Assessing Suitable Habitats for Gerbera piloselloides (L.)Cass. in China Using an Optimized MaxEnt Model and Key Environmental Drivers
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Gathering and Analysis of Distribution Records
2.2. Environmental Factors’ Acquisition and Selection
2.3. Optimization Models
2.4. Model Building and Accuracy Assessment
2.5. Data Processing
3. Results
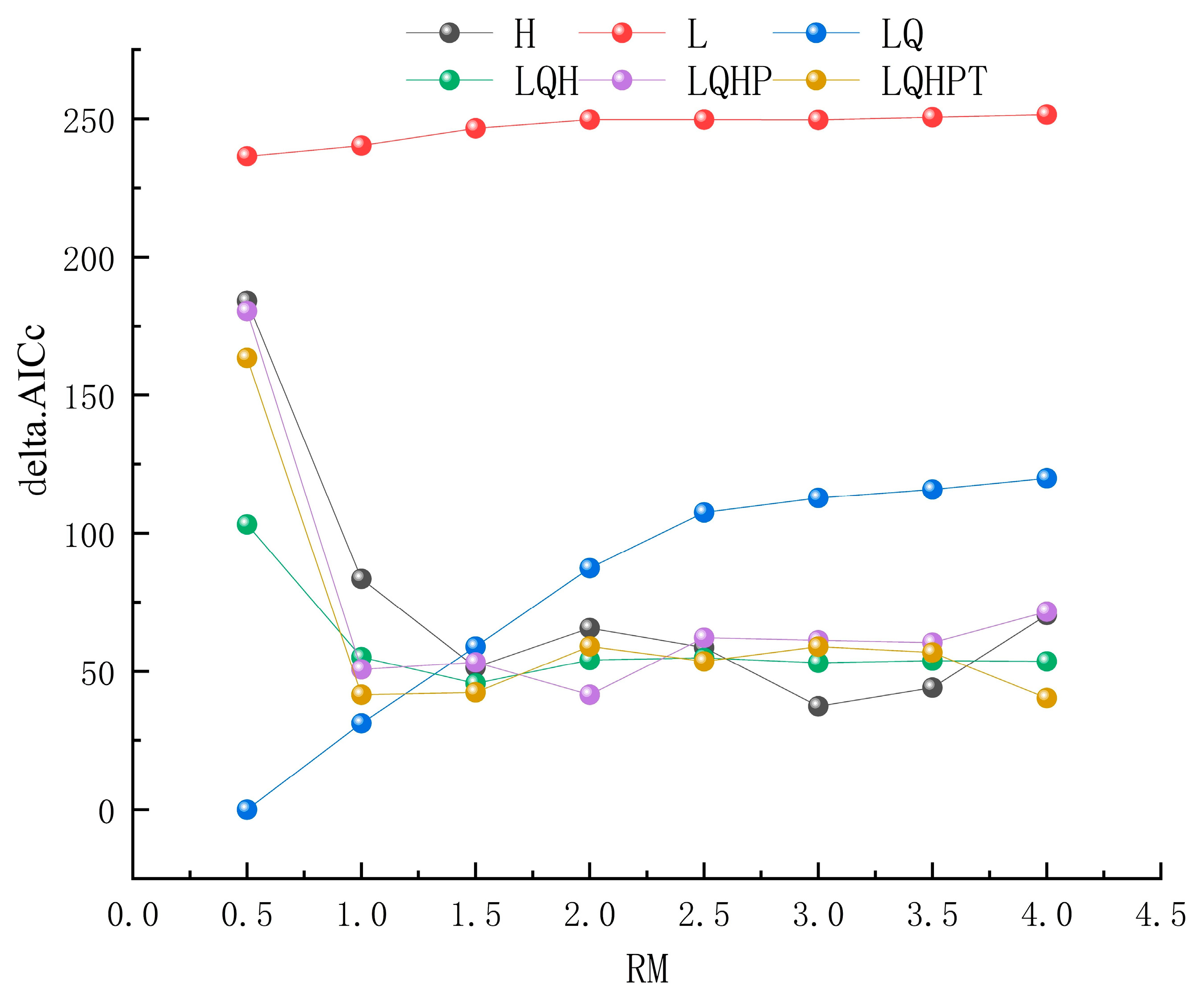
3.1. Results of Model Optimization and Accuracy Evaluation
3.2. Major Environmental Factors Affecting Gerbera piloselloides Distribution
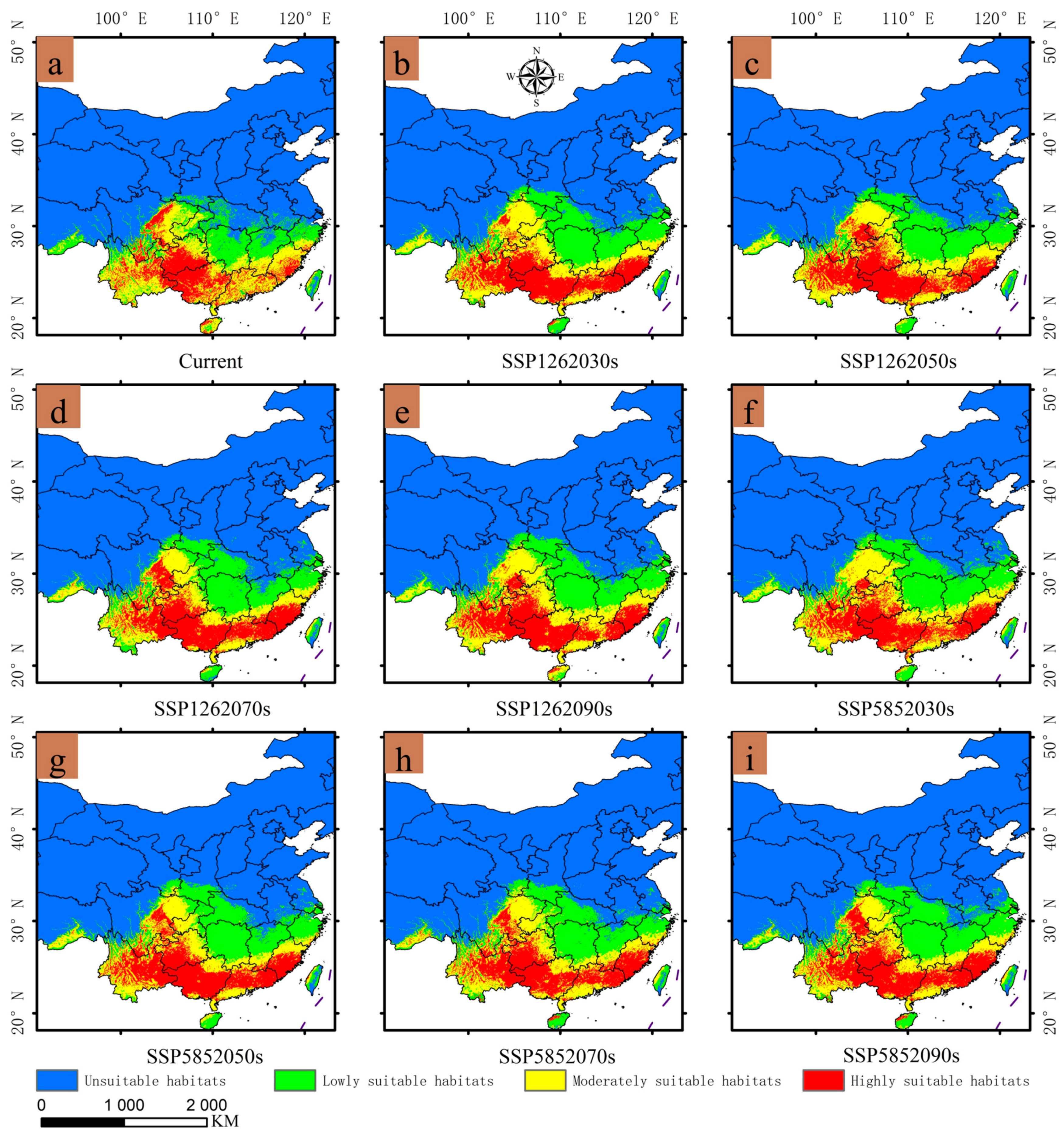
3.3. Existing Potential Distribution of Gerbera piloselloides
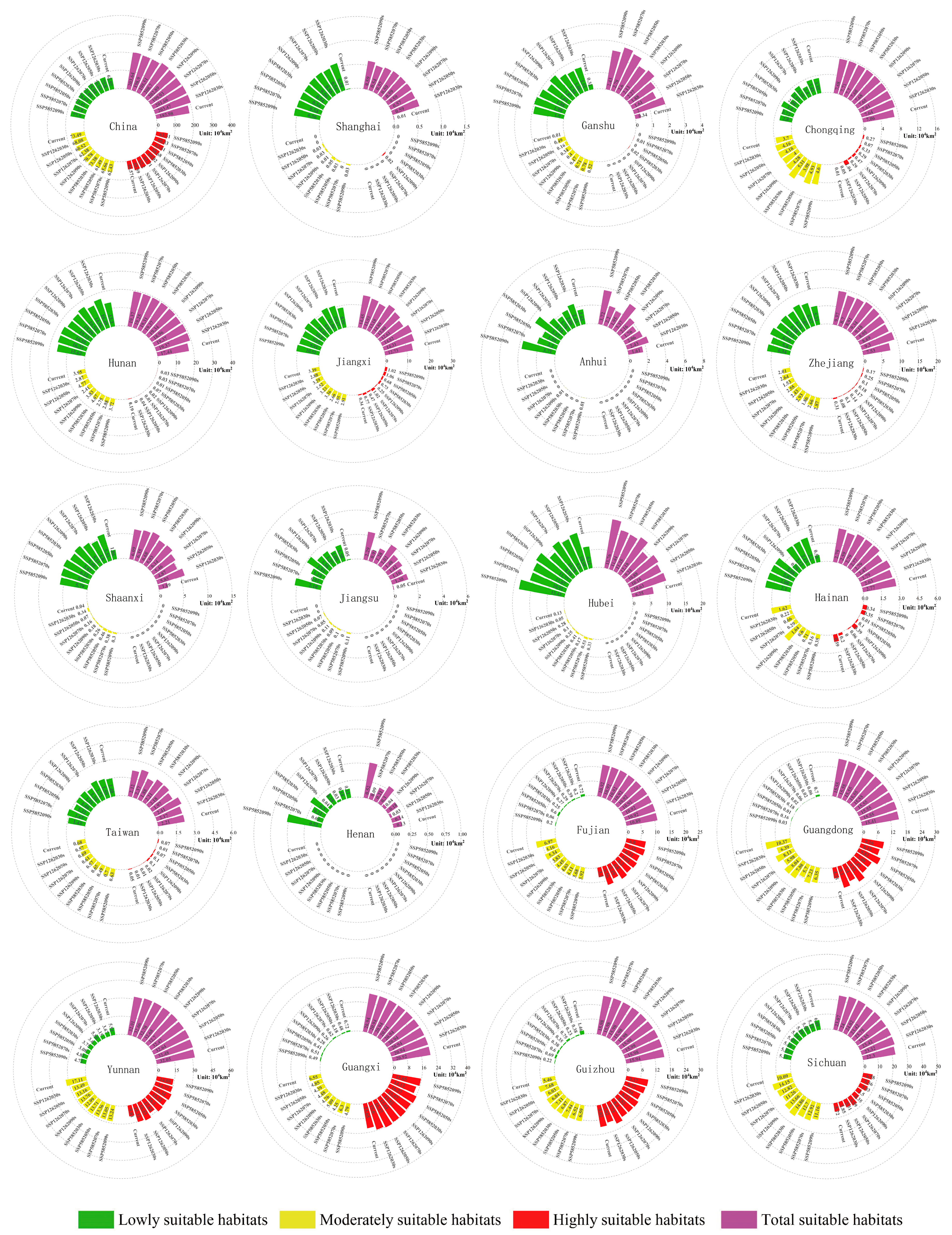
3.4. Expected Distribution Changes in Gerbera piloselloides in the Future
3.5. Migration Routes of the Suitable Habitat Centroid for Gerbera piloselloides
4. Discussion
4.1. Environmental Determinants of Gerbera piloselloides Distribution: Temperature Constraints and the Qinling–Huaihe Divide
4.2. Divergent Responses to Climate Change: Habitat Expansion in Northern Limits vs. Contraction in the Yangtze River Basin
4.3. Significance and Limitations of the Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, Y.R.; Hessen, D.O.; Samset, B.H.; Stordal, F. Evaluating Global and Regional Land Warming Trends in the Past Decades with Both MODIS and ERA5-Land Land Surface Temperature Data. Remote Sens. Environ. 2022, 280, 113181. [Google Scholar] [CrossRef]
- Peng, H.S.; Hao, J.D.; Huang, L.Q. Effect of Climate Change on Genuine Medicinal Materials Producing Areas during Last 2000 Years—Alisma orientale and Citrus aurantium as Examples. China J. Chin. Mater. Med. 2013, 38, 2218–2222. [Google Scholar]
- Pica, A.; Vela, D.; Magrini, S. Forest Orchids under Future Climate Scenarios: Habitat Suitability Modelling to Inform Conservation Strategies. Plants 2024, 13, 1810. [Google Scholar] [CrossRef]
- Franklin, J. Species Distribution Modelling Supports the Study of Past, Present and Future Biogeographies. J. Biogeogr. 2023, 50, 1533–1545. [Google Scholar] [CrossRef]
- Yue, C.; Li, H.; Shi, X. Geographical Distribution Dynamics of Acorus calamus in China under Climate Change. Plants 2024, 13, 3352. [Google Scholar] [CrossRef]
- Xu, J.; Cao, B.; Bai, C. Prediction of Potential Suitable Distribution of Endangered Plant Kingdonia uniflora in China with MaxEnt. Chin. J. Ecol. 2015, 34, 3354–3359. [Google Scholar]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum Entropy Modeling of Species Geographic Distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef]
- Li, Y.; Shao, W.; Huang, S.; Zhang, Y.; Fang, H.; Jiang, J. Prediction of Suitable Habitats for Sapindus delavayi Based on the MaxEnt Model. Forests 2022, 13, 1611. [Google Scholar] [CrossRef]
- Wang, J.J. Research on Elephantopus scaber L. and Gerbera piloselloides Cass. in the Jingxi Zhuang Dragon Boat Festival Herb Market. Master’s Thesis, Minzu University of China, Beijing, China, 2013. [Google Scholar]
- Editorial Committee of Flora of China, Chinese Academy of Sciences. Flora of China; Science Press: Beijing, China, 1996; Volume 79, pp. 94–96. [Google Scholar]
- Jiangsu New Medical College. Encyclopedia of Traditional Chinese Medicine. Part I.; Shanghai Scientific and Technical Publishers: Shanghai, China, 1997; pp. 446–447. [Google Scholar]
- Tian, D.L.; Cai, G. Traditional Chinese Internal Medicine, 7th ed.; Shanghai Scientific and Technical Publishers: Shanghai, China, 2011; pp. 281–282. [Google Scholar]
- Ma, H.Z.; Wang, X.F. Progress of Clinical Application of Xingpi Yang’er Granules. Chin. J. Ethnomed. Ethnopharm. 2015, 24, 3. [Google Scholar]
- Long, C.; Wang, J.; Shi, Y.; Hong, L.; Kennelly, E. Ethnobotany of and Antioxidants from Gerbera piloselloides. Planta Med. 2014, 80, 839. [Google Scholar] [CrossRef]
- Xiao, Y.; Ding, Y.; Li, J.B.; Nohara, T. Two Novel Dicoumaro-p-menthanes from Gerbera piloselloides (L.) Cass. Chem. Pharm. Bull. 2004, 52, 1362–1364. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Yang, J.C.F.; Cheng, X.H.; Wang, R.; Qu, H.C.; Jiang, H.C.; Bai, Y.H.; Cao, W.W. 8-Methoxysmyrindiol from Gerbera piloselloides (L.) Cass. and Its Vasodilation Effects on Isolated Rat Mesenteric Arteries. Fitoterapia 2019, 138, 104299. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Li, Q.J.; Yang, L.; Yang, J.; Sun, C. Optimization of Extraction Process of Total Coumarins from Piloselloides Hirsuta by Response Surface Methodology and its Antioxidant Effect. Chem. Reagents 2023, 45, 114–120. [Google Scholar]
- Yang, G.X.; Wu, Y.Y.; Ma, X.; Liu, C.H.; Zheng, L.; Li, Y.J.; Wang, Y.L. Improvement of the Quality Standard of Gerbera piloselloides. China Pharm. 2022, 33, 408–412+424. [Google Scholar]
- Wang, Q.; Su, R.; Wang, H.H. HPLC Fingerprint and Chemical Pattern Recognition of Xingpi Yang’er Granules. Chin. J. Pharm. Anal. 2021, 41, 1083–1090. [Google Scholar]
- Elith, J.; Leathwick, J.R. Species Distribution Models: Ecological Explanation and Prediction Across Space and Time. Annu. Rev. Ecol. Evol. Syst. 2009, 40, 677–697. [Google Scholar] [CrossRef]
- Zhao, G.; Cui, X.; Sun, J.; Li, T.; Wang, Q.; Ye, X.; Fan, B. Analysis of the Distribution Pattern of Chinese Ziziphus jujuba under Climate Change Based on Optimized biomod2 and MaxEnt Models. Ecol. Indic. 2021, 132, 108256. [Google Scholar] [CrossRef]
- Liang, Y.Y.; Chen, K.; Cui, K.; Fang, T.; Yang, K.; Li, J.; Zhao, X.X.; Yin, F.; Guo, W.; Lu, W.X. Potential Geographical Distribution of Purple Eel Goby Taenioides cirratus in China under Climate Change Scenarios. J. Dalian Ocean Univ. 2022, 37, 739–746. [Google Scholar]
- Warren, D.L.; Matzke, N.J.; Cardillo, M.; Baumgartner, J.B.; Beaumont, L.J.; Turelli, M.; Glor, R.E.; Huron, N.A.; Simões, M.; Iglesias, T.L.; et al. ENMTools 1.0: An R Package for Comparative Ecological Biogeography. Ecography 2021, 44, 504–511. [Google Scholar] [CrossRef]
- Bai, J.; Wang, H.; Hu, Y. Prediction of Potential Suitable Distribution of Liriodendron chinense (Hemsl.) Sarg. in China Based on Future Climate Change Using the Optimized MaxEnt Model. Forests 2024, 15, 988. [Google Scholar] [CrossRef]
- Syfert, M.M.; Smith, M.J.; Coomest, D.A.; Roberts, D.L. The Effects of Sampling Bias and Model Complexity on the Predictive Performance of MaxEnt Species Distribution Models. PLoS ONE 2013, 8, e55158. [Google Scholar] [CrossRef]
- Su, Q.; Du, Z.; Luo, Y.; Zhou, B.; Xiao, Y.; Zou, Z. MaxEnt Modeling for Predicting the Potential Geographical Distribution of Hydrocera triflora since the Last Interglacial and under Future Climate Scenarios. Biology 2024, 13, 745. [Google Scholar] [CrossRef]
- Wang, Y.; Xie, L.; Zhou, G.Z.F. Prediction of the Potentially Suitable Areas of Leonurus japonicus in China Based on Future Climate Change Using the Optimized MaxEnt Model. Ecol. Evol. 2023, 13, e10597. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.B.; Liu, Y.L.; Qin, H.; Meng, Q.X. Prediction on Spatial Migration of Suitable Distribution of Elaeagnus mollis under Climate Change Conditions in Shanxi Province, China. Chin. J. Appl. Ecol. 2019, 30, 496–502. [Google Scholar]
- Kong, F.; Tang, L.; He, H.; Yang, F.; Tao, J.; Wang, W. Assessing the Impact of Climate Change on the Distribution of Osmanthus fragrans Using Maxent. Environ. Sci. Pollut. Res. 2021, 28, 34655–34663. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Li, Y.; Zhao, J.; Weng, H.; Ye, X.; Liu, S.; Zhao, Z.; Ahmad, S.; Zhan, C. The Potential Habitat Response of Cyclobalanopsis gilva to Climate Change. Plants 2024, 13, 2336. [Google Scholar] [CrossRef]
- Yu, M.; Luobu, Z.; Zhuoga, D.; Wei, X.; Tang, Y. Advances in Plant Response to Low-Temperature Stress. Plant Growth Regul. 2025, 105, 167–185. [Google Scholar] [CrossRef]
- Chen, L.; Song, Y.L.; Xu, S.F. The Boundary of Palaearctic and Oriental Realms in Western China. Prog. Nat. Sci. 2008, 18, 833–841. [Google Scholar] [CrossRef]
- Qian, C.; Zhang, X. Changes in Temperature Seasonality in China: Human Influences and Internal Variability. J. Clim. 2019, 32, 6237–6249. [Google Scholar] [CrossRef]
- Tang, M. Spatio-Temporal Changes and the Risk Zoning of Frost in Huaihe River Basin from 1960 to 2015. Master’s Thesis, Northwest Normal University, Lanzhou, China, 2017. [Google Scholar]
- Long, G.Y. Cloning and Expression Analysis of Cold-Regulated Genes in Citrus Plants. Ph.D. Thesis, Hunan Agricultural University, Changsha, China, 2007. [Google Scholar]
- Ao, Y.J.; Hu, L.H.; Li, Y.; Rao, W.X.; Deng, J.R.; Zhang, B. Hanzhong Citrus Summer Management Technology Points. Fruit Grow Friend 2022, 8, 30–32. [Google Scholar]
- Deng, J.R.; Ao, Y.J.; Y, X.L.; Ding, D.K.; Zheng, Q.F.; Jiang, J.L. Analysis of the Causes of Citrus Leaf Yellowing in Hanzhong and Countermeasure Suggestions. Fruit Grow Friend 2022, 7, 61–63. [Google Scholar]
- Zhou, T.J.; Chen, Z.M.; Chen, X.L.; Zuo, M.; Jiang, J.; Hu, S. Under Scenarios and on Near-Term Information Interpreting IPCC AR6: Future Global Climate Based on Projection. Adv. Clim. Change Res. 2021, 17, 652–663. [Google Scholar]
- Wang, G.; Zhang, Q.; Yu, H.Q.; Shen, Z.X.; Sun, P. Double Increase in Precipitation Extremes Across China in a 1.5 °C/2.0 °C Warmer Climate. Sci. Total Environ. 2020, 746, 140807. [Google Scholar] [CrossRef] [PubMed]
- Cai, D.P.; Tao, L.F.; Yang, X.Q.; Sang, X.Z.; Fang, J.B.; Sun, X.G.; Wang, W.P.; Yan, H.M. A Climate Perspective of the Quasi-Stationary Front in Southwestern China: Structure, Variation and Impact. Clim. Dyn. 2022, 59, 547–560. [Google Scholar] [CrossRef]
- Su, B.; Zhang, J.Y.; Yang, J.S.; Yang, D.Q. Key Technology of Gerbera jamesonii Bolus Facility Cultivation in Anhui Province. J. Anhui Agric. Sci. 2010, 38, 20574–20575+20673. [Google Scholar]
- Shao, X.B.; Yuan, Z.C.; Zhu, P.B.; Zhao, T.L. Cut Flower Gerbera Sunlight Greenhouse Cultivation Technology. J. Jiangsu Agric. Sci. 2007, 6, 160–162. [Google Scholar]
- Tang, L.H.; Yu, J.; Shi, L.J. Key Points of Gerbera Cultivation Techniques in Greenhouses during Winter in Guizhou Region. Agric. Technol. Serv. 2024, 41, 74–77. [Google Scholar]
- Wu, J.; Li, X.W.; Huang, L.F.; Meng, X.X.; Hu, H.Y.; Luo, L.; Chen, S.L. A New GIS Model for Ecologically Suitable Distributions of Medicinal Plants. Chin. Med. 2019, 14, 4. [Google Scholar] [CrossRef]
- Mota-Vargas, C.; Rojas-Soto, O.R. The Importance of Defining the Geographic Distribution of Species for Conservation: The Case of the Bearded Wood-Partridge. J. Nat. Conserv. 2012, 20, 10–17. [Google Scholar] [CrossRef]
- Belinda, G.; Alexandra, Z.; Aldridge, D.C.; Eric, S. The Importance of the Human Footprint in Shaping the Global Distribution of Terrestrial, Freshwater and Marine Invaders. PLoS ONE 2015, 10, e0125801. [Google Scholar]
- Venter, O.; Sanderson, E.W.; Magrach, A.; Allan, J.R.; Beher, J.; Jones, K.R.; Possingham, H.P.; Laurance, W.F.; Wood, P.; Fekete, B.M. Global terrestrial Human Footprint maps for 1993 and 2009. Sci. Data. 2016, 3, 160067. [Google Scholar] [CrossRef] [PubMed]



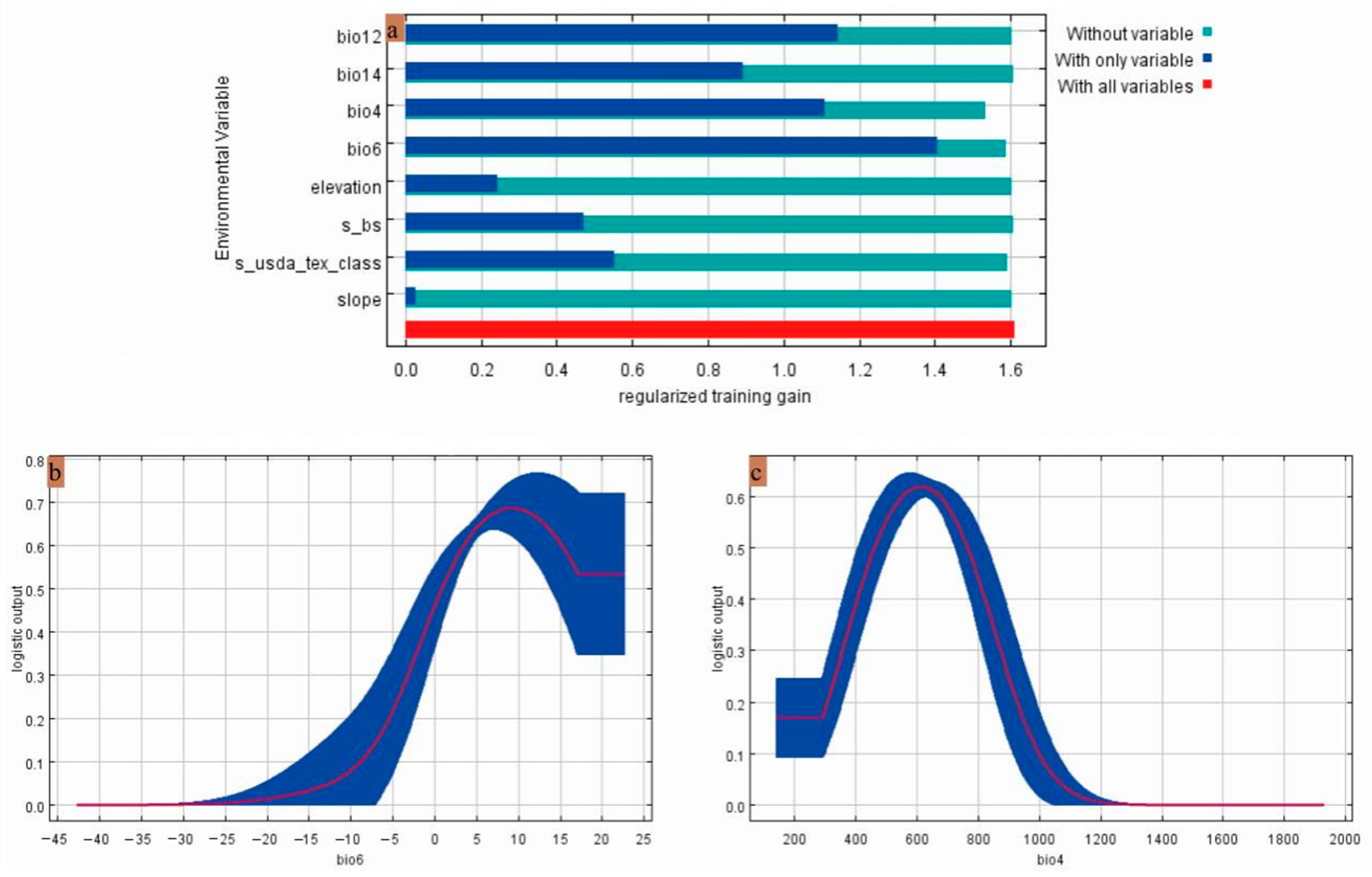

| Factor Name | Description | Percent Contribution |
|---|---|---|
| bio12 | Annual precipitation | 37.8 |
| bio6 | Minimum temperature of the coldest month | 19.8 |
| bio14 | Precipitation of the driest month | 14.1 |
| bio4 | Temperature seasonality | 4.9 |
| Elevation | Elevation | 3.2 |
| s_usda_tex_class | Subsoil USDA texture classification | 1.3 |
| Slope | Slope | 1.2 |
| s_bs | Subsoil base saturation | 1 |
| Factor Name | Description | Percent Contribution | Permutation Importance | Highly Suitable Habitat | Most Suitable Habitat | Unit |
|---|---|---|---|---|---|---|
| bio6 | Minimum temperature of the coldest month | 59.6 | 50.8 | 0.88–22.58 | 9.24 | °C |
| bio4 | Temperature seasonality | 19.7 | 39.7 | 461.54–763.96 | 613.66 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xue, J.; Liu, L.; Li, Y.; Zhang, Y.; Liang, S.; Chai, H. Assessing Suitable Habitats for Gerbera piloselloides (L.)Cass. in China Using an Optimized MaxEnt Model and Key Environmental Drivers. Biology 2025, 14, 769. https://doi.org/10.3390/biology14070769
Xue J, Liu L, Li Y, Zhang Y, Liang S, Chai H. Assessing Suitable Habitats for Gerbera piloselloides (L.)Cass. in China Using an Optimized MaxEnt Model and Key Environmental Drivers. Biology. 2025; 14(7):769. https://doi.org/10.3390/biology14070769
Chicago/Turabian StyleXue, Juan, Longjiang Liu, Yan Li, Yan Zhang, Shanshan Liang, and Huifang Chai. 2025. "Assessing Suitable Habitats for Gerbera piloselloides (L.)Cass. in China Using an Optimized MaxEnt Model and Key Environmental Drivers" Biology 14, no. 7: 769. https://doi.org/10.3390/biology14070769
APA StyleXue, J., Liu, L., Li, Y., Zhang, Y., Liang, S., & Chai, H. (2025). Assessing Suitable Habitats for Gerbera piloselloides (L.)Cass. in China Using an Optimized MaxEnt Model and Key Environmental Drivers. Biology, 14(7), 769. https://doi.org/10.3390/biology14070769





