Transcriptomic and Functional Validation Reveals PAQR3/P6-55 as Potential Therapeutic Targets in Colon Cancer
Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Cell Culture, Lentiviral Packaging, and Stable Strain Construction
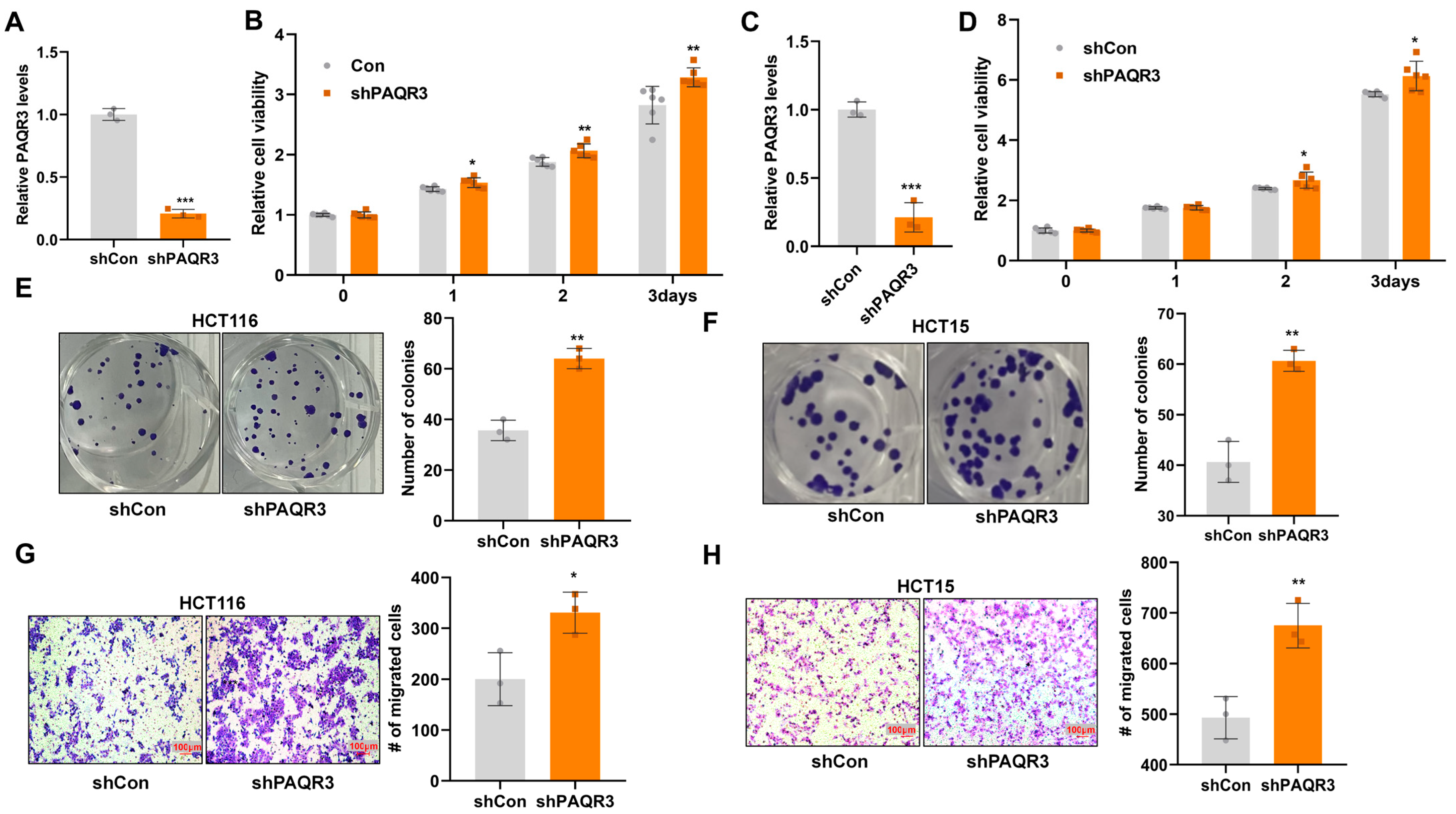
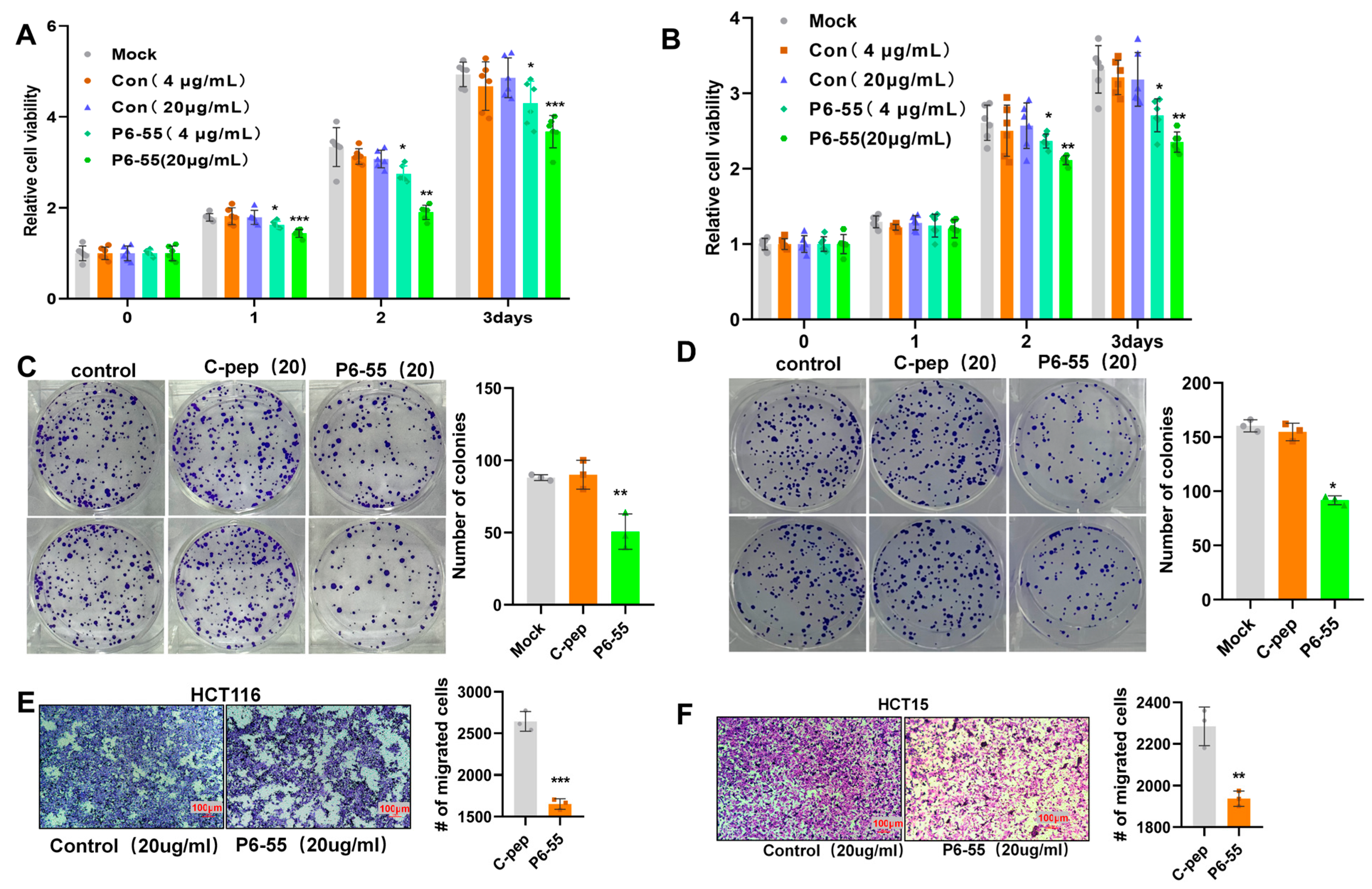
2.2. CCK8 Experiment
2.3. Colony Formation Assay
2.4. Cell Migration Assay
2.5. RNA Extraction, Reverse Transcription PCR, and qRT-PCR
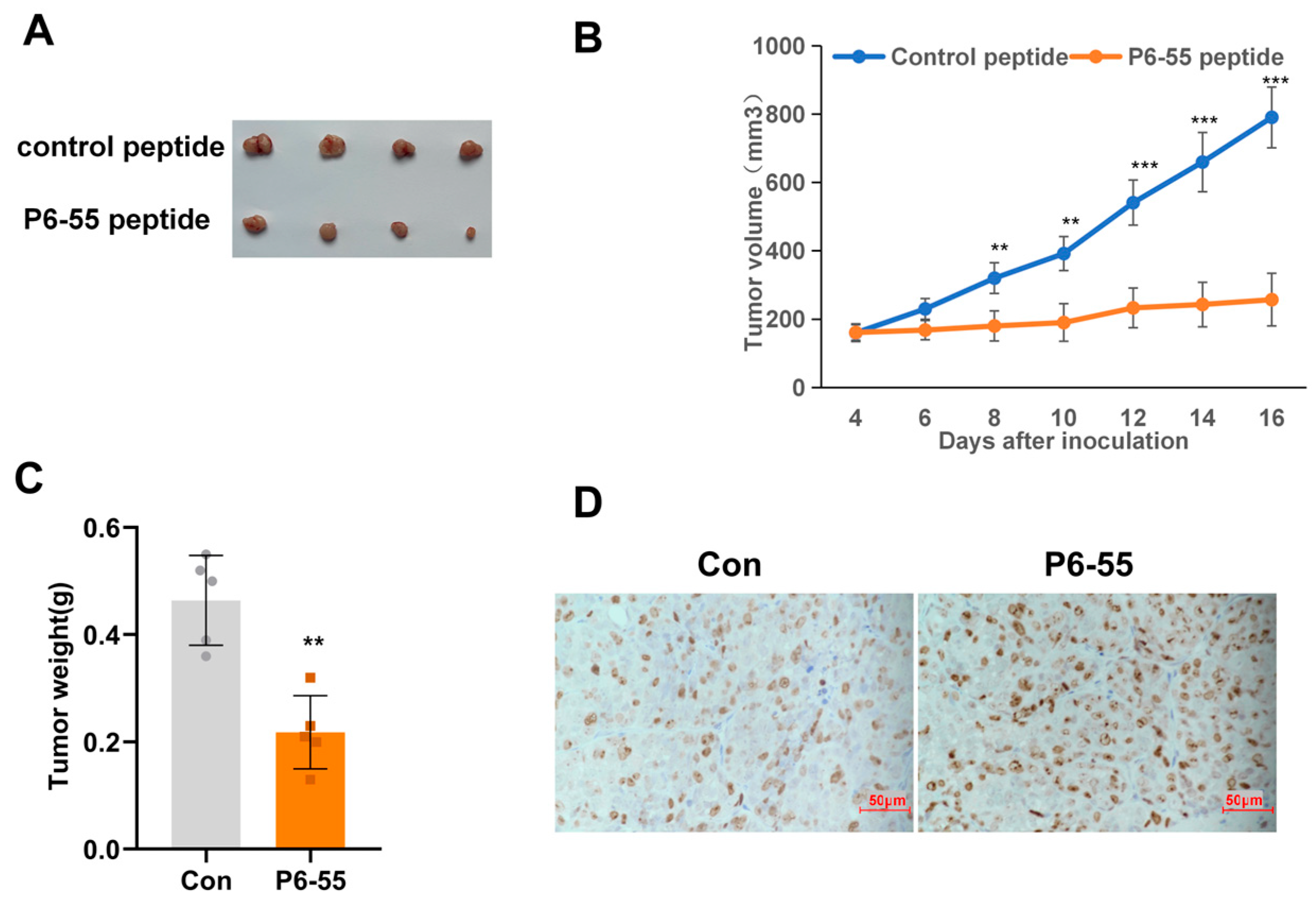
2.6. Subcutaneous Tumor Formation Experiment in Nude Mice
2.7. Western Blot Analysis
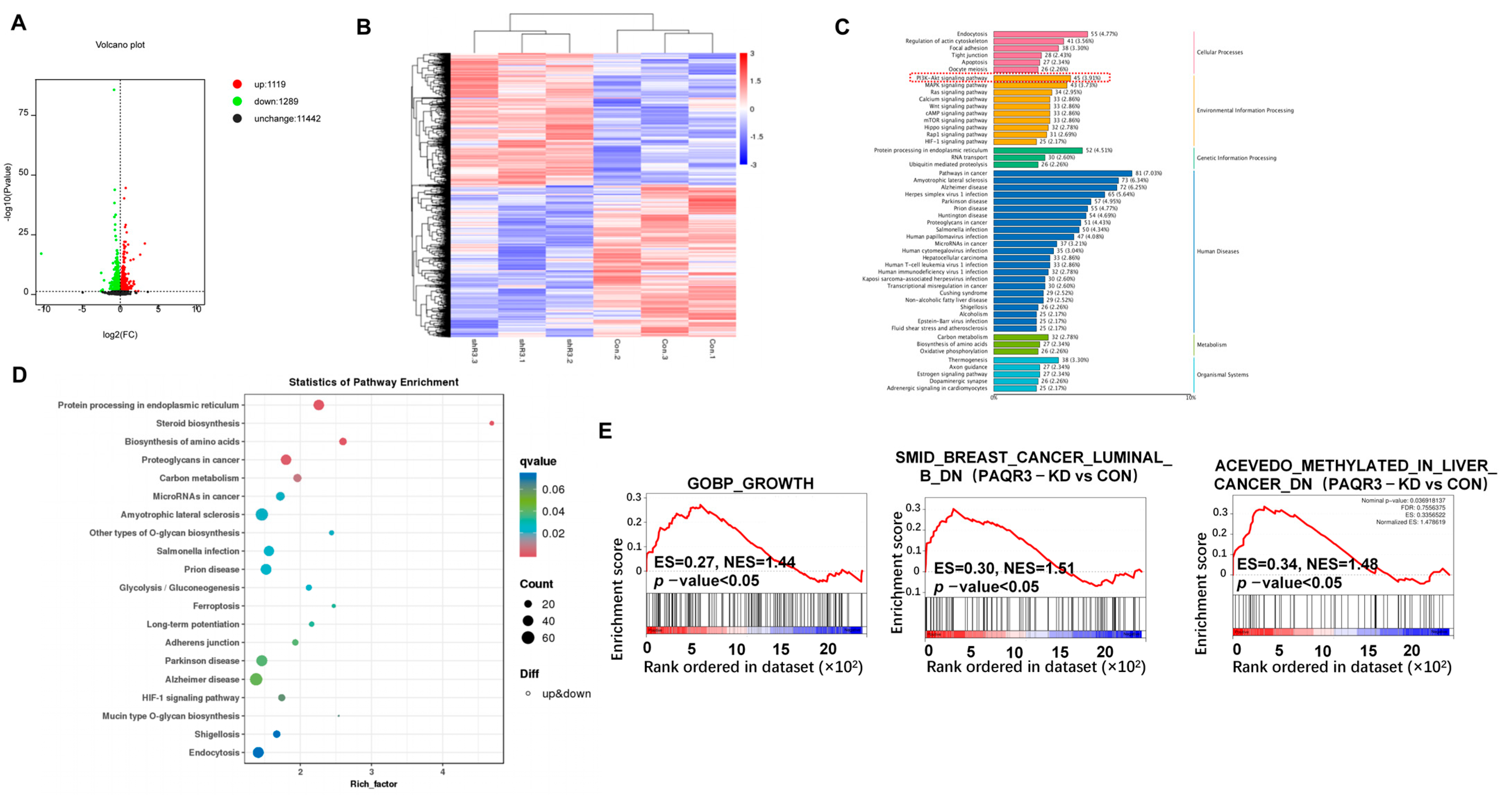
2.8. RNA Sequencing
2.9. GO Analysis, KEGG Pathway Enrichment Analysis, and GSEA
2.10. Statistical Analysis
3. Results
3.1. PAQR3 Knockdown Promotes the Proliferation and Migration of Colon Cancer Cells
3.2. PAQR3 Overexpression Inhibits the Proliferation and Migration of Colon Cancer Cells
3.3. P6-55 Inhibits the Proliferation and Migration of Colon Cancer Cells
3.4. P6-55 Inhibits the Growth of Colon Cancer in a Nude Mouse Tumor Model
3.5. RNA-Seq and Differential Gene Enrichment Analysis
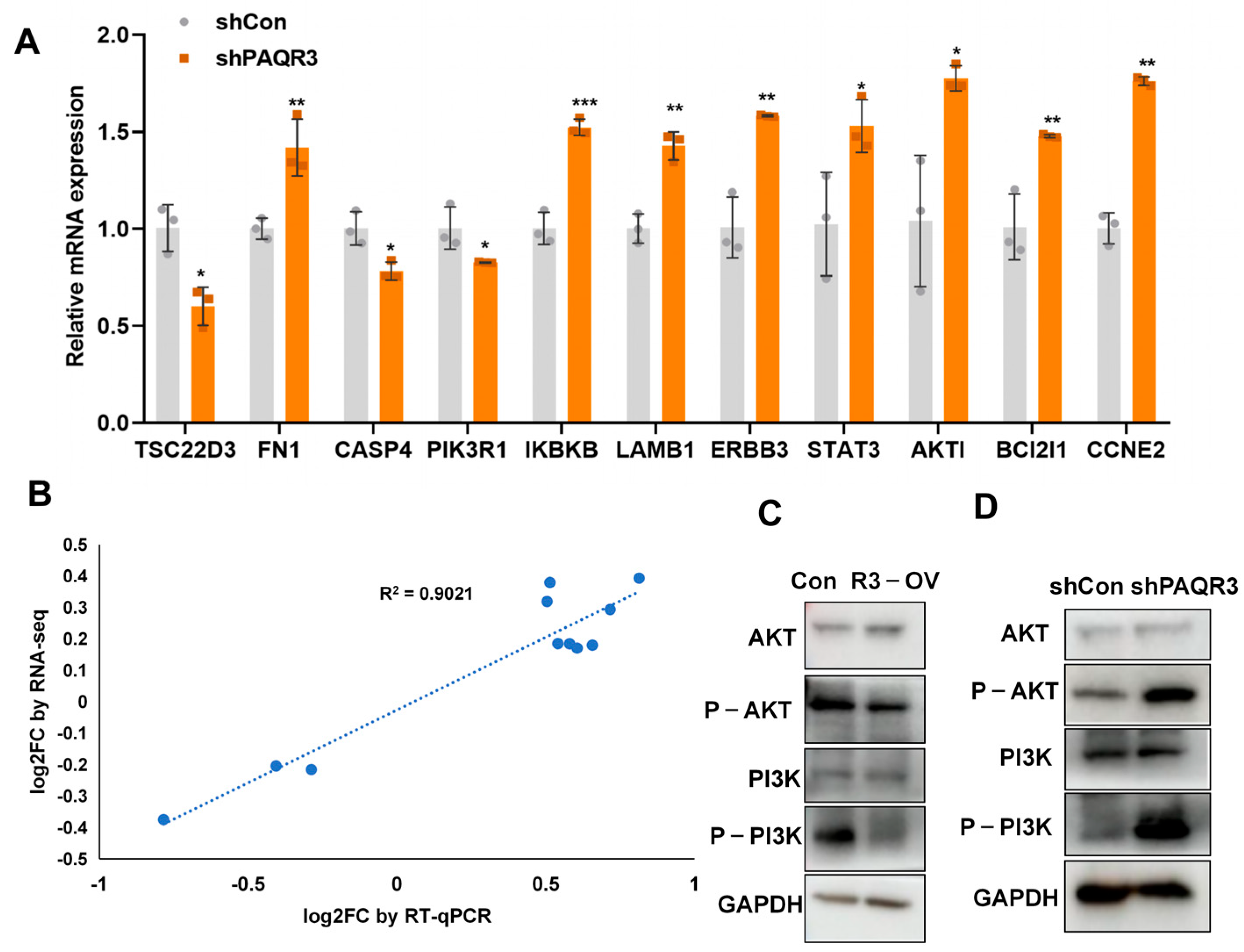
3.6. PAQR3 Regulates the PI3K-AKT Signaling Pathway in Colon Cancer
4. Discussion
5. Conclusions
- Multi-omics profiling: Conduct proteomic and phosphoproteomic analyses to map PAQR3-regulated effectors and modulators, with a focus on its PI3K-AKT interaction hub, thus refining its tumor-suppressive network.
- Pharmacological evaluation: Assess the pharmacokinetics, tumor-targeting efficiency, and pharmacodynamics of P6-55 in orthotopic and patient-derived xenograft models.
- Evolutionary conservation: Use comparative genomics in non-mammalian models to identify the conserved therapeutic potential of PAQR3.
- Prognostic analysis: Integrate genomic and proteomic datasets to evaluate the prognostic significance of PAQR3 in colorectal cancer cohorts.
- Combination therapy: Test the synergistic effects of P6-55 with PI3K-AKT inhibitors or immune checkpoint blockers in immunocompetent models to develop precision treatments.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Morris, V.K.; Bandey, I.N.; Hong, D.S.; Kopetz, S. Advancements in combining targeted therapy and immunotherapy for colorectal cancer. Trends Cancer 2024, 10, 598–609. [Google Scholar] [CrossRef] [PubMed]
- Fabregas, J.C.; Ramnaraign, B.; George, T.J. Clinical Updates for Colon Cancer Care in 2022. Clin. Colorectal Cancer 2022, 21, 198–203. [Google Scholar] [CrossRef]
- Feng, L.; Xie, X.; Ding, Q.; Luo, X.; He, J.; Fan, F.; Liu, W.; Wang, Z.; Chen, Y. Spatial regulation of Raf kinase signaling by RKTG. Proc. Natl. Acad. Sci. USA 2007, 104, 14348–14353. [Google Scholar] [CrossRef]
- Li, X.; Li, M.; Chen, D.; Shi, G.; Zhao, H. PAQR3 inhibits proliferation via suppressing PI3K/AKT signaling pathway in non-small cell lung cancer. Arch. Med. Sci. 2018, 14, 1289–1297. [Google Scholar] [CrossRef]
- Guo, Q.; Ke, X.X.; Fang, S.X.; Gao, W.L.; Song, Y.X.; Chen, C.; Lu, H.L.; Xu, G. PAQR3 Inhibits Non-small Cell Lung Cancer Growth by Regulating the NF-kappaB/p53/Bax Axis. Front. Cell Dev. Biol. 2020, 8, 581919. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.L.; Hong, L.L.; Ling, Z.N.; Hu, X.Y.; Liu, Z.; Li, P.; Ling, Z.Q. Golgi scaffold protein PAQR3 as a candidate suppressor of gastric cardia adenocarcinoma via regulating TGF-beta/Smad pathway. J. Clin. Lab. Anal. 2022, 36, e24617. [Google Scholar] [CrossRef]
- Guo, W.; You, X.; Xu, D.; Zhang, Y.; Wang, Z.; Man, K.; Wang, Z.; Chen, Y. PAQR3 enhances Twist1 degradation to suppress epithelial-mesenchymal transition and metastasis of gastric cancer cells. Carcinogenesis 2016, 37, 397–407. [Google Scholar] [CrossRef]
- Ling, Z.Q.; Guo, W.; Lu, X.X.; Zhu, X.; Hong, L.L.; Wang, Z.; Wang, Z.; Chen, Y. A Golgi-specific protein PAQR3 is closely associated with the progression, metastasis and prognosis of human gastric cancers. Ann. Oncol. 2014, 25, 1363–1372. [Google Scholar] [CrossRef]
- Cao, Q.; You, X.; Xu, L.; Wang, L.; Chen, Y. PAQR3 suppresses the growth of non-small cell lung cancer cells via modulation of EGFR-mediated autophagy. Autophagy 2020, 16, 1236–1247. [Google Scholar] [CrossRef]
- Song, X.; Zhang, W.; Yu, N.; Zhong, X. PAQR3 facilitates the ferroptosis of diffuse large B-cell lymphoma via the regulation of LDLR-mediated PI3K/AKT pathway. Hematol. Oncol. 2024, 42, e3219. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Tong, L. PAQR3 inhibits proliferation and aggravates ferroptosis in acute lymphoblastic leukemia through modulation Nrf2 stability. Immun. Inflamm. Dis. 2021, 9, 827–839. [Google Scholar] [CrossRef] [PubMed]
- Paskeh, M.D.A.; Ghadyani, F.; Hashemi, M.; Abbaspour, A.; Zabolian, A.; Javanshir, S.; Razzazan, M.; Mirzaei, S.; Entezari, M.; Goharrizi, M.; et al. Biological impact and therapeutic perspective of targeting PI3K/Akt signaling in hepatocellular carcinoma: Promises and Challenges. Pharmacol. Res. 2023, 187, 106553. [Google Scholar] [CrossRef]
- He, Y.; Sun, M.M.; Zhang, G.G.; Yang, J.; Chen, K.S.; Xu, W.W.; Li, B. Targeting PI3K/Akt signal transduction for cancer therapy. Signal Transduct. Target. Ther. 2021, 6, 425. [Google Scholar] [CrossRef]
- Maharati, A.; Moghbeli, M. PI3K/AKT signaling pathway as a critical regulator of epithelial-mesenchymal transition in colorectal tumor cells. Cell Commun. Signal 2023, 21, 201. [Google Scholar] [CrossRef] [PubMed]
- Fontana, F.; Giannitti, G.; Marchesi, S.; Limonta, P. The PI3K/Akt Pathway and Glucose Metabolism: A Dangerous Liaison in Cancer. Int. J. Biol. Sci. 2024, 20, 3113–3125. [Google Scholar] [CrossRef]
- Erickson, E.C.; Toker, A. Can Improved Use of Biomarkers Alter the Fate of PI3K Pathway Inhibitors in the Clinic? Cancer Res. 2021, 81, 6083–6086. [Google Scholar] [CrossRef]
- Guo, W.; You, X.; Wang, X.; Wang, L.; Chen, Y. A synthetic peptide hijacks the catalytic subunit of class I PI3K to suppress the growth of cancer cells. Cancer Lett. 2017, 405, 1–9. [Google Scholar] [CrossRef]
- Dibble, C.C.; Barritt, S.A.; Perry, G.E.; Lien, E.C.; Geck, R.C.; DuBois-Coyne, S.E.; Bartee, D.; Zengeya, T.T.; Cohen, E.B.; Yuan, M.; et al. PI3K drives the de novo synthesis of coenzyme A from vitamin B5. Nature 2022, 608, 192–198. [Google Scholar] [CrossRef]
- Wang, L.B.; Karpova, A.; Gritsenko, M.A.; Kyle, J.E.; Cao, S.; Li, Y.; Rykunov, D.; Colaprico, A.; Rothstein, J.H.; Hong, R.; et al. Proteogenomic and metabolomic characterization of human glioblastoma. Cancer Cell 2021, 39, 509–528.e20. [Google Scholar] [CrossRef]
- Gu, L.; Zhu, Y.; Lin, X.; Lu, B.; Zhou, X.; Zhou, F.; Zhao, Q.; Prochownik, E.V.; Li, Y. The IKKbeta-USP30-ACLY Axis Controls Lipogenesis and Tumorigenesis. Hepatology 2021, 73, 160–174. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Wang, J.; Li, J.; Xiao, J.; Liu, C.; Tan, L.; Tu, Y.; Yang, R.; Pei, Y.; Wang, M.; et al. L3MBTL3 and STAT3 collaboratively upregulate SNAIL expression to promote metastasis in female breast cancer. Nat. Commun. 2025, 16, 231. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Sun, Q.; Zhang, C.; Zheng, Q.; Liu, Y.; Xu, H.; He, Y.; Yao, C.; Chen, J.; Xia, H. RHOJ Induces Epithelial-to-Mesenchymal Transition by IL-6/STAT3 to Promote Invasion and Metastasis in Gastric Cancer. Int. J. Biol. Sci. 2023, 19, 4411–4426. [Google Scholar] [CrossRef]
- Jia, L.; Wang, Y.; Wang, C.Y. circFAT1 Promotes Cancer Stemness and Immune Evasion by Promoting STAT3 Activation. Adv. Sci. 2021, 8, 2003376. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Xia, L.; Chen, J.; Zhang, S.; Martin, V.; Li, Q.; Lin, S.; Chen, J.; Calmette, J.; Lu, M.; et al. Stress-glucocorticoid-TSC22D3 axis compromises therapy-induced antitumor immunity. Nat. Med. 2019, 25, 1428–1441. [Google Scholar] [CrossRef]
- Chakraborty, G.; Nandakumar, S.; Hirani, R.; Nguyen, B.; Stopsack, K.H.; Kreitzer, C.; Rajanala, S.H.; Ghale, R.; Mazzu, Y.Z.; Pillarsetty, N.V.K.; et al. The Impact of PIK3R1 Mutations and Insulin-PI3K-Glycolytic Pathway Regulation in Prostate Cancer. Clin. Cancer Res. 2022, 28, 3603–3617. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, D.; Li, Z.; Li, X.; Jin, M.; Jia, N.; Cui, X.; Hu, G.; Tang, T.; Yu, Q. Pan-cancer analysis on the role of PIK3R1 and PIK3R2 in human tumors. Sci. Rep. 2022, 12, 5924. [Google Scholar] [CrossRef]
- Zhang, X.; Luo, Y.; Cen, Y.; Qiu, X.; Li, J.; Jie, M.; Yang, S.; Qin, S. MACC1 promotes pancreatic cancer metastasis by interacting with the EMT regulator SNAI1. Cell Death Dis. 2022, 13, 923. [Google Scholar] [CrossRef]
- Liu, T.; Gan, H.; He, S.; Deng, J.; Hu, X.; Li, L.; Cai, L.; He, J.; Long, H.; Cai, J.; et al. RNA Helicase DDX24 Stabilizes LAMB1 to Promote Hepatocellular Carcinoma Progression. Cancer Res. 2022, 82, 3074–3087. [Google Scholar] [CrossRef]
- Ran, T.; Chen, Z.; Zhao, L.; Ran, W.; Fan, J.; Hong, S.; Yang, Z. LAMB1 Is Related to the T Stage and Indicates Poor Prognosis in Gastric Cancer. Technol. Cancer Res. Treat. 2021, 20, 15330338211004944. [Google Scholar] [CrossRef]
- Hanna, R.E.; Hegde, M.; Fagre, C.R.; DeWeirdt, P.C.; Sangree, A.K.; Szegletes, Z.; Griffith, A.; Feeley, M.N.; Sanson, K.R.; Baidi, Y.; et al. Massively parallel assessment of human variants with base editor screens. Cell 2021, 184, 1064–1080.e20. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.T.; Sansone, A.; Smirnov, A.; Stallings, C.L.; Orvedahl, A. Myeloid autophagy genes protect mice against fatal TNF- and LPS-induced cytokine storm syndromes. Autophagy 2023, 19, 1114–1127. [Google Scholar] [CrossRef]
- Basu, A. The interplay between apoptosis and cellular senescence: Bcl-2 family proteins as targets for cancer therapy. Pharmacol. Ther. 2022, 230, 107943. [Google Scholar] [CrossRef]
- Li, H.; Feng, H.; Zhang, T.; Wu, J.; Shen, X.; Xu, S.; Xu, L.; Wang, S.; Zhang, Y.; Jia, W.; et al. CircHAS2 activates CCNE2 to promote cell proliferation and sensitizes the response of colorectal cancer to anlotinib. Mol. Cancer 2024, 23, 59. [Google Scholar] [CrossRef]
- Wu, L.; Yang, K.; Gui, Y.; Wang, X. Nicotine-upregulated miR-30a arrests cell cycle in G1 phase by directly targeting CCNE2 in human periodontal ligament cells. Biochem. Cell Biol. 2020, 98, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Lu, A.; Hu, Z.; Li, J.; Lu, J. ERBB3 targeting: A promising approach to overcoming cancer therapeutic resistance. Cancer Lett. 2024, 599, 217146. [Google Scholar] [CrossRef] [PubMed]
- Miete, C.; Solis, G.P.; Koval, A.; Bruckner, M.; Katanaev, V.L.; Behrens, J.; Bernkopf, D.B. Galphai2-induced conductin/axin2 condensates inhibit Wnt/beta-catenin signaling and suppress cancer growth. Nat. Commun. 2022, 13, 674. [Google Scholar] [CrossRef]
- Xue, W.; Yang, L.; Chen, C.; Ashrafizadeh, M.; Tian, Y.; Sun, R. Wnt/beta-catenin-driven EMT regulation in human cancers. Cell Mol. Life Sci. 2024, 81, 79. [Google Scholar] [CrossRef]
- Ersahin, T.; Tuncbag, N.; Cetin-Atalay, R. The PI3K/AKT/mTOR interactive pathway. Mol. Biosyst. 2015, 11, 1946–1954. [Google Scholar] [CrossRef]
- Ren, J.; Sui, H.; Fang, F.; Li, Q.; Li, B. The application of Apc(Min/+) mouse model in colorectal tumor researches. J. Cancer Res. Clin. Oncol. 2019, 145, 1111–1122. [Google Scholar] [CrossRef]
- Cui, W.; Guo, M.; Liu, D.; Xiao, P.; Yang, C.; Huang, H.; Liang, C.; Yang, Y.; Fu, X.; Zhang, Y.; et al. Gut microbial metabolite facilitates colorectal cancer development via ferroptosis inhibition. Nat. Cell Biol. 2024, 26, 124–137. [Google Scholar] [CrossRef] [PubMed]
- Tiedt, R.; King, F.J.; Stamm, C.; Niederst, M.J.; Delach, S.; Zumstein-Mecker, S.; Meltzer, J.; Mulford, I.J.; Labrot, E.; Engstler, B.S.; et al. Integrated CRISPR screening and drug profiling identifies combination opportunities for EGFR, ALK, and BRAF/MEK inhibitors. Cell Rep. 2023, 42, 112297. [Google Scholar] [CrossRef]
- Jalalvand, N.; Esmaeili, D.; Bashi, M.m.M.M.; Raiszadeh, M.; Naeimi, S. Evaluation of Physicochemical Activity of Anticancer Fusion Proteins; Enterocin A- R type pyocin-Lactocin-Ligand Against Gastric Cancer Cell Line by Real-Time RT PCR Technique. Int. J. Pept. Res. Ther. 2021, 27, 1167–1175. [Google Scholar] [CrossRef]
- Li, Y.; Zeng, C.; Hu, J.; Pan, Y.; Shan, Y.; Liu, B.; Jia, L. Long non-coding RNA-SNHG7 acts as a target of miR-34a to increase GALNT7 level and regulate PI3K/Akt/mTOR pathway in colorectal cancer progression. J. Hematol. Oncol. 2018, 11, 89. [Google Scholar] [CrossRef] [PubMed]
- Shakeri, F.; Mohamadynejad, P.; Moghanibashi, M. Identification of ASMTL-AS1 and LINC02604 lncRNAs as novel biomarkers for diagnosis of colorectal cancer. Int. J. Colorectal Dis. 2024, 39, 112. [Google Scholar] [CrossRef]
- Morad, G.; Helmink, B.A.; Sharma, P.; Wargo, J.A. Hallmarks of response, resistance, and toxicity to immune checkpoint blockade. Cell 2021, 184, 5309–5337. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Liu, X.; Jin, S.; Chen, Y.; Guo, R. Ferroptosis in cancer therapy: A novel approach to reversing drug resistance. Mol. Cancer 2022, 21, 47. [Google Scholar] [CrossRef]
- Fan, T.; Zhang, M.; Yang, J.; Zhu, Z.; Cao, W.; Dong, C. Therapeutic cancer vaccines: Advancements, challenges, and prospects. Signal Transduct. Target. Ther. 2023, 8, 450. [Google Scholar] [CrossRef]
- Lee, H.M.; Wright, W.C.; Pan, M.; Low, J.; Currier, D.; Fang, J.; Singh, S.; Nance, S.; Delahunty, I.; Kim, Y.; et al. A CRISPR-drug perturbational map for identifying compounds to combine with commonly used chemotherapeutics. Nat. Commun. 2023, 14, 7332. [Google Scholar] [CrossRef]

| Genes | Forward Primer | Reverse Primer |
|---|---|---|
| TSC22D3 | AACACCGAAATGTATCAGACCC | TGTCCAGCTTAACGGAAACCA |
| FN1 | CGGTGGCTGTCAGTCAAAG | AAACCTCGGCTTCCTCCATAA |
| CASP4 | CAAGAGAAGCAACGTATGGCA | AGGCAGATGGTCAAACTCTGTA |
| PIK3R1 | AAGAAGTTGAACGAGTGGTTGG | GCCCTGTTTACTGCTCTCCC |
| IKBKB | GGAAGTACCTGAACCAGTTTGAG | GCAGGACGATGTTTTCTGGCT |
| LAMB1 | AGGAACCCGAGTTCAGCTAC | CACGTCGAGGTCACCGAAAG |
| ERBB3 | GACCCAGGTCTACGATGGGAA | GTGAGCTGAGTCAAGCGGAG |
| STAT3 | CAGCAGCTTGACACACGGTA | AAACACCAAAGTGGCATGTGA |
| AKT1 | AGCGACGTGGCTATTGTGAAG | GCCATCATTCTTGAGGAGGAAGT |
| Bcl2l1 | GAGCTGGTGGTTGACTTTCTC | TCCATCTCCGATTCAGTCCCT |
| CCNE2 | TCAAGACGAAGTAGCCGTTTAC | TGACATCCTGGGTAGTTTTCCTC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
You, X.; Gai, Y.; Wang, Z.; Wang, Y.; Ye, J.; Cao, Y.; Zhang, H.; Zhang, Z.; Feng, Y. Transcriptomic and Functional Validation Reveals PAQR3/P6-55 as Potential Therapeutic Targets in Colon Cancer. Biology 2025, 14, 780. https://doi.org/10.3390/biology14070780
You X, Gai Y, Wang Z, Wang Y, Ye J, Cao Y, Zhang H, Zhang Z, Feng Y. Transcriptomic and Functional Validation Reveals PAQR3/P6-55 as Potential Therapeutic Targets in Colon Cancer. Biology. 2025; 14(7):780. https://doi.org/10.3390/biology14070780
Chicago/Turabian StyleYou, Xue, Yikuo Gai, Ziyun Wang, Yanqi Wang, Jingran Ye, Yujia Cao, Hengshuo Zhang, Ziyi Zhang, and Ying Feng. 2025. "Transcriptomic and Functional Validation Reveals PAQR3/P6-55 as Potential Therapeutic Targets in Colon Cancer" Biology 14, no. 7: 780. https://doi.org/10.3390/biology14070780
APA StyleYou, X., Gai, Y., Wang, Z., Wang, Y., Ye, J., Cao, Y., Zhang, H., Zhang, Z., & Feng, Y. (2025). Transcriptomic and Functional Validation Reveals PAQR3/P6-55 as Potential Therapeutic Targets in Colon Cancer. Biology, 14(7), 780. https://doi.org/10.3390/biology14070780






