Functional Feed for Tilapia: Exploring the Benefits of Aspalathus linearis Tea Extract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. The Plant Material
2.2. Analysis of Plant Metabolites
2.2.1. Fatty Acid Methyl Esters (FAMEs)
2.2.2. Amino Acids
2.2.3. Sugars and Phenolic Acids
2.2.4. Sugar Derivatization
2.2.5. Phenolic Derivatization
2.3. Chromatographic Separation
2.3.1. Fatty Acid Methyl Esters (FAMEs)
2.3.2. Sugars
2.3.3. Amino Acids
2.3.4. Phenolic Acids
2.4. Experimental Procedures
2.4.1. Preparation of Rooibos Tea Extracts and Feed
2.4.2. Experimental Feeding Groups
2.4.3. Experimental Setup for Feeding Trial
2.4.4. Proximate Composition of Diets
2.5. Measurement of Growth Performance Parameters
2.6. Tissue Fixation and Processing
2.7. The Micronucleus Assay (MN)
2.8. Statistical Analysis
2.8.1. Growth Performance Parameters
2.8.2. Plant Metabolite Analysis
3. Results
3.1. Sugars in GRT and FRT
3.2. Growth Response of Larval Fish to FRT and GRT Extracts and Histopathology of Selected Organs
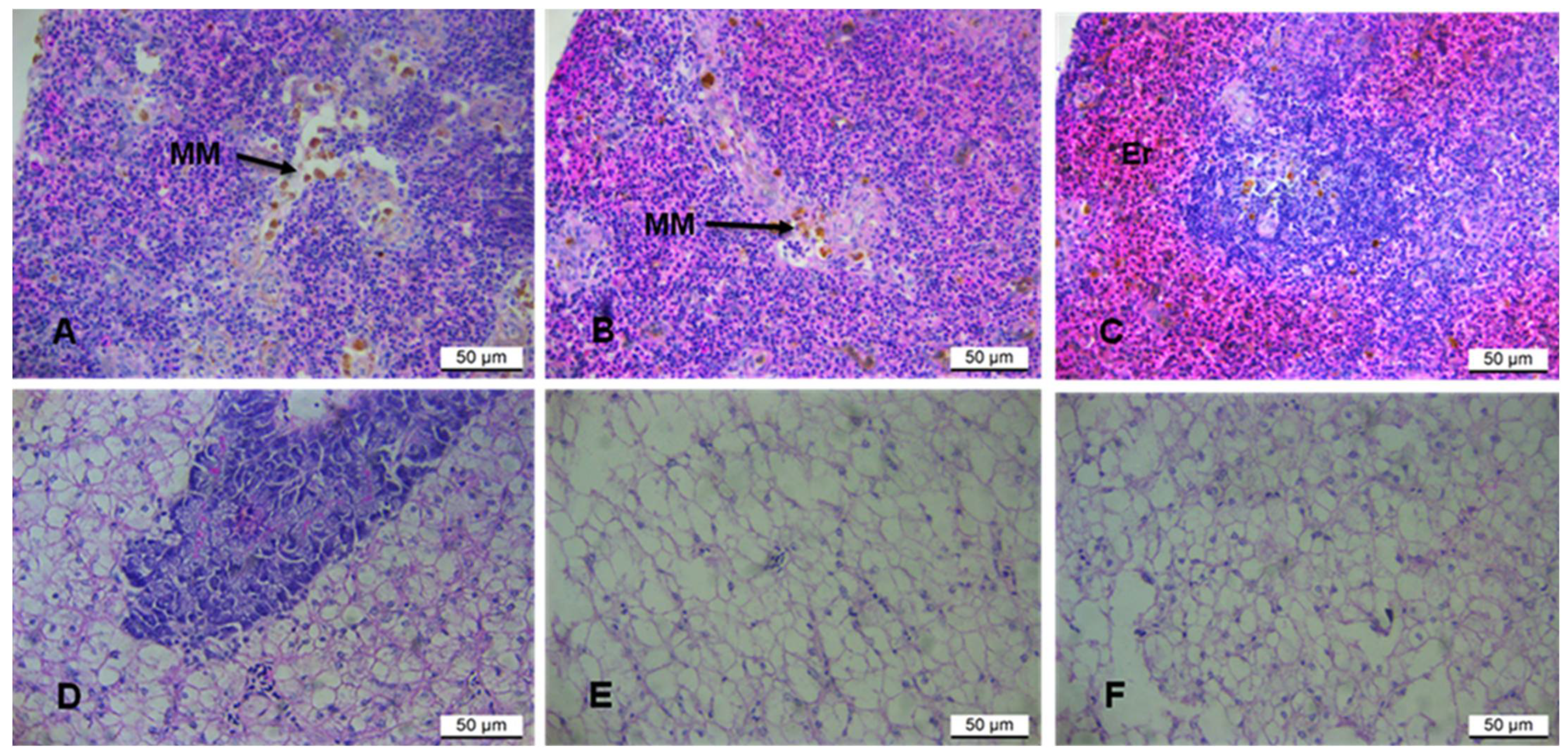
3.2.1. Liver and Spleen Histopathology
3.2.2. The Micronucleus (MN) Assay
4. Discussion
Larval Fish Growth Response to FRT- and GRT-Supplemented Diets
- Enhanced Phagocytic Activity: Rooibos extract can enhance the activity of phagocytes, which are crucial for the innate immune response and pathogen clearance. Dietary supplements like rooibos extract can enhance the phagocytic index in tilapia, similar to xanthones from mangosteen. Various dietary supplements positively influence tilapia immune responses, including phagocyte activity, suggesting rooibos extract’s similar role in enhancing innate immunity [96]. Natural extracts can improve overall health and disease resistance in tilapia, indicating rooibos’s broader potential in aquaculture.
- Modulation of Immunomodulatory Cytokines: The extracts may promote the expression of immunomodulatory cytokines, such as interleukin-1 beta (IL-1β) and interferon-gamma (INF-γ), which are vital for orchestrating immune responses.
- Increased Leukocyte Counts: The inclusion of functional ingredients like rooibos may lead to increased leukocyte counts, which is essential for effective phagocytosis [97].
- Reduction in Oxidative Stress: Compounds like aspalathin and nothofagin in rooibos exhibit strong antioxidant properties, reducing oxidative stress and inflammation in fish, which can enhance immune function [98].
- Improved Antioxidant Enzymes: The extract may elevate levels of antioxidant enzymes, such as catalase and glutathione peroxidase, further supporting immune health.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Aguado-Giménez, F. Effect of feed delivery rate and pellet size on rearing performance, feed wastage and economic profitability in gilthead seabream (Sparus aurata) ongrowing. Water 2020, 12, 954. [Google Scholar] [CrossRef]
- Riaz, D.; Hussain, S.M.; Hussain, M.; Arsalan, M.Z.-U.-H.; Naeem, E. Probiotics Supplementation for Improving Growth Performance, Nutrient Digestibility and Hematology of Catla catla Fingerlings Fed Sunflower Meal-Based Diet. Pak. J. Zool. 2024, 56, 1369–1377. [Google Scholar] [CrossRef]
- Burr, G.S.; Wolters, W.R.; Barrows, F.T.; Hardy, R.W. Replacing fishmeal with blends of alternative proteins on growth performance of rainbow trout (Oncorhynchus mykiss), and early or late stage juvenile Atlantic salmon (Salmo salar). Aquaculture 2012, 334, 110–116. [Google Scholar] [CrossRef]
- Colombo, S.M.; Turchini, G.M. ‘Aquafeed 3.0’: Creating a more resilient aquaculture industry with a circular bioeconomy framework. Rev. Aquac. 2021, 13, 1156–1158. [Google Scholar] [CrossRef]
- Tacon, A.G.; Metian, M.; McNevin, A.A. Future feeds: Suggested guidelines for sustainable development. Rev. Fish. Sci. Aquac. 2022, 30, 135–142. [Google Scholar] [CrossRef]
- Chahid, A.; N’Doye, I.; Majoris, J.E.; Berumen, M.L.; Laleg-Kirati, T.M. Model predictive control paradigms for fish growth reference tracking in precision aquaculture. J. Process Control 2021, 105, 160–168. [Google Scholar] [CrossRef]
- Cho, C.Y. Development of high nutrient-dense diets and fish feeding systems for optimum production and aquaculture waste reduction: A treatise. In Alternative Protein Sources in Aquaculture Diets; CRC Press: Boca Raton, FL, USA, 2023; pp. 17–50. [Google Scholar]
- Meltzer, H.M.; Abel, M.H.; Knutsen, H.K.; Amberntsson, A.; Brantsæter, A.L.; Budin-Ljøsne, I.; Husøy, T.; Iszatt, N.; Lund-Iversen, K.; Paulsen, M.M. What is a sustainable diet in the Norwegian context? Scand. J. Public Health 2025, 53, 195–206. [Google Scholar] [CrossRef]
- Waagbø, R. Feeding and disease resistance in fish. In Biology of Growing Animals; Elsevier: Amsterdam, The Netherlands, 2006; Volume 4, pp. 387–415. [Google Scholar] [CrossRef]
- Rohani, M.F.; Islam, S.M.; Hossain, M.K.; Ferdous, Z.; Siddik, M.A.; Nuruzzaman, M.; Padeniya, U.; Brown, C.; Shahjahan, M. Probiotics, prebiotics and synbiotics improved the functionality of aquafeed: Upgrading growth, reproduction, immunity and disease resistance in fish. Fish Shellfish Immunol. 2022, 120, 569–589. [Google Scholar] [CrossRef]
- Bae, J.; Hamidoghli, A.; Won, S.; Choi, W.; Lim, S.-G.; Kim, K.-W.; Lee, B.-J.; Hur, S.-W.; Bai, S.C. Evaluation of seven different functional feed additives in a low fish meal diet for olive flounder, Paralichthys olivaceus. Aquaculture 2020, 525, 735333. [Google Scholar] [CrossRef]
- Choi, W.; Moniruzzaman, M.; Bae, J.; Hamidoghli, A.; Lee, S.; Choi, Y.-H.; Min, T.; Bai, S.C. Evaluation of dietary probiotic bacteria and processed yeast (GroPro-Aqua) as the alternative of antibiotics in juvenile olive flounder Paralichthys olivaceus. Antibiotics 2022, 11, 129. [Google Scholar] [CrossRef]
- Dawood, M.A.; El Basuini, M.F.; Yilmaz, S.; Abdel-Latif, H.M.; Alagawany, M.; Kari, Z.A.; Abdul Razab, M.K.A.; Hamid, N.K.A.; Moonmanee, T.; Van Doan, H. Exploring the roles of dietary herbal essential oils in aquaculture: A review. Animals 2022, 12, 823. [Google Scholar] [CrossRef] [PubMed]
- Hodar, A.R.; Vasava, R.; Mahavadiya, D.; Joshi, N.; Nandaniya, V.; Solanki, H. Herbs and herbal medicines: A prominent source for sustainable aquaculture. J. Exp. Zool. India 2021, 24, 719–732. [Google Scholar]
- Sumana, S.L.; Xue, T.; Hu, H.; Abdullateef, M.M.; Shui, Y.; Ayana, G.U.; Kayiira, J.C.; Zhang, C.; Samwel, B.J.; Zhu, J. Medicinal Plants as Ecological Solutions for Fish Growth and Immunostimulatory Effects in Aquaculture. Aquac. Res. 2025, 2025, 9778623. [Google Scholar] [CrossRef]
- Tiamiyu, A.M.; Olatoye, I.O.; Olayemi, O.A.; Ekundayo, T.C.; Adedeji, O.B.; Okocha, R.C. Medicinal plant feed additives enhanced survivability and growth performance of Clarias gariepinus (African catfish) against bacterial infection. Microbiol. Res. 2021, 12, 744–752. [Google Scholar] [CrossRef]
- Hoseinifar, S.H.; Sun, Y.-Z.; Zhou, Z.; Van Doan, H.; Davies, S.J.; Harikrishnan, R. Boosting immune function and disease bio-control through environment-friendly and sustainable approaches in finfish aquaculture: Herbal therapy scenarios. Rev. Fish. Sci. Aquac. 2020, 28, 303–321. [Google Scholar] [CrossRef]
- Caipang, C.M.A.; Lazado, C.C. Nutritional impacts on fish mucosa: Immunostimulants, pre-and probiotics. In Mucosal Health in Aquaculture; Elsevier: Amsterdam, The Netherlands, 2015; pp. 211–272. [Google Scholar]
- Siddik, M.A.; Howieson, J.; Fotedar, R.; Partridge, G.J. Enzymatic fish protein hydrolysates in finfish aquaculture: A review. Rev. Aquac. 2021, 13, 406–430. [Google Scholar] [CrossRef]
- Abid, A.; Davies, S.; Waines, P.; Emery, M.; Castex, M.; Gioacchini, G.; Carnevali, O.; Bickerdike, R.; Romero, J.; Merrifield, D. Dietary synbiotic application modulates Atlantic salmon (Salmo salar) intestinal microbial communities and intestinal immunity. Fish Shellfish Immunol. 2013, 35, 1948–1956. [Google Scholar] [CrossRef] [PubMed]
- Kiron, V. Fish immune system and its nutritional modulation for preventive health care. Anim. Feed Sci. Technol. 2012, 173, 111–133. [Google Scholar] [CrossRef]
- Lu, Z.-Y.; Feng, L.; Jiang, W.-D.; Wu, P.; Liu, Y.; Jin, X.-W.; Ren, H.-M.; Kuang, S.-Y.; Li, S.-W.; Tang, L. An antioxidant supplement function exploration: Rescue of intestinal structure injury by mannan oligosaccharides after aeromonas hydrophila infection in grass carp (Ctenopharyngodon idella). Antioxidants 2022, 11, 806. [Google Scholar] [CrossRef]
- El-Saadony, M.T.; Alagawany, M.; Patra, A.K.; Kar, I.; Tiwari, R.; Dawood, M.A.; Dhama, K.; Abdel-Latif, H.M. The functionality of probiotics in aquaculture: An overview. Fish Shellfish Immunol. 2021, 117, 36–52. [Google Scholar] [CrossRef]
- Anane, A.Y.; Apalata, T.; Vasaikar, S.; Okuthe, G.E.; Songca, S. Prevalence and molecular analysis of multidrug-resistant Acinetobacter baumannii in the extra-hospital environment in Mthatha, South Africa. Braz. J. Infect. Dis. 2019, 23, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Dube, E.; Okuthe, G.E. Applications of antimicrobial photodynamic therapy in aquaculture: Effect on fish pathogenic bacteria. Fishes 2024, 9, 99. [Google Scholar] [CrossRef]
- Hosu, M.C.; Vasaikar, S.; Okuthe, G.E.; Apalata, T. Molecular detection of antibiotic-resistant genes in Pseudomonas aeruginosa from nonclinical environment: Public Health Implications in Mthatha, Eastern Cape Province, South Africa. Int. J. Microbiol. 2021, 2021, 8861074. [Google Scholar] [CrossRef]
- Miranda, C.D.; Tello, A.; Keen, P.L. Mechanisms of antimicrobial resistance in finfish aquaculture environments. Front. Microbiol. 2013, 4, 233. [Google Scholar] [CrossRef]
- Henriksson, P.J.; Rico, A.; Troell, M.; Klinger, D.H.; Buschmann, A.H.; Saksida, S.; Chadag, M.V.; Zhang, W. Unpacking factors influencing antimicrobial use in global aquaculture and their implication for management: A review from a systems perspective. Sustain. Sci. 2018, 13, 1105–1120. [Google Scholar] [CrossRef] [PubMed]
- Bondad-Reantaso, M.G.; MacKinnon, B.; Karunasagar, I.; Fridman, S.; Alday-Sanz, V.; Brun, E.; Le Groumellec, M.; Li, A.; Surachetpong, W.; Karunasagar, I.; et al. Review of alternatives to antibiotic use in aquaculture. Rev. Aquac. 2023, 15, 1421–1451. [Google Scholar] [CrossRef]
- Kuebutornye, F.K.A.; Roy, K.; Folorunso, E.A.; Mraz, J. Plant-based feed additives in Cyprinus carpio aquaculture. Rev. Aquac. 2024, 16, 309–336. [Google Scholar] [CrossRef]
- Song, Y.; Hu, Z.; Liu, S.; Luo, S.; He, R.; Yang, X.; Li, S.; Yang, X.; An, Y.; Lu, Y. Utilization of Microalgae and Duckweed as Sustainable Protein Sources for Food and Feed: Nutritional Potential and Functional Applications. J. Agric. Food Chem. 2025, 73, 4466–4482. [Google Scholar] [CrossRef]
- Okocha, R.C.; Olatoye, I.O.; Adedeji, O.B. Food safety impacts of antimicrobial use and their residues in aquaculture. Public Health Rev. 2018, 39, 21. [Google Scholar] [CrossRef]
- Castex, M.; Daniels, C.; Chim, L. Probiotic applications in crustaceans. In Aquaculture Nutrition: Gut Health, Probiotics and Prebiotics; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2014; pp. 290–327. [Google Scholar] [CrossRef]
- Makała, H. Impact of selected feed additives in broiler nutrition on breeding and the meat quality features. In Advanced Studies in the 21st Century Animal Nutrition; IntechOpen: London, UK, 2021. [Google Scholar]
- Alem, W.T. Effect of herbal extracts in animal nutrition as feed additives. Heliyon 2024, 10, e24973. [Google Scholar] [CrossRef]
- Lambo, M.; Ma, H.; Liu, R.; Dai, B.; Zhang, Y.; Li, Y. Mechanism, effectiveness, and the prospects of medicinal plants and their bioactive compounds in lowering ruminants’ enteric methane emission. Animal 2024, 18, 101134. [Google Scholar] [CrossRef] [PubMed]
- Okuthe, G. Valorizing fruit and vegetable waste: The untapped potential for entrepreneurship in sub-Saharan africa—A systematic review. Recycling 2024, 9, 40. [Google Scholar] [CrossRef]
- Riar, C.S.; Panesar, P.S. Bioactive compounds and nutraceuticals: Classification, potential sources, and application status. In Bioactive Compounds and Nutraceuticals from Dairy, Marine, and Nonconventional Sources; Apple Academic Press: Palm Bay, FL, USA, 2024; pp. 3–60. [Google Scholar]
- Hu, Y.; Zhang, X.; Shan, L.P.; Liu, L.; Chen, J. The antiviral activity of currently used medicinal plants in aquaculture and structure–activity relationship of their active ingredients. Rev. Aquac. 2024, 16, 154–173. [Google Scholar] [CrossRef]
- Iqbal, T.; Salma, U.; Umair, M.; Iqbal, H.; Khalid, T.; Hyder, S. Utilizing Medicinal Plants for Disease Treatment in Aquaculture: An Approach to Improve Fish Health: Medicinal Plants in Aquaculture. MARKHOR (J. Zool.) 2024, 5, 03–10. [Google Scholar] [CrossRef]
- Di Matteo, P.; Petrucci, R. A Novel Selective and Sensitive HPLC-ESI-Tandem MS/MS Method for Indole Structure-Retaining Metabolites of Tryptophan: Application in Beverages. Beverages 2025, 11, 37. [Google Scholar] [CrossRef]
- Rudrapal, M.; Rakshit, G.; Singh, R.P.; Garse, S.; Khan, J.; Chakraborty, S. Dietary polyphenols: Review on chemistry/sources, bioavailability/metabolism, antioxidant effects, and their role in disease management. Antioxidants 2024, 13, 429. [Google Scholar] [CrossRef] [PubMed]
- Sharma, K.K.; Sharma, M.; Devi, D.; Gupta, A.; Singh, S. Phytochemicals Present In Vegetables For Health Promotion. In Plant Metabolites and Vegetables as Nutraceuticals; Apple Academic Press: Palm Bay, FL, USA, 2024; pp. 61–94. [Google Scholar]
- Sharma, R.; Sharma, K.D.; Kumar, S.; Thakur, A. Phytochemicals: The Functional Food Ingredients and Their Health Benefits. In Functional Compounds and Foods of Plant Origin; Apple Academic Press: Palm Bay, FL, USA, 2025; pp. 3–38. [Google Scholar]
- Ahmadifar, E.; Yousefi, M.; Karimi, M.; Fadaei Raieni, R.; Dadar, M.; Yilmaz, S.; Dawood, M.A.; Abdel-Latif, H.M. Benefits of dietary polyphenols and polyphenol-rich additives to aquatic animal health: An overview. Rev. Fish. Sci. Aquac. 2021, 29, 478–511. [Google Scholar] [CrossRef]
- Ma, Y.-B.; Zhang, J.-X.; Zhou, X.-Q.; Jiang, W.-D.; Wu, P.; Liu, Y.; Kuang, S.-Y.; Tang, L.; Feng, L. Effect of tea polyphenols on flavour, healthcare components, physicochemical properties, and mechanisms of collagen synthesis in growing grass carp (Ctenopharyngodon idella) muscle. Aquaculture 2021, 534, 736237. [Google Scholar] [CrossRef]
- Liu, A.; Ka Makhaya, S.C.; Osewe, M. Factors influencing Rooibos tea certification and quality control for smallholder farmers in South Africa. Foods 2022, 11, 3495. [Google Scholar] [CrossRef]
- Koch, I.; Muller, M.; Joubert, E.; Van der Rijst, M.; Næs, T. Sensory characterization of rooibos tea and the development of a rooibos sensory wheel and lexicon. Food Res. Int. 2012, 46, 217–228. [Google Scholar] [CrossRef]
- Deka, H.; Sarmah, P.P.; Chowdhury, P.; Rajkhowa, K.; Sabhapondit, S.; Panja, S.; Karak, T. Impact of the season on total polyphenol and antioxidant properties of tea cultivars of industrial importance in Northeast India. Foods 2023, 12, 3196. [Google Scholar] [CrossRef] [PubMed]
- Tang, G.-Y.; Meng, X.; Gan, R.-Y.; Zhao, C.-N.; Liu, Q.; Feng, Y.-B.; Li, S.; Wei, X.-L.; Atanasov, A.G.; Corke, H.; et al. Health functions and related molecular mechanisms of tea components: An update review. Int. J. Mol. Sci. 2019, 20, 6196. [Google Scholar] [CrossRef] [PubMed]
- Hayat, K.; Iqbal, H.; Malik, U.; Bilal, U.; Mushtaq, S. Tea and its consumption: Benefits and risks. Crit. Rev. Food Sci. Nutr. 2015, 55, 939–954. [Google Scholar] [CrossRef]
- Naveed, M.; BiBi, J.; Kamboh, A.A.; Suheryani, I.; Kakar, I.; Fazlani, S.A.; FangFang, X.; Yunjuan, L.; Kakar, M.U.; Abd El-Hack, M.E. Pharmacological values and therapeutic properties of black tea (Camellia sinensis): A comprehensive overview. Biomed. Pharmacother. 2018, 100, 521–531. [Google Scholar] [CrossRef] [PubMed]
- Lukman, H.; Aribisala, J.; Akoonjee, A.; Sulyman, A.; Wudil, A.; Sabiu, S. Modulation of dipeptidyl peptidase IV by Rooibos tea metabolites towards type 2 diabetes care: Evidence from molecular dynamics simulation and density functional theory. Sci. Afr. 2024, 24, e02173. [Google Scholar] [CrossRef]
- Pou, K.J.; Paul, S.K.; Malakar, S. Industrial processing of CTC black tea. In Caffeinated and Cocoa Based Beverages; Elsevier: Amsterdam, The Netherlands, 2019; pp. 131–162. [Google Scholar] [CrossRef]
- Chipofya, E.; Docrat, T.F.; Marnewick, J.L. The Neuroprotective Effect of Rooibos Herbal Tea Against Alzheimer’s Disease: A Review. Mol. Nutr. Food Res. 2025, 69, e202400670. [Google Scholar] [CrossRef]
- Pretorius, L.; Smith, C. Green rooibos (Aspalathus linearis) promotes gut health: Insight into mechanisms. J. Ethnopharmacol. 2024, 319, 117379. [Google Scholar] [CrossRef]
- Nkukwana, T. Poultry production for food security: The South African perspective. Farmlink Afr. 2014, 4, 15–17. [Google Scholar]
- Dambuza, N.S.; Koekemoer, T.; van de Venter, M. In vitro bioavailability assessment of antioxidants from rooibos extracts. Pharmacology 2024, 28, 19–27. [Google Scholar]
- Ahmad, M.; Baba, W.N.; Shah, U.; Gani, A.; Gani, A.; Masoodi, F. Nutraceutical properties of the green tea polyphenols. J. Food Process. Technol. 2014, 5, 11. [Google Scholar] [CrossRef]
- Joubert, E.; Richards, E.S.; Merwe, J.D.V.D.; De Beer, D.; Manley, M.; Gelderblom, W.C. Effect of species variation and processing on phenolic composition and in vitro antioxidant activity of aqueous extracts of Cyclopia spp. (honeybush tea). J. Agric. Food Chem. 2008, 56, 954–963. [Google Scholar] [CrossRef] [PubMed]
- Shimamura, N.; Miyase, T.; Umehara, K.; Warashina, T.; Fujii, S. Phytoestrogens from Aspalathus linearis. Biol. Pharm. Bull. 2006, 29, 1271–1274. [Google Scholar] [CrossRef] [PubMed]
- Abasubong, K.P.; Gabriel, N.N.; Adjoumani, J.-J.Y. Ferulic Acid as Feed Additives in Aquaculture: A Review on Growth, Immune Response, and Antioxidant Status of Finfish. In Emerging Sustainable Aquaculture Innovations in Africa; Springer Nature: Berlin, Germany, 2023; pp. 251–272. [Google Scholar]
- Bramati, L.; Minoggio, M.; Gardana, C.; Simonetti, P.; Mauri, P.; Pietta, P. Quantitative characterization of flavonoid compounds in rooibos tea (Aspalathus linearis) by LC− UV/DAD. J. Agric. Food Chem. 2002, 50, 5513–5519. [Google Scholar] [CrossRef]
- Joubert, E. HPLC quantification of the dihydrochalcones, aspalathin and nothofagin in rooibos tea (Aspalathus linearis) as affected by processing. Food Chem. 1996, 55, 403–411. [Google Scholar] [CrossRef]
- Akinyede, K.A.; Hughes, G.D.; Ekpo, O.E.; Oguntibeju, O.O. Comparative study of the antioxidant constituents, activities and the GC-MS quantification and identification of fatty acids of four selected Helichrysum species. Plants 2022, 11, 998. [Google Scholar] [CrossRef]
- Amelo, A.A.; Mergo, W.Y.; Chemere, E.B.; Mhike, W.; Mavhungu, M.L. Phytochemicals and GC-MS analysis of fatty acids in leaves of Acmella caulirhiza traditional medicinal plant, evaluating efficiencies of extracting solvents and methods. Spectrosc. Lett. 2025, 40, 1–18. [Google Scholar] [CrossRef]
- Nasser, N.; Abiad, M.G.; Babikian, J.; Monzer, S.; Saoud, I.P. Using restaurant food waste as feed for Nile tilapia produc-tion. Aquac. Res. 2018, 49, 3142–3150. [Google Scholar] [CrossRef]
- Garling, D.L., Jr.; Wilson, R.P. Optimum dietary protein to energy ratio for channel catfish fingerlings, Ictalurus punctatus. J. Nutr. 1976, 106, 1368–1375. [Google Scholar] [CrossRef]
- AOAC INTERNATIONAL. AOAC Official Methods Program Manual on Development, Study, Review, and Approval Process for AOAC Official Methods; AOAC INTERNATIONAL: Gaithersburg, MD, USA, 1997. [Google Scholar]
- Griesh, A.S.; El-Nahla, A.M.; Aly, S.M.; Badran, M.F. Role of vitamin E supplementation on the reproductive and growth performance, hormonal profile and biochemical parameters of female hybrid red tilapia. Thalassas: Int. J. Mar. Sci. 2024, 40, 1169–1178. [Google Scholar] [CrossRef]
- Li, L.; Shen, Y.; Yang, W.; Xu, X.; Li, J. Effect of different stocking densities on fish growth performance: A meta-analysis. Aquaculture 2021, 544, 737152. [Google Scholar] [CrossRef]
- Okuthe, G.E.; Siguba, B. Silver nanoparticle-induced nephrotoxicity in Zebrafish (Danio rerio). Int. J. Mol. Sci. 2025, 26, 4216. [Google Scholar] [CrossRef]
- Okuthe, G. Investigation of apoptosis as a biomarker for earlier detection of impending sex inversion in zebrafish (Danio rerio). Trans. R. Soc. S. Afr. 2016, 71, 129–135. [Google Scholar] [CrossRef]
- Minissi, S.; Ciccotti, E.; Rizzoni, M. Micronucleus test in erythrocytes of Barbus plebejus (Teleostei, Pisces) from two natural environments: A bioassay for the in situ detection of mutagens in freshwater. Mutat. Res. Genet. Toxicol. 1996, 367, 245–251. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Volkoff, H. The effects of environmental changes on the endocrine regulation of feeding in fishes. Philos. Trans. R. Soc. B 2024, 379, 20220503. [Google Scholar] [CrossRef]
- Hasanpour, S.; Salati, A.P.; Falahatkar, B.; Azarm, H.M. Effects of dietary green tea (Camellia sinensis L.) supplementation on growth performance, lipid metabolism, and antioxidant status in a sturgeon hybrid of Sterlet (Huso huso♂× Acipenser ruthenus♀) fed oxidized fish oil. Fish Physiol. Biochem. 2017, 43, 1315–1323. [Google Scholar] [CrossRef]
- Sun, B.; Zhu, Z.; Cao, P.; Chen, H.; Chen, C.; Zhou, X.; Mao, Y.; Lei, J.; Jiang, Y.; Meng, W. Purple foliage coloration in tea (Camellia sinensis L.) arises from activation of the R2R3-MYB transcription factor CsAN1. Sci. Rep. 2016, 6, 32534. [Google Scholar] [CrossRef] [PubMed]
- Mbokane, E.M.; Moyo, N.A.G. Use of medicinal plants as feed additives in the diets of Mozambique tilapia (Oreochromis mossambicus) and the African Sharptooth catfish (Clarias gariepinus) in Southern Africa. Front. Vet. Sci. 2022, 9, 1072369. [Google Scholar] [CrossRef]
- Shahabuddin, A.; Hannan, M.A.; Hossain, M.F.; Hemal, S.; Khanam, R.; Afroz, T.; Mustafa, A. Evaluation of Ethanolic Extract of Red Seaweed (Gracilariopsis lemaneiformis) on Growth and Haematological Parameters of Nile Tilapia (Oreochromis niloticus). Aquaculture, Fish Fish. 2024, 4, e70011. [Google Scholar] [CrossRef]
- Wigraiboon, S.; Panchan, R.; Luang-In, V.; Ounjit, W.; Panase, P.; Sookying, S.; Sutthi, N. Effects of dietary tuber ethanolic extract of nut grass (Cyperus rotundus Linn.) on growth, immune response, and disease resistance in Nile tilapia (Oreochromis niloticus). Animals 2024, 14, 503. [Google Scholar] [CrossRef]
- Abd El-Naby, A.S.; El Asely, A.M.; Hussein, M.N.; Khattaby, A.E.-R.A.; Sabry, E.A.; Abdelsalam, M.; Samir, F. Effects of dietary fermented Saccharomyces cerevisiae extract (Hilyses) supplementation on growth, hematology, immunity, antioxidants, and intestinal health in Nile tilapia. Sci. Rep. 2024, 14, 12583. [Google Scholar] [CrossRef]
- Kamble, M.T.; Yostawonkul, J.; Medhe, S.V.; Chavan, B.R.; Kumar, A.; Palekar, G.R.; Daunde, V.Y.; Tayade, S.H.; Gabriel, N.N.; Ataguba, G.A. Innovative Feed Additives for Sustainable Aquaculture: Phytobiotics Encapsulated in Organic Nanoparticles. In Sustainable Feed Ingredients and Additives for Aquaculture Farming: Perspectives from Africa and Asia; Springer Nature: Berlin, Germany, 2024; pp. 501–520. [Google Scholar]
- Liu, C.; Hua, L.; Liu, H.; Wang, L.; Zhu, X.; Rebours, C.; Harding, K.G.; Tan, L.; Hu, Q.; Xie, S. Biotransformation of aquaculture wastewater into aquatic feed protein source: Chlorella sorokiniana nutritional value and safety risk assessment. J. Environ. Manag. 2024, 370, 122510. [Google Scholar] [CrossRef] [PubMed]
- Joubert, E.; Gelderblom, W.C.A.; Louw, A.; De Beer, D. South African herbal teas: Aspalathus linearis, Cyclopia spp. and Athrixia phylicoides—A review. J. Ethnopharmacol. 2008, 119, 376–412. [Google Scholar] [CrossRef]
- Kay, C.D.; Kroon, P.A.; Cassidy, A. The bioactivity of dietary anthocyanins is likely to be mediated by their degradation products. Mol. Nutr. Food Res. 2009, 53, S92–S101. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-Y.; Tsai, S.-J.; Huang, C.-S.; Yin, M.-C. Antiglycative effects of protocatechuic acid in the kidneys of diabetic mice. J. Agric. Food Chem. 2011, 59, 5117–5124. [Google Scholar] [CrossRef]
- Mahfuz, S.; Shang, Q.; Piao, X. Phenolic compounds as natural feed additives in poultry and swine diets: A review. J. Anim. Sci. Biotechnol. 2021, 12, 48. [Google Scholar] [CrossRef]
- Kumar, N.; Goel, N. Phenolic acids: Natural versatile molecules with promising therapeutic applications. Biotechnol. Rep. 2019, 24, e00370. [Google Scholar] [CrossRef] [PubMed]
- Mahfuz, S.; Mun, H.-S.; Dilawar, M.A.; Ampode, K.M.B.; Yang, C.-J. Potential role of protocatechuic acid as natural feed additives in farm animal production. Animals 2022, 12, 741. [Google Scholar] [CrossRef]
- Mountzouris, K.C.; Paraskevas, V.; Tsirtsikos, P.; Palamidi, I.; Steiner, T.; Schatzmayr, G.; Fegeros, K. Assessment of a phytogenic feed additive effect on broiler growth performance, nutrient digestibility and caecal microflora composition. Anim. Feed Sci. Technol. 2011, 168, 223–231. [Google Scholar] [CrossRef]
- Viveros, A.; Chamorro, S.; Pizarro, M.; Arija, I.; Centeno, C.; Brenes, A. Effects of dietary polyphenol-rich grape products on intestinal microflora and gut morphology in broiler chicks. Poult. Sci. 2011, 90, 566–578. [Google Scholar] [CrossRef]
- Christaki, E.; Giannenas, I.; Bonos, E.; Florou-Paneri, P. Innovative uses of aromatic plants as natural supplements in nutrition. In Feed Additives; Elsevier: Amsterdam, The Netherlands, 2020; pp. 19–34. [Google Scholar]
- Yilmaz, E. Effect of dietary carob (Ceratonia siliqua) syrup on blood parameters, gene expression responses and ammonia resistance in tilapia (Oreochromis niloticus). Aquac. Res. 2020, 51, 1903–1912. [Google Scholar] [CrossRef]
- Nehme, R.; Chervet, A.; Decombat, C.; Habanjar, O.; Longechamp, L.; Rousset, A.; Chalard, P.; Gainche, M.; Senejoux, F.; Fraisse, D. Selected Plant Extracts Regulating the Inflammatory Immune Response and Oxidative Stress: Focus on Quercus robur. Nutrients 2025, 17, 510. [Google Scholar] [CrossRef] [PubMed]
- García-León, M.Á.; Pérez-Mármol, J.M.; Gonzalez-Pérez, R.; del Carmen García-Ríos, M.; Peralta-Ramírez, M.I. Relationship between resilience and stress: Perceived stress, stressful life events, HPA axis response during a stressful task and hair cortisol. Physiol. Behav. 2019, 202, 87–93. [Google Scholar] [CrossRef]
- Kumar, S.; Choubey, A.K.; Srivastava, P.K. The effects of dietary immunostimulants on the innate immune response of Indian major carp: A review. Fish Shellfish Immunol. 2022, 123, 36–49. [Google Scholar] [CrossRef]
- Zubaidah, A.; Faidah, K.R.; Samsundari, S. Effectiveness of Strychnos ligustrina Bl. extract as feed supplementation to increase the immune system of Nile Tilapia (Oreochromis niloticus) wich againts Streptococcus agalactiae. IJOTA (Indones. J. Trop. Aquat.) 2018, 1, 1–8. [Google Scholar] [CrossRef]
- Lee, W.; Bae, J.-S. Anti-inflammatory effects of aspalathin and nothofagin from rooibos (Aspalathus linearis) in vitro and in vivo. Inflammation 2015, 38, 1502–1516. [Google Scholar] [CrossRef] [PubMed]
- Costa, P.M. Current aspects of DNA damage and repair in ecotoxicology: A mini-review. Ecotoxicology 2022, 31, 1–11. [Google Scholar] [CrossRef]
- Ergenler, A.; Turan, F. DNA Damage in Fish Due to Pesticide Pollution. Nat. Eng. Sci. 2023, 8, 195–201. [Google Scholar] [CrossRef]
- Cruz-Esquivel, Á.; Díez, S.; Marrugo-Negrete, J.L. Genotoxicity effects in freshwater fish species associated with gold mining activities in tropical aquatic ecosystems. Ecotoxicol. Environ. Saf. 2023, 253, 114670. [Google Scholar] [CrossRef] [PubMed]
- Kousar, S.; Javed, M.; Ambreen, F.; Abbas, S. Determination of genotoxic effects of aluminum on Cirrhinus mrigala and Ctenopharyngodon idella using comet assay and micronucleus test. Iran. J. Fish. Sci. 2022, 21, 545–567. [Google Scholar]
- Rabelo, J.C.; Hanusch, A.L.; de Jesus, L.W.O.; Mesquita, L.A.; Franco, F.C.; Silva, R.A.; Sabóia-Morais, S.M. DNA damage induced by cylindrospermopsin on different tissues of the biomonitor fish Poecilia reticulata. Environ. Toxicol. 2021, 36, 1125–1134. [Google Scholar] [CrossRef]
- Araldi, R.P.; de Melo, T.C.; Mendes, T.B.; de Sá Júnior, P.L.; Nozima, B.H.N.; Ito, E.T.; de Carvalho, R.F.; de Souza, E.B.; de Cassia Stocco, R. Using the comet and micronucleus assays for genotoxicity studies: A review. Biomed. Pharmacother. 2015, 72, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Benvindo-Souza, M.; Oliveira, E.A.S.; Assis, R.A.; Santos, C.G.A.; Borges, R.E.; e Silva, D.D.M.; de Souza Santos, L.R. Micronucleus test in tadpole erythrocytes: Trends in studies and new paths. Chemosphere 2020, 240, 124910. [Google Scholar] [CrossRef] [PubMed]
- Zapata, A.; Diez, B.; Cejalvo, T.; Gutierrez-de Frias, C.; Cortés, A. Ontogeny of the immune system of fish. Fish Shellfish Immunol. 2006, 20, 126–136. [Google Scholar] [CrossRef]
- Marnewick, J.L.; Rautenbach, F.; Venter, I.; Neethling, H.; Blackhurst, D.M.; Wolmarans, P.; Macharia, M. Effects of rooibos (Aspalathus linearis) on oxidative stress and biochemical parameters in adults at risk for cardiovascular disease. J. Ethnopharmacol. 2011, 133, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Zapata, A.G. The fish spleen. Fish Shellfish Immunol. 2024, 144, 109280. [Google Scholar] [CrossRef]
- Li, X.; Han, T.; Zheng, S.; Wu, G. Hepatic glucose metabolism and its disorders in fish. In Recent Advances in Animal Nutrition and Metabolism; Springer Nature: Berlin, Germany, 2022; pp. 207–236. [Google Scholar]
- Naiel, M.A.; Negm, S.S.; Ghazanfar, S.; Shukry, M.; Abdelnour, S.A. The risk assessment of high-fat diet in farmed fish and its mitigation approaches: A review. J. Anim. Physiol. Anim. Nutr. 2023, 107, 948–969. [Google Scholar] [CrossRef]
- Geng, H.; Yang, P.; Chen, Y.; Qin, Y.; Li, X.; He, C.; Mai, K.; Song, F. Dietary choline can partially spare methionine to improve the feeds utilization and immune response in juvenile largemouth bass (Micropterus salmoides): Based on phenotypic response to gene expression. Aquac. Rep. 2023, 30, 101546. [Google Scholar] [CrossRef]
- Tang, L.; Hao, G.; Zhou, D.; Fan, Y.; Wei, Z.; Li, D.; Shen, Y.; Fang, H.; Lin, F.; Zhao, M. Hepatotoxicity in Carp (Carassius auratus) Exposed to Perfluorooctane Sulfonate (PFOS): Integrative Histopathology and Transcriptomics Analysis. Animals 2025, 15, 610. [Google Scholar] [CrossRef]

| Diets | CBD | FRT | GRT | |
|---|---|---|---|---|
| Proximate Composition | % | |||
| Dry matter | 95.28 | 95.29 | 95.32 | |
| Moisture | 4.72 | 4.71 | 4.68 | |
| Protein | 32.99 | 32.8 | 33.17 | |
| Fat | 4.94 | 4.75 | 4.81 | |
| Ash | 7.03 | 6.98 | 7.03 | |
| Fiber | 3.32 | 2.69 | 3.87 | |
| Carbohydrates | 50.01 | 50.73 | 50.31 |
| Client | L_Rhamnose | D_Fructose | D-Glucose | Sucrose | Alpha_Lactose | D_Maltose | Raffinose |
|---|---|---|---|---|---|---|---|
| SFRT | 7.44 | 1.95 | 75.01 | 90.54 | 6.92 | 5.58 | 5.66 |
| SGRT | 5.86 | 21.20 | 44.25 | 90.54 | 27.19 | 5.85 | 13.31 |
| Amino Acid Profile (%) | ||
|---|---|---|
| Amino Acid | FRT * (%) | GRT * (%) |
| Alanine | 8.62 | 8.18 |
| Glycine | 6.07 | 5.98 |
| Valine | 5.57 | 5.36 |
| Leucine | 11.12 | 10.99 |
| Isoleucine | 4.35 | 4.22 |
| Proline | 9.13 | 9.42 |
| Methionine | 2.41 | 2.15 |
| Serine | 4.05 | 3.93 |
| Threonine | 8.33 | 7.64 |
| Phenylalanine | 6.31 | 6.22 |
| Aspartic acid | 11.04 | 13.44 |
| Glutamic acid | 15.14 | 14.56 |
| Asparagine | 3.89 | 3.77 |
| Tyrosine | 3.97 | 3.88 |
| Phenolic Acid Profile (μg/L) | ||
| Phenolic Acid | FRT * (%) | GRT * (%) |
| 4-Hydroxybenzoic_acid | 183.93 | 71.72 |
| Vanillic acid | 752.47 | 161.62 |
| Protocatechuic acid | 1243 | 920.2 |
| m-coumaric acid | n/d | n/d |
| p-coumaric acid | 129.43 | 77.75 |
| Syringic acid | 1404.98 | 135.83 |
| Growth Parameters | Basal Diet | FRT * | GRT * |
|---|---|---|---|
| Initial number of fish—NI | 300 | 300 | 300 |
| Initial body weight (g)—IBL | 0.55 ± 0.02 | 0.55 ± 0.02 | 0.54 ± 0.03 |
| Initial body length (cm)—IBL | 2.22 ± 0.41 | 2.24 ± 0.30 | 2.21 ± 0.45 |
| Final body weight (cm)—FBW | 2.86 ± 0.60 a | 4.01 ± 0.88 b | 4.13 ± 0.75 b |
| Final body length (cm)—FBL | 5.25 ± 0.51 a | 5.51 ± 0.50 ab | 5.84 ± 0.46 bc |
| SGR (%) | 3.65 ± 0.40 a | 4.38 ± 0.44 b | 4.48 ± 0.42 b |
| Condition factor (%)—CF | 13.14 ± 4.87 a | 6.92 ± 2.45 b | 6.70 ± 2.53 b |
| Growth (%) | 5.15 ± 1.32 a | 7.69 ± 1.95 b | 7.98 ± 1.65 b |
| HIS (%) | 6.59 ± 1.91 | 6.75 ± 1.96 | 7.02 ± 2.10 |
| VSI (%) | 21.06 ± 7.10 a | 22.91 ± 11.87 b | 26.64 ± 12.52 c * |
| Rate of weight gain (g)—RWG | 2.32 ± 0.59 a | 3.46 ± 0.88 b | 3.59 ± 0.74 b |
| Food conversion ratio (%)—FCR | 2.32± 0.57 a | 1.50± 0.25 b | 1.41± 0.07 b |
| Survival Rate (%)—SR | 95.30 | 96.70 | 96.30 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okuthe, G.; Bhomela, B.; Vundisa, N. Functional Feed for Tilapia: Exploring the Benefits of Aspalathus linearis Tea Extract. Biology 2025, 14, 778. https://doi.org/10.3390/biology14070778
Okuthe G, Bhomela B, Vundisa N. Functional Feed for Tilapia: Exploring the Benefits of Aspalathus linearis Tea Extract. Biology. 2025; 14(7):778. https://doi.org/10.3390/biology14070778
Chicago/Turabian StyleOkuthe, Grace, Bongile Bhomela, and Noluyolo Vundisa. 2025. "Functional Feed for Tilapia: Exploring the Benefits of Aspalathus linearis Tea Extract" Biology 14, no. 7: 778. https://doi.org/10.3390/biology14070778
APA StyleOkuthe, G., Bhomela, B., & Vundisa, N. (2025). Functional Feed for Tilapia: Exploring the Benefits of Aspalathus linearis Tea Extract. Biology, 14(7), 778. https://doi.org/10.3390/biology14070778






