Urolithin A Protects Ovarian Reserve Via Inhibiting PI3K/Akt Signaling and Preventing Chemotherapy-Induced Follicle Apoptosis
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Mouse Ovary Culture
2.3. Establishment of In Vivo Chemotherapy Model in Newborn Mice
2.4. Histological and Morphological Analysis
2.5. RNA Sequencing
2.6. RNA Isolation and Quantitative Real-Time PCR (qRT-PCR)
2.7. Immunofluorescent Staining
2.8. Bromodeoxyuridine (BrdU) Incorporation Assay
2.9. Western Blot Analysis
2.10. Statistical Analysis
3. Results
3.1. Urolithin A Maintains Primordial Follicle Dormancy In Vitro
3.2. Urolithin A Treatment Inhibits Granulosa Cell Proliferation
3.3. Urolithin A Maintains Primordial Follicle Dormancy Via Inhibiting PI3K/Akt Signaling
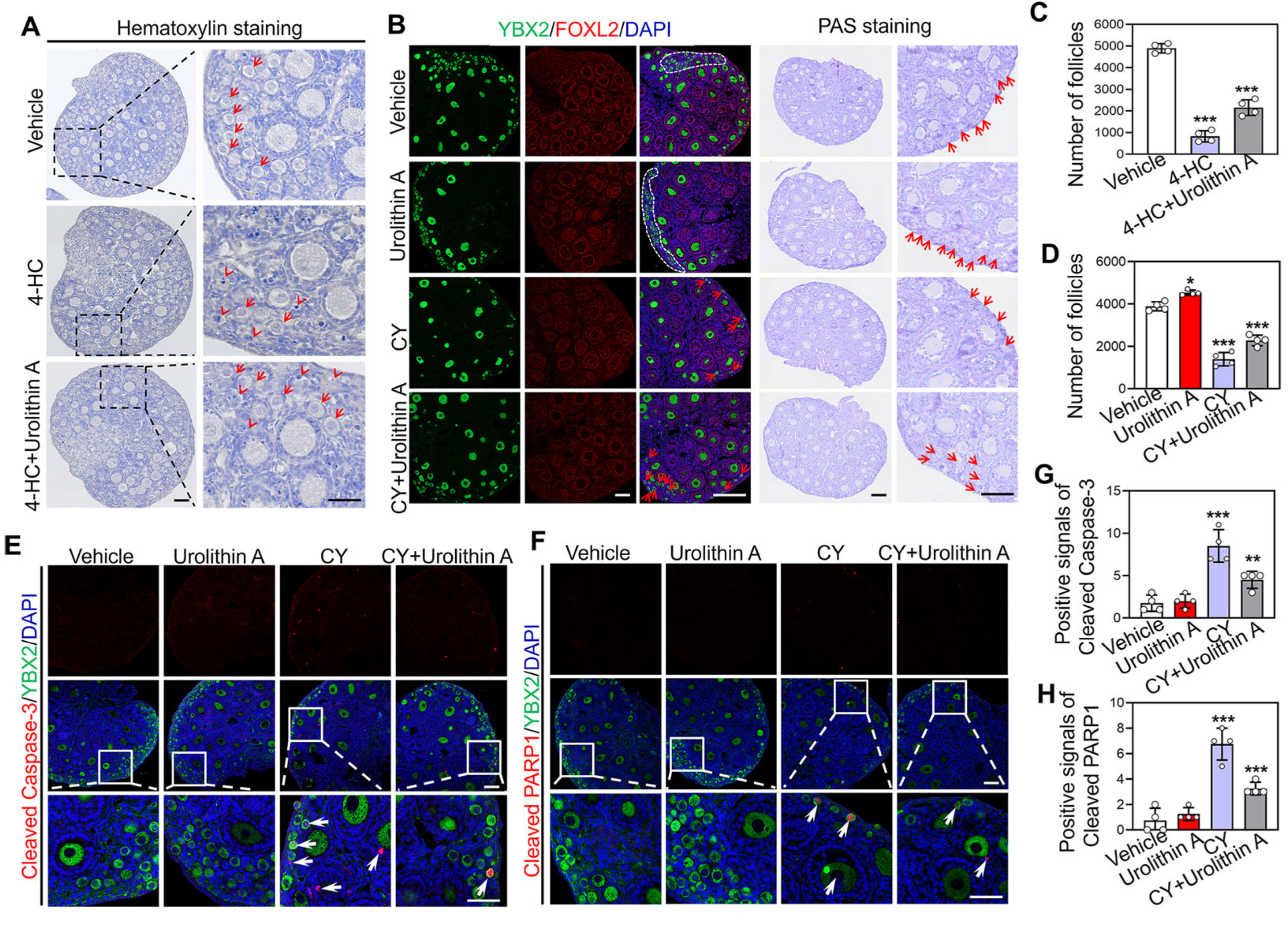
3.4. Urolithin A Reverses Cyclophosphamide-Induced Loss of Primordial Follicles
3.5. Transcriptome Reveals the Protective Effect of Urolithin A on Cyclophosphamide Induced Ovarian Damage
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Akt | Protein kinase B |
| Aldh3b2 | Aldehyde dehydrogenase 3 family, member B2 |
| AMPK | AMP-activated protein kinase |
| Bax | BCL-2-associated X protein |
| BrdU | Bromodeoxyuridine |
| BSA | Bovine serum albumin |
| Caspase-3 | Cysteinyl aspartate specific proteinase |
| Ccn1 | Cellular communication network factor 1 |
| Cdkn1a | Cyclin-dependent kinase inhibitor 1A |
| CY | cyclophosphamide |
| DAPI | 4,6-Diamidino-2-phenylindole |
| DDX4 | DEAD-box helicase 4 |
| DMSO | Dimethyl sulfoxide |
| dpp | Days postpartum |
| EA | Ellagic acid |
| ETs | Ellagitannins |
| Fas | Factor-related apoptosis |
| FOXO3a | Forkhead Box O3a |
| Gdf9 | Growth differentiation factor 9 |
| GO | Gene ontology |
| Grp68 | G protein-coupled receptor 68 |
| Hsd17b2 | hydroxysteroid 17-beta dehydrogenase 2 |
| KEGG | Kyoto encyclopedia of genes and genomes |
| KITL | Proto-oncogenic receptor tyrosine kinase ligand |
| Mdm2 | Mouse double minute 2 homolog |
| mTOR | Mammalian target of rapamycin |
| PARP1 | poly (ADP-ribose) polymerase family, member 1 |
| PCNA | Proliferating cell nuclear antigen |
| PFA | Paraformaldehyde |
| PGC-1α | Peroxisome proliferator-activated receptor γ coactivator α |
| PI3K | Phosphatidylinositol-3-kinase |
| PIP2 | Phosphatidylinositol 4,5-bisphosphate |
| PIP3 | Phosphatidylinositol 3,4,5-trisphosphate |
| PIK3CG | Phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit γ |
| Plcg2 | Phospholipase C gamma 2 |
| PMSF | Phenylmethanesulfonyl fluoride |
| POF | Premature ovarian failure |
| POI | Premature ovarian insufficiency |
| Prkcq | Protein kinase C theta |
| PVDF | Polyvinylidene fluoride |
| RIPA | Radio Immunoprecipitation assay lysis buffer |
| TBST | Tris buffer saline Tween-20 |
| Trim29 | Tripartite motif-containing 29 |
| Trp73 | Transformation related-protein 73 |
| Zp3 | Zona pellucida 3 |
| 4-HC | 4-Hydroperoxycyclophosphamide |
References
- Wang, W.; Liu, H.; Liu, S.; Hao, T.; Wei, Y.; Wei, H.; Zhou, W.; Zhang, X.; Hao, X.; Zhang, M. Oocyte-specific deletion of eukaryotic translation initiation factor 5 causes apoptosis of mouse oocytes within the early-growing follicles by mitochondrial fission defect-reactive oxygen species-DNA damage. Clin. Transl. Med. 2024, 14, e1791. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liu, K. Cellular and molecular regulation of the activation of mammalian primordial follicles: Somatic cells initiate follicle activation in adulthood. Hum. Reprod. Update 2015, 21, 779–786. [Google Scholar] [CrossRef]
- Kallen, A.; Polotsky, A.J.; Johnson, J. Untapped Reserves: Controlling Primordial Follicle Growth Activation. Trends Mol. Med. 2018, 24, 319–331. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, Y.L.; Zhao, L.W.; Guo, J.X.; Yu, J.L.; Ji, S.Y.; Cao, L.R.; Zhang, S.Y.; Shen, L.; Ou, X.H.; et al. Mammalian nucleolar protein DCAF13 is essential for ovarian follicle maintenance and oocyte growth by mediating rRNA processing. Cell Death Differ. 2019, 26, 1251–1266. [Google Scholar] [CrossRef]
- Telfer, E.E.; Grosbois, J.; Odey, Y.L.; Rosario, R.; Anderson, R.A. Making a good egg: Human oocyte health, aging, and in vitro development. Physiol. Rev. 2023, 103, 2623–2677. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Risal, S.; Gorre, N.; Busayavalasa, K.; Li, X.; Shen, Y.; Bosbach, B.; Brannstrom, M.; Liu, K. Somatic cells initiate primordial follicle activation and govern the development of dormant oocytes in mice. Curr. Biol. 2014, 24, 2501–2508. [Google Scholar] [CrossRef]
- Terren, C.; Nisolle, M.; Munaut, C. Pharmacological inhibition of the PI3K/PTEN/Akt and mTOR signalling pathways limits follicle activation induced by ovarian cryopreservation and in vitro culture. J. Ovarian Res. 2021, 14, 95. [Google Scholar] [CrossRef]
- Vo, K.C.T.; Kawamura, K. In Vitro Activation Early Follicles: From the Basic Science to the Clinical Perspectives. Int. J. Mol. Sci. 2021, 22, 3785. [Google Scholar] [CrossRef]
- Liu, S.; Wang, W.; Liu, H.; Wei, H.; Weng, Y.; Zhou, W.; Zhang, X.; He, S.; Chen, Y.; Wang, Y.; et al. Berberine promotes primordial follicle activation and increases ovulated oocyte quantity in aged mice. Mol. Med. Camb. Mass. 2024, 30, 251. [Google Scholar] [CrossRef]
- Reddy, P.; Liu, L.; Adhikari, D.; Jagarlamudi, K.; Rajareddy, S.; Shen, Y.; Du, C.; Tang, W.; Hamalainen, T.; Peng, S.L.; et al. Oocyte-specific deletion of Pten causes premature activation of the primordial follicle pool. Science 2008, 319, 611–613. [Google Scholar] [CrossRef]
- Stuenkel, C.A.; Gompel, A. Primary Ovarian Insufficiency. New Engl. J. Med. 2023, 388, 154–163. [Google Scholar] [CrossRef]
- Wang, Y.; Fu, Y.; Ghazi, P.; Gao, Q.; Tian, T.; Kong, F.; Zhan, S.; Liu, C.; Bloom, D.E.; Qiao, J. Prevalence of intimate partner violence against infertile women in low-income and middle-income countries: A systematic review and meta-analysis. Lancet Glob. Health 2022, 10, e820–e830. [Google Scholar] [CrossRef] [PubMed]
- Ke, H.; Tang, S.; Guo, T.; Hou, D.; Jiao, X.; Li, S.; Luo, W.; Xu, B.; Zhao, S.; Li, G.; et al. Landscape of pathogenic mutations in premature ovarian insufficiency. Nat. Med. 2023, 29, 483–492. [Google Scholar] [CrossRef]
- Touraine, P.; Chabbert-Buffet, N.; Plu-Bureau, G.; Duranteau, L.; Sinclair, A.H.; Tucker, E.J. Premature ovarian insufficiency. Nat. Rev. Dis. Primers 2024, 10, 63. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Xue, L.; Tang, W.; Xiong, J.; Chen, D.; Dai, Y.; Wu, C.; Wei, S.; Dai, J.; Wu, M.; et al. Ovarian microenvironment: Challenges and opportunities in protecting against chemotherapy-associated ovarian damage. Hum. Reprod. Update 2024, 30, 614–647. [Google Scholar] [CrossRef] [PubMed]
- Emadi, A.; Jones, R.J.; Brodsky, R.A. Cyclophosphamide and cancer: Golden anniversary. Nat. Rev. Clin. Oncol. 2009, 6, 638–647. [Google Scholar] [CrossRef] [PubMed]
- Su, C.; Zhang, R.; Zhang, X.; Lv, M.; Liu, X.; Ao, K.; Hao, J.; Mu, Y.L. Dingkun Pill modulate ovarian function in chemotherapy-induced premature ovarian insufficiency mice by regulating PTEN/PI3K/AKT/FOXO3a signaling pathway. J. Ethnopharmacol. 2023, 315, 116703. [Google Scholar] [CrossRef]
- Bhardwaj, J.K.; Bikal, P.; Sachdeva, S.N. Chemotherapeutic drugs induced female reproductive toxicity and treatment strategies. J. Biochem. Mol. Toxicol. 2023, 37, e23371. [Google Scholar] [CrossRef]
- Zou, Y.; Li, Z.; Lin, Y.; Zheng, Y.; Liu, Z.; Li, Y.; Huang, L.; Chen, Z.; Zhu, L. Shanyao regulates the PI3K/AKT/P21 pathway to promote oogonial stem cell proliferation and stemness restoration to alleviate premature ovarian insufficiency. J. Ethnopharmacol. 2025, 340, 119168. [Google Scholar] [CrossRef]
- Xie, Q.; Liao, Q.; Wang, L.; Zhang, Y.; Chen, J.; Bai, H.; Li, K.; Ai, J. The Dominant Mechanism of Cyclophosphamide-Induced Damage to Ovarian Reserve: Premature Activation or Apoptosis of Primordial Follicles? Reprod. Sci. Thousand Oaks Calif. 2024, 31, 30–44. [Google Scholar] [CrossRef]
- Huang, C.C.; Chou, C.H.; Yang, Y.S.; Ho, H.N.; Shun, C.T.; Wen, W.F.; Chen, S.U.; Chen, M.J. Metformin: A novel promising option for fertility preservation during cyclophosphamide-based chemotherapy. Mol. Hum. Reprod. 2021, 27, gaaa084. [Google Scholar] [CrossRef] [PubMed]
- Kashi, O.; Roness, H.; Spector, I.; Derech-Haim, S.; Meirow, D. Dual suppression of follicle activation pathways completely prevents the cyclophosphamide-induced loss of ovarian reserve. Hum. Reprod. 2023, 38, 1086–1098. [Google Scholar] [CrossRef] [PubMed]
- Oktay, K.; Harvey, B.E.; Partridge, A.H.; Quinn, G.P.; Reinecke, J.; Taylor, H.S.; Wallace, W.H.; Wang, E.T.; Loren, A.W. Fertility Preservation in Patients With Cancer: ASCO Clinical Practice Guideline Update. J. Clin. Oncol. 2018, 36, 1994–2001. [Google Scholar] [CrossRef]
- Christianson, M.S.; Oktay, K. Advances in fertility-preservation surgery: Navigating new frontiers. Fertil. Steril. 2019, 112, 438–445. [Google Scholar] [CrossRef]
- Zhang, M.; Cui, S.; Mao, B.; Zhang, Q.; Zhao, J.; Zhang, H.; Tang, X.; Chen, W. Ellagic acid and intestinal microflora metabolite urolithin A: A review on its sources, metabolic distribution, health benefits, and biotransformation. Crit. Rev. Food Sci. Nutr. 2023, 63, 6900–6922. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, M.; Alvarenga, L.; Cardozo, L.; Baptista, B.G.; Nascimento, D.; Esgalhado, M.; Mafra, D. Urolithin as a Metabolite of Ellagitannins and Ellagic Acid from Fruits and Nuts Produced by the Gut Microbiota: Its Role on Non-Communicable Diseases. Curr. Nutr. Rep. 2025, 14, 55. [Google Scholar] [CrossRef]
- D’Amico, D.; Andreux, P.A.; Valdes, P.; Singh, A.; Rinsch, C.; Auwerx, J. Impact of the Natural Compound Urolithin A on Health, Disease, and Aging. Trends Mol. Med. 2021, 27, 687–699. [Google Scholar] [CrossRef]
- Andreux, P.A.; Blanco-Bose, W.; Ryu, D.; Burdet, F.; Ibberson, M.; Aebischer, P.; Auwerx, J.; Singh, A.; Rinsch, C. The mitophagy activator urolithin A is safe and induces a molecular signature of improved mitochondrial and cellular health in humans. Nat. Metab. 2019, 1, 595–603. [Google Scholar] [CrossRef]
- Liu, S.; D’Amico, D.; Shankland, E.; Bhayana, S.; Garcia, J.M.; Aebischer, P.; Rinsch, C.; Singh, A.; Marcinek, D.J. Effect of Urolithin A Supplementation on Muscle Endurance and Mitochondrial Health in Older Adults: A Randomized Clinical Trial. JAMA Netw. Open 2022, 5, e2144279. [Google Scholar] [CrossRef]
- Avila-Galvez, M.A.; Gimenez-Bastida, J.A.; Gonzalez-Sarrias, A.; Espin, J.C. Tissue deconjugation of urolithin A glucuronide to free urolithin A in systemic inflammation. Food Funct. 2019, 10, 3135–3141. [Google Scholar] [CrossRef]
- Xiao, Y.; Li, K.; Zhang, H.; Li, Y.; Han, L.; Liu, H.; Wang, M. The profile of buckwheat tannins based on widely targeted metabolome analysis and pharmacokinetic study of ellagitannin metabolite urolithin A. Lwt 2022, 156, 113069. [Google Scholar] [CrossRef]
- Lin, I.C.; Wu, J.Y.; Fang, C.Y.; Wang, S.C.; Liu, Y.W.; Ho, S.T. Absorption and Metabolism of Urolithin A and Ellagic Acid in Mice and Their Cytotoxicity in Human Colorectal Cancer Cells. Evid. Based Complement. Altern. Med. 2023, 2023, 8264716. [Google Scholar] [CrossRef] [PubMed]
- Luan, P.; D’Amico, D.; Andreux, P.A.; Laurila, P.P.; Wohlwend, M.; Li, H.; Imamura de Lima, T.; Place, N.; Rinsch, C.; Zanou, N.; et al. Urolithin A improves muscle function by inducing mitophagy in muscular dystrophy. Sci. Transl. Med. 2021, 13, abb0319. [Google Scholar] [CrossRef]
- Zhao, H.; Song, G.; Zhu, H.; Qian, H.; Pan, X.; Song, X.; Xie, Y.; Liu, C. Pharmacological Effects of Urolithin A and Its Role in Muscle Health and Performance: Current Knowledge and Prospects. Nutrients 2023, 15, 4441. [Google Scholar] [CrossRef]
- Liu, J.; Jiang, J.; Qiu, J.; Wang, L.; Zhuo, J.; Wang, B.; Sun, D.; Yu, S.; Lou, H. Urolithin A protects dopaminergic neurons in experimental models of Parkinson’s disease by promoting mitochondrial biogenesis through the SIRT1/PGC-1alpha signaling pathway. Food Funct. 2022, 13, 375–385. [Google Scholar] [CrossRef]
- Cota, V.; Sohrabi, S.; Kaletsky, R.; Murphy, C.T. Oocyte mitophagy is critical for extended reproductive longevity. PLoS Genet. 2022, 18, e1010400. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Mu, X.; Ji, X.; Chen, X.; Geng, Y.; Zhang, Y.; Liu, Q.; Li, F.; Wang, Y.; He, J. In-utero exposure to HT-2 toxin affects meiotic progression and early oogenesis in foetal oocytes by increasing oxidative stress. Environ. Pollut. 2021, 279, 116917. [Google Scholar] [CrossRef]
- Gao, Z.; Yi, W.; Tang, J.; Sun, Y.; Huang, J.; Lan, T.; Dai, X.; Xu, S.; Jin, Z.G.; Wu, X. Urolithin A protects against acetaminophen-induced liver injury in mice via sustained activation of Nrf2. Int. J. Biol. Sci. 2022, 18, 2146–2162. [Google Scholar] [CrossRef]
- Casedas, G.; Les, F.; Choya-Foces, C.; Hugo, M.; Lopez, V. The Metabolite Urolithin-A Ameliorates Oxidative Stress in Neuro-2a Cells, Becoming a Potential Neuroprotective Agent. Antioxidants 2020, 9, 177. [Google Scholar] [CrossRef]
- Ma, S.; Wu, Q.; Wu, W.; Tian, Y.; Zhang, J.; Chen, C.; Sheng, X.; Zhao, F.; Ding, L.; Wang, T.; et al. Urolithin A Hijacks ERK1/2-ULK1 Cascade to Improve CD8(+) T Cell Fitness for Antitumor Immunity. Adv. Sci. 2024, 11, e2310065. [Google Scholar] [CrossRef]
- Gong, Q.Y.; Cai, L.; Jing, Y.; Wang, W.; Yang, D.X.; Chen, S.W.; Tian, H.L. Urolithin A alleviates blood-brain barrier disruption and attenuates neuronal apoptosis following traumatic brain injury in mice. Neural Regen. Res. 2022, 17, 2007–2013. [Google Scholar] [CrossRef] [PubMed]
- Remadevi, V.; Jaikumar, V.S.; Vini, R.; Krishnendhu, B.; Azeez, J.M.; Sundaram, S.; Sreeja, S. Urolithin A, induces apoptosis and autophagy crosstalk in Oral Squamous Cell Carcinoma via mTOR /AKT/ERK1/2 pathway. Phytomedicine 2024, 130, 155721. [Google Scholar] [CrossRef]
- Rodriguez-Garcia, A.; Ancos-Pintado, R.; Garcia-Vicente, R.; Ortiz-Ruiz, A.; Arroyo, A.; Navarro, M.A.; Morales, M.L.; Guevara-Ramirez, P.; Justo, P.; Lopez-Munoz, N.; et al. Microbiota-derived urolithin A in monoclonal gammopathies and multiple myeloma therapy. Microbiome 2025, 13, 56. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Pei, J.; Wang, X.; Tai, S.; Tang, L.; Hu, X. Gut bacterial metabolite Urolithin A inhibits myocardial fibrosis through activation of Nrf2 pathway in vitro and in vivo. Mol. Med. Camb. Mass. 2022, 28, 19. [Google Scholar] [CrossRef] [PubMed]
- El-Wetidy, M.S.; Ahmad, R.; Rady, I.; Helal, H.; Rady, M.I.; Vaali-Mohammed, M.A.; Al-Khayal, K.; Traiki, T.B.; Abdulla, M.H. Urolithin A induces cell cycle arrest and apoptosis by inhibiting Bcl-2, increasing p53-p21 proteins and reactive oxygen species production in colorectal cancer cells. Cell Stress Chaperones 2021, 26, 473–493. [Google Scholar] [CrossRef]
- Liang, Y.; Zheng, Y.; Yang, J.; Ke, J.; Cheng, K. Design, synthesis and bioactivity evaluation of a series of quinazolinone derivatives as potent PI3Kgamma antagonist. Bioorg Med. Chem. 2023, 84, 117261. [Google Scholar] [CrossRef]
- Yin, X.; Wang, J.; Ge, M.; Feng, X.; Zhang, G. Designing Small Molecule PI3Kgamma Inhibitors: A Review of Structure-Based Methods and Computational Approaches. J. Med. Chem. 2024, 67, 10530–10547. [Google Scholar] [CrossRef]
- Liu, R.; Huang, J.; Ge, Y.; Liu, S.; Huang, T.; Cai, H.; Pan, B.; Zhang, Q.; Yang, P.; Liao, M.; et al. Inhibition of Phosphatidylinositol 3-Kinase gamma by IPI-549 Attenuates Abdominal Aortic Aneurysm Formation in Mice. Eur. J. Vasc. Endovasc. Surg. 2020, 60, 254–263. [Google Scholar] [CrossRef]
- Gutfreund, N.; Tuppi, M.; Schafer, B.; Klinger, F.G.; Kirchhof, A.; Hofmann, A.; Dikic, I.; Dotsch, V. Mechanisms of chemotherapy-induced oocyte death through activation of TAp63alpha. Reproduction 2025, 169. [Google Scholar] [CrossRef]
- Erden, M.; Oktay, K.H. Does gonadotoxic chemotherapy deplete the ovarian reserve through activation of primordial follicles? Hum. Reprod. 2025, 40, 571–579. [Google Scholar] [CrossRef]
- Emori, C.; Boucher, Z.; Bolcun-Filas, E. CHEK2 signaling is the key regulator of oocyte survival after chemotherapy. Sci. Adv. 2023, 9, eadg0898. [Google Scholar] [CrossRef]
- Ma, K.; Chen, Y.; Fan, X.; Yuan, Y.; Wang, K.; Tian, C.; Li, M. Dingkun Pill replenishes diminished ovarian reserve through the PI3K/AKT/mTOR signaling pathway in TWP-induced mice. J. Ethnopharmacol. 2020, 262, 112993. [Google Scholar] [CrossRef]
- Totiger, T.M.; Srinivasan, S.; Jala, V.R.; Lamichhane, P.; Dosch, A.R.; Gaidarski, A.A., 3rd; Joshi, C.; Rangappa, S.; Castellanos, J.; Vemula, P.K.; et al. Urolithin A, a Novel Natural Compound to Target PI3K/AKT/mTOR Pathway in Pancreatic Cancer. Mol. Cancer Ther. 2019, 18, 301–311. [Google Scholar] [CrossRef]
- Wang, X.; Ma, C.; Mi, K.; Cao, X.; Tan, Y.; Yuan, H.; Ren, J.; Liang, X. Urolithin A attenuates Doxorubicin-induced cardiotoxicity by enhancing PINK1-regulated mitophagy via Ambra1. Chem. Biol. Interact. 2025, 406, 111363. [Google Scholar] [CrossRef]
- Gonfloni, S.; Jodice, C.; Gustavino, B.; Valentini, E. DNA Damage Stress Response and Follicle Activation: Signaling Routes of Mammalian Ovarian Reserve. Int. J. Mol. Sci. 2022, 23, 14379. [Google Scholar] [CrossRef] [PubMed]
- Szymanska, K.J.; Tan, X.; Oktay, K. Unraveling the mechanisms of chemotherapy-induced damage to human primordial follicle reserve: Road to developing therapeutics for fertility preservation and reversing ovarian aging. Mol. Hum. Reprod. 2020, 26, 553–566. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Cordeiro, M.H.; Serna, V.A.; Ebbert, K.; Butler, L.M.; Sinha, S.; Mills, A.A.; Woodruff, T.K.; Kurita, T. Rescue of platinum-damaged oocytes from programmed cell death through inactivation of the p53 family signaling network. Cell Death Differ. 2013, 20, 987–997. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Xue, L.; Chen, Y.; Tang, W.; Guo, Y.; Xiong, J.; Chen, D.; Zhu, Q.; Fu, F.; Wang, S. Inhibition of checkpoint kinase prevents human oocyte apoptosis induced by chemotherapy and allows enhanced tumour chemotherapeutic efficacy. Hum. Reprod. 2023, 38, 1769–1783. [Google Scholar] [CrossRef]
- Luan, Y.; Yu, S.Y.; Abazarikia, A.; Dong, R.; Kim, S.Y. TAp63 determines the fate of oocytes against DNA damage. Sci. Adv. 2022, 8, eade1846. [Google Scholar] [CrossRef]
- Jeong, J.H.; An, J.Y.; Kwon, Y.T.; Rhee, J.G.; Lee, Y.J. Effects of low dose quercetin: Cancer cell-specific inhibition of cell cycle progression. J. Cell Biochem. 2009, 106, 73–82. [Google Scholar] [CrossRef]
- Li, J.; Long, H.; Cong, Y.; Gao, H.; Lyu, Q.; Yu, S.; Kuang, Y. Quercetin prevents primordial follicle loss via suppression of PI3K/Akt/Foxo3a pathway activation in cyclophosphamide-treated mice. Reprod. Biol. Endocrinol. 2021, 19, 63. [Google Scholar] [CrossRef] [PubMed]
- Aghababaei, F.; Hadidi, M. Recent Advances in Potential Health Benefits of Quercetin. Pharmaceuticals 2023, 16, 1020. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.; Zhou, R.; Ruan, Y.; Fan, S. Urolithin A Protects Ovarian Reserve Via Inhibiting PI3K/Akt Signaling and Preventing Chemotherapy-Induced Follicle Apoptosis. Biology 2025, 14, 829. https://doi.org/10.3390/biology14070829
Wang W, Zhou R, Ruan Y, Fan S. Urolithin A Protects Ovarian Reserve Via Inhibiting PI3K/Akt Signaling and Preventing Chemotherapy-Induced Follicle Apoptosis. Biology. 2025; 14(7):829. https://doi.org/10.3390/biology14070829
Chicago/Turabian StyleWang, Weiyong, Ren Zhou, Yong Ruan, and Shuhao Fan. 2025. "Urolithin A Protects Ovarian Reserve Via Inhibiting PI3K/Akt Signaling and Preventing Chemotherapy-Induced Follicle Apoptosis" Biology 14, no. 7: 829. https://doi.org/10.3390/biology14070829
APA StyleWang, W., Zhou, R., Ruan, Y., & Fan, S. (2025). Urolithin A Protects Ovarian Reserve Via Inhibiting PI3K/Akt Signaling and Preventing Chemotherapy-Induced Follicle Apoptosis. Biology, 14(7), 829. https://doi.org/10.3390/biology14070829




