Effects of the Invasive Common Myna (Acridotheres tristis) on Nest Site Competition and Predation in Native Birds: A Before-After-Control-Impact Study
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Studied Species
2.3. Before-After-Control-Impact (BACI)
2.4. Do Common Myna Predate and Usurp Great Tit Nests? Field Experiment
2.5. Statistical Analysis
3. Results
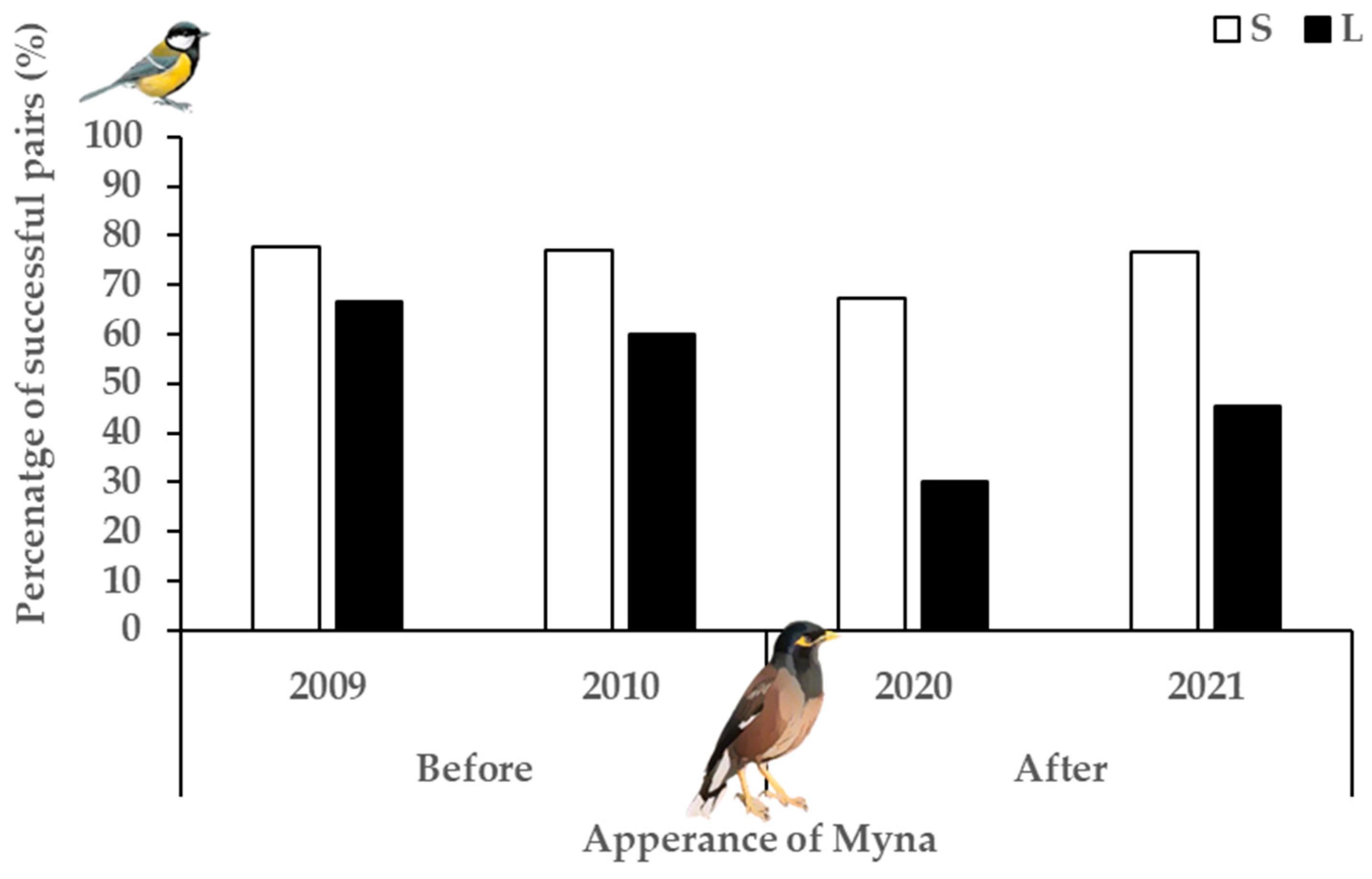
3.1. Myna Impact on House Sparrow (BACI)
3.2. Myna Impact on Great Tits (BACI)
3.3. Do Common Myna Predate and Usurp Great Tit Nests?
4. Discussion
4.1. Impact on House Sparrows (BACI)
4.2. Impact on Great Tit (BACI)
4.3. Predation and Nest Usurpation (Interference Competition)
4.4. Implications for Management and Conservation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Berglund, H.; Järemo, J.; Bengtsson, G. Associations of Invasive Alien Species and Other Threats to IUCN Red List Species (Chordata: Vertebrates). Biol. Invasions 2013, 15, 1169–1180. [Google Scholar] [CrossRef]
- Ancillotto, L.; Studer, V.; Howard, T.; Smith, V.S.; McAlister, E.; Beccaloni, J.; Manzia, F.; Renzopaoli, F.; Bosso, L.; Russo, D.; et al. Environmental Drivers of Parasite Load and Species Richness in Introduced Parakeets in an Urban Landscape. Parasitol. Res. 2018, 117, 3591–3599. [Google Scholar] [CrossRef] [PubMed]
- Baker, J.; Harvey, K.J.; French, K. Threats from Introduced Birds to Native Birds. Emu-Austral Ornithol. 2014, 114, 1–12. [Google Scholar] [CrossRef]
- Blackburn, T.M.; Essl, F.; Evans, T.; Hulme, P.E.; Jeschke, J.M.; Kühn, I.; Kumschick, S.; Marková, Z.; Mrugała, A.; Nentwig, W.; et al. A Unified Classification of Alien Species Based on the Magnitude of Their Environmental Impacts. PLoS Biol. 2014, 12, e1001850. [Google Scholar] [CrossRef]
- Dueñas, M.A.; Ruffhead, H.J.; Wakefield, N.H.; Roberts, P.D.; Hemming, D.J.; Diaz-Soltero, H. The Role Played by Invasive Species in Interactions with Endangered and Threatened Species in the United States: A Systematic Review. Biodivers. Conserv. 2018, 27, 3171–3183. [Google Scholar] [CrossRef]
- Colléony, A.; Shwartz, A. When the Winners Are the Losers: Invasive Alien Bird Species Outcompete the Native Winners in the Biotic Homogenization Process. Biol. Conserv. 2020, 241, 108314. [Google Scholar] [CrossRef]
- Inger, R.; Gregory, R.; Duffy, J.P.; Stott, I.; Voříšek, P.; Gaston, K.J. Common European Birds Are Declining Rapidly While Less Abundant Species’ Numbers Are Rising. Ecol. Lett. 2015, 18, 28–36. [Google Scholar] [CrossRef]
- Evans, T.; Jeschke, J.M.; Liu, C.; Redding, D.W.; Şekercioğlu, Ç.H.; Blackburn, T.M. What Factors Increase the Vulnerability of Native Birds to the Impacts of Alien Birds? Ecography 2021, 44, 727–739. [Google Scholar] [CrossRef]
- Feare, C.J.; Bristol, R.M.; Van De Crommenacker, J. Eradication of a Highly Invasive Bird, the Common Myna Acridotheres Tristis, Facilitates the Establishment of Insurance Populations of Island Endemic Birds. Bird Conserv. Int. 2022, 32, 439–459. [Google Scholar] [CrossRef]
- Grarock, K.; Tidemann, C.R.; Wood, J.; Lindenmayer, D.B. Is It Benign or Is It a Pariah? Empirical Evidence for the Impact of the Common Myna (Acridotheres Tristis) on Australian Birds. PLoS ONE 2012, 7, e40622. [Google Scholar] [CrossRef]
- Tindall, S.D.; Ralph, C.J.; Clout, M.N. Changes in Bird Abundance Following Common Myna Control on ANew Zealand Island. Pacific Conserv. Biol. 2007, 13, 202–212. [Google Scholar] [CrossRef]
- Newton, I. Population Limitation in Birds; Academic Press: London, UK, 1998; ISBN 9780125173667. [Google Scholar]
- Dhondt, A.A. Interspecific Competition in Birds; Oxford University Press: Oxford, UK, 2011; ISBN 9780199589029. [Google Scholar]
- Koenig, W.D. European Starlings and Their Effect on Native Cavity-Nesting Birds. Conserv. Biol. 2003, 17, 1134–1140. [Google Scholar] [CrossRef]
- Diamond, J.M.; Ross, M.S. Exotic Parrots Breeding in Urban Tree Cavities: Nesting Requirements, Geographic Distribution, and Potential Impacts on Cavity Nesting Birds in Southeast Florida. Avian Res. 2019, 10, 39. [Google Scholar] [CrossRef]
- Cooper, C.B.; Hochachka, W.M.; Dhondt, A.A. Contrasting Natural Experiments Confirm Competition Between House Finches and House Sparrows. Ecology 2007, 88, 864–870. [Google Scholar] [CrossRef] [PubMed]
- Grarock, K.; Lindenmayer, D.B.; Wood, J.T.; Tidemann, C.R. Does Human-Induced Habitat Modification Influence the Impact of Introduced Species? A Case Study on Cavity-Nesting by the Introduced Common Myna (Acridotheres Tristis) and Two Australian Native Parrots. Environ. Manag. 2013, 52, 958–970. [Google Scholar] [CrossRef]
- Charter, M.; Izhaki, I.; Ben Mocha, Y.; Kark, S. Nest-Site Competition between Invasive and Native Cavity Nesting Birds and Its Implication for Conservation. J. Environ. Manag. 2016, 181, 129–134. [Google Scholar] [CrossRef]
- Strubbe, D.; Matthysen, E.; Strubbe, D.; Matthysen, E. Invasive Ring-Necked Parakeets Psittacula Krameri in Belgium: Habitat Selection and Impact on Native Birds. Ecography 2007, 30, 578–588. [Google Scholar] [CrossRef]
- van Balen, J.H. Relationship Between Nest-Box Size, Occupation and Breeding Parameters of the Great Tit Parus Major and Some Other Hole-Nesting Species. Ardea 1984, 72, 163–175. [Google Scholar]
- Pell, A.S.; Tidemann, C.R. The Impact of Two Exotic Hollow-Nesting Birds on Two Native Parrots in Savannah and Woodland in Eastern Australia. Biol. Conserv. 1997, 79, 145–153. [Google Scholar] [CrossRef]
- Davis, A.; Major, R.E.; Taylor, C.E. Housing Shortages in Urban Regions: Aggressive Interactions at Tree Hollows in Forest Remnants. PLoS ONE 2013, 8, e59332. [Google Scholar] [CrossRef]
- Ingold, D.J. The Influence of Starlings on Flicker Reproduction When Both Naturally Excavated Cavities and Artificial Nest Boxes Are Available. Wilson Bull. 1998, 110, 218–225. [Google Scholar]
- Van Balen, J.H.; Booy, C.J.H.; Van Franeker, J.A.; Osieck, E.R. Studies on Hole-Nesting Birds in Natural Nest Sites. Ardea 1982, 70, 1–24. [Google Scholar] [CrossRef]
- de Meireles, R.C.; Lopes, L.E.; Pichorim, M.; Machado, T.L.d.S.S.; Duca, C.; Solar, R. Nest Survival of the Threatened Campo Miner Geositta poeciloptera: A Tropical Cavity-Nesting Grassland Bird. Austral Ecol. 2021, 46, 1236–1245. [Google Scholar] [CrossRef]
- Minot, E.O.; Perrins, C.M. Interspecific Interference Competition--Nest Sites for Blue and Great Tits. J. Anim. Ecol. 1986, 55, 331–350. [Google Scholar] [CrossRef]
- Goldshtein, A.; Markman, S.; Leshem, Y.; Puchinsky, M.; Charter, M. Nest-Site Interference Competition with House Sparrows Affects Breeding Success and Parental Care in Great Tits. J. Ornithol. 2018, 159, 667–673. [Google Scholar] [CrossRef]
- Charter, M.; Leshem, Y.; Izhaki, I. Asymmetric Seasonal Nest Site Competition between Great Tits and House Sparrows. J. Ornithol. 2013, 154, 173–181. [Google Scholar] [CrossRef]
- Charter, M.; Izhaki, I.; Leshem, Y. Effects of the Risk of Competition and Predation on Large Secondary Cavity Breeders. J. Ornithol. 2010, 151, 791–795. [Google Scholar] [CrossRef]
- Ye, P.; Yang, C.; Liang, W. Nest Site Availability and Niche Differentiation between Two Cavity-Nesting Birds in Time and Space. Ecol. Evol. 2019, 9, 11904–11910. [Google Scholar] [CrossRef]
- Grether, G.F.; Losin, N.; Anderson, C.N.; Okamoto, K. The Role of Interspecific Interference Competition in Character Displacement and the Evolution of Competitor Recognition. Biol. Rev. 2009, 84, 617–635. [Google Scholar] [CrossRef]
- Sarà, M.; Milazzo, A.; Falletta, W.; Bellia, E. Exploitation Competition between Hole-Nesters (Muscardinus Avellanarius, Mammalia and Parus Caeruleus, Aves) in Mediterranean Woodlands. J. Zool. 2005, 265, 347–357. [Google Scholar] [CrossRef]
- Le Roux, D.S.; Ikin, K.; Lindenmayer, D.B.; Bistricer, G.; Manning, A.D.; Gibbons, P. Effects of Entrance Size, Tree Size and Landscape Context on Nest Box Occupancy: Considerations for Management and Biodiversity Offsets. For. Ecol. Manag. 2016, 366, 135–142. [Google Scholar] [CrossRef]
- Orchan, Y.; Chiron, F.; Shwartz, A.; Kark, S. The Complex Interaction Network among Multiple Invasive Bird Species in a Cavity-Nesting Community. Biol. Invasions 2013, 15, 429–445. [Google Scholar] [CrossRef]
- Diamond, J.M.; Ross, M.S.; Diamond, J.M.; Ross, M.S. Overlap in Reproductive Phenology Increases the Likelihood of Cavity Nest Usurpation by Invasive Species in a Tropical City. Condor 2020, 122, duaa013. [Google Scholar] [CrossRef]
- Hernández-Brito, D.; Carrete, M.; Ibáñez, C.; Juste, J.; Tella, J.L. Nest-Site Competition and Killing by Invasive Parakeets Cause the Decline of a Threatened Bat Population. R. Soc. Open Sci. 2018, 5, 172477. [Google Scholar] [CrossRef]
- Hernández-Brito, D.; Carrete, M.; Popa-Lisseanu, A.G.; Ibáñez, C.; Tella, J.L. Crowding in the City: Losing and Winning Competitors of an Invasive Bird. PLoS ONE 2014, 9, e100593. [Google Scholar] [CrossRef] [PubMed]
- Bailey, R.L.; Faulkner-Grant, H.A.; Martin, V.Y.; Phillips, T.B.; Bonter, D.N. Nest Usurpation by Non-Native Birds and the Role of People in Nest Box Management. Conserv. Sci. Pract. 2020, 2, e185. [Google Scholar] [CrossRef]
- Ingold, D.J. Influence of Nest-Site Competition between European Starlings and Woodpeckers. Wilson Bull. 1994, 206, 227–241. [Google Scholar]
- Rogers, A.M.; Griffin, A.S.; van Rensburg, B.J.; Kark, S. Noisy Neighbours and Myna Problems: Interaction Webs and Aggression around Tree Hollows in Urban Habitats. J. Appl. Ecol. 2020, 57, 1891–1901. [Google Scholar] [CrossRef]
- Lahti, D.C.; David Lahti, C.C. Why We Have Been Unable to Generalize About Bird Nest Predation. Anim. Conserv. 2009, 12, 279–281. [Google Scholar] [CrossRef]
- Sih, A.; Crowley, P.; McPeek, M.; Petranka, J.; Strohmeier, K. Predation, Competition, and Prey Communities: A Review of Field Experiments. Annu. Rev. Ecol. Syst. 1985, 16, 269–311. [Google Scholar] [CrossRef]
- Parejo, D.; Avilés, J.M. Predation Risk Determines Breeding Territory Choice in a Mediterranean Cavity-Nesting Bird Community. Oecologia 2011, 165, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Mouton, J.C.; Martin, T.E. Fitness Consequences of Interspecific Nesting Associations among Cavity-Nesting Birds. Am. Nat. 2018, 192, 389–396. [Google Scholar] [CrossRef] [PubMed]
- Remm, J.; Lõhmus, A.; Remm, K. Tree Cavities in Riverine Forests: What Determines Their Occurrence and Use by Hole-Nesting Passerines? For. Ecol. Manag. 2006, 221, 267–277. [Google Scholar] [CrossRef]
- Rogers, A.M.; Lermite, F.; Griffin, A.S.; van Rensburg, B.J.; Kark, S. Alien vs. Predator: Impacts of Invasive Species and Native Predators on Urban Nest Box Use by Native Birds. Animals 2023, 13, 1807. [Google Scholar] [CrossRef]
- Doherty, T.S.; Glen, A.S.; Nimmo, D.G.; Ritchie, E.G.; Dickman, C.R. Invasive Predators and Global Biodiversity Loss. Proc. Natl. Acad. Sci. USA 2016, 113, 11261–11265. [Google Scholar] [CrossRef] [PubMed]
- Massaro, M.; Stanbury, M.; Briskie, J.V. Nest Site Selection by the Endangered Black Robin Increases Vulnerability to Predation by an Invasive Bird. Anim. Conserv. 2013, 16, 404–411. [Google Scholar] [CrossRef]
- Bissessur, P.; Florens, F.V. Predation of the Mauritius Endemic Macchabé Skink, Gongylomorphus Bojerii Fontenayi, by the Common Myna, Acridotheres Tristis. Bull. Phaethon 2018, 48, 91–93. [Google Scholar]
- Feare, C.J.; Lebarbenchon, C.; Dietrich, M.; Larose, C.S. Predation of Seabird Eggs by Common Mynas on Bird Island, Seychelles, and Its Broader Implications. Bull. Afr. Bird Club 2015, 22, 162–170. [Google Scholar] [CrossRef]
- Hughes, B.; Dickey, R.; Reynolds, S. Predation Pressures on Sooty Terns by Cats, Rats and Common Mynas on Ascension Island in the South Atlantic. In Occasional Paper of the IUCN Species Survival Commission No. 62, Proceedings of the Invasives: Scaling Up to Meet the Challenge, Dundee, UK, 10–14 July 2017; Veitch, G., Clout, M., Martin, A., Russell, J., West, C., Eds.; International Union for Conservation of Nature and Natural Resources: Gland, Switzerland, 2019; pp. 295–301. [Google Scholar]
- Israel Central Bureau of Statistics Israel Central Bureau of Statistics. Available online: https://www.cbs.gov.il/ (accessed on 24 February 2025).
- Yom-Tov, Y.; Hatzofe, O.; Geffen, E. Israel’s Breeding Avifauna: A Century of Dramatic Change. Biol. Conserv. 2012, 147, 13–21. [Google Scholar] [CrossRef]
- Charter, M.; Leshem, Y.; Halevi, S.; Izhaki, I. Nest Box Use by Great Tits in Semi–Arid Rural Residential Gardens. Wilson J. Ornithol. 2010, 122, 604–608. [Google Scholar] [CrossRef]
- Charter, M.; Izhaki, I.; Leshem, Y. Predation or Facilitation? An Experimental Assessment of Whether Generalist Predators Affect the Breeding Success of Passerines. J. Ornithol. 2011, 152, 533–539. [Google Scholar] [CrossRef]
- Holzapfel, C.; Levin, N.; Hatzofe, O.; Kark, S. Colonisation of the Middle East by the Invasive Common Myna Acridotheres Tristis L., with Special Reference to Israel. Sandgrouse 2006, 28, 44–51. [Google Scholar]
- Magory Cohen, T.; Hauber, M.E.; Akriotis, T.; Crochet, P.A.; Karris, G.; Kirschel, A.N.G.; Khoury, F.; Menchetti, M.; Mori, E.; Per, E.; et al. Accelerated Avian Invasion into the Mediterranean Region Endangers Biodiversity and Mandates International Collaboration. J. Appl. Ecol. 2022, 59, 1440–1455. [Google Scholar] [CrossRef]
- Wesołowski, T. Nest-Site Re-Use: Marsh Tit Poecile Palustris Decisions in a Primeval Forest. Bird Study 2006, 53, 199–204. [Google Scholar] [CrossRef]
- Haas, C.A. Effects of Prior Nesting Success on Site Fidelity and Breeding Dispersal: An Experimental Approach. Auk 1998, 115, 929–936. [Google Scholar] [CrossRef]
- Dodaro, G.; Battisti, C. Rose-Ringed Parakeet (Psittacula Krameri) and Starling (Sturnus Vulgaris) Syntopics in a Mediterranean Urban Park: Evidence for Competition in Nest-Site Selection? Belgian J. Zool. 2014, 144, 5–14. [Google Scholar] [CrossRef]
- Le Louarn, M.; Couillens, B.; Deschamps-Cottin, M.; Clergeau, P. Interference Competition Between an Invasive Parakeet and Native Bird Species at Feeding Sites. J. Ethol. 2016, 34, 291–298. [Google Scholar] [CrossRef]
- Peck, H.L.; Pringle, H.E.; Marshall, H.H.; Owens, I.P.F.; Lord, A.M. Experimental Evidence of Impacts of an Invasive Parakeet on Foraging Behavior of Native Birds. Behav. Ecol. 2014, 25, 582–590. [Google Scholar] [CrossRef]
- Yin, D.; Yu, J.; Jin, J.; Shen, C.; Zhang, L.; Li, X.; Zhang, K.; Wang, H. Nest Box Entrance Hole Size Can Influence Nest Site Selection and Nest Defence Behaviour in Japanese Tits. Anim. Cogn. 2023, 26, 1423–1430. [Google Scholar] [CrossRef]
- Xue, B.; She, H.; Zuo, L.; Bates, J.M.; Cheviron, Z.A.; Wu, Y.; Li, D.; DuBay, S.G.; Qu, Y. Phenotypic Plasticity Co-Varies with Elevational Range in Two Avian Species of Elevational Migrants in the Himalayas. Nat. Commun. 2025, 16, 5316. [Google Scholar] [CrossRef]
- Strubbe, D.; Matthysen, E. Experimental Evidence for Nest-Site Competition between Invasive Ring-Necked Parakeets (Psittacula krameri) and Native Nuthatches (Sitta europaea). Biol. Conserv. 2009, 142, 1588–1594. [Google Scholar] [CrossRef]
- Jauregui, A.; Gonzalez, E.; Segura, L.N. Impacts of the Invasive European Starling on Two Neotropical Woodpecker Species: Agonistic Responses and Reproductive Interactions. Emu 2021, 121, 223–230. [Google Scholar] [CrossRef]
- Miller, K.E.; Leonard, D.L. Partial Predation at Cavity Nests in Southern Pine Forests. Southeast. Nat. 2010, 9, 395–402. [Google Scholar] [CrossRef]
- Skórka, P.; Martyka, R.; Wójcik, J.D.; Lenda, M. An Invasive Gull Displaces Native Waterbirds to Breeding Habitats More Exposed to Native Predators. Popul. Ecol. 2014, 56, 359–374. [Google Scholar] [CrossRef]
- Brown, T.J.; Hellicar, M.; Accouche, W.; van der Woude, J.; Dugdale, H.; Komdeur, J.; Richardson, D.S. A Major Myna Problem; Invasive Predator Removal Benefits Female Survival and Population Growth of a Translocated Island Endemic. Glob. Ecol. Conserv. 2023, 46, e02584. [Google Scholar] [CrossRef]
- Magory Cohen, T.; Kumar, R.S.; Nair, M.; Hauber, M.E.; Dor, R. Innovation and Decreased Neophobia Drive Invasion Success in a Widespread Avian Invader. Anim. Behav. 2020, 163, 61–72. [Google Scholar] [CrossRef]
- Brazill-Boast, J.; Pryke, S.R.; Griffith, S.C. Provisioning Habitat with Custom-Designed Nest-Boxes Increases Reproductive Success in an Endangered Finch. Austral Ecol. 2013, 38, 405–412. [Google Scholar] [CrossRef]
- Tatayah, R.V.V.; Malham, J.; Haverson, P.; Reuleaux, A.; Van de Wetering, J. Design and Provision of Nest Boxes for Echo Parakeets Psittacula Eques in Black River Georges National Park, Mauritius. Conserv. Evid. 2007, 4, 16–19. [Google Scholar]
- Lambrechts, M.M.; Wiebe, K.L.; Sunde, P.; Solonen, T.; Sergio, F.; Roulin, A.; Møller, A.P.; López, B.C.; Fargallo, J.A.; Exo, K.M.; et al. Nest Box Design for the Study of Diurnal Raptors and Owls Is Still an Overlooked Point in Ecological, Evolutionary and Conservation Studies: A Review. J. Ornithol. 2012, 153, 23–34. [Google Scholar] [CrossRef]
- Bailey, R.L.; Bonter, D.N. Predator Guards on Nest Boxes Improve Nesting Success of Birds. Wildl. Soc. Bull. 2017, 41, 434–441. [Google Scholar] [CrossRef]
- James Reynolds, S.; Ibáñez-Álamo, J.D.; Sumasgutner, P.; Mainwaring, M.C. Urbanisation and Nest Building in Birds: A Review of Threats and Opportunities. J. Ornithol. 2019, 160, 841–860. [Google Scholar] [CrossRef]
- Bremner, A.; Park, K. Public Attitudes to the Management of Invasive Non-Native Species in Scotland. Biol. Conserv. 2007, 139, 306–314. [Google Scholar] [CrossRef]
- Steele, Z.T.; Pienaar, E.F. Knowledge, Reason and Emotion: Using Behavioral Theories to Understand People’s Support for Invasive Animal Management. Biol. Invasions 2021, 23, 3513–3527. [Google Scholar] [CrossRef]
- Jaatinen, K.; Hermansson, I.; Mohring, B.; Steele, B.B.; Öst, M. Mitigating Impacts of Invasive Alien Predators on an Endangered Sea Duck amidst High Native Predation Pressure. Oecologia 2022, 198, 543–552. [Google Scholar] [CrossRef]
- Weston, K.A.; O’Donnell, C.F.J.; van dam-Bates, P.; Monks, J.M. Control of Invasive Predators Improves Breeding Success of an Endangered Alpine Passerine. Ibis 2018, 160, 892–899. [Google Scholar] [CrossRef]
- Smith, R.K.; Pullin, A.S.; Stewart, G.B.; Sutherland, W.J. Is Nest Predator Exclusion an Effective Strategy for Enhancing Bird Populations? Biol. Conserv. 2011, 144, 1–10. [Google Scholar] [CrossRef]
- Jones, H.P.; Holmes, N.D.; Butchart, S.H.M.; Tershy, B.R.; Kappes, P.J.; Corkery, I.; Aguirre-Muñoz, A.; Armstrong, D.P.; Bonnaud, E.; Burbidge, A.A.; et al. Invasive Mammal Eradication on Islands Results in Substantial Conservation Gains. Proc. Natl. Acad. Sci. USA 2016, 113, 4033–4038. [Google Scholar] [CrossRef] [PubMed]
- Charter, M.; Angel, I. Where Have the WILD Birds Gone? The Answer Is with the Invasive Myna Bird. Available online: https://www.youtube.com/watch?v=gLgaM9sNqbU (accessed on 23 February 2023).
- Castillo-Huitrón, N.M.; Naranjo, E.J.; Santos-Fita, D.; Estrada-Lugo, E. The Importance of Human Emotions for Wildlife Conservation. Front. Psychol. 2020, 11, 1277. [Google Scholar] [CrossRef]
- Grarock, K.; Tidemann, C.R.; Wood, J.T.; Lindenmayer, D.B. Are Invasive Species Drivers of Native Species Decline or Passengers of Habitat Modification? A Case Study of the Impact of the Common Myna (Acridotheres Tristis) on Australian Bird Species. Austral Ecol. 2014, 39, 106–114. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Engel, I.F.; Izhaki, I.; Charter, M. Effects of the Invasive Common Myna (Acridotheres tristis) on Nest Site Competition and Predation in Native Birds: A Before-After-Control-Impact Study. Biology 2025, 14, 828. https://doi.org/10.3390/biology14070828
Engel IF, Izhaki I, Charter M. Effects of the Invasive Common Myna (Acridotheres tristis) on Nest Site Competition and Predation in Native Birds: A Before-After-Control-Impact Study. Biology. 2025; 14(7):828. https://doi.org/10.3390/biology14070828
Chicago/Turabian StyleEngel, Iris Fortoune, Ido Izhaki, and Motti Charter. 2025. "Effects of the Invasive Common Myna (Acridotheres tristis) on Nest Site Competition and Predation in Native Birds: A Before-After-Control-Impact Study" Biology 14, no. 7: 828. https://doi.org/10.3390/biology14070828
APA StyleEngel, I. F., Izhaki, I., & Charter, M. (2025). Effects of the Invasive Common Myna (Acridotheres tristis) on Nest Site Competition and Predation in Native Birds: A Before-After-Control-Impact Study. Biology, 14(7), 828. https://doi.org/10.3390/biology14070828







