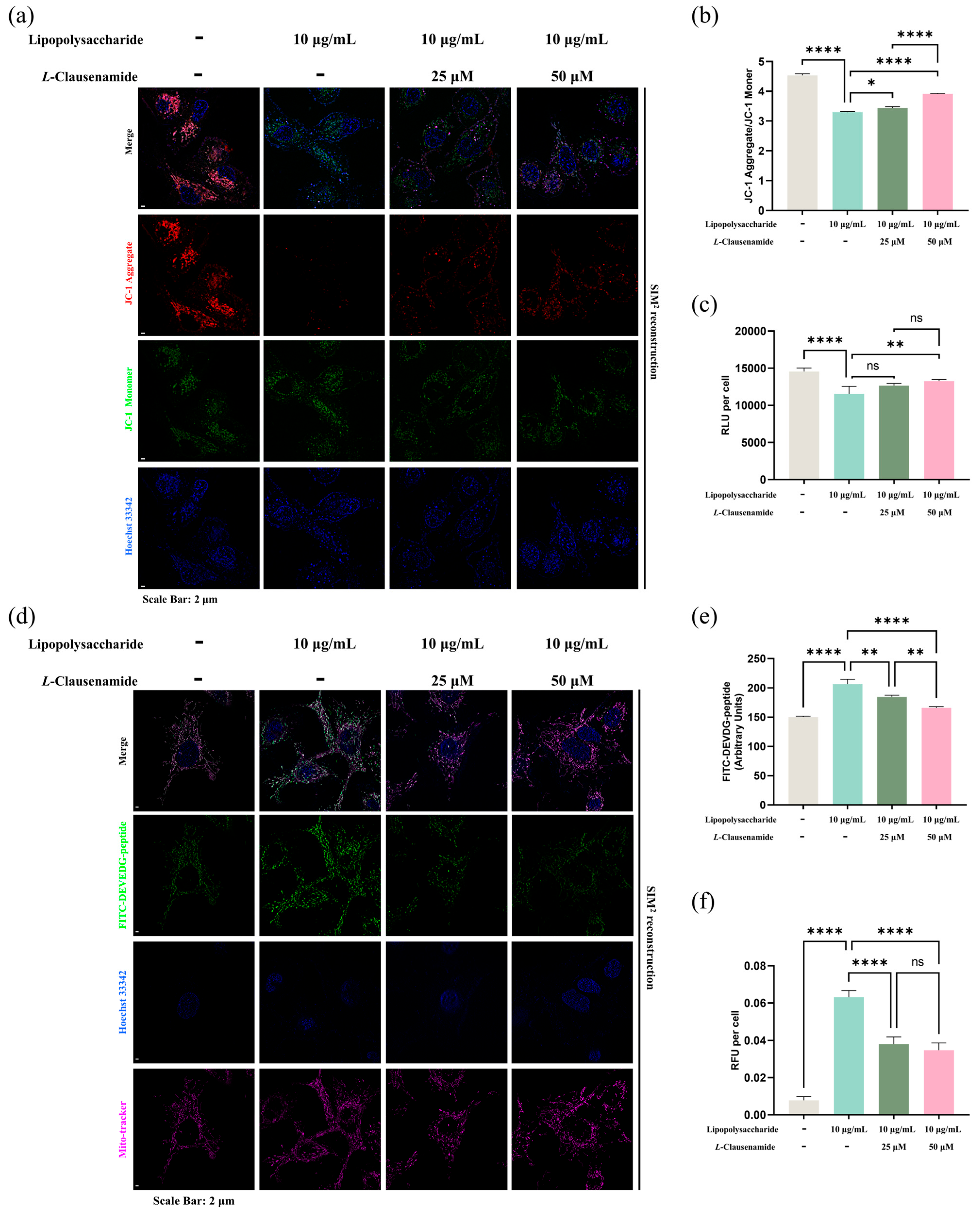
3.9. L-Clausenamide Inhibits LPS-Facilitated Mitochondrial Membrane Potential Loss and ATP Decrease
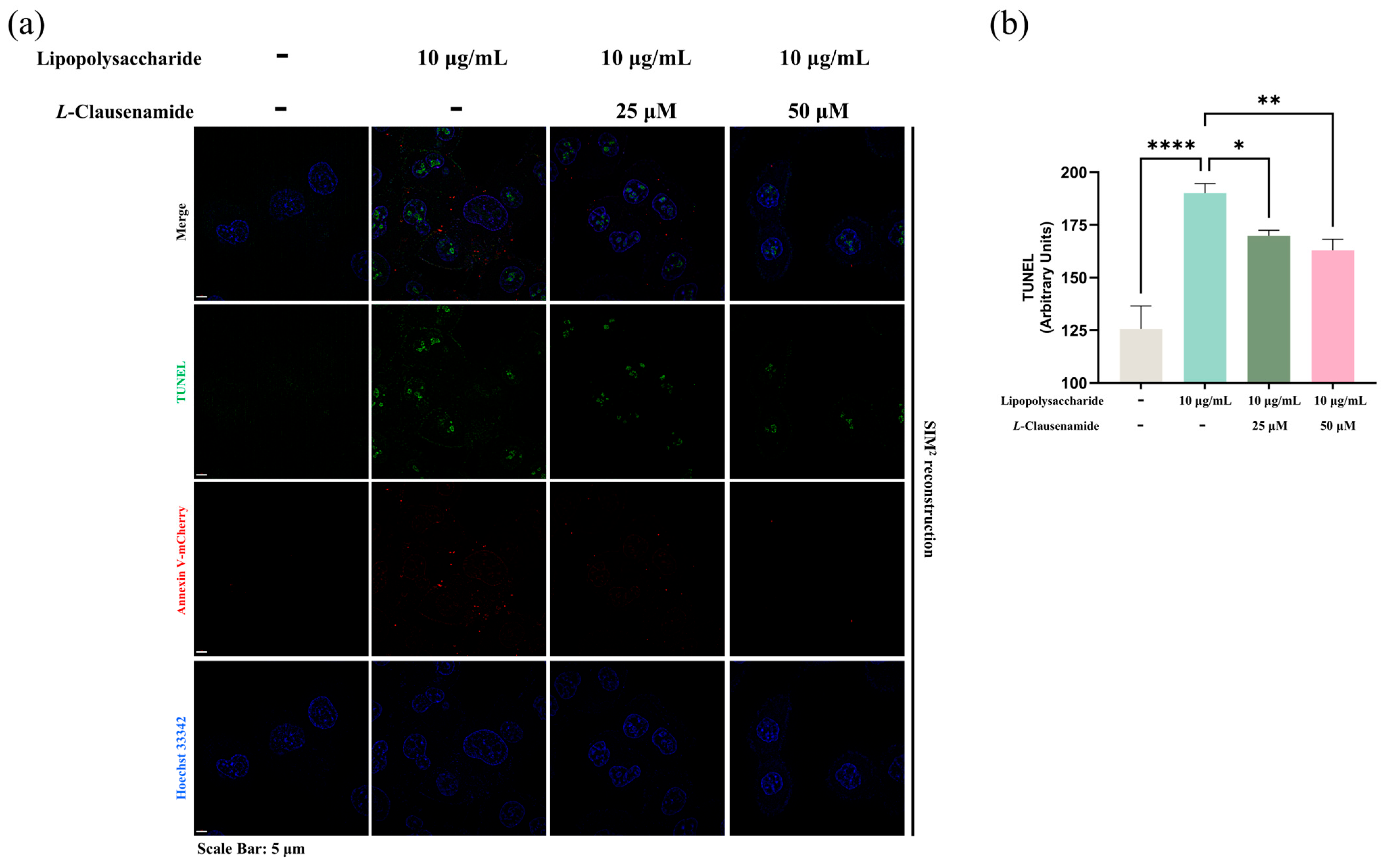
3.10. L-Clausenamide Inhibits LPS-Facilitated Mitochondrial Morphological Abnormality, Caspase-3 Activity, and DNA Fragmentation
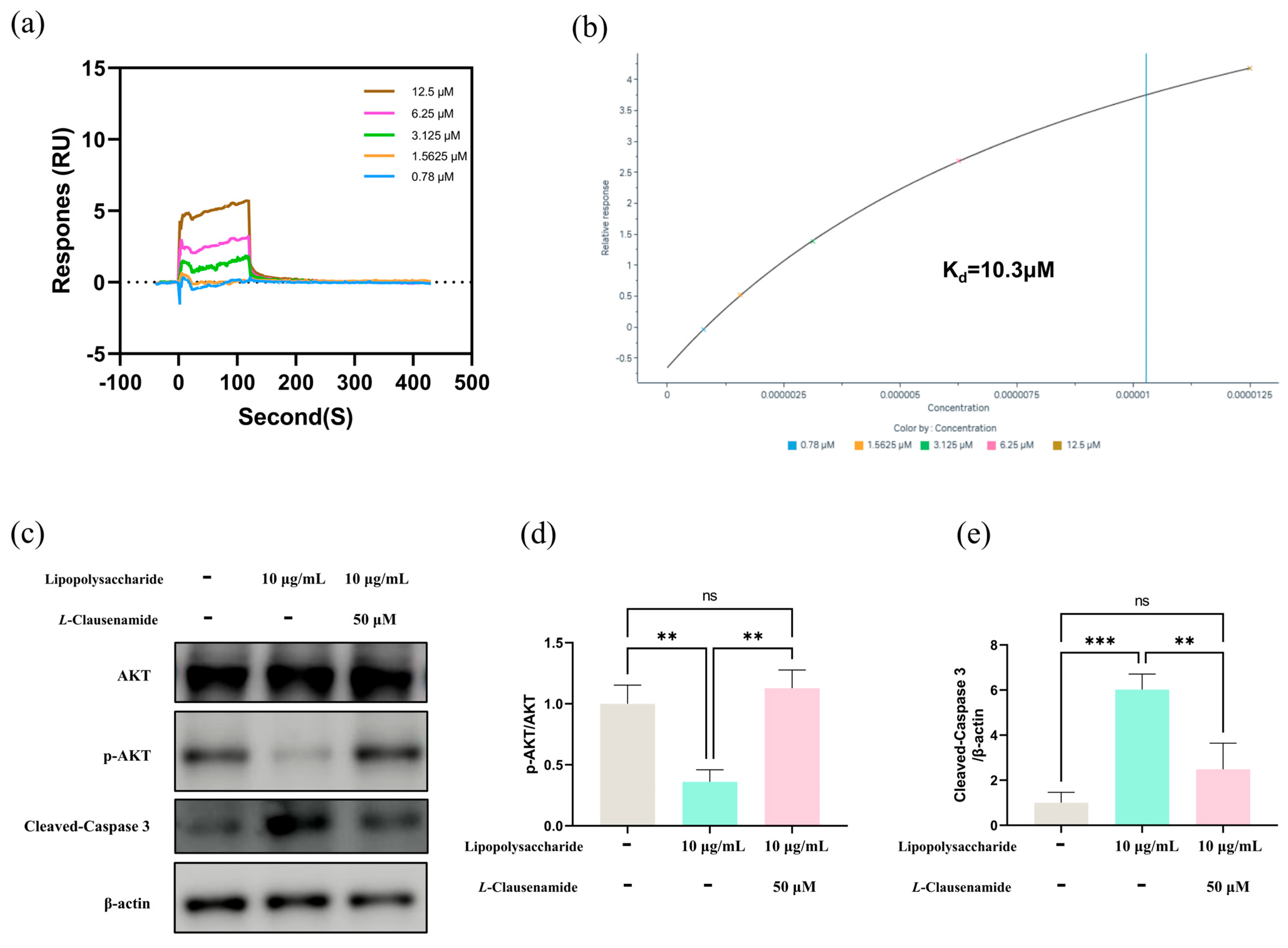
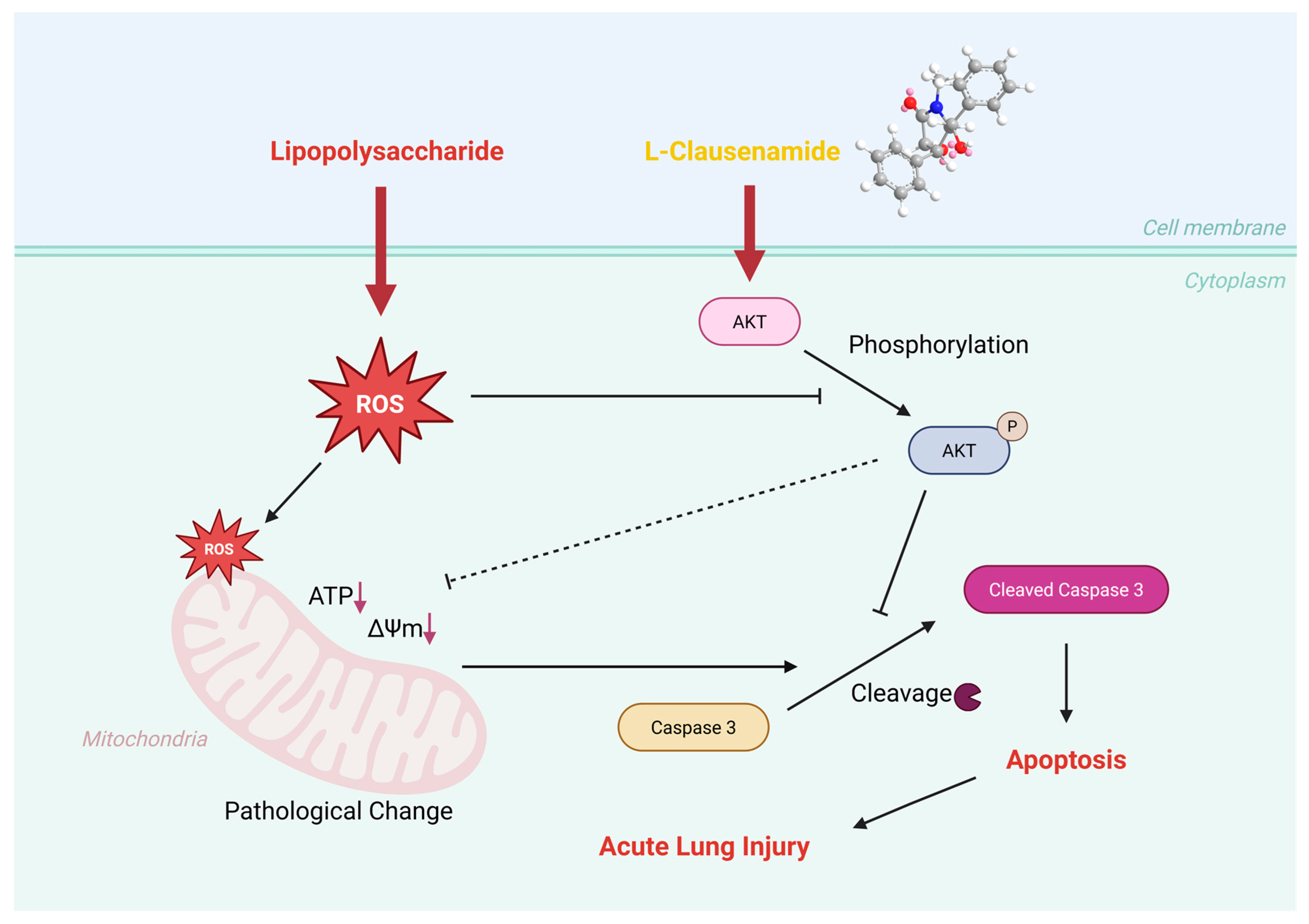
3.11. Molecular Mechanism of L-Clausenamide Improving the LPS-Induced Acute Lung Injury
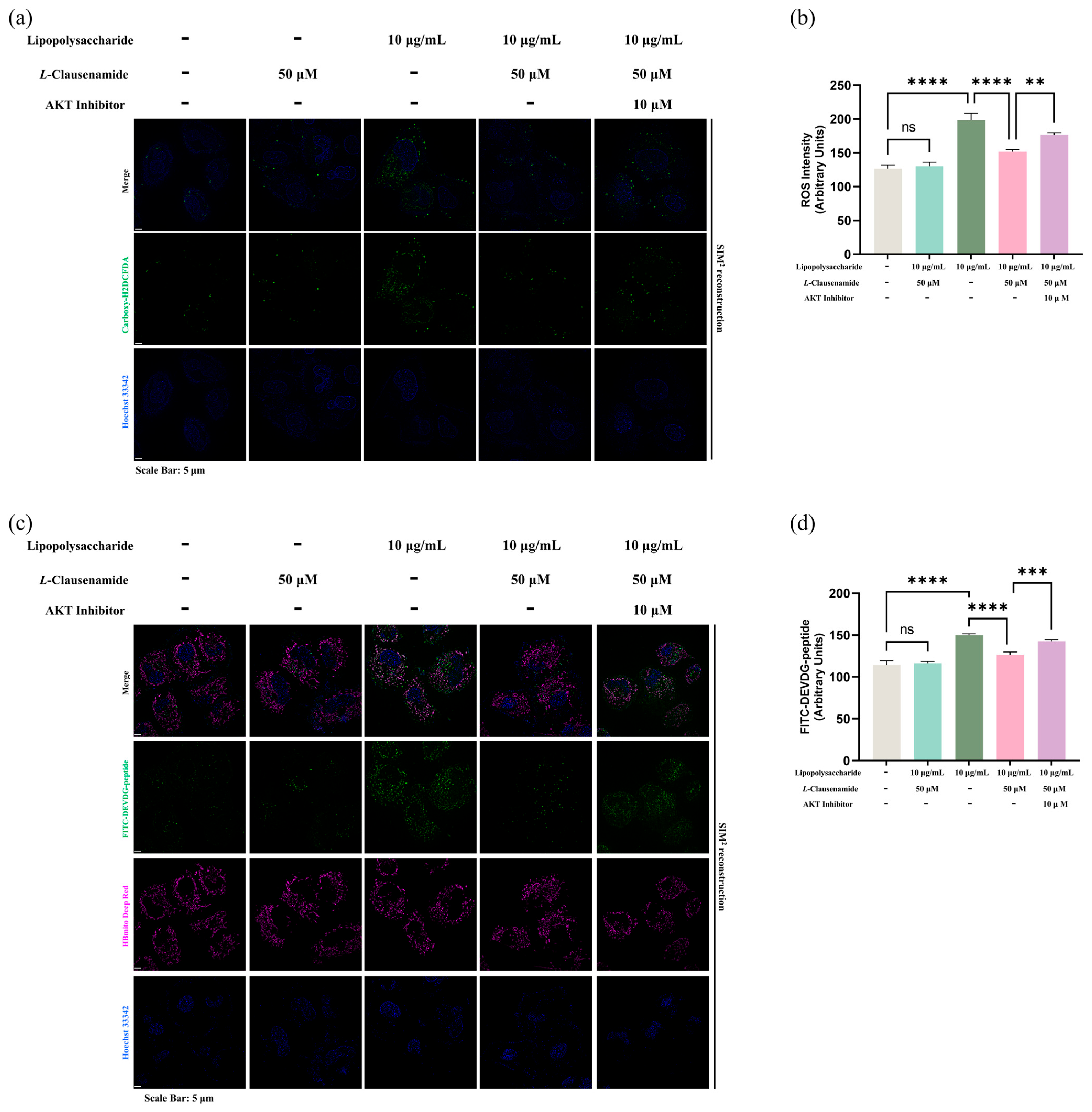
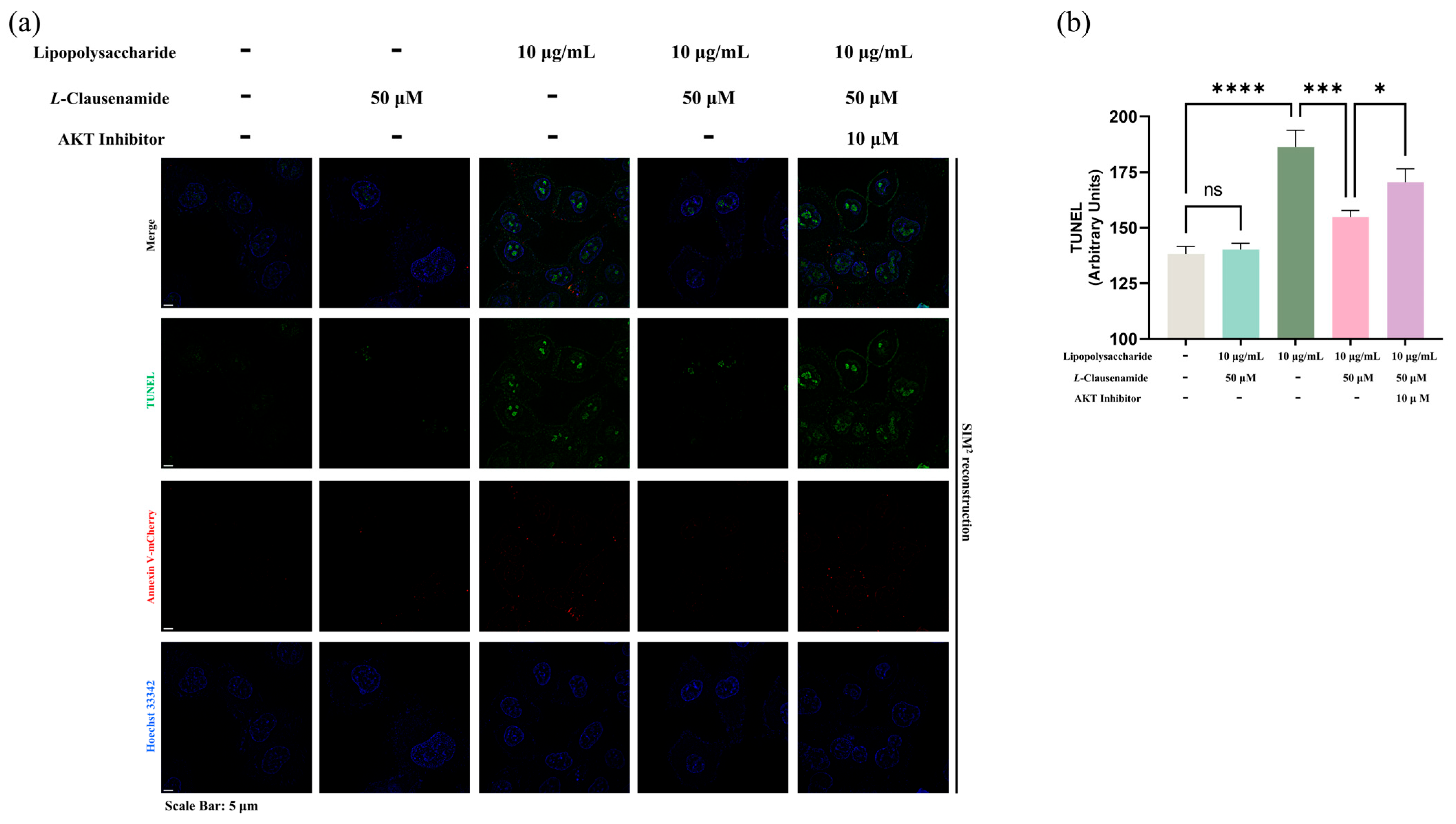
3.12. AKT Inhibitor Reduces the Apoptotic Alleviation Effect of L-Clausenamide on LPS-Induced A549 Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AKT1 | RAC-alpha serine/threonine-protein kinase |
| ALB | serum albumin |
| ALI | acute lung injury |
| Arg | arginine |
| ATP | Adenosine 5′-triphosphate |
| BC | betweenness centrality |
| CASP3 | Caspase-3 |
| CC | closeness centrality |
| CC | cellular components |
| Cys | cystine |
| em | emission |
| DC | degree centrality |
| DEVDG | Aspartate-Glutamate-Valine-Aspartate-Glycine |
| DiBaC4 | bis-(1,3-dibutylbarbituric acid) trimethine oxonol |
| DNA | deoxyriboNucleic Acid |
| EGFR | epidermal growth factor receptor |
| ESR1 | estrogen receptors |
| FITC | Fluorescein Isothiocyanate |
| GO | Gene Ontology |
| HSP90AA1 | heat shock protein 90 alpha family class A member 1 |
| JC-1 | 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimi-dazolylcarbocyanine iodide |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| LPS | Lipopolysaccharide |
| MCC | Maximal Clique Centrality |
| MF | molecular functions |
| MMP-9 | matrix metalloproteinase-9 |
| MNC | Maximum Neighborhood Component |
| nm | nanometer |
| OMIM | Online Mendelian Inheritance in Man |
| PCD | programmed cell death |
| PMF | proton motive force |
| PPI | protein-protein interaction |
| RLU | relative luminescent unit |
| RFU | relative fluorescent unit |
| ROS | reactive oxygen species |
| RMSD | root mean square deviation plot |
| RMSF | root mean square fluctuation plot |
| Rg | radius of gyration plot |
| SPR | Surface Plasmon Resonance |
| SASA | solvent accessible surface area plot |
| SIM | Structured Illumination Microscopy |
| SRC | proto-oncogene tyrosine-protein kinase |
| 2D | 2-Dimensional |
| 3D | 3-Dimensional |
References
- Li, Y.; Chen, X.; Zhang, H.; Xiao, J.; Yang, C.; Chen, W.; Wei, Z.; Chen, X.; Liu, J. 4-Octyl Itaconate alleviates lipopolysaccharide-induced acute lung injury in mice by inhibiting oxidative stress and inflammation. Drug Des. Dev. Ther. 2020, 14, 5547–5558. [Google Scholar] [CrossRef]
- Wu, B.; Xu, M.M.; Fan, C.; Feng, C.L.; Lu, Q.K.; Lu, H.M.; Xiang, C.G.; Bai, F.; Wang, H.Y.; Wu, Y.W.; et al. STING inhibitor ameliorates LPS-induced ALI by preventing vascular endothelial cells-mediated immune cells chemotaxis and adhesion. Acta Pharmacol. Sin. 2022, 43, 2055–2066. [Google Scholar] [CrossRef] [PubMed]
- Vatti, R.R.; Ali, F.; Teuber, S.; Chang, C.; Gershwin, M.E. Hypersensitivity reactions to corticosteroids. Clin. Rev. Allergy Immunol. 2014, 47, 26–37. [Google Scholar] [CrossRef]
- Goswami, D.G.; Kant, R.; Ammar, D.A.; Kumar, D.; Enzenauer, R.W.; Petrash, J.M.; Tewari-Singh, N.; Agarwal, R. Acute corneal injury in rabbits following nitrogen mustard ocular exposure. Exp. Mol. Pathol. 2019, 110, 104275. [Google Scholar] [CrossRef]
- Zhao, J.; Nan, P.; Zhong, Y.; Zhao, J.; Nan, P.; Zhong, Y. Chemical composition of the essential oils of Clausena lansium from Hainan Island, China. Z. Naturforsch C J. Biosci. 2004, 59, 153–156. [Google Scholar] [CrossRef]
- Lim, T.; Lim, T. Clausena lansium. In Edible Medicinal and Non-Medicinal Plants: Volume 4, Fruits; Springer: Dordrecht, The Netherlands, 2012; pp. 871–883. [Google Scholar]
- Pedras, M.S.C.; Taylor, J.L.; Morales, V.M. Phomaligin a and other yellow pigments in Phoma-lingam and P-wasabiae. Phytochemistry 1995, 38, 1215–1222. [Google Scholar] [CrossRef]
- Hu, J.F.; Chu, S.F.; Ning, N.; Yuan, Y.H.; Xue, W.; Chen, N.H.; Zhang, J.T. Protective effect of (-)clausenamide against Abeta-induced neurotoxicity in differentiated PC12 cells. Neurosci. Lett. 2010, 483, 78–82. [Google Scholar] [CrossRef]
- Chebaiki, M.; Delfourne, E.; Tamhaev, R.; Danoun, S.; Rodriguez, F.; Hoffmann, P.; Grosjean, E.; Goncalves, F.; Azéma-Despeyroux, J.; Pál, A.; et al. Discovery of new diaryl ether inhibitors against Mycobacterium tuberculosis targeting the minor portal of InhA. Eur. J. Med. Chem. 2023, 259, 115646. [Google Scholar] [CrossRef]
- Han, B.; Luo, J.; Xu, B. Revealing Molecular Mechanisms of the bioactive saponins from edible root of Platycodon grandiflorum in combating obesity. Plants 2024, 13, 1123. [Google Scholar] [CrossRef]
- Wu, Y.Z.; Luo, J.H.; Xu, B.J. Network pharmacology and bioinformatics study of geniposide regulating oxidative stress in colorectal cancer. Int. J. Mol. Sci. 2023, 24, 15222. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef]
- Coudert, E.; Gehant, S.; de Castro, E.; Pozzato, M.; Baratin, D.; Neto, T.; Sigrist, C.J.A.; Redaschi, N.; Bridge, A.; UniProt, C. Annotation of biologically relevant ligands in UniProtKB using ChEBI. Bioinformatics 2023, 39, btac793. [Google Scholar] [CrossRef]
- Amberger, J.S.; Bocchini, C.A.; Schiettecatte, F.; Scott, A.F.; Hamosh, A. OMIM.org: Online Mendelian Inheritance in Man (OMIM(R)), an online catalog of human genes and genetic disorders. Nucleic Acids Res. 2015, 43, D789–D798. [Google Scholar] [CrossRef] [PubMed]
- Stelzer, G.; Rosen, N.; Plaschkes, I.; Zimmerman, S.; Twik, M.; Fishilevich, S.; Stein, T.I.; Nudel, R.; Lieder, I.; Mazor, Y.; et al. The GeneCards Suite: From gene data mining to disease genome sequence analyses. Curr. Protoc. Bioinform. 2016, 54, 1.30.1–1.30.33. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Kirsch, R.; Koutrouli, M.; Nastou, K.; Mehryary, F.; Hachilif, R.; Gable, A.L.; Fang, T.; Doncheva, N.T.; Pyysalo, S.; et al. The STRING database in 2023: Protein-protein association networks and functional enrichment analyses for any sequenced genome of interest. Nucleic Acids Res. 2023, 51, D638–D646. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhou, B.; Pache, L.; Chang, M.; Khodabakhshi, A.H.; Tanaseichuk, O.; Benner, C.; Chanda, S.K. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat. Commun. 2019, 10, 1523. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.H.; Wu, Y.Z.; Moussa, A.Y.; Huang, Y.Y.; Fu, Y.; Wei, Y.; Luo, J.C.; Xu, B.J. Unveiling the molecular mechanisms of adzuki bean (Vigna angularis) derived bioactive phytochemicals in combating obesity. Food Biosci. 2024, 62, 105515. [Google Scholar] [CrossRef]
- Shen, X.; He, L.; Cai, W. Role of lipopolysaccharides in the inflammation and pyroptosis of alveolar epithelial cells in acute lung injury and acute respiratory distress syndrome. J. Inflamm. Res. 2024, 17, 5855–5869. [Google Scholar] [CrossRef]
- Chuang, C.Y.; Chen, T.L.; Cherng, Y.G.; Tai, Y.T.; Chen, T.G.; Chen, R.M. Lipopolysaccharide induces apoptotic insults to human alveolar epithelial A549 cells through reactive oxygen species-mediated activation of an intrinsic mitochondrion-dependent pathway. Arch. Toxicol. 2011, 85, 209–218. [Google Scholar] [CrossRef]
- Bardou, P.; Mariette, J.; Escudie, F.; Djemiel, C.; Klopp, C. jvenn: An interactive Venn diagram viewer. BMC Bioinform. 2014, 15, 293. [Google Scholar] [CrossRef]
- Luo, J.; Luo, J.; Wu, Y.; Fu, Y.; Fang, Z.; Han, B.; Du, B.; Yang, Z.; Xu, B. Anti-Obesity Effects of adzuki bean saponins in improving lipid metabolism through reducing oxidative stress and alleviating mitochondrial abnormality by activating the PI3K/Akt/GSK3beta/beta-Catenin signaling pathway. Antioxidants 2024, 13, 1380. [Google Scholar] [CrossRef]
- Shankaranarayanan, P.; Nigam, S. IL-4 induces apoptosis in A549 lung adenocarcinoma cells: Evidence for the pivotal role of 15-hydroxyeicosatetraenoic acid binding to activated peroxisome proliferator-activated receptor gamma transcription factor. J. Immunol. 2003, 170, 887–894. [Google Scholar] [CrossRef]
- Ren, W.; Ge, X.; Li, M.; Sun, J.; Li, S.; Gao, S.; Shan, C.; Gao, B.; Xi, P. Author Correction: Visualization of cristae and mtDNA interactions via STED nanoscopy using a low saturation power probe. Light. Sci. Appl. 2024, 13, 235. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, H.; Zhou, Q.; Xia, W.; Zhao, X.; Li, L.; Wang, X.; Yang, J.; Ren, X.; Wu, J.; et al. VP5 protein of oncolytic herpes simplex virus type 2 induces apoptosis in A549 cells through TP53I3 protein. Virology 2024, 595, 110093. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Xiao, Y.; Kong, F.; Zhang, X.; Xu, H.; Zhu, A.; Liu, Y.; Lei, J.; Tian, B.; Yuan, Y.; et al. Molecular basis of human noradrenaline transporter reuptake and inhibition. Nature 2024, 632, 921–929. [Google Scholar] [CrossRef] [PubMed]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 2001, 46, 3–26. [Google Scholar] [CrossRef]
- Li, M.; Lu, Y.; Wang, J.; Wu, F.X.; Pan, Y. A Topology potential-based method for identifying essential proteins from PPI networks. IEEE/ACM Trans. Comput. Biol. Bioinform. 2015, 12, 372–383. [Google Scholar] [CrossRef]
- Huang, C.; Zheng, H.; He, W.; Lu, G.; Li, X.; Deng, Y.; Zeng, M. Ghrelin ameliorates the human alveolar epithelial A549 cell apoptosis induced by lipopolysaccharide. Biochem. Biophys. Res. Commun. 2016, 474, 83–90. [Google Scholar] [CrossRef]
- Chen, W.; Zhao, H.K.; Li, Y.S. Mitochondrial dynamics in health and disease: Mechanisms and potential targets. Signal Transduct. Target. Ther. 2023, 8, 333. [Google Scholar] [CrossRef]
- Adams, D.S.; Levin, M. Measuring resting membrane potential using the fluorescent voltage reporters DiBAC4(3) and CC2-DMPE. Cold Spring Harb. Protoc. 2012, 2012, 459–464. [Google Scholar] [CrossRef]
- Perelman, A.; Wachtel, C.; Cohen, M.; Haupt, S.; Shapiro, H.; Tzur, A. JC-1: Alternative excitation wavelengths facilitate mitochondrial membrane potential cytometry. Cell Death Dis. 2012, 3, e430. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; He, L.; Wu, J.; Xie, C.; Geng, S.; Wu, J.; Zhong, C.; Li, X. Bisphenol A-induced cancer-associated adipocytes promotes breast carcinogenesis via CXCL12/AKT signaling. Mol. Cell Endocrinol. 2025, 599, 112473. [Google Scholar] [CrossRef]
- Domscheit, H.; Hegeman, M.A.; Carvalho, N.; Spieth, P.M. Molecular dynamics of lipopolysaccharide-induced lung injury in rodents. Front. Physiol. 2020, 11, 36. [Google Scholar] [CrossRef]
- Chu, S.F.; Zhang, J.T. Recent advances in the study of (-)clausenamide: Chemistry, biological activities and mechanism of action. Acta Pharm. Sin. B 2014, 4, 417–423. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.J.; Zhang, J.T. Identification of rat cytochrome P450 forms involved in the metabolism of clausenamide enantiomers. Chirality 2003, 15, 448–455. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.J.; Zhang, J.T. Stereoselective plasma protein binding and target tissue distribution of clausenamide enantiomers in rats. Chirality 2009, 21, 402–406. [Google Scholar] [CrossRef]
- Zhu, C.J.; Zhang, J.T. Stereoselective pharmacokinetics of clausenamide enantiomers and their major metabolites after single intravenous and oral administration to rats. Chirality 2003, 15, 668–673. [Google Scholar] [CrossRef]
- Porter, A.G.; Janicke, R.U. Emerging roles of caspase-3 in apoptosis. Cell Death Differ. 1999, 6, 99–104. [Google Scholar] [CrossRef]
- Yu, F.; Tan, W.; Chen, Z.; Shen, X.; Mo, X.; Mo, X.; He, J.; Deng, Z.; Wang, J.; Luo, Z.; et al. Nitidine chloride induces caspase 3/GSDME-dependent pyroptosis by inhibting PI3K/Akt pathway in lung cancer. Chin. Med. 2022, 17, 115. [Google Scholar] [CrossRef]
- Luo, X.; Lin, B.; Gao, Y.; Lei, X.; Wang, X.; Li, Y.; Li, T. Genipin attenuates mitochondrial-dependent apoptosis, endoplasmic reticulum stress, and inflammation via the PI3K/AKT pathway in acute lung injury. Int. Immunopharmacol. 2019, 76, 105842. [Google Scholar] [CrossRef]
- Khan, I.; Yousif, A.; Chesnokov, M.; Hong, L.; Chefetz, I. A decade of cell death studies: Breathing new life into necroptosis. Pharmacol. Ther. 2021, 220, 107717. [Google Scholar] [CrossRef] [PubMed]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.Y.; Condict, L.; Richardson, S.J.; Brennan, C.S.; Kasapis, S. Exploring the inhibitory mechanism of p-coumaric acid on α-amylase via multi-spectroscopic analysis, enzymatic inhibition assay and molecular docking. Food Hydrocoll. 2023, 139, 108524. [Google Scholar] [CrossRef]
- Ferenczy, G.G.; Kellermayer, M. Contribution of hydrophobic interactions to protein mechanical stability. Comput. Struct. Biotechnol. J. 2022, 20, 1946–1956. [Google Scholar] [CrossRef]
- Barabutis, N. Heat shock protein 90 inhibition in the inflamed lungs. Cell Stress Chaperon 2020, 25, 195–197. [Google Scholar] [CrossRef]
- Hsu, L.H.; Chu, N.M.; Kao, S.H. Estrogen, Estrogen Receptor and Lung Cancer. Int. J. Mol. Sci. 2017, 18, 1713. [Google Scholar] [CrossRef]
- Xing, J.; Wang, Q.; Coughlan, K.; Viollet, B.; Moriasi, C.; Zou, M.H. Inhibition of AMP-activated protein kinase accentuates lipopolysaccharide-induced lung endothelial barrier dysfunction and lung injury in vivo. Am. J. Pathol. 2013, 182, 1021–1030. [Google Scholar] [CrossRef]
- Yabluchanskiy, A.; Ma, Y.G.; Iyer, R.P.; Hall, M.E.; Lindsey, M.L. Matrix metalloproteinase-9: Many shades of function in cardiovascular disease. Physiology 2013, 28, 391–403. [Google Scholar] [CrossRef]
- Alnababteh, M.H.; Huang, S.S.; Ryan, A.; McGowan, K.M.; Yohannes, S. A multimodal sepsis quality-improvement initiative including 24/7 screening and a dedicated sepsis response team-reduced readmissions and mortality. Crit. Care Explor. 2020, 2, e0251. [Google Scholar] [CrossRef]
- Finigan, J.H.; Downey, G.P.; Kern, J.A. Human epidermal growth factor receptor signaling in acute lung injury. Am. J. Respir. Cell Mol. Biol. 2012, 47, 395–404. [Google Scholar] [CrossRef]
- Zhang, N.; Shan, W.; Gao, L.; Kou, S.H.; Lu, C.; Yang, H.; Peng, B.; Tam, K.Y.; Lee, L.T.O.; Zheng, J. Repurposing the Hedgehog pathway inhibitor, BMS-833923, as a phosphatidylglycerol-selective membrane-disruptive colistin adjuvant against ESKAPE pathogens. Int. J. Antimicrob. Agents 2023, 62, 106888. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.N.; Tan, Y.; Xiao, X.C.; Li, Q.; Wu, Q.; Peng, Y.Y.; Ren, J.; Dong, M.L. Deletion of TLR4 attenuates lipopolysaccharide-induced acute liver injury by inhibiting inflammation and apoptosis. Acta Pharmacol. Sin. 2021, 42, 1610–1619. [Google Scholar] [CrossRef]
- Huang, C.Y.; Deng, J.S.; Huang, W.C.; Jiang, W.P.; Huang, G.J. Attenuation of lipopolysaccharide-induced acute lung injury by hispolon in mice, through regulating the TLR4/PI3K/Akt/mTOR and Keap1/Nrf2/HO-1 pathways, and suppressing oxidative stress-mediated ER stress-induced apoptosis and autophagy. Nutrients 2020, 12, 1742. [Google Scholar] [CrossRef]
- Higuchi, M.; Honda, T.; Proske, R.J.; Yeh, E.T.H. Regulation of reactive oxygen species-induced apoptosis and necrosis by caspase 3-like proteases. Oncogene 1998, 17, 2753–2760. [Google Scholar] [CrossRef] [PubMed]
- Anderson, G.; Kubera, M.; Duda, W.; Lason, W.; Berk, M.; Maes, M. Increased IL-6 trans-signaling in depression: Focus on the tryptophan catabolite pathway, melatonin and neuroprogression. Pharmacol. Rep. 2013, 65, 1647–1654. [Google Scholar] [CrossRef]
- Ly, J.D.; Grubb, D.R.; Lawen, A. The mitochondrial membrane potential (deltapsi(m)) in apoptosis; an update. Apoptosis 2003, 8, 115–128. [Google Scholar] [CrossRef]
- Choi, E.H.; Kim, M.H.; Park, S.J. Targeting mitochondrial dysfunction and reactive oxygen species for neurodegenerative disease treatment. Int. J. Mol. Sci. 2024, 25, 7952. [Google Scholar] [CrossRef]
- Li, L.; Li, W.; Liu, Y.; Han, B.; Yu, Y.; Lin, H. MEHP induced mitochondrial damage by promoting ROS production in CIK cells, leading to apoptosis, autophagy, cell cycle arrest. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2025, 288, 110064. [Google Scholar] [CrossRef]
- Gorrini, C.; Harris, I.S.; Mak, T.W. Modulation of oxidative stress as an anticancer strategy. Nat. Rev. Drug Discov. 2013, 12, 931–947. [Google Scholar] [CrossRef]
- Sabharwal, S.S.; Schumacker, P.T. Mitochondrial ROS in cancer: Initiators, amplifiers or an Achilles’ heel? Nat. Rev. Cancer 2014, 14, 709–721. [Google Scholar] [CrossRef]
- Dixon, S.J.; Stockwell, B.R. The role of iron and reactive oxygen species in cell death. Nat. Chem. Biol. 2014, 10, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Hou, L.; Zhang, Y.; Guo, T.; Wang, Y.; Xing, M. Polystyrene microplastics mediate cell cycle arrest, apoptosis, and autophagy in the G2/M phase through ROS in grass carp kidney cells. Environ. Toxicol. 2024, 39, 1923–1935. [Google Scholar] [CrossRef] [PubMed]
- Cervellati, C.; Trentini, A.; Pecorelli, A.; Valacchi, G. Inflammation in neurological disorders: The thin boundary between brain and periphery. Antioxid. Redox Signal 2020, 33, 191–210. [Google Scholar] [CrossRef]
- Green, D.R.; Amarante-Mendes, G.P. The point of no return: Mitochondria, caspases, and the commitment to cell death. Results Probl. Cell Differ. 1998, 24, 45–61. [Google Scholar] [CrossRef]
- Li, J.; Yin, K.; Hou, L.; Zhang, Y.; Lu, H.; Ma, C.; Xing, M. Polystyrene microplastics mediate inflammatory responses in the chicken thymus by Nrf2/NF-kappaB pathway and trigger autophagy and apoptosis. Environ. Toxicol. Pharmacol. 2023, 100, 104136. [Google Scholar] [CrossRef]
- Dwyer, D.J.; Camacho, D.M.; Kohanski, M.A.; Callura, J.M.; Collins, J.J. Antibiotic-induced bacterial cell death exhibits physiological and biochemical hallmarks of apoptosis. Mol. Cell 2012, 46, 561–572. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, R.; Xiu, Z.; Yang, X.; Zhu, Y.; Han, J.; Li, S.; Li, Y.; Sun, L.; Li, X.; et al. Neobavaisoflavone induces pyroptosis of liver cancer cells via Tom20 sensing the activated ROS signal. Phytomedicine 2023, 116, 154869. [Google Scholar] [CrossRef] [PubMed]
- Suski, J.M.; Lebiedzinska, M.; Bonora, M.; Pinton, P.; Duszynski, J.; Wieckowski, M.R. Relation between mitochondrial membrane potential and ROS formation. Methods Mol. Biol. 2012, 810, 183–205. [Google Scholar] [CrossRef]
- Lu, H.; Yin, K.; Su, H.; Wang, D.; Zhang, Y.; Hou, L.; Li, J.B.; Wang, Y.; Xing, M. Polystyrene microplastics induce autophagy and apoptosis in birds lungs via PTEN/PI3K/AKT/mTOR. Environ. Toxicol. 2023, 38, 78–89. [Google Scholar] [CrossRef]
- Wang, Y.; Qi, H.; Liu, Y.; Duan, C.; Liu, X.; Xia, T.; Chen, D.; Piao, H.L.; Liu, H.X. The double-edged roles of ROS in cancer prevention and therapy. Theranostics 2021, 11, 4839–4857. [Google Scholar] [CrossRef]
- Antico Arciuch, V.G.; Galli, S.; Franco, M.C.; Lam, P.Y.; Cadenas, E.; Carreras, M.C.; Poderoso, J.J. Akt1 intramitochondrial cycling is a crucial step in the redox modulation of cell cycle progression. PLoS ONE 2009, 4, e7523. [Google Scholar] [CrossRef]
- Chen, Y.H.; Su, C.C.; Deng, W.; Lock, L.F.; Donovan, P.J.; Kayala, M.A.; Baldi, P.; Lee, H.C.; Chen, Y.; Wang, P.H. Mitochondrial Akt signaling modulated reprogramming of somatic cells. Sci. Rep. 2019, 9, 9919. [Google Scholar] [CrossRef]
- Kelley, T.W.; Graham, M.M.; Doseff, A.I.; Pomerantz, R.W.; Lau, S.M.; Ostrowski, M.C.; Franke, T.F.; Marsh, C.B. Macrophage colony-stimulating factor promotes cell survival through Akt/protein kinase B. J. Biol. Chem. 1999, 274, 26393–26398. [Google Scholar] [CrossRef] [PubMed]
- Goyal, A.; Wang, Y.; Graham, M.M.; Doseff, A.I.; Bhatt, N.Y.; Marsh, C.B. Monocyte survival factors induce Akt activation and suppress caspase-3. Am. J. Respir. Cell Mol. Biol. 2002, 26, 224–230. [Google Scholar] [CrossRef]
- Andjelkovic, M.; Alessi, D.R.; Meier, R.; Fernandez, A.; Lamb, N.J.; Frech, M.; Cron, P.; Cohen, P.; Lucocq, J.M.; Hemmings, B.A. Role of translocation in the activation and function of protein kinase B. J. Biol. Chem. 1997, 272, 31515–31524. [Google Scholar] [CrossRef]
- Liu, Y.; Pan, Y.; Xu, Y. Binding investigation of integrin alphavbeta3 with its inhibitors by SPR technology and molecular docking simulation. J. Biomol. Screen. 2010, 15, 131–137. [Google Scholar] [CrossRef]
- Liu, L.; Jiao, Y.; Yang, M.; Wu, L.; Long, G.; Hu, W. Network pharmacology, molecular docking and molecular dynamics to explore the potential immunomodulatory mechanisms of deer antler. Int. J. Mol. Sci. 2023, 24, 10370. [Google Scholar] [CrossRef]
- Patching, S.G. Surface plasmon resonance spectroscopy for characterisation of membrane protein-ligand interactions and its potential for drug discovery. Biochim. Biophys. Acta 2014, 1838, 43–55. [Google Scholar] [CrossRef]

| Compound Name | L-Clausenamide | |
|---|---|---|
| PubChem ID | 9904294 | |
| Lipinski rules | Molecular weight (<500) | 297.35 |
| H-bond acceptors (<10) | 3 | |
| H-bond donors (<5) | 2 | |
| MLogP (Log Po/w) (<4.15) | 1.60 | |
| Lipinski’s violations (<1) | 0 | |
| Blood–Brain Barrier (BBB) permeant | Yes | |
| Gastrointestinal (GI) absorption | High | |
| Bioavailability Score (>0.1) | 0.55 | |
| Log Kp (skin permeation) | −7.02 cm/s | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, Y.; Wang, N.; Luo, J.; Huang, Y.; Liu, B.; Brennan, C.S.; Xu, B.; Luo, J. Revealing the Improving Effect and Molecular Mechanism of L-Clausenamide in Combating the Acute Lung Injury: Insights from Network Pharmacology, Molecular Docking, and In Vitro Validation. Biology 2025, 14, 836. https://doi.org/10.3390/biology14070836
Fu Y, Wang N, Luo J, Huang Y, Liu B, Brennan CS, Xu B, Luo J. Revealing the Improving Effect and Molecular Mechanism of L-Clausenamide in Combating the Acute Lung Injury: Insights from Network Pharmacology, Molecular Docking, and In Vitro Validation. Biology. 2025; 14(7):836. https://doi.org/10.3390/biology14070836
Chicago/Turabian StyleFu, Yu, Nannan Wang, Jinhai Luo, Yanyi Huang, Baoning Liu, Charles S. Brennan, Baojun Xu, and Jincan Luo. 2025. "Revealing the Improving Effect and Molecular Mechanism of L-Clausenamide in Combating the Acute Lung Injury: Insights from Network Pharmacology, Molecular Docking, and In Vitro Validation" Biology 14, no. 7: 836. https://doi.org/10.3390/biology14070836
APA StyleFu, Y., Wang, N., Luo, J., Huang, Y., Liu, B., Brennan, C. S., Xu, B., & Luo, J. (2025). Revealing the Improving Effect and Molecular Mechanism of L-Clausenamide in Combating the Acute Lung Injury: Insights from Network Pharmacology, Molecular Docking, and In Vitro Validation. Biology, 14(7), 836. https://doi.org/10.3390/biology14070836









