Endocranial Morphology in Metopism
Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Material
2.2. Methods
2.2.1. Data Collection
2.2.2. Data Analyses
Intraobserver Measurement Error
Descriptive and Test Statistics
Machine Learning
- Classifiers
- Classifier Evaluation Schema
- Attribute Selection
- Attribute selection was performed using the Correlation-based Feature Selection method [25], implemented as the CfsSubsetEval algorithm in the Weka environment (https://www.cs.waikato.ac.nz/mL/weka/, accessed on 5 June 2025). Searching for the best subset of attributes was conducted via the Weka BestFirst algorithm [26];
- The attribute selection method was tested in 5-fold cross-validation mode. Thus, the attribute selection was performed on 5 different subsets of the training sets containing 80% of the examples;
- The previous step was repeated 10 times with randomly selected initial conditions. Thus, for each training dataset, 50 different subsets of selected attributes were learned from different subsets of the training datasets;
- Attribute importance (AI) for a classification task was assessed based on the frequency of selection of the attributes;
- According to the AI, values were assembled as subsets of attributes by intervals;
- The selected ML algorithms were applied to the attribute subsets defined by the AI intervals in 10 × 5-fold cross-validation mode.
3. Results
3.1. Intraobserver Measurement Error
3.2. Morphometry
3.3. Machine Learning
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Weinzweig, J.; Kirschner, R.E.; Farley, A.; Reiss, P.; Hunter, J.; Whitaker, L.A.; Bartlett, S.P. Metopic synostosis: Defining the temporal sequence of normal suture fusion and differentiating it from synostosis on the basis of computed tomography images. Plast. Reconst. Surg. 2003, 112, 1211–1218. [Google Scholar] [CrossRef] [PubMed]
- Ashley-Montagu, M.F. The Medio-Frontal Suture and the Problem of Metopism in the Primates. J. R. Anthropol. Inst. Gt Br. Irel. 1937, 67, 157–201. [Google Scholar]
- Vinchon, M. The metopic suture: Natural history. Neurochirurgie 2019, 65, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Torgersen, J. A roentgenological study of the metopic suture. Acta Radiol. 1950, 33, 1–11. [Google Scholar] [CrossRef]
- Berry, A.C. Factors affecting the incidence of non-metrical skeletal variants. J. Anat. 1975, 120, 519–535. [Google Scholar] [PubMed]
- Zdilla, M.J.; Russell, M.L.; Koons, A.W.; Bliss, K.N.; Mangus, K.R. Metopism: A study of the persistent metopic suture. J. Craniofac Surg. 2018, 29, 204–208. [Google Scholar] [CrossRef]
- Bryce, T.H. Observations on Metopism. J. Anat. 1917, 51, 153–166. [Google Scholar]
- Woo, J.-K. Racial and sexual differences in the frontal curvature and its relation to metopism. Am. J. Phys. Anthropol. 1949, 7, 215–226. [Google Scholar] [CrossRef]
- Nikolova, S.; Toneva, D.; Agre, G.; Lazarov, N. Data mining for peculiarities in the configuration of neurocranium when the metopic suture persists. Anthropol. Anz. 2020, 77, 89–107. [Google Scholar] [CrossRef] [PubMed]
- Nikolova, S.; Toneva, D.; Tasheva-Terzieva, E.; Lazarov, N. Cranial morphology in metopism: A comparative geometric morphometric study. Ann. Anat. 2022, 243, 151951. [Google Scholar] [CrossRef] [PubMed]
- Nikolova, S.; Toneva, D.; Lazarov, N. Neurocranium morphology in the presence of anatomic variations in the frontal and occipital bone. Anthropol. Sci. 2023, 131, 79–88. [Google Scholar] [CrossRef]
- Nikolova, S.; Toneva, D.; Agre, G.; Lazarov, N. Influence of persistent metopic suture on sagittal suture closure. Ann. Anat. 2022, 239, 151811. [Google Scholar] [CrossRef] [PubMed]
- Nikolova, S.; Toneva, D.; Georgiev, I.; Lazarov, N. Digital radiomorphometric analysis of the frontal sinus and assessment of the relation between persistent metopic suture and frontal sinus development. Am. J. Phys. Anthropol. 2018, 165, 492–506. [Google Scholar] [CrossRef] [PubMed]
- Castriota-Scanderbeg, A.; Dallapiccola, B. Abnormal Skeletal Phenotypes; Springer: Berlin/Heidelberg, Germany, 2005. [Google Scholar]
- Nikolova, S.; Toneva, D.; Georgiev, I. Cranial Base angulation in metopic and non-metopic cranial series. Acta Morphol. Anthropol. 2017, 24, 45–49. [Google Scholar]
- Nikolova, S.; Toneva, D.; Georgiev, I.; Lazarov, N. Sagittal suture maturation: Morphological reorganization, relation to aging, and reliability as an age-at-death indicator. Am. J. Phys. Anthropol. 2019, 169, 78–92. [Google Scholar] [CrossRef] [PubMed]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 9–18. Available online: http://palaeo-electronica.org/2001_1/past/issue1_01.htm (accessed on 5 June 2025).
- Domingos, P. The Master Algorithm: How the Quest for the Ultimate Learning Machine Will Remake Our World; Basic Books: New York, NY, USA, 2015. [Google Scholar]
- Clark, P.; Niblett, T. The CN2 induction algorithm. Mach. Learn. 1989, 3, 261–283. [Google Scholar] [CrossRef]
- Hosmer, D.; Lemeshow, S. Applied Logistic Regression; John Wiley & Sons: New York, NY, USA, 2000. [Google Scholar] [CrossRef]
- Mitchell, T. Machine Learning; McGraw Hill: New York, NY, USA, 1997. [Google Scholar]
- Cortes, C.; Vapnik, V. Support-vector networks. Mach. Learn. 1995, 20, 273–297. [Google Scholar] [CrossRef]
- Domingos, P.; Pazzani, M. Beyond independence: Conditions for optimality of the simple Bayesian classifier. In Proceedings of the 13th International Conference on Machine Learning, Bari, Italy, 28 June–1 July 1996; pp. 105–112. [Google Scholar]
- Breiman, L. Random Forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Hall, M. Correlation-Based Feature Selection for Machine Learning. Ph.D. Thesis, University of Waikato, Waikato, New Zealand, 1999. [Google Scholar]
- Witten, I.; Eibe, F.; Hall, M. Data Mining: Practical Machine Learning Tools and Techniques; Morgan Kaufmann: San Mateo, CA, USA, 2011. [Google Scholar]
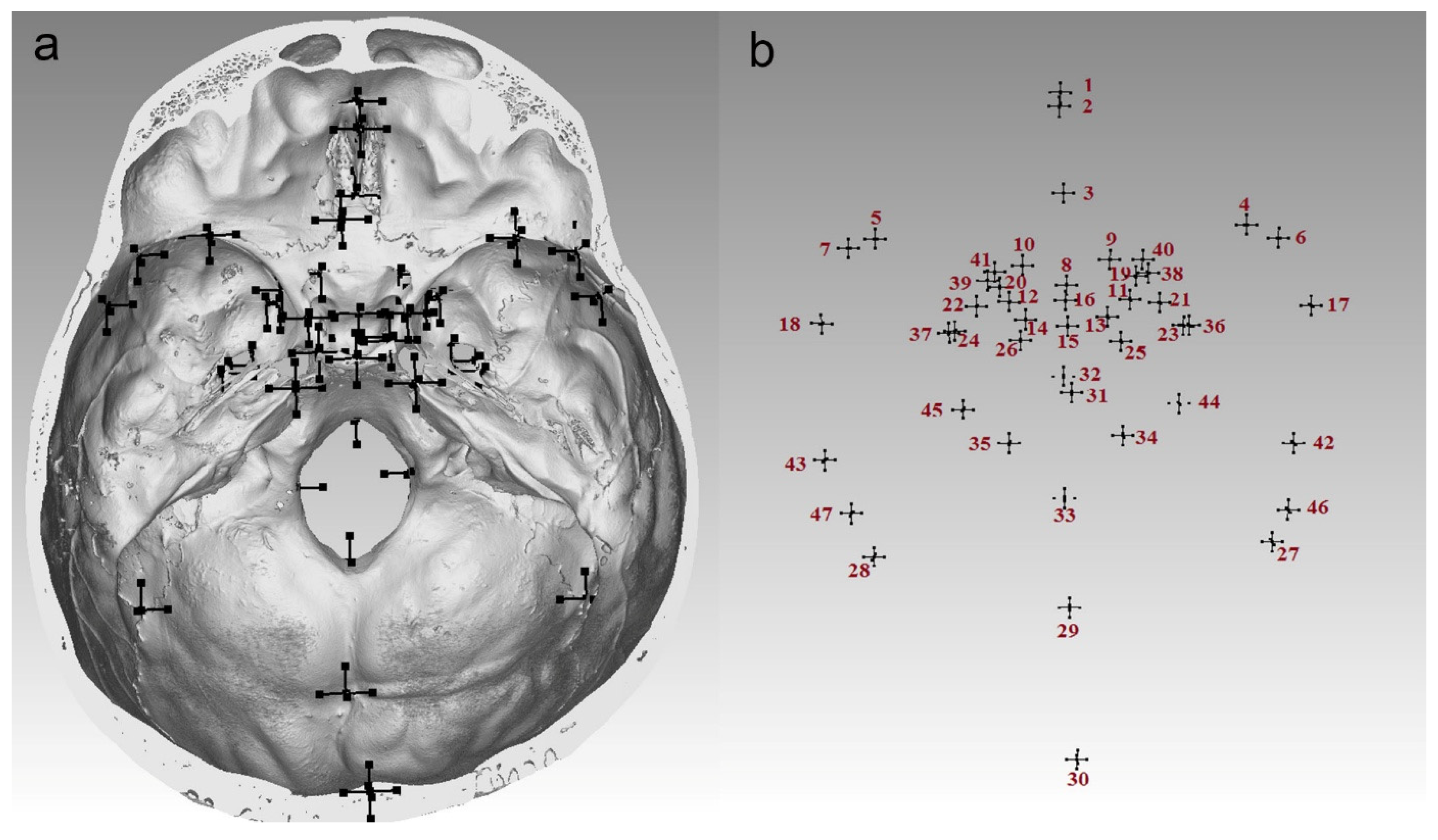
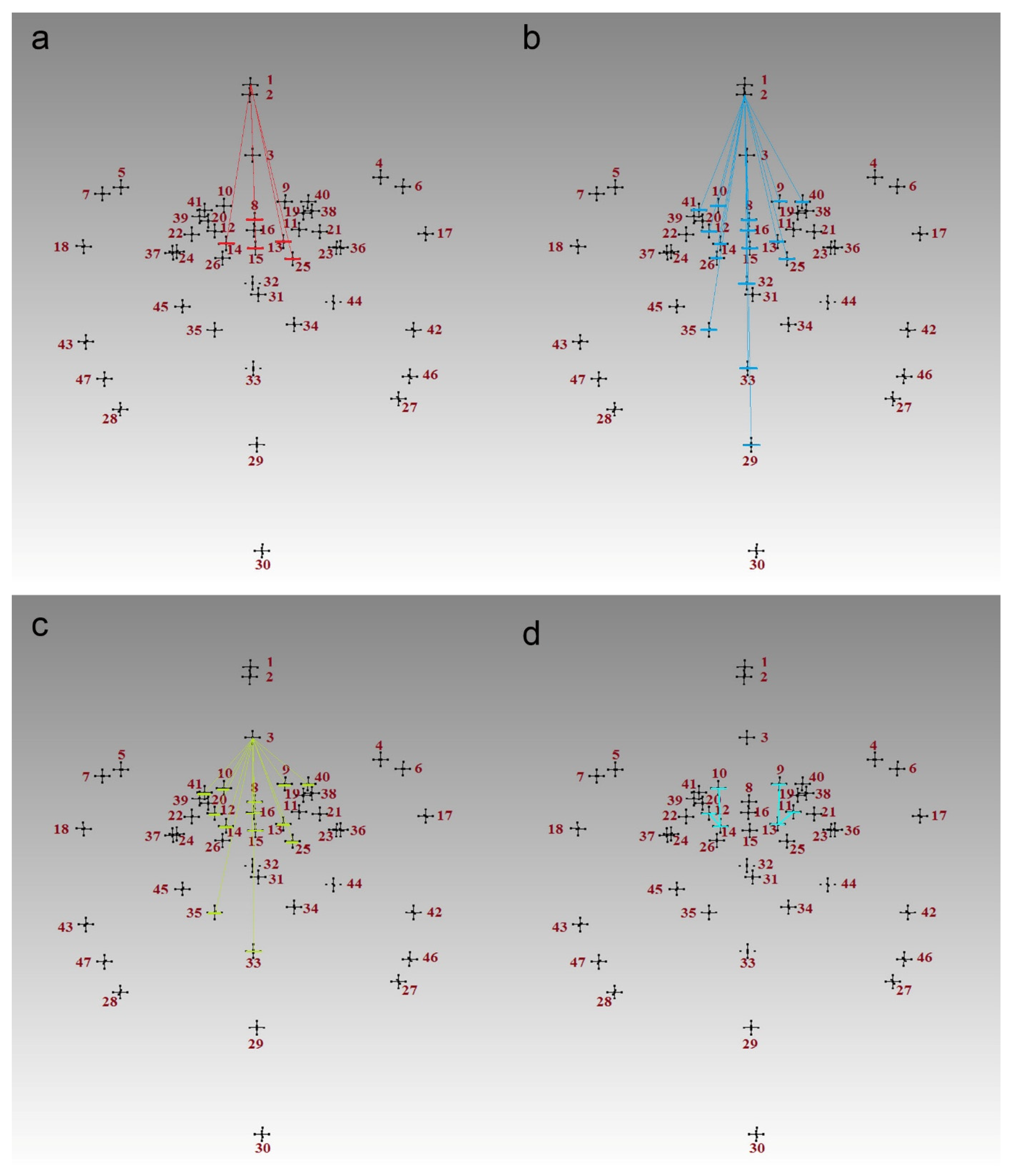
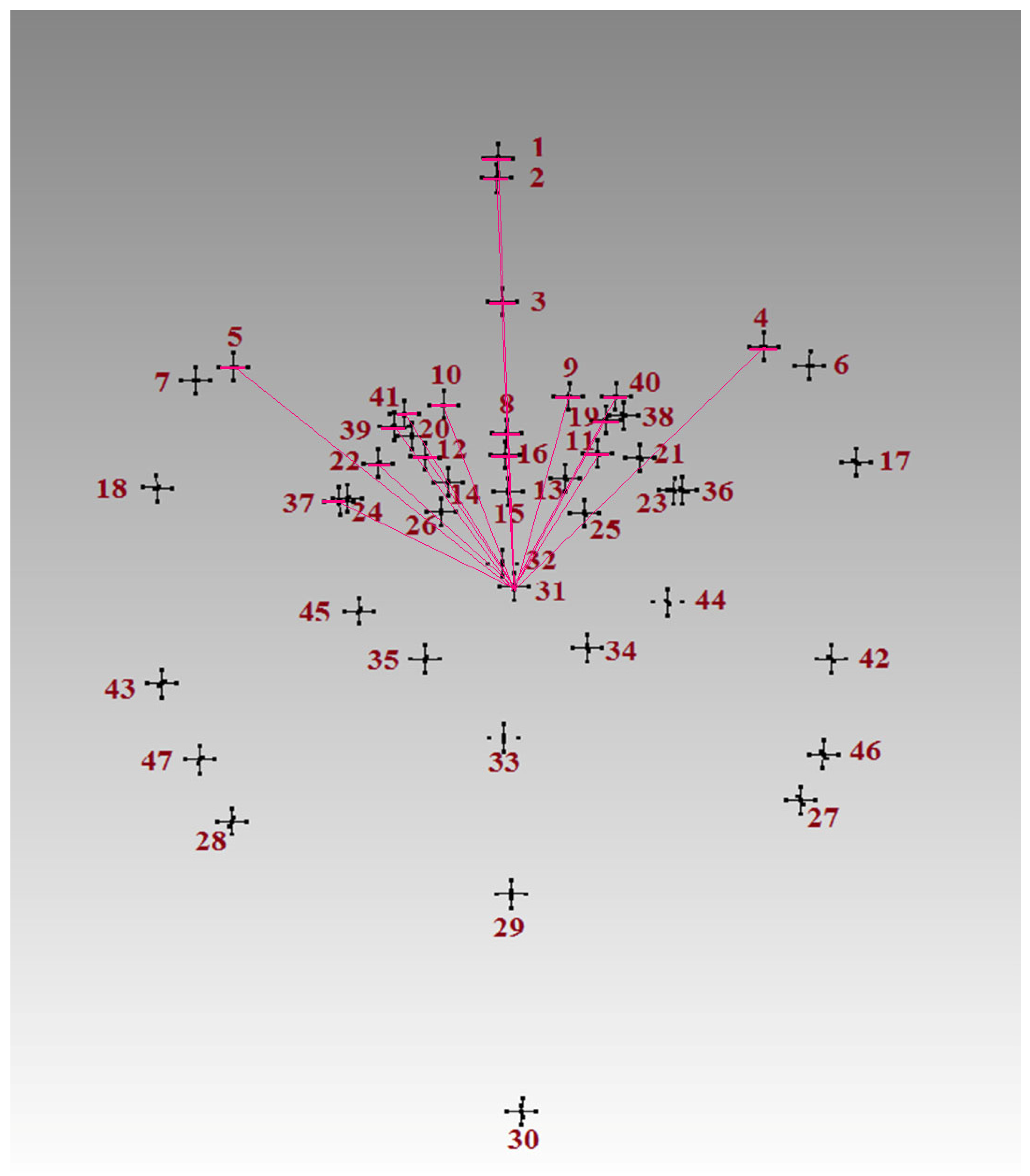

| Landmark | Description |
|---|---|
| Midsagittal | |
| Basion (ba) | A point on the anterior margin of the foramen magnum in the midsagittal plane |
| Crista galli (cg) | The most prominent point on the crista galli |
| Dorsum sellae (ds) | A point at the dorsum sellae in the midsagittal plane |
| Endobregma (eb) | The point of intersection of the coronal and sagittal sutures on the endocranial surface |
| Endolambda (el) | The point of intersection of the sagittal and lambdoid sutures on the endocranial surface |
| Crista galli base (cgb) | A point placed at the posterior ridge of the crista galli in its base |
| Foramen cecum (fc) | A point on the posterior margin of the foramen cecum in the midsagittal plane |
| Internal occipital protuberance (iop) | A point on the internal occipital protuberance |
| Opisthion (o) | A point on the posterior margin of the foramen magnum in the midsagittal plane |
| Sella (s) | A point at the center of the sella turcica in the midsagittal plane |
| Tuberculum sellae (ts) | A point on the tuberculum sellae in the midsagittal plane |
| Bilateral | |
| Anterior clinoid process (acp) | The most prominent point on the anterior clinoid process |
| Endoasterion (ea) | The point of intersection of the lambdoid, occipitomastoid and parietomastoid sutures on the endocranial surface |
| Endopterion (ept) | The meeting point between the greater wing of the sphenoid, the parietal bone and the temporal squama on the endocranial surface |
| Foramen magnum laterale (fml) | The most lateral point on the margin of the foramen magnum |
| Foramen ovale mediale (fom) | The most medial point on the foramen ovale on the endocranial surface |
| Foramen ovale laterale (fol) | The most lateral point on the foramen ovale on the endocranial surface |
| Foramen rotundum mediale (frm) | A point on the medial ridge of the foramen rotundum |
| Foramen rotundum laterale (frl) | A point on the lateral ridge of the foramen rotundum |
| Foramen spinosum (fs) | A point on the foramen spinosum margin on the endocranial surface |
| Greater wing of the sphenoid (gw) | The meeting point between the greater wing of the sphenoid, the parietal bone and the frontal bone on the endocranial surface |
| Lesser wing of the sphenoid (lw) | The sharpest point at the site of the articulation with the frontal bone |
| Occipitomastoid suture (oms) | A point at the occipitomastoid suture, posterolateral to the jugular foramen on the endocranial surface |
| Optic canal (oc) | A point on the medial ridge of the optic canal |
| Posterior clinoid process (pcp) | The most prominent point on the posterior clinoid process |
| Parietal notch (pn) | The point of intersection of the squamous suture and the parietomastoid suture on the endocranial surface |
| Petrous apex (pa) | A point at the apex of the petrous part of the temporal bone |
| Superior orbital fissure (sof) | The most inferior point on the superior orbital fissure on the endocranial surface |
| Superior petrous margin (spm) | A point at the intersection of the superior margin of the petrous part of the temporal bone, formed between the anterior and posterior surfaces, with the parietomastoid suture, on the endocranial surface |
| Algorithm | CA | Class 1 | Class 2 |
|---|---|---|---|
| Majority | 80.00 | 100.00 | 0.00 |
| Naive Bayes 69 attributes with AI > 0 | 85.31 ± 1.12 | 94.00 ± 1.44 | 50.44 ± 3.52 |
| Neural Network 69 attributes with AI > 0 | 82.35 ± 2.06 | 91.72 ± 1.52 | 50.36 ± 5.35 |
| SVM 69 attributes with AI > 0 | 83.09 ± 0.97 | 98.27 ± 0.50 | 22.38 ± 4.49 |
| Logistic regression 69 attributes with AI > 0 | 81.87 ± 1.93 | 90.42 ± 1.83 | 47.61 ± 5.26 |
| Random Forest 18 attributes with AI > 0.04 | 81.48 ± 1.05 | 95.92 ± 0.82 | 23.69 ± 3.30 |
| CN2 Rules 18 attributes with AI > 0.04 | 79.87 ± 1.74 | 93.77 ± 1.92 | 24.37 ± 5.86 |
| Algorithm | CA | Class 1 | Class 2 |
|---|---|---|---|
| Majority | 80.00 | 100.00 | 0.00 |
| Naive Bayes | 81.35 ± 1.49 | 90.82 ± 1.60 | 43.72 ± 4.75 |
| Neural Network | 79.23 ± 1.65 | 90.05 ± 1.00 | 35.86 ± 5.86 |
| SVM | 80.31 ± 1.12 | 97.61 ± 1.04 | 9.57 ± 2.78 |
| Logistic regression | 77.87 ± 1.14 | 88.75 ± 1.17 | 34.35 ± 4.10 |
| Random Forest | 78.74 ± 0.66 | 94.24 ± 0.61 | 16.74 ± 2.18 |
| CN2 Rules | 77.65 ± 1.70 | 93.44 ± 1.93 | 13.70 ± 2.93 |
| Rule Length | Rule Quality | Predicted Class | Distribution * Class 1:Class 2 | Rule |
|---|---|---|---|---|
| 3 | 0.98 | 1 | 62:0 | IF 13-6 ≤ 46.0 AND 31-10 > 85.0 AND 2-35 > 80.0 THEN Class = 1 |
| 4 | 0.98 | 1 | 43:0 | IF 8-3 ≤ 25.0 AND 31-10 > 86.0 AND 13-2 > 50.0 AND 12-2 ≤ 75.0 THEN Class = 1 |
| 3 | 0.96 | 1 | 26:0 | IF 13-2 ≤ 52.0 AND 32-45 > 35.0 AND 23-44 ≤ 35.0 THEN Class = 1 |
| 2 | 0.94 | 1 | 16:0 | IF 25-1 ≤ 65.0 AND 21-4 > 38.0 THEN Class = 1 |
| 2 | 0.93 | 1 | 12:0 | IF 31-1 > 97.0 AND 31-1 ≤ 106.0 THEN Class = 1 |
| 3 | 0.93 | 2 | 0:12 | IF 13-3 > 36.0 AND 21-4 ≤ 37.0 AND 8-3 > 25.0 THEN Class = 2 |
| 4 | 0.91 | 2 | 0:9 | IF 10-41 > 12.0 AND 2-31 ≤ 85.0 AND 13-3 > 34.0 AND 25-1 > 66.0 THEN Class = 2 |
| 4 | 0.89 | 2 | 0:7 | IF 2-1 ≤ 5.0 AND 13-6 > 50.0 AND 2-1 > 3.0 AND 13-6 ≤ 53.0 THEN Class = 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nikolova, S.; Toneva, D.; Agre, G. Endocranial Morphology in Metopism. Biology 2025, 14, 835. https://doi.org/10.3390/biology14070835
Nikolova S, Toneva D, Agre G. Endocranial Morphology in Metopism. Biology. 2025; 14(7):835. https://doi.org/10.3390/biology14070835
Chicago/Turabian StyleNikolova, Silviya, Diana Toneva, and Gennady Agre. 2025. "Endocranial Morphology in Metopism" Biology 14, no. 7: 835. https://doi.org/10.3390/biology14070835
APA StyleNikolova, S., Toneva, D., & Agre, G. (2025). Endocranial Morphology in Metopism. Biology, 14(7), 835. https://doi.org/10.3390/biology14070835








